Abstract
Background
Glioblastoma (GBM) is the most common and most malignant glioma. Nonglioblastoma (non-GBM) gliomas (WHO Grades II and III) are invasive and also often fatal. The goal of this study is to determine whether sex differences exist in glioma survival.
Methods
Data were obtained from the National Cancer Database (NCDB) for years 2010 to 2014. GBM (WHO Grade IV; N = 2073) and non-GBM (WHO Grades II and III; N = 2963) were defined using the histology grouping of the Central Brain Tumor Registry of the United States. Non-GBM was divided into oligodendrogliomas/mixed gliomas and astrocytomas. Sex differences in survival were analyzed using Kaplan–Meier and multivariable Cox proportional hazards models adjusted for known prognostic variables.
Results
There was a female survival advantage in patients with GBM both in the unadjusted (P = .048) and adjusted (P = .003) models. Unadjusted, median survival was 20.1 months (95% CI: 18.7-21.3 months) for women and 17.8 months (95% CI: 16.9-18.7 months) for men. Adjusted, median survival was 20.4 months (95% CI: 18.9-21.6 months) for women and 17.5 months (95% CI: 16.7-18.3 months) for men. When stratifying by age group (18-55 vs 56+ years at diagnosis), this female survival advantage appeared only in the older group, adjusting for covariates (P = .017). Women (44.1%) had a higher proportion of methylated MGMT (O6-methylguanine-DNA methyltransferase) than men (38.4%). No sex differences were found for non-GBM.
Conclusions
Using the NCDB data, there was a statistically significant female survival advantage in GBM, but not in non-GBM.
Keywords: glioma, glioblastoma, NCDB, sex differences, survival
Gliomas are the most common form of primary brain tumor, representing about 80% of malignant brain and other CNS tumors.1–11 The World Health Organization (WHO) classification system groups gliomas into 4 histological grades based on level of malignancy; malignant gliomas are defined as Grade II, III and IV tumors.1–9,12–14 Nonglioblastoma (non-GBM), WHO Grades II and III gliomas, usually also evolve into high-grade gliomas, which are universally fatal.1,12,15,16 Glioblastoma (GBM), WHO Grade IV, is the most common, aggressive, and deadly glioma, accounting for approximately 15% of all primary brain and CNS tumors and 50% of all malignant primary brain and CNS tumors.1–4,17–21 Standard-of-care treatment consists of maximum safe surgical resection followed by radiotherapy in addition to concomitant and adjuvant chemotherapy with the alkylating agent temozolomide (TMZ).13,17–19 Despite this aggressive therapeutic regimen, patients with GBM have an extremely poor prognosis,1,3,4,10,13,17,20 with a 5-year survival rate of 5.6%.2 Non-GBMs generally affect patients at a younger age than GBM, with a peak incidence occurring between age 35 and 44 years in patients with non-GBM, compared with between age 75 and 84 years in those with GBM.2,12,15 Standard treatment for non-GBM gliomas begins with maximal safe resection with subsequent radiotherapy and/or chemotherapy depending on the tumor lineage and grade.12,15,16
Sex disparities in cancer incidence, survival, and prevalence have been well established for a variety of cancers, including oral and oropharyngeal cancer, liver cancer, cloacogenic anal cancer, and brain tumors.3,5,11,22–28 Previous studies have shown that men not only develop cancers more frequently, they have poorer responses to therapy as measured by progression-free and overall survival compared with women.5,22 Overall, cancer incidence rates are about 20% higher in men, while mortality rates are about 40% higher.22 The EUROCARE-2 study analyzed survival in 1 million European cancer cases diagnosed in 1985-1989, finding that sex was a significant predictor of survival and suggesting that women had a biological advantage over men in coping with cancer.29 In general, gliomas are more common in men than women.2,5–7,11,13,15,20,23,24,30–32 Primary GBM is about 1.58 times more common in men than in women.2,17,20,21,25 A study by Ostrom et al found that sex was significantly associated with survival in 2 independent datasets, confirming a female survival advantage among patients with GBM who received standard-of-care treatment.33 Whether the female survival advantage exists in non-GBM is unclear. Thus, the goals of this study are to confirm the sex differences in survival findings of Ostrom and colleagues in patients with GBM who received standard-of-care and assess this potential sex disparity in survival in primary non-GBM using data from the National Cancer Database (NCDB).
Materials and Methods
This study was approved as an exempt study by the University Hospitals Institutional Review Board. Data were obtained from the NCDB for the diagnosis years of 2010 to 2014, which collects data from more than 1500 hospitals in the United States, covering more than 70% of all newly diagnosed cases of cancer.34 GBM and non-GBM were defined using the histology grouping scheme for malignant gliomas (WHO Grades II, III, and IV) consistent with that of the Central Brain Tumor Registry of the United States (CBTRUS).2 GBM was defined using ICD-O-3 codes 9440/3, 9441/3, and 9442/3. Non-GBM was divided into 2 categories: oligodendrogliomas/mixed gliomas and astrocytomas. The oligodendrogliomas/mixed gliomas were defined using the following ICD-O-3 codes: oligodendroglioma (9450/3), anaplastic oligodendroglioma (9451/3, 9460/3), and oligoastrocytic tumors (9382/3). The astrocytomas were defined using the following ICD-O-3 codes: diffuse astrocytoma (9400/3, 9410/3, 9411/3, 9420/3), anaplastic astrocytoma (9401/3), and unique astrocytoma variants (9381/3, 9424/3).
For the primary GBM dataset, patients were included if they were diagnosed in 2010-2014, were at least age 18 years at diagnosis, either had subtotal or gross total resection, had radiotherapy (beam or combination) following their surgery, had chemotherapy (defined as chemotherapy administered as first course of therapy, single-agent chemotherapy administered as first course of therapy, or multiagent chemotherapy administered as first course of therapy), had a histologic diagnostic confirmation, had a KPS between 10 and 100 (0 excluded), had known promoter methylation status of O6-methylguanine-DNA methyltransferase (MGMT), and had unifocal GBM. The final dataset included 2073 patients (1250 men and 823 women). A CONSORT diagram depicting the GBM patient selection process is presented in Supplemental Figure 1. For the primary non-GBM dataset, patients were included if they were diagnosed in 2010-2014, were at least age 18 years at diagnosis, had a histologic diagnostic confirmation, had KPS between 10 and 100 (0 excluded), known loss of heterozygosity status of chromosomes 1p/19q (1p/19q codeletion), and had unifocal non-GBM. The final dataset included 2963 patients (1676 men and 1287 women). A CONSORT diagram depicting the non-GBM patient selection process is presented in Supplemental Figure 2. Owing to the level of missing data for MGMT in the GBM dataset and 1p/19q in the non-GBM dataset, sensitivity analyses were run to assess any difference in distribution of these variables between men and women. Two methods were used: Pearson’s chi-squared test and calculating the area under the receiver operating characteristic (ROC) curve.
The following variables were included in the analyses: sex (men, women), age at diagnosis (continuous; years), primary payer (not insured, private insurance/managed care, Medicaid, Medicare, other government), urban/rural (metro, urban, rural), CBTRUS histology (diffuse astrocytoma, anaplastic astrocytoma, unique astrocytoma variants, oligodendroglioma, anaplastic oligodendroglioma, oligoastrocytic tumors) [non-GBM only], tumor size (continuous; mm), KPS (continuous; score from 0–100 by tens), MGMT methylation (yes, no) [GBM only], 1p/19q codeletion (yes, no) [non-GBM only], surgical resection (no surgery, biopsy, subtotal resection, gross total resection), radiation therapy (yes, no) [non-GBM only], chemotherapy (yes, no) [derived from as defined above for “yes” for non-GBM], follow-up time (continuous; months), and vital status (alive, dead). Because of the amount of missing data for KPS (76.2% missing for GBM and 81.3% for non-GBM), this variable was imputed using multivariate imputation by chained equations using the R package “mice.”35 The raw, unimputed values for KPS for GBM and non-GBM are displayed in Supplemental Table 1.
Descriptive statistics were used to assess sex differences in important prognostic variables, using t-tests for continuous variables and chi-squared tests for categorical variables. Sex differences in survival were analyzed using R version 3.5.036 by Kaplan–Meier and multivariable Cox proportional hazards models, including adjusted Kaplan–Meier survival curves using the “survival” R package (R code available on request following pages 28–31, 68–70, 98–99, and 119–120).37 For both GBM and non-GBM, unadjusted Kaplan–Meier survival curves were generated stratified by sex and tested for differences using the log-rank test. P values less than .05 were considered statistically significant. For GBM only, adjusted Kaplan–Meier survival curves were generated by sex, controlling for (1) age at diagnosis and surgical resection, (2) age at diagnosis, surgical resection, and KPS, and (3) age at diagnosis, surgical resection, KPS, MGMT promoter methylation, primary payer status, urban/rural, and tumor size. The first 2 adjusted curves (1) and (2) were generated to compare the results with past literature.33 Similarly, multivariable Cox proportional hazards models were developed for the same models. To examine the potential effect of menopause and sex hormones on survival, the GBM dataset was stratified by age (18-55 years and 56+ years). The cutoff of 55 years was chosen because the most common age range at which women experience menopause in the United States is 48-55 years.38 For non-GBM, the oligodendrogliomas/mixed gliomas and astrocytomas were analyzed separately. Adjusted Kaplan–Meier survival curves were generated stratified by sex, controlling for age at diagnosis, surgical resection, KPS, 1p/19q codeletion, radiotherapy, chemotherapy, primary payer status, urban/rural, tumor size, and CBTRUS histology. Corresponding multivariable Cox proportional hazards models were developed both for oligodendrogliomas/mixed gliomas and astrocytomas.
Results
Patient demographics both for the GBM dataset (N = 2073) and the non-GBM dataset (N = 2963) for the study years of 2010-2014 are described in Table 1. For GBM, the majority of patients were male (60.3%), had unmethylated MGMT (59.3%), and had gross total resection of their tumor (54.3%). The average age at diagnosis was 58.56 years. Men were significantly more likely to have private insurance/managed care, and women were significantly more likely to use Medicaid (P = .027). Women were more likely to have methylated MGMT than men (44.1% women vs 38.4% men) (P = .011). No other significant differences between men and women were found on the other prognostic factors examined. For non-GBM, the majority of patients were male (56.6%), did not have the 1p/19q codeletion (54.2%), had gross total resection (38.3%), had radiation therapy (54.5%), and had chemotherapy (56.5%). The average age at diagnosis was 43.71 years. There were no significant differences between men and women in the prognostic factors examined.
Table 1.
Patient characteristics for glioblastoma (GBM) and non-GBM data sets, National Cancer Database 2010-2014
| Glioblastoma (GBM) | Nonglioblastoma (Non-GBM) | |||||
|---|---|---|---|---|---|---|
| Variable | Male (N = 1250) | Female (N = 823) | P Value | Male (N = 1676) | Female (N = 1287) | P Value |
| Age at diagnosisa (mean [SD]) | 58.47 (11.99) | 58.71 (12.43) | 0.654 | 43.53 (14.08) | 43.96 (14.42) | .412 |
| Primary Payerb (N [%]) | (Missing N = 18; 0.9%) | 0.027 | (Missing N = 29; 1.0%) | .266 | ||
| Not insured | 129 (11.0%) | 91 (11.9%) | 97 (5.9%) | 65 (5.1%) | ||
| Private insurance/managed care | 211 (18.0%) | 121 (15.9%) | 1154 (69.6%) | 910 (71.3%) | ||
| Medicaid | 195 (16.7%) | 143 (18.8%) | 204 (12.3%) | 142 (11.1%) | ||
| Medicare | 241 (20.6%) | 159 (20.9%) | 164 (9.9%) | 141 (11.0%) | ||
| Other government | 40 (3.4%) | 23 (3.0%) | 38 (2.3%) | 19 (1.5%) | ||
| Urban/Ruralb (N [%]) | (Missing N = 67; 3.2%) | 0.279 | (Missing N = 88; 3.0%) | .166 | ||
| Metro | 1021 (84.1%) | 680 (85.9%) | 1356 (83.1%) | 1050 (84.4%) | ||
| Urban | 171 (14.1%) | 104 (13.1%) | 244 (15.0%) | 181 (14.5%) | ||
| Rural | 22 (1.8%) | 8 (1.0%) | 31 (1.9%) | 13 (1.0%) | ||
| CBTRUS Histologyb (N (%)) | NA | .086 | ||||
| Diffuse astrocytoma | 179 (10.7%) | 172 (13.4%) | ||||
| Anaplastic astrocytoma | 246 (14.7%) | 199 (15.5%) | ||||
| Unique astrocytoma variants | 3 (0.2%) | 6 (0.5%) | ||||
| Oligodendroglioma | 550 (32.8%) | 396 (30.8%) | ||||
| Anaplastic oligodendroglioma | 264 (15.8%) | 177 (13.8%) | ||||
| Oligoastrocytic tumors | 434 (25.9%) | 337 (26.2%) | ||||
| Size of Tumora (mm) (mean [SD]) | (Missing N = 286; 13.8%) | 0.313 | (Missing N = 747; 25.2%) | .282 | ||
| 48.44 (42.76) | 46.35 (42.44) | 52.45 (59.83) | 49.76 (55.69) | |||
| KPSb (imputedc) (N [%]) | 0.702 | .103 | ||||
| 10 | 7 (0.6%) | 4 (0.5%) | 6 (0.4%) | 8 (0.6%) | ||
| 20 | 5 (0.4%) | 4 (0.5%) | 3 (0.2%) | 7 (0.5%) | ||
| 30 | 5 (0.4%) | 2 (0.2%) | 8 (0.5%) | 9 (0.7%) | ||
| 40 | 18 (1.4%) | 20 (2.4%) | 12 (0.7%) | 6 (0.5%) | ||
| 50 | 27 (2.2%) | 23 (2.8%) | 14 (0.8%) | 25 (1.9%) | ||
| 60 | 81 (6.5%) | 47 (5.7%) | 58 (3.5%) | 51 (4.0%) | ||
| 70 | 150 (12.0%) | 113 (13.7%) | 221 (13.2%) | 170 (13.2%) | ||
| 80 | 376 (30.1%) | 236 (28.7%) | 367 (21.9%) | 296 (23.0%) | ||
| 90 | 438 (35.0%) | 276 (33.5%) | 665 (39.7%) | 489 (38.0%) | ||
| 100 | 143 (11.4%) | 98 (11.9%) | 322 (19.2%) | 226 (17.6%) | ||
| MGMT Promoter Methylationb (N [%]) | 0.011 | NA | ||||
| Methylated | 480 (38.4%) | 363 (44.1%) | ||||
| Unmethylated | 770 (61.6%) | 460 (55.9%) | ||||
| 1p/19q Codeletionb (N [%]) | NA | .912 | ||||
| Yes codeletion | 769 (45.9%) | 587 (45.6%) | ||||
| No codeletion | 907 (54.1%) | 700 (54.4%) | ||||
| Surgical Resectionb (N [%]) | 0.824 | .489 | ||||
| No surgery | NA | 165 (9.8%) | 126 (9.8%) | |||
| Biopsy | NA | 344 (20.5%) | 263 (20.4%) | |||
| Subtotal resection | 574 (45.9%) | 373 (45.3%) | 387 (30.1%) | 543 (32.4%) | ||
| Gross total resection | 676 (54.1%) | 450 (54.7%) | 511 (39.7%) | 624 (37.2%) | ||
| Radiation Therapyb (N [%]) | NA—all GBM patients had radiotherapy because of inclusion criteria | .603 | ||||
| Yes | 921 (55.0%) | 694 (53.9%) | ||||
| No | 755 (45.0%) | 593 (46.1%) | ||||
| Chemotherapyb (N [%]) | NA—all GBM patients had chemotherapy because of inclusion criteria | .214 | ||||
| Yes | 964 (57.5%) | 710 (55.2%) | ||||
| No | 712 (42.5%) | 577 (44.8%) | ||||
Abbreviation: CBTRUS, Central Brain Tumor Registry of the United States; MGMT, O6-methylguanine-DNA methyltransferase; NA, not available.
a P value calculated by t-test.
b P value calculated by chi-squared test.
cKPS had 2410 (76.2%) missing values for the GBM data set and 1580 (81.3%) missing values in the non-GBM data set. Therefore, KPS was imputed using multivariate imputation by chained equations using the “mice” R package.33
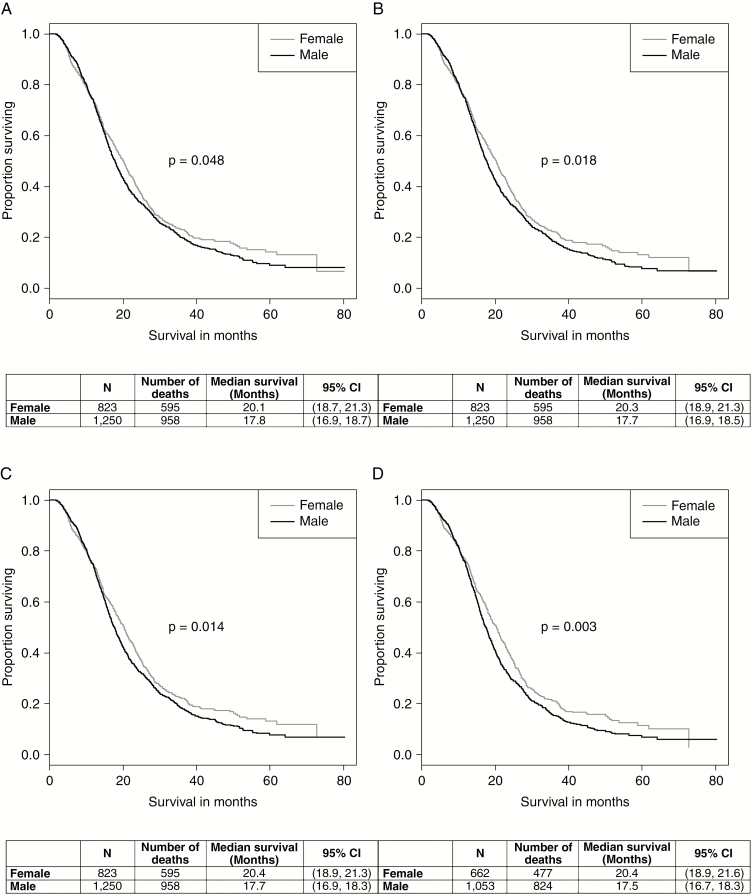
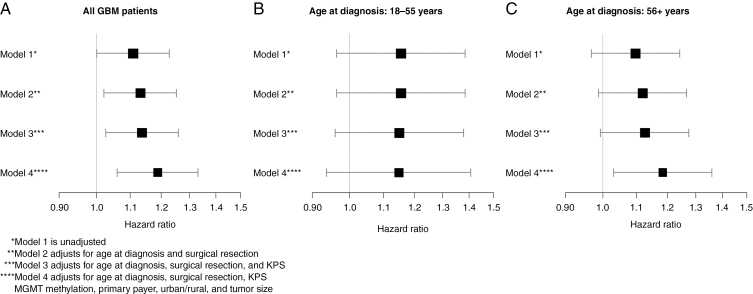
Without adjusting for covariates, the Kaplan–Meier curve (Fig. 1A) shows a significant sex difference for GBM patients, with women having better overall survival than men (P = .048). The median overall survival for women was 20.1 months (95% CI: 18.7-21.3 months), and 17.8 months for men (95% CI: 16.9-18.7 months). This difference gets larger with additional adjustments for covariates. A past study showed a significant sex difference for GBM patients adjusting for age at diagnosis and surgical resection, as well as adjusting for age at diagnosis, surgical resection, and KPS,33 so these Kaplan–Meier curves were drawn using NCDB data to see whether this difference still existed. When adjusting for age at diagnosis and surgical resection, the log-rank P value assessing the difference between men and women decreased to .018 (Fig. 1B), and adjusting further for KPS decreased the P value to .014 (Fig. 1C). After adjusting for all covariates of interest (age at diagnosis, surgical resection, KPS, MGMT methylation, primary payer status, urban/rural, and tumor size), the log-rank P value decreased to .003 (Fig. 1D). The median overall survival for women was 20.4 months (95% CI: 18.9-21.6 months), and 17.5 months for men (95% CI: 16.7-18.3 months). The corresponding hazard ratios (HRs) for sex, presented as men vs women, along with their 95% confidence intervals (CI) for each of the 4 models, are presented in Fig. 2A. The plot shows that the male:female HR shifts farther away from 1 with more covariates added. The values for the HRs, 95% CIs, and P values can be found in Supplemental Table 2. All covariates were statistically significant at the .05 level, except for tumor size. The Cox proportional hazards assumption was not violated (P = .308).
Fig. 1.
Kaplan–Meier curves for glioblastoma survival by sex A, unadjusted, B, adjusted for age at diagnosis and surgical resection, C, adjusted for age at diagnosis, surgical resection, and KPS, and D, adjusted for age at diagnosis, surgical resection, KPS, O6-methylguanine-DNA methyltransferase (MGMT) methylation, primary payer, urban/rural, and tumor size, National Cancer Database 2010-2014.
Fig. 2.
Cox proportional hazards models’ hazard ratios with corresponding 95% CIs for glioblastoma patients A, overall, B, in those age 18–55 years, and C, in those age 56+ years, National Cancer Database 2010-2014.
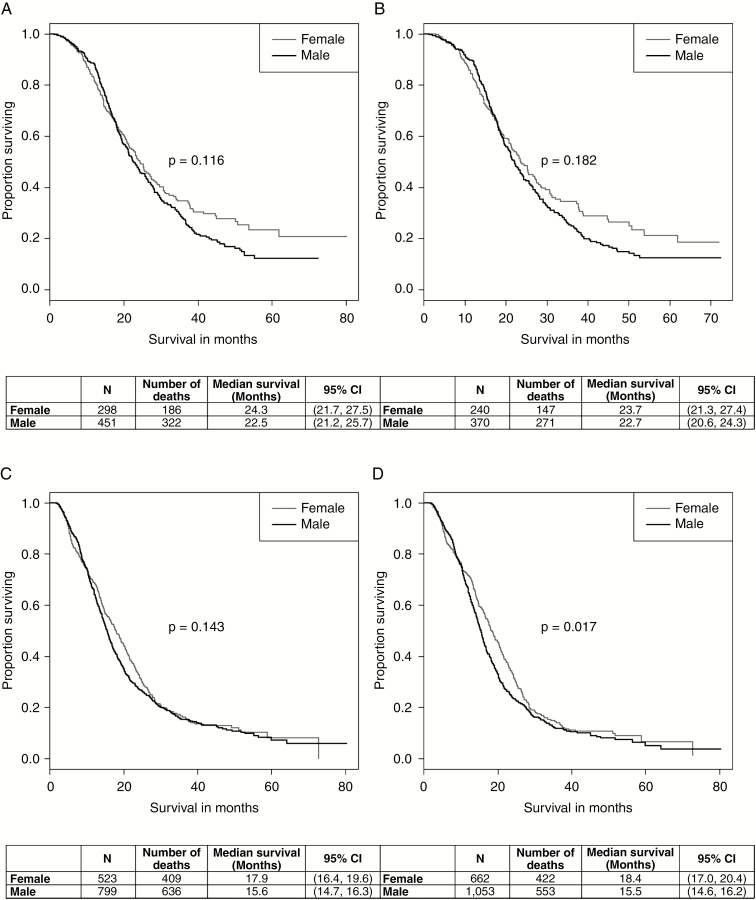
To assess whether sex hormones contributed to the female survival advantage in GBM patients, the data were stratified into those age 18–55 years at diagnosis (premenopausal, N = 749) and those 56 years and older at diagnosis (postmenopausal, N = 1324). In the younger age group, without adjustment for any covariates, the log-rank P value was not statistically significant (P = .116), although the Kaplan–Meier curve does seem to show a favorable outcome for women with long-term survival (Fig. 3A). The median survival for women was 24.3 months (95% CI: 21.7-27.5 months), and 22.5 months for men (95% CI: 21.2-25.7 months). After adjusting for all covariates of interest, the results remained similar (P = .182) (Fig. 3B). The median survival for women was 23.7 months (95% CI: 21.3-27.4 months), and 22.1 months for men (95% CI: 20.6-24.3 months). The corresponding HRs and 95% CIs are shown in Fig. 2B (models 1 and 4). The plot shows that the male:female HRs do not change significantly with additional covariates. In the older age group, without adjustment for any covariates, the log-rank P value was not statistically significant (P = .143) (Fig. 3C). The median survival for women was 17.9 months (95% CI: 16.4-19.6 months), and 15.6 months for men (95% CI: 14.7-16.3 months). After adjusting for all covariates of interest, however, the female survival advantage resurfaced (P = .017), although the Kaplan–Meier curves appeared to be similar (Fig. 3D). The median survival for women was 18.4 months (95% CI: 17.0-20.4 months), and 15.5 months for men (95% CI: 14.6-16.2 months). The corresponding HRs and 95% CIs are shown in Fig. 2C (models 1 and 4). The plot shows that the male:female HR shifts farther away from 1 with more covariates added. The values for the HRs, 95% CIs, and P values can be found in Supplemental Table 2.
Fig. 3.
Kaplan–Meier curves for glioblastoma survival by sex and stratified by age at diagnosis A, unadjusted (age 18-55), B, adjusted for age at diagnosis, surgical resection, KPS, O6-methylguanine-DNA methyltransferase (MGMT) methylation, primary payer, urban/rural, and tumor size (age 18-55), C, unadjusted (age 56+), and D, adjusted for age at diagnosis, surgical resection, KPS, MGMT methylation, primary payer, urban/rural, and tumor size (age 56+), National Cancer Database 2010-2014.
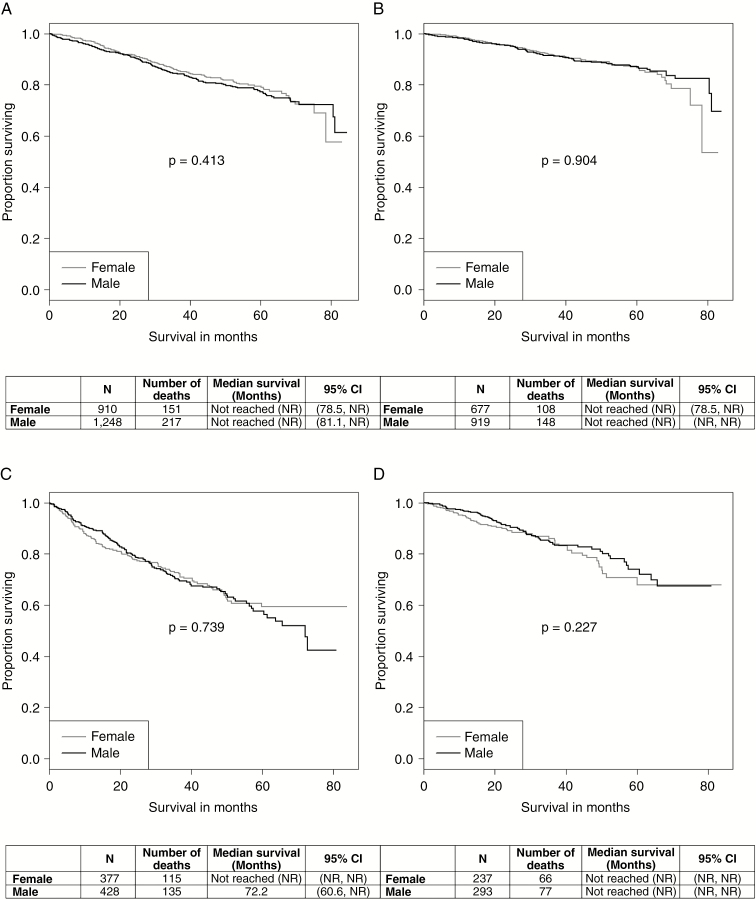
Kaplan–Meier analysis was performed for non-GBM patients, stratified by histology (oligodendroglioma/mixed glioma and astrocytomas), unadjusted and adjusted for covariates. For oligodendroglioma/mixed glioma, the unadjusted log-rank P value was .413 (Fig. 4A), and the adjusted log-rank P value was .904 (Fig. 4B), showing no sex difference in overall survival. Unadjusted, the median overall survival for women and men could not be obtained, since the proportion of patients who died did not reach 0.5; however, the 95% CI lower bound was 78.5 months for women and 81.1 months for men. Adjusted, the 95% CI lower bound stayed the same for women (78.5 months), but could not be calculated for men, as their overall survival improved after adjustment. For astrocytomas, the unadjusted log-rank P value was .739 (Fig. 4C), and the adjusted log-rank P value was .227 (Fig. 4D), again demonstrating no sex difference in overall survival. Unadjusted, the median overall survival and corresponding 95% CI for women could not be obtained; however, the median overall survival for men was 72.2 months, with a 95% CI lower bound of 60.6 months. Adjusted, none of the median survival estimates could be obtained, which could be because of the short period of follow-up. The corresponding values for the HRs, 95% CIs, and P values from the Cox proportional hazards models can be found in Supplemental Table 3. The Cox proportional hazards assumption was not violated (P = .687).
Fig. 4.
Kaplan–Meier curves for nonglioblastoma survival by sex A, unadjusted (oligodendroglioma/mixed glioma), B, adjusted for age at diagnosis, surgical resection, KPS, 1p/19q codeletion, radiotherapy, chemotherapy, primary payer, urban/rural, tumor size, and Central Brain Tumor Registry of the United States (CBTRUS) histology (oligodendroglioma/mixed glioma), C, unadjusted (astrocytoma), and D, adjusted for age at diagnosis, surgical resection, KPS, 1p/19q codeletion, radiotherapy, chemotherapy, primary payer, urban/rural, tumor size, and CBTRUS histology (astrocytoma), National Cancer Database 2010-2014.
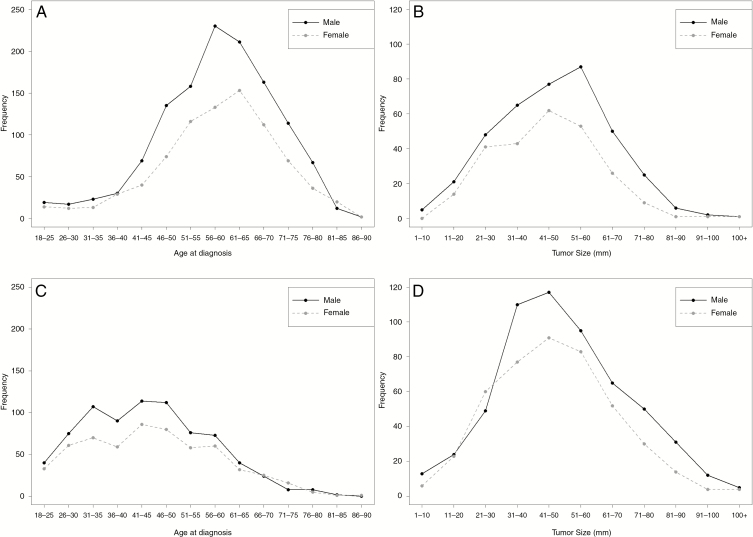
The mean ages at diagnosis were about the same for men and women both for GBM (men: 58.47 years; women: 58.71 years) (P = .654) and non-GBM (men: 43.53 years; women: 43.96 years) (P = .412). Similarly, the mean tumor sizes at diagnosis were about the same for men and women both for GBM (men: 48.44 mm; women: 46.35 mm) (P = .313) and non-GBM (men: 52.45 mm; women: 49.76 mm) (P = .282). To determine any differences in the underlying distributions of patient age at diagnosis by sex and tumor size by sex, plots of these distributions were generated both for GBM and non-GBM. For GBM, women were diagnosed at later ages than men (Fig. 5A), and women had smaller tumor sizes at diagnosis than men (Fig. 5B). Conversely, in non-GBM, there was no significant difference in age (Fig. 5C) or tumor size (Fig. 5D) by sex at the time of diagnosis.
Fig. 5.
Distribution by sex of A, glioblastoma (GBM) patient age at diagnosis, B, GBM patient tumor size, C, non-GBM patient age at diagnosis, and D, non-GBM patient tumor size, National Cancer Database 2010-2014.
Discussion
We have described overall survival by sex in patients with GBM and non-GBM (malignant gliomas, WHO Grades II, III, and IV) for the diagnosis years 2010-2014 using NCDB data. There is a notable female survival advantage in GBM, but not in non-GBM. Our study validates a previous study by Ostrom et al that reported a significant female survival advantage in patients with GBM diagnosed in 2007 or later who received standard-of-care using 2 data sources: (1) the Ohio Brain Tumor Study (OBTS), a multicenter study in Ohio, and (2) the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.33 The study reported a significant log-rank P value, with women having better overall survival than men when adjusting for age at diagnosis and extent of resection (P = .0017) using the SEER 18 data, and adjusting for age at diagnosis, extent of resection, and postsurgical KPS (P = .0006) using the OBTS data.33 Our study built on the previous analyses by including a more robust sample size (N = 2073) than that of OBTS (N = 228), and including additional prognostic factors in our survival models, such as MGMT methylation status, primary payer status, urban/rural location, and tumor size. Moreover, the cohort of patients with GBM from NCDB is more comprehensive across the United States (covering more than 70% of cancer diagnoses in the United States), as the SEER cohort represents patients from the 18 SEER registries (covering ~30% of the United States), and the OBTS cohort represents patients from Ohio only. It is important to note that the analyses by Ostrom and colleagues did not include biopsy-only patients with GBM; therefore, to directly compare our results with those of Ostrom et al, we limited our cohort to patients who received either subtotal or gross total surgical resection.
Previous literature has suggested that endogenous hormones may play a role in cancer incidence and survival.22,29 A study of liver cancer found that estrogens, at concentrations present in women but not in men, suppressed IL-6 production, thereby inhibiting chemically induced liver carcinogenesis.26 To determine the potential role of menopause and sex hormones in survival in our GBM patient population, patients were stratified into 2 age groups: 18-55 years at diagnosis and 56+ years at diagnosis. Within the younger group, there were no sex differences in survival. The older group demonstrated a female survival advantage on multivariable analysis, contradicting the hypothesis that the female survival advantage in GBM patients is due to elevated estrogen or other sex hormones alone.
There is a paucity of literature assessing sex differences in survival in patients with non-GBM (WHO Grades II and III), and the results have been inconclusive thus far. One study found that men were 1.18 times more likely than women to die of their non-GBM,10 while another study found that sex did not significantly influence survival in non-GBM (log-rank test P value >.09).39 Our study results agree with the latter finding. We did not find a significant sex difference in survival for patients with non-GBM both in the oligodendroglioma/mixed glioma patients and astrocytoma patients, even after adjusting for known prognostic factors. The current study used the most up-to-date data available at the population-level in the United States, as previous studies of sex differences in non-GBM used data from 1979 to 200110 and from 1980 to 199539. Because the definition of non-GBM has likely changed since those studies were conducted, our results are most relevant to current diagnosis of non-GBM.
The female survival advantage in cancer has been previously attributed to a variety of explanations, including lower prevalence of comorbidity, earlier diagnosis, greater resistance to disease, more attentiveness to health, and a lower incidence in risky behaviors in women relative to men.28,29,40 Another study found that adult height was positively associated with cancer incidence and death both in men and women and accounts for about one-third of the sex disparity in cancer risk by sex.22 Although height and certain environmental or behavioral risks are not included in the NCDB and thus cannot be addressed in this study, the covariates chosen for our survival models were chosen strategically to account for some of these potential explanations. Assessment of primary payer status and urban/rural location were added to control for patient access to care and to serve as a marker of socioeconomic status, and both of these variables were found to be significant predictors of glioma survival (urban/rural was not significant in non-GBM).
A genetic component may contribute to the female survival advantage found in patients with GBM. Numerous studies have confirmed that MGMT promoter methylation is correlated with an improved overall survival benefit in response to TMZ, irrespective of other treatment choices.2,3,7,13,18–20 Methylation of the MGMT promoter has been demonstrated in 30% to 60% of patients with GBM.19,20 In our GBM dataset, women had a significantly greater proportion of MGMT methylation (women: 44.1% methylated MGMT; men: 38.4% methylated MGMT; P = .011), which may contribute to their improved survival. One study found that MGMT methylation was significantly associated with longer survival in the entire cohort of patients with GBM (P = .009), but was significantly associated with longer survival only in female patients (P = .003) and not in male patients (P = .603).17 For patients with non-GBM, the most common genetic mutation and characteristic is loss of heterozygosity at chromosomes 1p and 19q.2,3,13 Like MGMT promoter methylation, 1p/19q codeletion is associated with sensitivity to chemotherapy with alkylating agents and improved survival.7,15,16,41 Codeletion of 1p/19q is also far more common in oligodendrogliomas than in astrocytic gliomas.3,9 In our oligodendroglioma/mixed glioma dataset, women had a higher proportion of 1p/19q codeletion (61.5%) than men (59.2%), though this difference was not significant (P = .296). In our astrocytoma dataset, women had the same proportion of 1p/19q codeletion (7.2%) as men (7.0%) (P = 1.000).
There are several limitations to this study. First, the NCDB data are not population based, as the results cannot be generalized beyond the 1500 US hospitals included in the data collection. Nonetheless, because more than 70% of new patients with cancer are captured in the dataset, the results are representative across the United States and cover a higher proportion of the country than SEER. Second, there was a fair amount of missing data on important variables, such as KPS, MGMT promoter methylation status, and 1p/19q codeletion in the overall NCDB dataset. Thus GBM and non-GBM analyses were limited to patients with complete MGMT methylation and 1p/19q codeletion information, respectively, which restricted the sample sizes of both datasets. However, sensitivity analyses were conducted to assess any potential difference in the distribution of missing data between men and women in the MGMT and 1p/19q variables. For GBM, the level of missingness in MGMT was not differentially distributed by sex (Pearson’s chi-squared test P = .824 and area under the ROC curve 0.499 [95% CI: 0.492-0.507]). For non-GBM, the level of missingness of 1p/19q deletions was not differentially distributed by sex (Pearson’s chi-squared test P = .796 and area under the ROC curve 0.501 [95% CI: 0.493-0.509]). KPS had 76.2% missing data in the GBM set and 81.3% in the non-GBM set. We imputed KPS using multivariate imputation by chained equations using the “mice” R package,35 which is powerful enough to handle this level of missing data in 1 variable. Third, unfortunately, we do not have information as to which agents (such as TMZ) were given; however, because the patients were all treated between 2010 and 2014, we assume the vast majority of these patients were given TMZ since this is the standard chemotherapy given for glioma. We do not know based on the coding of these data whether radiation therapy and chemotherapy were given concomitantly; however, they were both given as part of first course of treatment so we assume standard-of-care guidelines for glioma were followed for the majority of patients. Fourth, the biggest study limitation is that the NCDB does not provide any data on isocitrate dehydrogenase (IDH) mutation, which is an important prognostic variable. The 2016 CNS WHO restructured the definition of diffuse gliomas, incorporating new entities that are defined both by histology and molecular features, including GBM as either GBM IDH-wild type or GBM IDH-mutant.42 However, although IDH mutations are associated with favorable outcome,2,3,7,8,16,20 even IDH-mutant tumors show notable variability in overall survival, suggesting the likely contribution of additional influential molecular markers.9 Moreover, IDH mutations have fairly low prevalence in GBM, with estimates ranging from about 5% to 12%,1,3,9,13,15,20 so the vast majority of patients in our GBM dataset are likely IDH-wild type. For non-GBM, on the other hand, IDH mutations are found in a substantial majority of these cases,1,9 presenting in approximately 50% to 80% of patients with non-GBM.3,7,13,20 It is possible that adding IDH mutation to the non-GBM survival models would have changed the null results. It is also important to note that these hallmark genetic markers are neither mutually exclusive nor entirely independent.13 For example, patients who have IDH mutations tend to be younger individuals.3MGMT methylation has been found to be highly associated with 1p/19q codeletion and IDH mutations.13,20 To what extent the interaction of these molecular markers predict responsiveness to specific therapies or overall prognosis remains unclear.
In conclusion, the NCDB data demonstrate a notable female survival advantage in GBM, but not in non-GBM. Since the survival advantage occurred in the patients age ≥56 years, female survival advantage is not likely due to estrogen levels at the time of diagnosis. The reasons for this sex difference in survival for GBM are beyond the scope of this study, but will hopefully provoke future research into these devastating tumors.
Funding
This work was supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas [RP160097T to Q.T.O.]; the National Institutes of Health [CA217956], the Peter D Cristal Chair & the Center of Excellence for Translational Neuro-Oncology, and the Gerald Kaufman Fund for Glioma Research at University Hospitals of Cleveland, the Kimble Family Foundation, and the Ferry Family Foundation to A.E.S; the Central Brain Tumor Registry of the United States, funded by the Centers for Disease Control and Prevention [Contract No. 2016-M-9030], the American Brain Tumor Association, The Sontag Foundation, Novocure, AbbVie, the Musella Foundation, National Brain Tumor Society, and the National Cancer Institute [Contract No. HHSN261201800176P], the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations to C.K. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or NCI.
Conflict of interest statement.
None declared.
Supplementary Material
References
- 1. Yan H, Parsons DW, Jin G, et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):v1–v86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. [DOI] [PubMed] [Google Scholar]
- 4. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72(17):3323–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 7. Ostrom QT, Bauchet L, Davis FG, et al. . The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceccarelli M, Barthel FP, Malta TM, et al. . Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorovets D, Kannan K, Shen R, et al. . IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18(9):2490–2501. [DOI] [PubMed] [Google Scholar]
- 10. Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Liao J, Zhang X, et al. . Sex difference of mutation clonality in diffuse glioma evolution [published online ahead of print September 25, 2018]. Neuro Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cavaliere R, Lopes MB, Schiff D. Low-grade gliomas: an update on pathology and therapy. Lancet Neurol. 2005;4(11):760–770. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G;. ESMO Guidelines Working Group. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii93–101. [DOI] [PubMed] [Google Scholar]
- 14. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503; quiz 1 p following 516. [DOI] [PubMed] [Google Scholar]
- 15. Pouratian N, Schiff D. Management of low-grade glioma. Curr Neurol Neurosci Rep. 2010;10(3):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, et al. . Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiffgens S, Wilkens L, Brandes AA, et al. . Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget. 2016;7(34):55169–55180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kickingereder P, Neuberger U, Bonekamp D, et al. . Radiomic subtyping improves disease stratification beyond key molecular, clinical, and standard imaging characteristics in patients with glioblastoma. Neuro Oncol. 2018;20(6):848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preusser M, de Ribaupierre S, Wöhrer A, et al. . Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. [DOI] [PubMed] [Google Scholar]
- 20. Thakkar JP, Dolecek TA, Horbinski C, et al. . Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song W, Ruder AM, Hu L, et al. . Genetic epidemiology of glioblastoma multiforme: confirmatory and new findings from analyses of human leukocyte antigen alleles and motifs. PLoS One. 2009;4(9):e7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 23. Ostrom QT, McCulloh C, Chen Y, et al. . Family history of cancer in benign brain tumor subtypes versus gliomas. Front Oncol. 2012;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974-1999. Cancer. 2005;104(12):2798–2806. [DOI] [PubMed] [Google Scholar]
- 26. Naugler WE, Sakurai T, Kim S, et al. . Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. [DOI] [PubMed] [Google Scholar]
- 27. Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the Surveillance, Epidemiology, and End Results experience, 1973-2000. Cancer. 2004;101(2):281–288. [DOI] [PubMed] [Google Scholar]
- 28. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–316. [DOI] [PubMed] [Google Scholar]
- 29. Micheli A, Ciampichini R, Oberaigner W, et al. . The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45(6):1017–1027. [DOI] [PubMed] [Google Scholar]
- 30. Dubrow R, Darefsky AS. Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer. 2011;11:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004;101(10):2293–2299. [DOI] [PubMed] [Google Scholar]
- 32. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. [DOI] [PubMed] [Google Scholar]
- 33. Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018;20(4):576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merkow RP, Rademaker AW, Bilimoria KY. Practical guide to surgical data sets: National Cancer Database (NCDB). JAMA Surg. 2018;153(9):850–851. [DOI] [PubMed] [Google Scholar]
- 35. van Buuren S, Groothuis-Oudshoorn CG.. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45(3):1–67. https://www.jstatsoft.org/v45/i03/. Accessed November 29, 2018. [Google Scholar]
- 36. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org/. Accessed November 27, 2018. [Google Scholar]
- 37. Therneau T. A Package for Survival Analysis in S. version 2.38, https://CRAN.R-project.org/package=survival. https://cran.r-project.org/web/packages/survival/survival.pdf.2015. Accessed December 10, 2018.
- 38. eMedicineHealth. WebMD, Inc. Menopause (symptoms, remedies, and treatment medications) 2018. https://www.emedicinehealth.com/menopause/article_em.htm#definition_and_facts_about_menopause. Accessed December 4, 2018.
- 39. Lote K, Egeland T, Hager B, et al. . Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15(9):3129–3140. [DOI] [PubMed] [Google Scholar]
- 40. Sant M, Allemani C, Santaquilani M, et al. . Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45(6):931–991. [DOI] [PubMed] [Google Scholar]
- 41. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. . Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6): 803–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.