Abstract
Background
1H-magnetic resonance spectroscopy (MRS) facilitates noninvasive diagnosis of pediatric brain tumors by providing metabolite profiles. Prospective studies of diagnostic accuracy and comparisons with conventional MRI are lacking. We aimed to evaluate diagnostic accuracy of MRS for childhood brain tumors and determine added clinical value compared with conventional MRI.
Methods
Children presenting to a tertiary pediatric center with brain lesions from December 2015 through 2017 were included. MRI and single-voxel MRS were acquired on 52 tumors and sequentially interpreted by 3 radiologists, blinded to histopathology. Proportions of correct diagnoses and interrater agreement at each stage were compared. Cases were reviewed to determine added value of qualitative radiological review of MRS through increased certainty of correct diagnosis, reduced number of differentials, or diagnosis following spectroscopist evaluation. Final diagnosis was agreed by the tumor board at study end.
Results
Radiologists’ principal MRI diagnosis was correct in 69%, increasing to 77% with MRS. MRI + MRS resulted in significantly more additional correct diagnoses than MRI alone (P = .035). There was a significant increase in interrater agreement when correct with MRS (P = .046). Added value following radiologist interpretation of MRS occurred in 73% of cases, increasing to 83% with additional spectroscopist review. First histopathological diagnosis was available a median of 9.5 days following imaging, with 25% of all patients managed without conclusive histopathology.
Conclusions
MRS can improve the accuracy of noninvasive diagnosis of pediatric brain tumors and add value in the diagnostic pathway. Incorporation into practice has the potential to facilitate early diagnosis, guide treatment planning, and improve patient care.
Keywords: diagnostic accuracy, 1H-magnetic resonance spectroscopy (MRS), MRI, metabolite profiles, pediatric brain tumors
Brain tumors are the most prevalent type of solid cancer in childhood, a significant cause of disability, and the predominant cause of pediatric cancer death.1 Treatment options and outcomes are dependent on tumor type, location, and age. Histopathology following biopsy or resection is the current diagnostic gold standard.2 This has associated risks of morbidity and sampling error,3 and definitive histopathological diagnosis is unavailable for several days. Accurate, early, noninvasive diagnosis could improve patient care by guiding surgical decision making, allowing timely treatment planning, and informing family discussions.
MRI is the standard imaging investigation, although there is a paucity of evidence for diagnostic accuracy.4,5 The pathology of childhood brain tumors is diverse,6 with different histological tumor types displaying overlapping imaging characteristics.7,8 Conventional MRI cannot always allow accurate identification of tumor type or grade4,5,8–11 or conclusive differentiation of neoplastic from indolent lesions.7,8,12
1H-magnetic resonance spectroscopy (MRS) is an advanced imaging technique with the potential to improve diagnosis and characterization of brain tumors through noninvasive measurement of metabolite profiles.5,13,14 Although different tumor types can appear morphologically similar on MRI, they display different key metabolic features.8,15,16 MRS can be performed following MRI in 5 minutes, allowing incorporation of sequences into standard radiological evaluation. MRS is not currently used routinely in all pediatric centers, partly because there is little formal evaluation of its diagnostic utility and few radiologists are trained in quantitative evaluation.
Despite evidence for technical feasibility and diagnostic accuracy,9,13,17,18 few studies have evaluated how MRS can add value to conventional pediatric radiological reporting11,19–21 or assessed its impact on patient management in clinical practice.12,22 Most evidence involves pattern recognition using computer-based classifiers, with retrospective single- and multicenter studies reporting accurate discrimination of medulloblastoma, pilocytic astrocytoma, and ependymoma.9,17,18,23,24 As pattern recognition is clinically unavailable and technically challenging, it is important to explore simpler interpretation modalities. Visual interpretation of MRS significantly improved radiologists’ diagnostic accuracy retrospectively11,25 and provided valuable information to facilitate clinical decision making, identify indolent and high-grade lesions, guide biopsy, and differentiate relapse from treatment-related changes.25 Prospective studies are lacking, with little systematic comparison with conventional MRI.
Studies are needed to prospectively evaluate the diagnostic impact of MRS over MRI alone in pediatric brain tumors. Further work is necessary to integrate the technique into the diagnostic pathway. Exploring methods of MRS interpretation that may be adopted by radiologists without complicated quantitative interpretation or sophisticated computer software could facilitate incorporation into practice. Standards for Reporting Diagnostic Accuracy Studies guidance26 should be adhered to in conducting research into diagnostic accuracy.
We aimed to evaluate added diagnostic value of MRS in combination with conventional radiological reporting in routine clinical pediatric practice. A further objective was to determine whether MRS is equally helpful for all brain tumor types and identify clinical scenarios with the potential to improve patient care.
Materials and Methods
Participants
Children younger than 16 years presenting to a tertiary children’s hospital between December 2015 and 2017 with radiological features of a brain tumor were eligible for inclusion. Participants formed a consecutive series with data collected prospectively and recruitment prior to 1H-MRS and pediatric neurooncology tumor board (TB) consensus diagnosis. All 52 cases with MRI and MRS available prior to histopathological diagnosis were included. Ethical approval was granted for functional imaging research and written informed parental consent obtained. Cases without diagnosis confidently established by the TB were excluded.
MRI and MRS
The index test was single-voxel 1H-MRS, acquired as standard diagnostic imaging as part of the tumor imaging protocol at our institution prior to treatment or surgical intervention. MRS was performed at 1.5T (GE Signa Excite, Siemens Avanto) or 3T (Phillips Interna Achieva) following conventional MRI, including axial T1- and T2-weighted, diffusion echo planar imaging and fluid-attenuated inversion recovery, and postcontrast T1-weighted sequences. At 1.5T, a single-voxel MRS protocol was used with point-resolved spectroscopy localization, a short echo time of 30 ms, and a repetition time of 1500 ms. Cubic voxels had 1.5 cm or 2 cm side length, acquiring 256 or 128 repetitions, respectively. At 3T, a short echo time of 35 ms and a repetition time of 2000 ms were used, with cubic voxels of side length 1.3 cm, 1.5 cm, or 2 cm and 196, 128, and 196 repetitions, respectively. MRI confirmed accurate voxel placement within solid tumor, avoiding cysts, necrosis and nontumoral tissue and located more than 3 mm from bone, scalp, and air. In case of large heterogeneous tumors, images on conventional MR sequences were used to guide voxel placement to the area of highest contrast enhancement or greatest restriction of diffusion. Voxel placement was determined by experienced radiographers trained in MRS acquisition, with adequate position checked by radiologists in difficult cases.
Spectroscopy processing was performed using standard scanner software exported to the hospital picture archiving and communication system (PACS) (Agfa IMPAX 6.5.2.2016). The majority of raw data were also processed using Totally Automatic Robust Quantitation in Nuclear Magnetic Resonance (TARQUIN), v3.2.2.27 Processing provided a graphical magnetic resonance spectrum and tissue metabolite profile automatically available through PACS. Spectra were inspected visually for baseline abnormalities, signal-to-noise ratio, acceptable line width, and major artifacts.
Reference Standard: TB Consensus Diagnosis
The reference standard was TB consensus diagnosis, incorporating clinical information, MRI, MRS, histopathology, second opinion, genetics, and follow-up information about treatment response and tumor behavior. The TB consisted of pediatric oncologists, pediatric radiologists in part specializing in neuroradiology, neurosurgeons, clinical oncologists, and histopathologists. Consensus diagnosis was confirmed once clinical course had been established at study end.
Subanalysis was performed using histopathology as an alternative reference standard, excluding cases without conclusive histopathology. Histopathology is an accepted but imperfect diagnostic gold standard: Sampling error may occur, particularly in heterogeneous tumors, with rare or atypical lesions misdiagnosed. Some remain unbiopsied or exhibit characteristic clinical or radiological features precluding the need for tissue diagnosis.
Conventional MRI and MRS Interpretation: Radiologists
Three consultant pediatric radiologists from the neurooncology TB reported imaging. Radiologist 1 (LM) had more than 15 years’ experience in pediatric neuroradiology and local and national brain tumor research, and Radiologists 2 (AO) and 3 (BP) had been members of the TB for 6 and 3 years, respectively. Reporting was undertaken blind to final histopathological and reference diagnoses, independently from the other 2 radiologists.
Radiologists attended face-to-face training in visual MRS interpretation and quality assurance, and were given a booklet containing mean spectra for common pediatric brain tumors (Supplement 1). Instructions were to review conventional imaging and formulate differential diagnoses, then match the index MRS to mean spectra of these tumors. Determining best match would confirm or refute potential diagnoses.
Information was provided about age, gender, and clinical presentation. Radiologists sequentially viewed conventional imaging followed by MRS processed using scanner software or TARQUIN. Each gave up to 4 differential diagnoses based on MRI alone, rating certainty on a scale of 1 to 10. This was repeated after visually interpreting MRS. Principal MRI/MRI + MRS diagnosis was that with highest certainty.
Independent MRS Interpretation: Spectroscopist
An expert spectroscopist and pediatric oncologist (AP, 15 years’ experience), blinded to radiological, histopathological, and reference diagnoses, independently sequentially interpreted MRS with no information other than voxel location images, followed by adding the radiologists’ differential diagnoses. Up to 4 diagnoses with certainty ratings were given at each stage.
All interpreters recorded whether MRS was suitable for analysis.
Histopathology
Histopathology was interpreted by a histopathologist, IN (10 years’ experience), as clinical practice, documented on an official hospital reporting system (Sunquest ICE Desktop Live, v.541). Intraoperative, first authorized reports, and second opinion or central review were recorded. Time from imaging and intervention to histopathological diagnosis was evaluated. Unbiopsied cases and those with inconclusive histopathology were reviewed.
Radiologist Interviews
Semistructured interviews (10-15 minutes’ duration) were performed at study end to determine the radiologists’ perceptions of the added value of MRS in the diagnostic pathway. Interviews were recorded, transcribed, and coded and thematic analysis performed.
Statistical Analysis
Comparison of post-MRI and post-MRS diagnosis with TB consensus determined how many were correctly diagnosed in accordance with the reference standard at each stage. The accuracy of MRI ± MRS for each category of brain tumor (all locations, and supratentorial, and infratentorial) was made through estimates of sensitivity. Results of individual radiologists were analyzed, with interobserver variability calculated (chi-squared test). McNemar test determined whether MRS significantly improved individual radiologists’ proportion of correct diagnoses. Significance of direction of change toward improvement with MRS (McNemar ordered category test of directional change) and increase in radiologists’ agreement on correct diagnoses (Bhapkar chi-squared test) were assessed.28
Cases were reviewed individually to determine the proportion for which MRS added value through radiologists changing an incorrect to a correct diagnosis, increasing the certainty of a correct diagnosis, or reducing the number of differentials considered. Added value of expert spectroscopist interpretation was ascertained through reviewing cases with correct diagnoses confirmed and those correctly rediagnosed. Incorrectly diagnosed cases were evaluated and reasons documented.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in this study.
Study Protocol
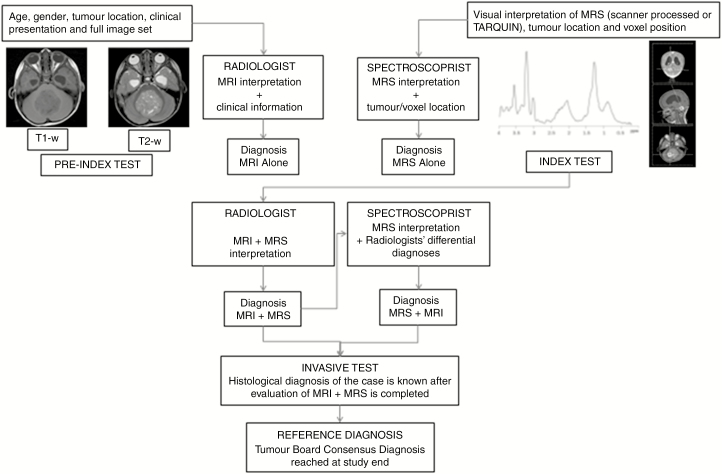
The study protocol and workflow is illustrated in Fig. 1.
Fig. 1.
Study Protocol and Workflow for Readers of 1H-Magnetic Resonance Spectroscopy. 1H-magnetic resonance spectroscopy (MRS) was interpreted independently by 1) radiologists and 2) an expert spectroscopist. All readers were blinded to the reference standard of the tumor board consensus diagnosis and final histopathology. Radiologists determined diagnosis in 2 stages: MRI interpretation in combination with clinical information, and MRS interpretation in combination with MRI results and clinical information (index test). The spectroscopist performed a similar process: MRS interpretation blinded to clinical and radiological information (but knowing tumor location and voxel position), and MRS interpretation with the differential diagnosis made by radiologists.
Results
Demographics
Diagnostic MRS was performed on 52 patients. One was excluded for lack of consensus diagnosis. Ages ranged from antenatal 36 weeks’ gestation to 15 years (median 6.9 years); 27 were male. Tumors were located in the posterior fossa (PF) (27), supratentorially (22), and brainstem (2). Breakdown of cases by reference diagnosis is in Supplement 2 with detailed clinical information in Supplement 3. Participant flow is depicted in Supplement 4.
Of our patient cohort, 35 (69%) individuals had undergone previous imaging that had detected a CNS lesion prior to routine reimaging including MRS using the standard diagnostic tumor protocol described. Of these 35, 24 (47%) were performed externally, of which 7 were CTs, 16 MRIs, and 1 an antenatal ultrasound. Of the 11 (22%) previously imaged at Birmingham Children’s Hospital, 6 were CTs and 5 MRIs, 2 of which were performed as investigations for epilepsy, 1 requested by endocrinology, and 2 emergency scans performed after hours.
Diagnostic Accuracy of MRI and MRI + MRS
Table 1 shows the radiologists’ combined and individual sequential diagnostic accuracy of principal diagnosis, followed by the accuracy of the spectroscopist’s interpretation.
Table 1.
Diagnostic Accuracy of Radiologists Following MRI Alone and MRS + MRS, and Spectroscopist Interpretation of MRS Alone and MRS + Differentials
| Number of Patients | Correct MRI Alone | Correct MRI + MRS | Correct MRS Alone | Correct MRS + Differentials | |
|---|---|---|---|---|---|
| Radiologists | Spectroscopist | ||||
| By Location (All Tumors) | |||||
| PF/BS | 29 | 80% | 88% | 78% | 78% |
| ST | 22 (18 analyzablea) | 62% | 68% | 59% (72% analyzablea) | 68% (83% analyzablea) |
| By Radiologist (All Tumors) | |||||
| Rad 1 | 51 | 73% | 78% | – | – |
| Rad 2 | 51 | 65% | 71% | – | – |
| Rad 3 | 51 | 71% | 83% | – | – |
| All Tumours | 51 (47 analyzable a) | 69% | 77% | 69% (75% analyzable a) | 75% (81% analyzable a) |
| Histopathology Only | |||||
| Tumours with Histopathological Diagnosis Only | 38 | 74% | 81% | 68% (72% analyzable a) | 73% (78% analyzable a) |
Abbreviations: MRS, 1H-magnetic resonance spectroscopy; PF, posterior fossa; ST, supratentorial; BS, brainstem.
aAnalyzable MRS: passed or borderline QC, or interpretable despite failing QC (n = 47).
Diagnostic Accuracy
Radiologists
There was no significant interobserver difference between radiologists’ diagnostic accuracy using conventional MRI (P = .67, chi-squared test). Principal MRI diagnosis was correct in 69% of cases, increasing to 77% with MRS.
MRI + MRS resulted in significantly more additional correct diagnoses than MRI alone (P = .03, McNemar ordered category test of directional change). Median changes in accuracy and 95% confidence intervals for Radiologist 1 (most experienced), 2, and 3 respectively following the addition of MRS were +5.8% (–0.03 to 0.14; P = .25), +5.8% (–0.06 to 0.18; P = .45), and +11.8% (0.01 to 0.26; P = .03) (McNemar test). The direction of change was toward improvement for all radiologists, reaching significance for Radiologist 3. There was significant increase in radiologists’ agreement on correct diagnosis with the addition of MRS (P = .046, Bhapkar chi-squared test28). There was no increase in radiologists’ agreement when the diagnosis was incorrect.
Spectroscopist
Four cases were excluded from independent spectroscopy analysis as MRS failed quality control (QC). Spectroscopist interpretation was correct in 69% all cases (75% of 47 suitable for analysis) increasing to 75% (81% with analyzable MRS) following provision of radiologists’ differentials.
Added Clinical Value of MRS
The radiologists’ review of MRS added value in 73% cases (37/51) by replacing an incorrect with a correct diagnosis, increasing certainty when correct, or reducing the number of differentials considered. The spectroscopist’s review accurately diagnosed a further 10%, with a total of 83% (42/51) incurring added value through a combination of radiologist and spectroscopist interpretation.
The 27% (14/51) for which MRS did not benefit radiologists had either failed QC, or were rare or atypical tumors without comparator mean MRS or conclusive local histopathology. Spectroscopist review correctly diagnosed 5 of these difficult cases.
Spectroscopist review confirmed correct diagnosis in 70% (36/51) of all cases, or 77% of 47 suitable for analysis. Diagnosis was not attempted in 4 (8%) failing QC. Of 11 (22%) for which the spectroscopist was incorrect, 5 were diagnosed after central review (inconclusive local histopathology), 2 had inconclusive histopathology postcentral review, and 1 was unbiopsied. Reasons for misdiagnosis of the remaining 3 were unclear, although each MRS was atypical. Radiologists diagnosed these 3 tumors correctly on MRI, suggesting clinical management would not have been adversely altered.
Review of tumors incorrectly diagnosed on MRI subsequently rediagnosed correctly following MRS suggests where added value may be incurred in the clinical pathway. In the PF, visual MRS interpretation improved radiologists’ diagnostic accuracy of medulloblastoma (89% to 96%), pilocytic astrocytoma (72% to 82%), atypical teratoid rhabdoid tumor (ATRT) (50% to 100%), and diffuse astrocytoma (67% to 100%), with further improvement in ependymoma (89% to 100%) following spectroscopist review. Supratentorially, radiologists’ diagnosis of ependymoma (0% to 33%) and tectal plate glioma (89% to 100%) improved after MRS, with spectroscopist review improving ATRT (0% to 100%) and diffuse astrocytoma (0% to 100%). The spectroscopist’s review increased the diagnostic accuracy of lesions that were not primary CNS tumors and nontumors (67% to 100%) in all locations.
Tumor types with improvement in accuracy of the principal diagnosis following radiologist or spectroscopist review of MRS may be seen in Table 2.
Table 2.
Tumor Types With Improvement in Accuracy of Principal Diagnosis Following 1H-Magnetic Resonance Spectroscopy
| Tumor Type | Correct MRI alone (R) | Correct MRI + MRS (R) | Correct MRS + DD (S) |
|---|---|---|---|
| Posterior fossa | |||
| Medulloblastoma | 89% | 96% | 67% |
| Ependymoma | 89% | 89% | 100% |
| Pilocytic astrocytoma | 72% | 82% | 82% |
| ATRT | 50% | 100% | 100% |
| Diffuse astrocytoma | 67% | 100% | 0% |
| Nontumor | 67% | 67% | 100% |
| Ewing sarcoma of occiputa | 33% | 33% | 100% |
| Supratentorial | |||
| ATRT | 0% | 0% | 100% |
| Diffuse astrocytoma | 0% | 0% | 100% |
| Tectal plate glioma | 89% | 100% | 100% |
| Ependymoma | 0% | 33% | 0% |
| Rhabdomyosarcoma skull basea | 67% | 67% | 100% |
| Nontumor | 67% | 67% | 100% |
Abbreviations: ATRT, atypical teratoid rhabdoid tumor; DD, differential diagnosis; R, radiologists; S, spectroscopist.
aCorrect diagnosis: not primary CNS lesion.
QC
Of 51 MRS, 36 (71%) met QC standards. Nine (17.5%) were borderline (normal brain within voxel, poor voxel placement, poor shimming, phasing or line width, unacceptable baseline, low signal-to-noise). Six (11.5%) failed QC (voxel placement over bone, poor phasing), of which 2 provided sufficient information for analysis. Interpretation was not attempted in the remaining 4.
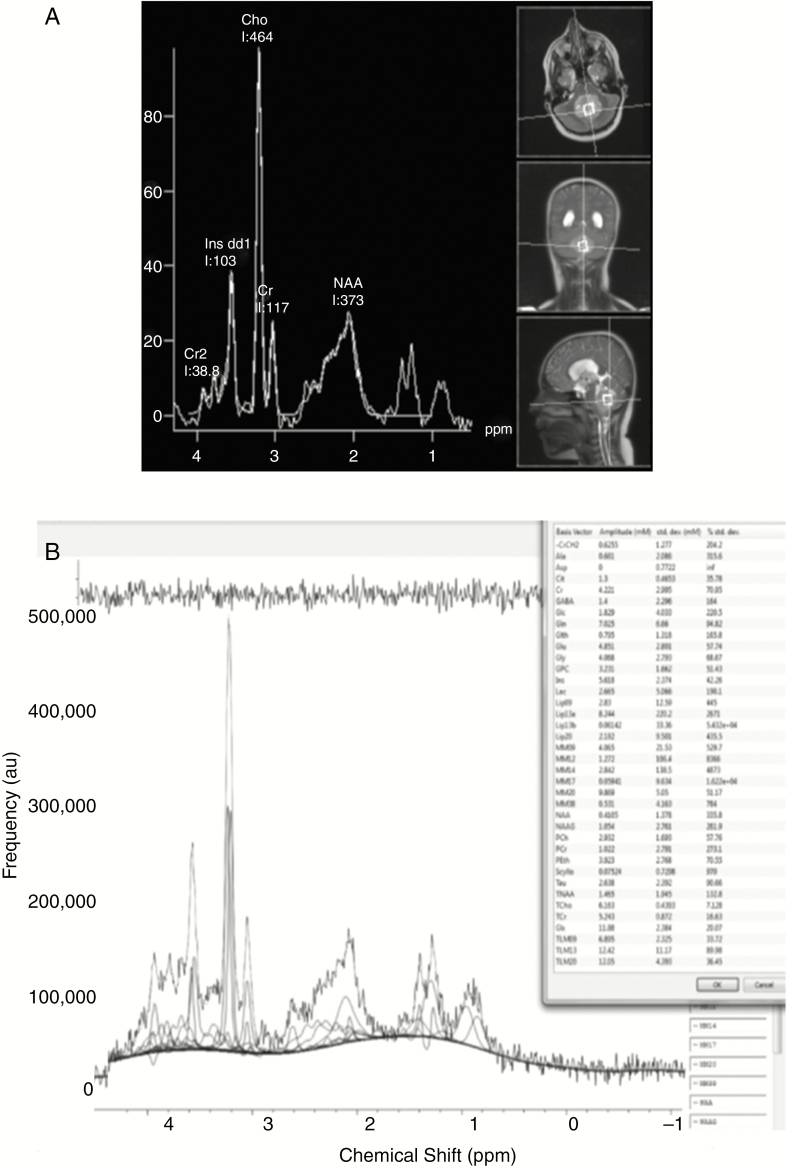
Of 45 spectra obtained at 1.5T, 32 (71%) passed QC, compared with 4 of 6 (67%) 3T spectra. TARQUIN processing was available in 47, leading to 4 additional MRS being of acceptable quality. One of 4 MRS processed by scanner software only failed QC. A comparison between MRS processed using scanner software and using TARQUIN may be seen in Fig. 2.
Fig. 2.
1H-Magnetic Resonance Spectroscopy Processed Using A, Scanner Software, and B, TARQUIN. 1H-magnetic resonance spectroscopy from a patient with medulloblastoma processed using A, scanner software, and B, Totally Automatic Robust Quantitation in Nuclear Magnetic Resonance (TARQUIN). Metabolite concentrations and their Crammer Rao lower bounds are listed in the box to the right of the TARQUIN output.
The spectroscopist correctly diagnosed 8 of 9 borderline quality spectra. Of 6 failing QC, 2 were correctly diagnosed, with a third identified as a hemorrhagic tumor. Uninterpretable MRS were germinoma, nongerminomatous germ cell tumors (NGGCTs), and a pituitary adenoma. All radiologists correctly diagnosed the germinoma and NGGCTs on conventional imaging, and 2 identified the pituitary adenoma.
Adverse Events Resulting from MRS
One case, a supratentorial central neurocytoma, was correctly diagnosed by 1 radiologist on MRI but subsequently misdiagnosed following MRS. No other correctly diagnosed tumors were misdiagnosed.
Histopathology
The availability of histopathology is shown in Supplement 5.
Of 51 patients, 80% (41/51) underwent surgical intervention. Histopathological diagnosis was achieved in 75% (38/51); the remaining 25% lacked tissue diagnosis. Intraoperative histopathology accorded with TB consensus diagnosis in 42% biopsied (33% all) cases. First histopathology was correct in 78% of those biopsied (63% all), incorrect in 7%, and inconclusive in 15%. Central review was requested in 27%, resulting in change of diagnosis in 20% with 7% remaining inconclusive.
First histopathology was available a median of 9.5 days (range, 1-611 days) following MRI, or 6 days (0-30) postsurgery. A second histological opinion yielded results a median of 25 days (range, 14-625 days) following imaging, with treatment commencing after 31 days (range, 11-78 days).
Histopathological Diagnosis Subgroup Analysis
Demographics
Subgroup analysis was performed in 38 patients with histopathological diagnosis using this alternative reference standard (Supplement 3). Radiologists correctly diagnosed 74% using MRI alone, increasing to 81% with MRS (Table 1). The 7% increase in accuracy was comparable to 8% observed overall. Spectroscopist MRS interpretation was accurate in 74%, or 78% excluding 2 uninterpretable spectra.
Case Example
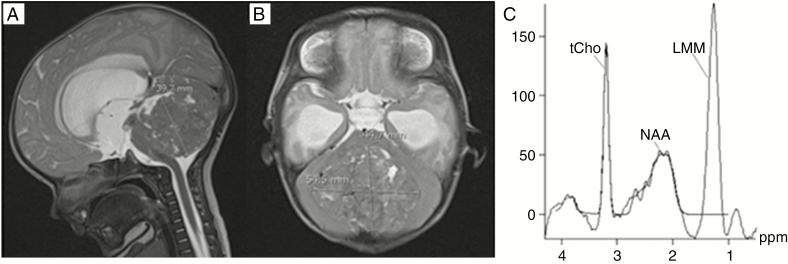
A case in which diagnostic accuracy and management was improved following MRS is presented in Fig. 3.
Fig. 3.
Case Example of Improvement in Diagnostic Accuracy and Management Following 1H-Magnetic Resonance Spectroscopy. A, T2-weighted sagittal, and B, axial MR, images of an 8-week-old patient with a metastatic posterior fossa lesion. Initial differential radiological diagnoses included ependymoma, medulloblastoma, and atypical teratoid rhabdoid tumor (ATRT). Spectroscopy favored ATRT with low N-acetylaspartate (NAA), high choline, and lipid, subsequently confirmed following open biopsy. Confident radiological diagnosis could have spared an invasive diagnostic procedure where palliation was appropriate. Using conventional MRI, radiologists 1 and 2 misdiagnosed ependymoma with 50% and 60% certainties, respectively. Radiologist 3 diagnosed ATRT with 60% certainty. Review of MRS resulted in diagnoses of ATRT alone with certainties of 80%, 90%, and 90%, confirmed on independent spectroscopy interpretation and subsequently by histopathology. LMM indicates lipids and macromolecules; tCho, total choline.
Interviews
Radiologists found MRS a supportive tool to refine diagnoses reached through conventional imaging, particularly in PF tumors with overlapping imaging characteristics. Total choline (tCho) facilitated classification of ambiguous lesions as malignant or indolent. Aggressive features in conservatively managed lesions were identified using tCho, lipids, and lactate, with usual MRS suggesting atypia. Radiologists found MRS increasingly helpful with experience, being confident to interpret spectra visually in 5-10 minutes. The main difficulty was QC due to poor voxel placement and artifact. MRS did not allow definitive diagnosis of rare supratentorial tumors lacking comparator mean spectra. The main comments from the spectroscopist were that TARQUIN processing led to additional MRS being of acceptable quality when scanner processing did not pass QC standards, and that formal discussion with radiologists (rather than just being provided with a differential diagnosis and MRS) would provide optimal diagnostic accuracy.
Discussion
Visual interpretation of MRS improved accuracy of pretherapeutic diagnosis for all participating radiologists, adding further value by confirming correct MRI diagnoses, narrowing broad differentials, and increasing interrater agreement. Expert spectroscopist review verified correct diagnoses, allowing characterization of more difficult cases. These are clinically important outcomes given that accurate early diagnosis of childhood brain tumors has potential to improve patient care and the challenge in formulating therapeutic decisions based on uncertainty.
Noninvasive diagnosis of PF tumors may be sufficiently robust to recommend treatment planning prior to histopathological confirmation. Radiologists correctly identified 88% PF tumors using MRS, similar to earlier reports from our institution,13,25 the majority misdiagnosed being rare lesions. Identification of medulloblastomas would allow early radiotherapy referral, with recognition of ependymomas guiding surgical planning for complete resection. Correct diagnosis of all indolent lesions after spectroscopist review suggests biopsy may be avoided if MRS indicates a typical nontumor.
All tumors irrespective of location, diagnosis, or quality of MRS were included to reflect clinical practice. Increased diagnostic accuracy was more marked in PF than supratentorial lesions. The former consist of a limited number of tumor types accurately discriminated using computer-based classifiers.9,17,18,23,24 The latter are histologically diverse, including rare subtypes without comparator mean spectra. Reflecting the proportion of rare tumors in the pediatric population accounts for lower diagnostic accuracy than studies of common PF lesions.9,17,18,23,24 Although quality issues limit implementation of MRS, our spectroscopist derived useful information from poorer-quality spectra. Spectroscopist visual interpretation of MRS passing QC was 81% accurate, comparable with an increase from 63% to 87% reported in the literature.11
The role of MRS in the diagnostic pathway is demonstrated through the sequence of imaging interpretation. Radiologists review conventional imaging followed by MRS as a supportive tool, seeking expert opinion if the diagnosis remains uncertain. Conventional imaging is sufficient to diagnose lesions with distinct radiological features (eg, craniopharyngioma). Radiological MRS review adds value in common tumors with overlapping imaging characteristics, such as PF medulloblastoma and ATRT. Expert spectroscopist review can further improve diagnosis of difficult cases, with atypical lesions including misdiagnosed ependymoma, pontine pilocytic astrocytoma, supratentorial ATRT, and nonprimary CNS tumors accurately identified at this stage.
Although MRS is intended to complement not replace tissue diagnosis, histopathology is an imperfect gold standard. Many pediatric tumors are in central vital structures, and providing representative tissue samples through biopsy is difficult. Awaiting histopathological confirmation can result in treatment delay. This is important to avoid, particularly with the emergence of proton therapy, for which time from decision to treatment is longer.
Advanced imaging would be particularly valuable in tumors not amenable to biopsy or with inconclusive histopathology. More than 25% of our cohort lacked a tissue diagnosis with management determined on radiological and clinical features alone. Although MRS may influence consensus diagnosis, with potential incorporation bias using this reference standard, subgroup analysis against histopathology revealed a similar net improvement in accuracy. TB diagnoses were verified through clinical course rather than simply accepted as correct following MRI and MRS.
Potential adverse effects of MRS should be considered. A central neurocytoma diagnosed correctly by MRI was subsequently misdiagnosed as diffuse astrocytoma. This rare tumor did not have comparator mean spectra, but was correctly identified following spectroscopist review. Spectroscopist analysis was incorrect in 3 tumors correctly diagnosed by radiologists (medulloblastoma, pilocytic astrocytoma, and craniopharyngioma). MRS were atypical of tumor type, highlighting the importance of interpretation in the context of all available information. Other lesions misdiagnosed were rare and difficult to diagnose histologically.
Making MRS widely clinically available is an important objective to improve patient care. Techniques described are readily applicable without additional infrastructure. Our MRS protocol involves single-voxel acquisition and visual interpretation, each adding around 5 minutes to examination and reporting times. It is generally accepted that optimal analysis of MRS data is provided by fitting to a linear combination of metabolite basis functions, and this was not available through the scanner software used in this study. One of the barriers to using platform-independent software has been the difficulty of handling MRS raw data. However, this is becoming easier and here we demonstrate the use of a PACS linked to a server running TARQUIN software, which made results available to users in real time. In this study, TARQUIN analysis was more robust than clinical data of variable quality, increasing the number of cases that provided useful information. Clinicians also appreciated clarification regarding metabolite assignment available through TARQUIN. Complex spectroscopy methods with longer acquisition times (eg, magnetic resonance spectroscopic imaging), sophisticated decision-support software, and automated classifiers may improve accuracy,9,13,17,18 but are not routinely available and are challenging to implement clinically.
As radiologists are not trained to quantitatively evaluate MRS, it is important to present easily interpretable information. Our radiologists incorporate visual MRS interpretation into routine practice, finding it a helpful tool to confirm or refute diagnoses made through conventional imaging. Expert spectroscopist support for difficult cases and centers with less experience could potentially be provided through central radiological review. Developing usable classifiers and decision-support systems for radiologists may improve MRS interpretation and is a key aim for the future.
It is important to acknowledge limitations of our study design. It is difficult in a single center to ensure complete blindness of radiologists to patients’ clinical course. Bias may have been introduced as not all cases were reported contemporaneously, although final histopathological and consensus diagnoses were not revealed until the study end. The results of this single-center study may not be generalizable to institutions with less spectroscopic or more pediatric neuroradiological expertise and prospective multicenter evaluation is required. Although our institution is one of the largest in the United Kingdom with an established neurooncology TB and imaging research program, participating radiologists were not all experienced in MRS interpretation.
In conclusion, this study demonstrates MRS can add diagnostic value in clinical practice, both through visual interpretation by radiologists and expert spectroscopy review. Further prospective multicenter studies are needed to validate these findings in a range of clinical settings and formulate recommendations to incorporate MRS into the diagnostic pathway to improve patient care.
Funding
This work was supported by National Institute for Health Research grant [code 13–0053].
Supplementary Material
Acknowledgments
We would like to thank Shaheen Lateef and Dr Heather Rose for their assistance in data collection.
Conflict of interest statement. None declared.
References
- 1. Childhood Cancer Research Group. National Registry of Childhood Tumours/Childhood Cancer Research Group. https://www.ccrg.ox.ac.uk. 2010. [Google Scholar]
- 2. Olsen ØE. Why measure tumours? Pediatr Radiol. 2015;45(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng WH, Lim T. Targeting regions with highest lipid content on MR spectroscopy may improve diagnostic yield in stereotactic biopsy. J Clin Neurosci. 2008;15(5):502–506. [DOI] [PubMed] [Google Scholar]
- 4. Orphanidou-Vlachou E, Auer D, Brundler MA, et al. (1)H magnetic resonance spectroscopy in the diagnosis of paediatric low grade brain tumours. Eur J Radiol. 2013;82(6):e295–e301. [DOI] [PubMed] [Google Scholar]
- 5. Panigrahy A, Krieger MD, Gonzalez-Gomez I, et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR Am J Neuroradiol. 2006;27(3):560–572. [PMC free article] [PubMed] [Google Scholar]
- 6. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panigrahy A, Blüml S. Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol. 2009;24(11):1343–1365. [DOI] [PubMed] [Google Scholar]
- 8. Panigrahy A, Nelson MD Jr, Blüml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol. 2010;40(1):3–30. [DOI] [PubMed] [Google Scholar]
- 9. Arle JE, Morriss C, Wang ZJ, Zimmerman RA, Phillips PG, Sutton LN.. Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J Neurosurg. 1997;86(5):755–761. [DOI] [PubMed] [Google Scholar]
- 10. Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 11. Shiroishi MS, Panigrahy A, Moore KR, et al. Combined MRI and MRS improves pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. Neuroradiology. 2015;57(9):951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Möller-Hartmann W, Herminghaus S, Krings T, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44(5):371–381. [DOI] [PubMed] [Google Scholar]
- 13. Davies NP, Wilson M, Harris LM, et al. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed. 2008;21(8):908–918. [DOI] [PubMed] [Google Scholar]
- 14. Davies NP, Wilson M, Natarajan K, et al. Non-invasive detection of glycine as a biomarker of malignancy in childhood brain tumours using in-vivo 1H MRS at 1.5 tesla confirmed by ex-vivo high-resolution magic-angle spinning NMR. NMR Biomed. 2010;23(1):80–87. [DOI] [PubMed] [Google Scholar]
- 15. Hollingworth W, Medina LS, Lenkinski RE, et al. A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. AJNR Am J Neuroradiol. 2006;27(7):1404–1411. [PMC free article] [PubMed] [Google Scholar]
- 16. Preul MC, Caramanos Z, Collins DL, et al. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nat Med. 1996;2(3):323–325. [DOI] [PubMed] [Google Scholar]
- 17. Wang Z, Sutton LN, Cnaan A, et al. Proton MR spectroscopy of pediatric cerebellar tumors. AJNR Am J Neuroradiol. 1995;16(9):1821–1833. [PMC free article] [PubMed] [Google Scholar]
- 18. Vicente J, Fuster-Garcia E, Tortajada S, et al. Accurate classification of childhood brain tumours by in vivo ¹H MRS—a multi-centre study. Eur J Cancer. 2013;49(3):658–667. [DOI] [PubMed] [Google Scholar]
- 19. Murphy M, Loosemore A, Clifton AG, et al. The contribution of proton magnetic resonance spectroscopy (1HMRS) to clinical brain tumour diagnosis. Br J Neurosurg. 2002;16(4):329–334. [DOI] [PubMed] [Google Scholar]
- 20. Julià-Sapé M, Coronel I, Majós C, et al. Prospective diagnostic performance evaluation of single-voxel 1H MRS for typing and grading of brain tumours. NMR Biomed. 2012;25(4):661–673. [DOI] [PubMed] [Google Scholar]
- 21. Galanaud D, Nicoli F, Chinot O, et al. Noninvasive diagnostic assessment of brain tumors using combined in vivo MR imaging and spectroscopy. Magn Reson Med. 2006;55(6):1236–1245. [DOI] [PubMed] [Google Scholar]
- 22. Lin A, Bluml S, Mamelak AN. Efficacy of proton magnetic resonance spectroscopy in clinical decision making for patients with suspected malignant brain tumors. J Neurooncol. 1999;45(1):69–81. [DOI] [PubMed] [Google Scholar]
- 23. Schneider JF, Confort-Gouny S, Viola A, et al. Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J Magn Reson Imaging. 2007;26(6):1390–1398. [DOI] [PubMed] [Google Scholar]
- 24. Davies NP, Arvanitis TN, Auer D, et al. Multicentre prospective classification of childhood brain tumours based on 1H MRS metabolite profiles. In: Neuro-Oncology. Oxford Univ Press Inc; 2010;II32-II32. [Google Scholar]
- 25. Manias K, Gill SK, Zarinabad N, et al. Evaluation of the added value of 1H-magnetic resonance spectroscopy for the diagnosis of pediatric brain lesions in clinical practice. Neurooncol Pract. 2018;5(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44(5):639–650. [PubMed] [Google Scholar]
- 27. Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC.. A constrained least-squares approach to the automated quantitation of in vivo ¹H magnetic resonance spectroscopy data. Magn Reson Med. 2011;65(1):1–12. [DOI] [PubMed] [Google Scholar]
- 28. Bhapkar VP. A note on the equivalence of two test criteria for hypotheses in categorical data. J Am Stat Assoc. 1966;61(313):228–235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.