Abstract
Background
Impairments in neurocognitive functioning (NCF) frequently occur in glioma patients. Both the tumor and its treatment contribute to these impairments. We aimed to quantify NCF in glioma patients before treatment and to investigate which factors influence NCF.
Methods
We performed a retrospective cohort study in diffuse glioma patients according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria. All patients had undergone neuropsychological assessment as part of routine clinical care, before awake surgery. We studied “overall NCF” and NCF in 5 neurocognitive domains separately. For “overall NCF” and per domain, we performed analyses at 2 different levels of outcome measures: (1) group level: mean cognitive functioning of the study sample, and (2) individual level: the percentage of impaired patients. We performed multivariable logistic regression analyses to investigate which factors were associated with the occurrence of cognitive impairments.
Results
From our cohort of glioma patients (2010-2016), 168 patients met all the inclusion criteria. All cognitive domains were significantly affected at the group level. The percentages of neurocognitive impairments (–2SD) were highest for Executive Functioning, Psychomotor Speed, and Memory (26.5%, 23.2%, and 19.3%, respectively). Patients with high-grade glioma were affected more severely than patients with low-grade glioma. Tumor volume, isocitrate dehydrogenase status, WHO grade, and histology were associated with the occurrence of domain-specific impairments.
Conclusions
Cognitive impairment occurs in the majority of treatment-naive glioma patients. The domains Executive Functioning, Speed, and Memory are involved most frequently. These impairments in NCF are explained not only by tumor location and volume, but also by other (biological) mechanisms.
Keywords: brain tumor, cognition, glioma, IDH, isocitrate dehydrogenase, neurocognitive functioning, neuropsychology
About one-third of patients with diffuse glioma (WHO grade II-IV) suffer from a deficit in 1 or more cognitive domains.1–4 The most common domains affected are Executive Functioning (EF), Psychomotor Speed, and Memory. These cognitive impairments have a marked impact on quality of life, both for patients and their caretakers.5
The brain maintains a complex network of interactions between local and distant areas to synthesize regional specialization with global integration. Cognition in particular relies on successful coordination between regions of specialized function. Consequently, cognitive dysfunction in glioma patients can be more generalized than might be expected based solely on tumor location and size when coordination has been disrupted.6 EF is particularly likely to be impaired in glioma patients.7,8 EF encompasses a set of higher-order neurocognitive processes that allow people to make choices and engage in goal-directed and future-oriented behavior. EF is an emergent property of a widespread brain network rather than a product of regional specialization, and is therefore vulnerable to the glioma’s effects.
The pathophysiological mechanisms underlying network disturbances and the resulting deficits in neurocognitive function (NCF) of patients have thus far not been clarified fully. Cognitive disruption by gliomas can be similar for tumors in quite different brain regions, suggesting that network dysfunction may be caused by more than just the structural nuance of the tumor mass.9 In fact, accumulating evidence demonstrates that brain tumors induce an array of metabolic changes in their environment that potentially influence neuronal signaling, which, in turn, impairs NCF.10
Given its central role in quality of life, preservation of NCF has become a primary goal in therapy. However, to predict cognitive functioning after treatment, it is important to quantify patients’ preoperative NCF, and to identify which factors influence cognitive functioning. At present, NCF in glioma patients has primarily been studied postoperatively, and data about the role of the tumor itself on neurocognitive status are scarce.8,11–14
To help fill this gap, we performed a retrospective study to evaluate the incidence of neurocognitive deficits in glioma patients before surgery, chemotherapy, or radiotherapy. We also investigated the role of patient-related and (mechanical or biomolecular) tumor-related factors in neurocognitive dysfunction.
Methods
Design
We performed a single-center retrospective study in a cohort of treatment-naive diffuse glioma patients who underwent neuropsychological testing as part of their preoperative work-up for awake brain surgery between 2010 and 2016.
In the study sample, we studied overall NCF as well as domain-specific NCF for 5 main neurocognitive domains. We also studied the influence of age, sex, tumor grade, tumor histology, isocitrate dehydrogenase (IDH) mutation, tumor lateralization, tumor volume, and tumor location on pretreatment NCF in diffuse glioma patients. We used the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria (Appendix 1) in the drafting of this manuscript.
Participants
Data were obtained between January 2010 and December 2016 from a database of consecutive patients with diffuse gliomas at the University Medical Center in Utrecht (UMCU) who underwent awake neurosurgery. All patients who underwent awake brain tumor surgery also underwent preoperative neuropsychological assessment, consisting of an elaborate neuropsychological test battery (except for patients with centrally located tumors, who underwent only pure motor function testing). Inclusion criteria for this study were the presence of a diffuse glioma according to the 2007 WHO criteria and a minimum age of 18 years. All patients were operated on in awake condition. Exclusion criteria were any form of tumor-directed treatment (operation [except biopsy before resection], chemotherapy, radiotherapy) before neuropsychological assessment and incomplete neuropsychological assessment (due to emergency surgery of tumors located in the motor strip, for instance). Data were considered complete if more than 50% of tasks within 1 domain were performed and if data on at least 4 out of 5 different domains (80%) were obtained. If not, neuropsychological assessment was considered insufficient and patients were excluded from further analysis (Supplementary Fig. 1). The UMCU institutional ethical review board approved the study; informed consent was not obtained for this observational study on data that were obtained as part of routine clinical care.
Neuropsychological Tests
The neuropsychological instruments that were used as part of our routine clinical care are listed in Table 1. These tests are internationally widely used, standardized psychometric instruments for assessing neurocognitive deficits in the major neurocognitive domains. All tests have normative data that take into account age and, when appropriate, educational level and sex. Neuropsychological tests often tap more than 1 cognitive domain, and classification into cognitive domains often varies in the literature. We made use of a predetermined test classification based on the available literature and local experience (Table 1). Owing to time or patient’s participation constraints, the selection of tests was sometimes tailored to the patient, depending on the patient’s complaints, or on tumor location.
Table 1.
Neuropsychological Tasks per Domain
| Attention and Executive Functioning |
| Wechsler Adult Intelligence Scale (WAIS-III) Digit Span Forward a |
| Trail Making Test (TMT) Switching ratio (TMTB/TMTA) b |
| Phonologic Fluency c |
| Stroop/Delis Kaplan Executive Function System (DKEFS) inhibition ratio d |
| Memory |
| Wechsler Adult Intelligence Scale (WAIS-III) Digit Span Backward |
| RAVLT-Dutch version immediate, delay, recognition e |
| Rey-Osterieth Complex Figure Test (ROCF) delay f |
| Semantic Fluency g |
| Language |
| Boston Naming Test h Token Test short version of AAT i |
| Visuospatial Functioning |
| Benton Judgment of Line Orientation (JULO) j |
| ROCF direct |
| Psychomotor Speed |
| Stroop/DKEFS I |
| Stroop/DKEFS II |
| TMTA |
Abbreviations: AAT, Aachen aphasia test; RAVLT, Rey Auditory Verbal Learning Test; TMTA, Trail Making Test part A; TMTB, Trail Making Test part B;
aWechsler Adult Intelligence Scale Third Edition Digit Span (WAIS-III) (WAIS-III Administration and scoring manual, 1997), Wechsler Adult Intelligence Scale Fourth Edition Digit Span (WAIS-IV) (WAIS-IV-NL Technische handleiding, 2013).
bGiovagnoli, Del Pesce, Mascheroni, Simoncelli, Laiacona, Capitani, 1996.
cPhonologic Verbal Fluency Test (Lexical Fluency) (Harrison, Buxton, Husain, Wise, 2010; Schmand, Groenink, Van Den Dungen, 2008).
dThe Stroop Color and Word Test (Stroop) (MacLeod, 1991), Color Word Interference Test (Benton, Sivan, Hamsher, Varney, Spreen, 1994).
e15 Words Test (15WT) (Saan, Deelman, 1986).
fBerry, Carpenter, 1992; Spreen, Strauss, 1998.
gSemantic Verbal Fluency Test, Harrison et al, 2010.
hBoston Naming Task, Heesbeen, Van Loon-Vervoorn, 2001.
iToken Test, Boller, Vignolo, 1966.
jBenton, Sivan, Hamsher, Varney, Spreen, 1994; Benton, Varney, Hamsher, 1978.
Procedure/Data Extraction
The neuropsychological evaluation was conducted shortly (1-7 days) before the awake brain tumor surgery. All evaluations were conducted under optimal testing conditions, with use of standardized test instructions. The neuropsychological evaluation took most patients approximately 2 hours to complete. At least 1 break was always inserted halfway to make the test battery less exhausting for the patient. Each neuropsychological test was scored according to standardized scoring criteria. For normative comparisons the uncorrected scores were transformed into Z-scores based on the mean and SD of control individuals derived from published norm data.
All neuropsychological data were prospectively collected between 2010 and 2016 in a database. We further extracted data on patient characteristics from the electronic patient file for all awake and nonawake operated on glioma patients in this period. Data included sex, age at first surgery, histology, WHO grade, lateralization, glioma location based on MRI, KPS score, IDH mutation (IDH1 R132H), occurrence of epileptic seizures, use of antiepileptic drugs (AEDs) and dexamethasone, and preoperative volume. In most cases IDH mutation was established by immunohistochemistry, and in a minority of cases additionally exon 4 DNA sequencing was performed. Methods of performing IDH1 R132H immunohistochemistry and exon 4 DNA sequencing have been described previously.15
For each patient the preoperative MRI (both T2/fluid-attenuation inversion recovery [FLAIR] and T1 with gadolinium) was reviewed and data were acquired by a junior clinical scientist (EvK) and reviewed by an experienced neuro-oncologist (TJS). Involvement of the following structures was registered for both hemispheres: frontal, parietal, temporal, occipital, hippocampus, insular, and multifocal involvement. Volumes were measured in 3 dimensions with use of Osirix Lite (v. 9.5.2) on T2-/FLAIR-weighted MRI scans, and the volume was defined as the whole area of hyperintensity. This represents the total lesion volume, including tumor and edema. Volumes were measured by a junior clinical scientist (EvK) and a neuro-oncological neurosurgeon (KMvB). Since this parameter is independent of enhancement (and thereby grade) of the lesion, it forms a widely usable representation of the extent of brain volume that is potentially hampered in its function.
Analyses
We performed analyses with SPSS (IBM SPSS Statistics, 25.0.0). Because our study sample represents a subset of all patients with diffuse glioma, we first compared baseline tumor- and patient-related characteristics of the included patients (who underwent awake surgery) with patients who underwent glioma surgery under general anesthesia in the same period of time. By comparing age, sex, KPS score, tumor grade, tumor histology, tumor lateralization, and tumor location, we investigated whether our study sample was representative for the whole glioma patient population.
We performed analyses of data for different outcome measures of NCF:
group-level: comparison of mean NCF Z scores of the patient sample compared with control data or normative data for each domain (“domain level”) and for overall NCF (“in any domain”); and
individual patient-level: percentage of patients with test performance below the threshold of impairment; this was calculated for each domain (“percentage impaired patients per domain”) for different thresholds and overall neurocognitive dysfunction (“number of domains affected per patient”).
Group-Level Analyses
Individual patients’ Z scores for given cognitive domains were calculated as the mean of the patients’ Z scores derived from all tests in this domain. Based on these individual mean Z scores, group domain scores were calculated for the following neurocognitive domains: EF and Attention, Memory, Language, Visuospatial Functioning, and Psychomotor Speed. Furthermore, overall NCF was computed as the mean of the Z scores of all 5 neurocognitive domains.
We performed 1-sample T-tests to statistically test patient performances (mean of Z scores per domain) against norm performance (with the null hypothesis being Z = 0 meaning no difference between patients and expected norm performance) for (a) each neuropsychological domain, and (b) overall NCF. For the domains in which data were not normally distributed, we performed a nonparametric Wilcoxon-signed-rank test. Both for this analysis and for the individual patient-level-analysis (below), we performed complete-case analyses and we did not impute for missing data because only a very small percentage of data was missing (as given in Supplementary Table 1). We performed subgroup analysis for low-grade glioma (LGG) and high-grade glioma (HGG) separately.
Individual Patient-Level Analysis
To determine the percentage of impaired patients at domain level, we counted the number of individual patients with an impaired performance per domain. A patient was considered impaired in a given domain if the patient performed below –2 SD on any of the administered tests within that domain. To identify severe as well as more-subtle abnormalities in NCF, we used different thresholds of –1 SD, –1.5 SD, and –2 SD. For overall NCF, we determined the number of domains affected per patient for the different thresholds.
Logistic univariable and multivariable regression analyses were conducted to study the influence of age, sex, tumor grade, histology, tumor lateralization, tumor volume, IDH mutation, and tumor location on the 5 neurocognitive domains separately. We constructed a multivariable model with all the variables mentioned in univariable analyses (Supplementary Table 2) with a P value < .25. We then performed a backward selection procedure, repeatedly excluding the variable with the lowest P value until all variables in the model had a P value < .05. Because grade and IDH mutations interact too strongly, we did not analyze them together in the same model.
Results
Clinical Characteristics
Clinical characteristics are presented in Table 2 by type of surgery (awake vs nonawake). In total 168 eligible patients underwent awake surgery, and 612 other diffuse glioma patients were operated on under general anesthesia between 2010 and 2016. The “awake” group and the general anesthesia group differed in various baseline characteristics, including, age, sex, KPS score, tumor grade, tumor histology, tumor lateralization, and brain regions involved, but not in the occurrence of epileptic seizures and use of AEDs. In the awake group, 39.3% received dexamethasone before surgery.
Table 2.
Baseline Characteristics in Awake and Nonawake Patients
| Determinants | Nonawake Surgery n = 612 (% of Nonawake)a | Awake Surgery n = 168 (% of Awake)a | P value for Pearson-Chi2/ t-test |
|---|---|---|---|
| Age (Mean) | 61.4 y | 51.5 y | <.0005 |
| Gender, Male | 361 (60.0) | 114 (67.9) | .035 |
| Biopsy vs Resection, Biopsy Only | 211 (34.5) | 0 (0) | <.0005 |
| Histology | <.0005 | ||
| Oligodendroglioma | 16 (2.6) | 8 (4.8) | |
| Astrocytoma | 78 (12.8) | 39 (23.2) | |
| Oligoastrocytoma | 23 (3.8) | 41 (24.4) | |
| Glioblastoma | 493 (80.6) | 78 (46.4) | |
| Ganglioglioma | 1 (0.2) | 2 (1.2) | |
| WHO Grade | <.0005 | ||
| II | 54 (8.8) | 62 (36.9) | |
| III | 65 (10.6) | 29 (17.3) | |
| IV | 493 (80.6) | 77 (45.8) | |
| Epileptic Seizure(S) Before Surgery | 231 (37.8) | 61 (36.3) | .600 |
| Use of Antiepileptic Drugs Before Surgery | 211 (34.5) | 58 (34.5) | .992 |
| KPS | .003 | ||
| 0-60 | 57 (9.3) | 11 (6.6) | |
| 70 or more | 307 (50.1) | 157 (93.5) | |
| Hemisphere Involvement on T2 (FLAIR) Measured | <.0005 | ||
| Left | 210 (34.3) | 118 (70.2) | |
| Right | 270 (44.1) | 44 (26.2) | |
| Both sided | 133 (21.7) | 6 (3.6) | |
| Left frontal (+) | 229 (37.4) | 85 (50.6) | .003 |
| Left parietal (+) | 148 (24.2) | 41 (24.4) | .977 |
| Left temporal (+) | 151 (24.7) | 62 (36.9) | .002 |
| Left occipital (+) | 60 (9.8) | 21 (12.5) | .337 |
| Left insula (+) | 143 (23.4) | 69 (41.1) | <.0005 |
| Left hippocampus (+) | 48 (7.8) | 30 (17.9) | <.0005 |
| Right frontal (+) | 271 (44.3) | 42 (25.0) | <.0005 |
| Right parietal (+) | 203 (33.2) | 23 (13.7) | <.0005 |
| Right temporal (+) | 222 (36.3) | 20 (11.9) | <.0005 |
| Right occipital (+) | 116 (20.0) | 5 (3.0) | <.0005 |
| Right insula (+) | 212 (34.6) | 27 (16.1) | <.0005 |
| Right hippocampus (–) | 87 (14.2) | 3 (1.8) | <.0005 |
| Multifocal (+) | 75 (12.3) | 3 (1.8) | <.0005 |
Abbreviation: FLAIR, fluid-attenuated inversion recovery.
aPercentages do not add up to 100% for certain variables because of missing values.
Neurocognitive Data
Neurocognitive functioning (group and individual level)
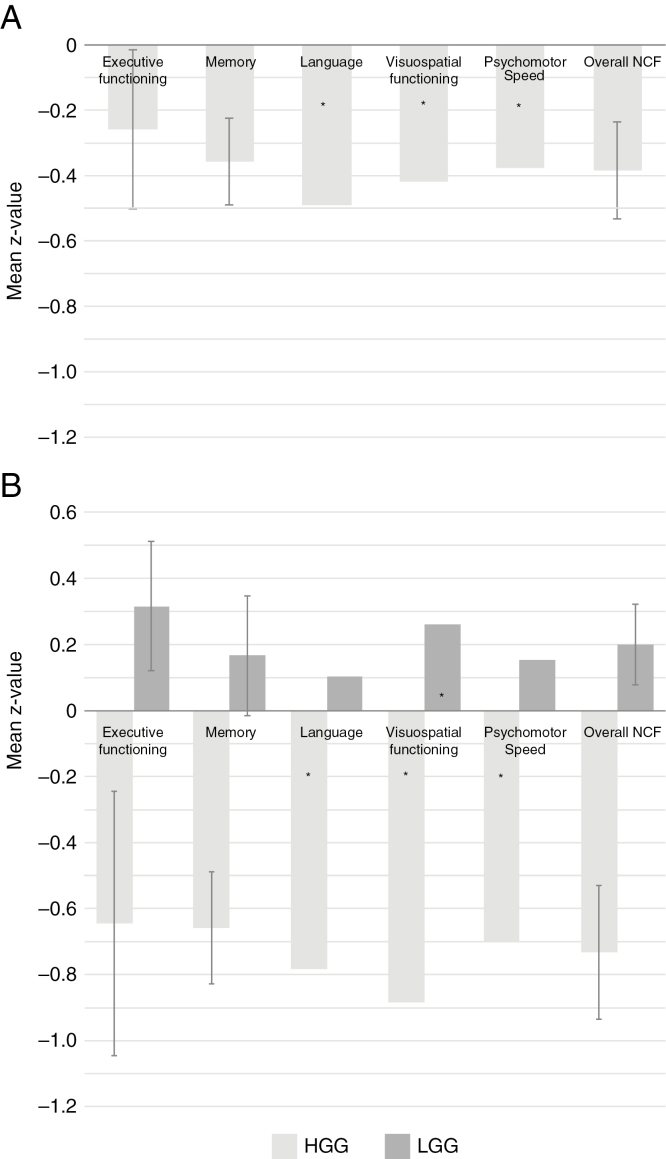
Supplementary Fig. 1 shows a flowchart of the selection of our study sample. Results of NCF analyzed at the group level are shown in Supplementary Table 1 and Fig. 1. Patients’ NCF scores were significantly lower than norm data at the group level, with mean Z scores lower than 0 on all domains. Subgroup analyses for HGG patients showed decreased scores (significantly lower than 0) for all different domains. Notably, LGG subgroup analyses showed increased mean NCF scores for any domain.
Fig. 1.
Group-Level Analyses.
Mean Z values per domain (error bars represent 95% confidence interval; these are not given for nonparametric test) with subgroup analyses for HGG and LGG. A, HGG and LGG; B, subgroup analyses for HGG and LGG. LGG indicates low-grade glioma, HGG, high-grade glioma; NCF, neurocognitive functioning. *P < .05 on nonparametric test.
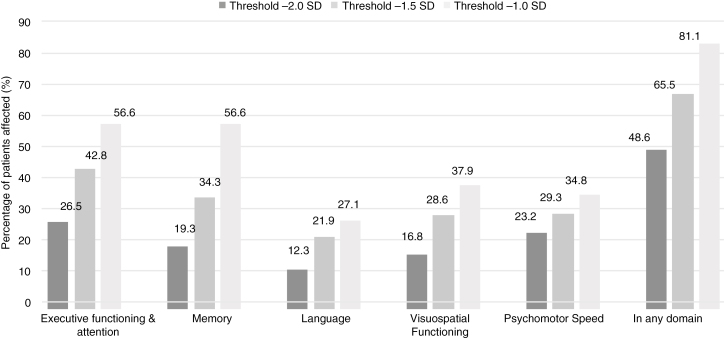
The proportion of individuals with a cognitive impairment at different thresholds is shown in Fig. 2. The percentages of severe neurocognitive impairments (–2SD) were highest in EF and Attention, Psychomotor Speed, and Memory (26.5%, 23.2%, and 19.3%, respectively). The percentage of more-subtle deficits (threshold –1.5SD and –1SD) were highest in EF and Memory (42.8% and 34.3%, respectively, for –1.5SD and 56.6% in both domains for –1SD). The percentages of impaired patients (–2SD) differed significantly between LGG and HGG for all domains and are shown in Table 3.
Fig. 2.
Individual-Level Analyses.
Percentage of impaired patients per domain for different thresholds.
Table 3.
Neurocognitive Functioning on Individual Level
| Domain | Grade | Percentage Impaired Patients for –2SD |
Relative Risk HGG vs LGG | 95% Confidence Interval |
|---|---|---|---|---|
| Executive Functioning and Attention | LGG | 11.5 | 3.1 | 1.7-10.2 |
| HGG | 35.2 | |||
| Memory | LGG | 3.3 | 8.7 | 2.7-51.4 |
| HGG | 28.6 | |||
| Language | LGG | 1.7 | 11.1 | 1.7-103.2 |
| HGG | 18.8 | |||
| Visuospatial Functioning | LGG | 6.6 | ||
| HGG | 23 | 3.5 | 1.4-13.0 | |
| Psychomotor Speed | LGG | 6.6 | 5.0 | 2.4-21.0 |
| HGG | 33.0 |
Abbreviations: HGG, high-grade glioma; LGG, low-grade glioma.
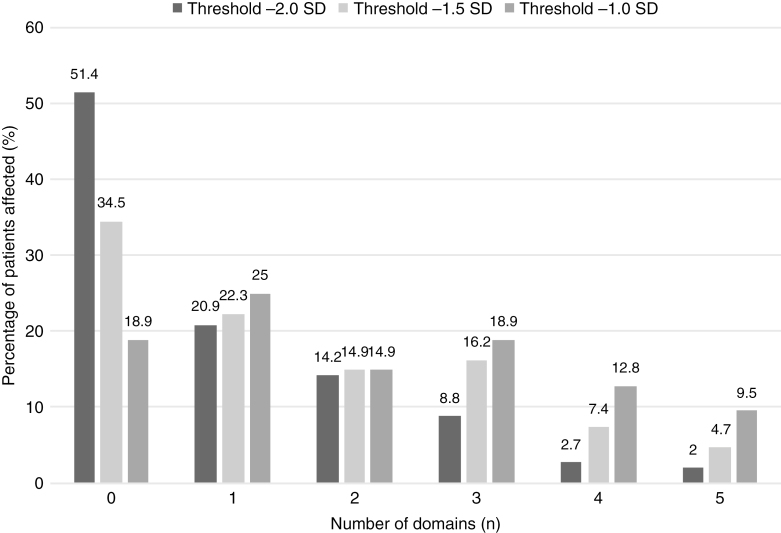
A closer look into the number of impaired domains per individual patient (Fig. 3) reveals that a severe impairment (–2SD) in at least 1 domain was found in 58.6% of patients. A more-subtle deficit in at least 1 domain was found in 65.5% and 81.1% for thresholds –1.5 and –1SD, respectively. The number of domains (for threshold –2SD) that were affected per patient was also higher for HGG than for LGG, as shown in Supplementary Fig. 2. None of the LGG patients had cognitive impairments in more than 3 domains.
Fig. 3.
Individual-Level Analyses.
Percentage of patients in different amount of affected domains for different thresholds.
The different number of tests performed (within a certain domain) between patients is a potential source of bias. To test whether such bias occurred, we performed a chi-squared test to estimate if the number of tests we performed was associated with the risk of impairment in the domain Memory. About 80% of patients underwent all 6 tests in this domain. Performing fewer than 6 tasks (2-5) was not of significant influence (P = .206) on the risk of impairment in memory.
Determinants of pretreatment neurocognitive functioning
Univariable logistic regression analysis showed that age, histology (glioblastoma), WHO grade (IV), presence of an IDH mutation, and preoperative T2/FLAIR volume correlated significantly with the risk of impairment in all 5 different domains (Supplementary Table 2). Tumor location also correlated to some extent with NCF impairments dependent on the location. For the domains EF and Attention, Psychomotor Speed, and Memory, locations in the left hemisphere, but not in the right hemisphere, were associated with the risk of impairment. A posterior lobe location correlated with impaired Visuospatial Functioning, as compared with anterior lobes.
In multivariable logistic regression, the presence of an IDH mutation negatively correlated with the risk of impairments (–2SD) in Memory, Psychomotor Speed, and Visuospatial Functioning, even when corrected for tumor volume (Table 4). IDH-mutated tumors also showed a trend for a lower risk of impairment in EF and language. EF showed the strongest association with tumor volume.
Table 4.
Multivariable Logistic Regression: Most Important Determinants of Influence on Cognitive Impairment (–2SD) for Specific Domains
| Domain | Executive Functioning | Memory | Language | Visuospatial Functioning | Psychomotor Speed | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95%CI) | P Value | OR (95% CI) | P Value | |
| Volumes T2-FLAIR (mm 3) | 1.000012 (1.000006-1.000018) | <.0005 | 1.000011 (1.000004-1.000018) | .001 | 1.000009 (1.000001-1.000016) | .026 | 1.000005 (0.999998-1.000011) | .141 | 1.000005 (0.999998-1.000011) | .143 |
| IDH-mutation | 0.463 (0.192-1.117) | 0.087 | 0.175(0.054-0.569) | 0.004 | 0.360 (0.104-1.248) | 0.107 | 0.185(0.063-0.542) | 0.002 | 0.338 (0.138-0.827) | 0.018 |
Abbreviations: FLAIR, fluid-attenuated inversion recovery; IDH, isocitrate dehydrogenase; OR, odds ratio.
Discussion
Impairments in NCF frequently occur in glioma patients. Treatment and the tumor itself contribute to these impairments, but NCF has mostly been studied postoperatively to date. In our series of 168 patients, 1 in every 2 patients had severe impairments in cognitive functioning in at least 1 cognitive domain before any treatment was given. Taking more subtle impairments into account, only 18.9% of the patients were not affected. In other words, nearly all treatment-naive glioma patients selected for awake surgery suffer from some degree of neurocognitive dysfunction.
In all domain-specific analyses, EF and Attention, Memory, and Psychomotor Speed appeared most frequently involved. These functions rely on widespread neural networks and can be altered by a mechanical conflict between the tumor and important nodes (hubs) or pathways of these networks.16,17 Indeed, in our patients, location of the tumor was found to be of influence: Left hemispheric tumors were associated in univariable analyses with the occurrence of impairments in EF, Memory, and Language, and EF appeared to be most vulnerable for the effects of tumor volume. However, these associations did not remain significant in multivariable analysis, possibly because of the relatively small size of our samples. Nevertheless, another possibility is that other factors also mediate neurocognitive dysfunction. In addition to their “mechanic” effects, tumors can indeed induce biochemical changes in the brain parenchyma. Such changes can occur from a distance of the epicenter of the tumor, and can be the consequence of the tumor itself or of the host response to the tumor.18 Also, cognitive dysfunction in breast cancer patients without a brain tumor is associated with degree of fatigue, suggesting that general factors also determine cognitive status.19
In our series of patients, IDH mutations were correlated with relatively good performance in Memory, Psychomotor Speed, and Visuospatial Functioning, independent of tumor volume. This supports the hypothesis that the biology of the tumor plays a role independent of the size and edema of the tumor. It is unlikely that the protective cognitive effect of IDH mutations is due to the gene’s metabolic product. IDH1 mutations result in the production of 2-hydroxyglutarate, the accumulation of which can cause neurodegeneration and cognitive decline, as in D-2-hydroxyglutaric aciduria, a neurometabolic disease caused by germline IDH1 mutations.20–22 It is more likely that the favorable cognitive profile in IDH1-mutated tumors is caused by associated molecular-genetic characteristics in IDH-mutated tumors, which speculatively result in increased interaction with neighboring cells and associated metabolic changes, less growth velocity, and more additional plasticity of the surrounding nervous tissue. The latter explanation is supported by a recent study by Wefel et al, who found a complex interrelationship between patients’ NCF, tumor growth velocity, and the presence or absence of an IDH mutation.18 The suspected preferred location of IDH-mutated tumors for less-eloquent areas is another explanation for their more favorable cognitive profile.23 Nevertheless, these differences in preferred locations can again be driven by the genetic profile of the tumor. Whether additional oncobiological and metabolic changes in the tumors can influence the NCF independent of tumor volume and location is matter for further research that could lead to better, NCF-oriented, therapies.
Because we established IDH mutation in most cases by immunohistochemistry, it is possible that we missed a small proportion of IDH mutations, as immunohistochemistry determines only the presence of an IDH1 R132H mutation. Based on the literature, patients with other IDH1/2 mutations represent less than 10% of patients with an IDH mutation,24 so less than 5.5% of our sample wherein an IDH mutation was found in approximately 50% of patients.
The problem of neurocognitive dysfunction in glioma is often considered to be most relevant in LGG patients, given their relatively long survival and (often) young age. For this reason, awake surgery is performed in LGG patients more frequently than in HGG patients. However, analyses for subgroups based on glioma grade showed that HGG patients suffered from cognitive impairments significantly more often than patients with LGG. The latter finding illustrates the importance of cognitive dysfunction in HGG, implying that awake surgery should also be considered for HGG to preserve NCF. An explanation for the higher degree of impairments in cognitive functioning in HGG can be growth velocity/lesion momentum of the tumor, specifically in IDH wild-type tumors.25
Although less severe than in HGG, patients with treatment-naive LGG frequently exhibit subtle neurocognitive problems (threshold –1SD) that can still lead to significant problems in daily life and thereby be of influence on quality of life both of patients and their social environment.5 Strikingly, we found mean NCF scores in LGG patients that were significantly higher than norm scores on the group level (for the different domains). This may be caused by the strong motivation that patients undergoing glioma surgery exhibit during neuropsychological testing, which may exceed the motivation of “normal healthy controls.”26 This explanation would imply that the cognitive problems we found in the HGG patients may even be an underestimation.
Limitations of our study should be mentioned. Most important, selection bias might have played a role, because only patients undergoing awake surgery were included in our study sample. For this reason we compared baseline tumor- and patient-related characteristics of the included patients with patients who underwent glioma surgery under general anesthesia in the same period of time. Results of this comparison showed that the awake patients are a specific group of patients with relatively good clinical performance and they are often selected based on the localization of the tumor. In addition, the percentage of LGG patients is higher in the group of awake surgery patients than in the total glioma population. The selection of patients for awake operation who are not too severely affected probably caused an underestimation of the cognitive problems in the complete spectrum of glioma patients at the population level. This specifically is the case for location-independent domains, such as Psychomotor Speed, EF, and Attention; the latter domains are less often monitored during awake surgery. On the other hand, this selection for awake patients with tumors in more-eloquent areas could have led to an overestimation of neurocognitive deficits in location-dependent domains. However, as shown in Table 2, a significant proportion of our patients had tumor involvement of the right hemisphere, making our data unique in this field of research. Furthermore, our results especially included problems in location-independent domains, making an overestimation in domains such as language less likely.
A possible source of bias is the selective loss of patients who had insufficient neuropsychological data to perform analyses on. The reason for having insufficient data was often emergency surgery in case of rapid clinical decline, so this could have led to an underestimation of neurocognitive problems. We could also not rule out an effect of medication (AEDs and dexamethasone) fully. However, the use of AEDs and dexamethasone are consequences of the tumor and thereby predictors, instead of baseline factors with a possible causal relation with cognitive problems. Besides this, AED and dexamethasone use interfere too strongly with grade (and thereby IDH mutation), making it difficult to distinguish their effects in multivariable analyses.
We decided to group tasks by their conceptual background (“domain”) to enhance power; analyses per task would add up to an undesirable number of analyses and could potentially obscure findings for the overarching cognitive domain. The question of which cognitive concept (or domain) is best represented by a specific task is always complicated since intrinsically more than 1 concept is tapped in any task. However, neuropsychologists do share common ground in the categorization of tasks across domains.27–29 The affected domains apparently differed between our group-level and individual-level analyses. This is likely because in individual-level analysis, we considered the domain affected as a whole when a patient showed impairment-level scores on any single test within that domain. For this reason an effect of the amount of tests within 1 domain on our outcome measure (at the individual level) cannot be ruled out. However, in our analysis for the domain Memory, we found no association between the amount of tests performed and the percentage of impairment, which argues against the existence of such an effect.
Overall, most of the abovementioned limitations may lead to an underestimation of the degree of cognitive dysfunction at the individual patient level and group level per domain. Keeping this possible underestimation in mind, our data show that neurocognitive dysfunction is very common in patients with a diffuse glioma prior to surgery or other antitumor treatment, underscoring the importance of quantifying pretreatment NCF in glioma patients.
These findings support the hypothesis that the tumor itself contributes significantly to neurocognitive dysfunction in diffuse glioma (prior to antimitotic treatment) and that tumor biology might play a role in causing widespread disturbances in functional cerebral networks. Deeper knowledge of the degree and origins of tumor-related cognitive dysfunction will likely facilitate the development of new strategies for treatment and rehabilitation.
Funding
This work was supported by the Ton & Patricia Bohnenn Fund for Neuro-Oncology.
Supplementary Material
Acknowledgment
We thank Dr K.J. Miller for valuable comments on a previous version of this manuscript.
Conflict of interest statement. None declared.
References
- 1. Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. [DOI] [PubMed] [Google Scholar]
- 2. Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC.. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):992–999. [DOI] [PubMed] [Google Scholar]
- 3. Weitzner MA, Meyers CA, Byrne K. Psychosocial functioning and quality of life in patients with primary brain tumors. J Neurosurg. 1996;84(1):29–34. [DOI] [PubMed] [Google Scholar]
- 4. Anderson SW, Damasio H, Tranel D. Neuropsychological impairments associated with lesions caused by tumor or stroke. Arch Neurol. 1990;47(4):397–405. [DOI] [PubMed] [Google Scholar]
- 5. Boele FW, Zant M, Heine EC, et al. The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neurooncol Pract. 2014;1(2):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosma I, Douw L, Bartolomei F, et al. Synchronized brain activity and neurocognitive function in patients with low-grade glioma: a magnetoencephalography study. Neuro Oncol. 2008;10(5):734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ.. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tucha O, Smely C, Preier M, Lange KW.. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333; discussion 333–334. [DOI] [PubMed] [Google Scholar]
- 9. Warren DE, Power JD, Bruss J, et al. Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci U S A. 2014;111(39):14247–14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kölker S, Pawlak V, Ahlemeyer B, et al. NMDA receptor activation and respiratory chain complex V inhibition contribute to neurodegeneration in d-2-hydroxyglutaric aciduria. Eur J Neurosci. 2002;16(1):21–28. [DOI] [PubMed] [Google Scholar]
- 11. Talacchi A, Santini B, Savazzi S, Gerosa M.. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541–549. [DOI] [PubMed] [Google Scholar]
- 12. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115–1125. [DOI] [PubMed] [Google Scholar]
- 13. Yoshii Y, Tominaga D, Sugimoto K, et al. Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg Neurol. 2008;69(1):51–61; discussion 61. [DOI] [PubMed] [Google Scholar]
- 14. Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. [DOI] [PubMed] [Google Scholar]
- 15. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hart MG, Ypma RJ, Romero-Garcia R, Price SJ, Suckling J.. Graph theory analysis of complex brain networks: new concepts in brain mapping applied to neurosurgery. J Neurosurg. 2016;124(6):1665–1678. [DOI] [PubMed] [Google Scholar]
- 17. Corbetta M, Siegel JS, Shulman GL, et al. On the low dimensionality of behavioral deficits and alterations of brain network connectivity after focal injury. Cortex. 2018;107:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wefel JS, Noll KR, Rao G, Cahill DP.. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18(12):1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menning S, de Ruiter MB, Veltman DJ, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—the role of fatigue. Neuroimage Clin. 2015;7:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akbay EA, Moslehi J, Christensen CL, et al. D-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Dev. 2014;28(5):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Zhang LZ, Yi Y, et al. Genome-wide DNA methylation changes associated with olfactory learning and memory in Apis mellifera. Sci Rep. 2017;7(1):17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi S, Yu L, Li H, et al. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014;7:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agarwal S, Sharma MC, Jha P, et al. Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing. Neuro Oncol. 2013;15(6):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 26. Belayachi S, Majerus S, Gendolla G, Salmon E, Peters F, Van der Linden M.. Are the carrot and the stick the two sides of same coin? A neural examination of approach/avoidance motivation during cognitive performance. Behav Brain Res. 2015;293:217–226. [DOI] [PubMed] [Google Scholar]
- 27. Doherty JM, Belletier C, Rhodes S, et al. Dual-task costs in working memory: an adversarial collaboration [published online ahead of print November 8, 2018]. J Exp Psychol Learn Mem Cogn.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biesbroek JM, van Zandvoort MJ, Kappelle LJ, Velthuis BK, Biessels GJ, Postma A.. Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Struct Funct. 2016;221(4):2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ.. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23(5-6):408–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.