Abstract
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors are currently used as second-line therapy for treatment of patients with type 2 diabetes mellitus (T2DM). Based on the results from dedicated cardiovascular outcome trials (CVOTs), current guidelines suggest the use of SGLT-2 inhibitors for patients with T2DM and established atherosclerotic cardiovascular disease (ASCVD) or heart failure. The cardiovascular safety profile of dapagliflozin, a novel SGLT-2 inhibitor, has been recently explored in large CVOTs. Treatment with dapagliflozin reduced the risk of the composite outcome of cardiovascular mortality or hospitalization for heart failure compared with placebo, both among patients with T2DM who had or were at risk of ASCVD, as well as among patients with heart failure and a reduced ejection fraction. The observed cardiovascular benefit was mainly attributed to the lower rate of hospitalization for heart failure. Additionally, treatment with dapagliflozin was associated with a lower rate of renal adverse events. The safety and efficacy of dapagliflozin on glycemic and non-glycemic endpoints has been also well established in a series of other clinical trials and real-word studies. The aim of the present review is to summarize the available evidence regarding the cardiovascular profile of dapagliflozin in patients with T2DM. Overall, by reducing the rate of hospitalization for heart failure and ameliorating renal adverse events, dapagliflozin is a valuable option for the management of patients with T2DM and multiple cardiovascular risk factors.
Keywords: dapagliflozin, sodium-glucose co-transporter 2 inhibitors, SGLT-2 inhibitors, type 2 diabetes, cardiovascular risk
Introduction
Type 2 diabetes mellitus (T2DM) is a global epidemic affecting more than 450 million adults and increasing healthcare expenditures.1 It is well established that patients with T2DM have a higher risk of cardiovascular complications compared with the general population,2,3 while cardiovascular disease remains the leading cause of death.4 Approval of multiple novel glucose-lowering agents has provided clinicians with a wide array of available treatment options. At the same time, while improved glycemic control has been associated with beneficial effects on microvascular outcomes, the effect on cardiovascular endpoints has not been conclusively established.5–8 Hence, multiple cardiovascular outcome trials (CVOTs) have been conducted to clarify the effect of novel antidiabetic agents on major cardiovascular endpoints. Accumulating evidence has shown that some agents are associated with a net reduction in the rate of cardiovascular events.9,10 Hence, it is now advocated that choice of antidiabetic treatment should not be based solely on the potential for improved glycemic control but also on the ability of antihyperglycemic agents to confer cardiovascular benefits.
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors are a relatively new class of antidiabetic agents that improve glycemic control by blocking glucose reabsorption at the proximal tubule of the kidney thereby promoting urinary glucose excretion.11 Table 1 summarizes the pharmacokinetic parameters of SGLT-2 inhibitors approved by regulatory authorities in the United States or Europe.12,13
Table 1.
| SGLT-2 Inhibitor | Bioavailability | Time to Peak Action (Hours) | Half-Life (Hours) | SGLT-2 Inhibitor Selectivity Over SGLT-1 |
|---|---|---|---|---|
| Canagliflozin | ~ 65% | 1–2 h | 11–13 h | 1:414 |
| Dapagliflozin | ~ 78% | 1–1.5 h | 13 h | 1:1200 |
| Empagliflozin | ~75% | 1.5 h | 13 h | 1:2500 |
| Ertugliflozin | 70–90% | 0.5–1.5 h | 11–17 h | 1:2000 |
Abbreviation: SGLT-2, sodium-glucose co-transporter 2.
Results from CVOTs have shown that treatment with empagliflozin and canagliflozin reduces the risk of major adverse cardiovascular events as well as the risk of the composite outcome of cardiovascular death or hospitalization for heart failure.14,15 The dedicated CVOT for dapagliflozin (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58, DECLARE-TIMI 58) has demonstrated its cardiovascular safety and its favorable effect on reducing the risk of hospitalization for heart failure and the occurrence of renal adverse events in patients with T2DM and high cardiovascular risk.16 Additionally, in a recently published long-term phase 3 trial treatment with dapagliflozin was associated with cardiovascular benefit in patients with heart failure irrespective of the presence of T2DM.17 The antihyperglycemic efficacy of dapagliflozin has been also well established in a large number of studies, which demonstrated its beneficial effect on multiple additional outcomes, including body weight and blood pressure.18 In light of emerging evidence, aim of the present review is to summarize the role of dapagliflozin in reducing the cardiovascular risk in patients with T2DM.
DECLARE-TIMI 58 Trial
DECLARE-TIMI 58 evaluated the effect of dapagliflozin on cardiovascular and renal outcomes in patients with type T2DM. In the largest CVOT in diabetes conducted to date, 17,160 T2DM patients with a creatine clearance of at least 60 mL/min were randomized in a double-blind manner to receive dapagliflozin or placebo. Among participants, 6974 (40.6%) had established atherosclerotic cardiovascular disease (ASCVD) while the rest (10,186 patients, 59.4%) had multiple risk factors for ASCVD. The primary outcomes included a composite outcome of major adverse cardiovascular events (MACE) defined as cardiovascular death, myocardial infarction or ischemic stroke, and a composite outcome of cardiovascular death or hospitalization for heart failure. Predefined secondary outcomes assessed included a renal composite outcome, defined as a sustained decrease of 40% or more in estimated glomerular filtration rate (eGFR) to less than 60 mL/min/1.73 m2, new end-stage renal disease or death from renal or cardiovascular causes, or death from any cause.19
After a median follow-up of 4.2 years, dapagliflozin was noninferior to placebo with respect to the risk of MACE. The incidence of MACE was 8.8% in the dapagliflozin group (756 patients with a MACE event out of a total of 8582) and 9.4% in the placebo group (803 patients out of a total of 8578). The hazard ratio (HR) for MACE between treatment arms was 0.93 (95% confidence interval [CI] 0.84 to 1.03; P=0.17). Treatment with dapagliflozin was associated with a 17% reduction in the composite outcome of cardiovascular death or hospitalization for heart failure (HR 0.83, 95% CI 0.73 to 0.95; P=0.005), with 417 patients experiencing an event in the dapagliflozin group compared with 496 patients in the placebo group. This finding was driven by the lower incidence of hospitalizations for heart failure in the dapagliflozin group (2.5% in the dapagliflozin group versus 3.3% in the placebo group, HR 0.73; 95% CI 0.61 to 0.88), while no difference between the two treatment groups was evident in the risk of cardiovascular death (HR 0.98; 95% CI 0.82 to 1.17).16 The favorable effect of dapagliflozin on the composite outcome was more prominent among patients with established ASCVD (7.8% in patients treated with dapagliflozin versus 9.3% among patients treated with placebo, HR 0.83; 95% CI 0.71 to 0.98) as opposed to patients with multiple risk factors (2.8% in the dapagliflozin arm versus 3.4% in the placebo arm, HR 0.84; 95% CI 0.67 to 1.04).
Among the 17,160 patients enrolled in the DECLARE-TIMI 58 trial, 1987 (11.6%) had a history of heart failure, 3.9% with reduced ejection fraction (<45%) and 7.7% without known reduced ejection fraction. Treatment with dapagliflozin reduced the risk of cardiovascular death or hospitalization for heart failure both in patients with or without heart failure (HRs 0.79; 95% CI 0.63 to 0.99 and 0.84; 95% CI 0.72 to 0.99 respectively). Furthermore, a subgroup analysis based on the left ventricular ejection fraction at baseline showed that in patients with heart failure and reduced ejection fraction treatment with dapagliflozin resulted in reduction of the relative risk of the composite outcome of cardiovascular death or hospitalization for heart failure compared with placebo (HR 0.62; 95% CI 0.45 to 0.86). Analyses for the individual outcomes showed that dapagliflozin reduced both the risk of hospitalization for heart failure (HR 0.64; 95% CI 0.43 to 0.95) as well as the risk of cardiovascular death and all-cause mortality (HR 0.55; 95% CI 0.34 to 0.90 and 0.59; 95% CI 0.40 to 0.88 respectively) in the aforementioned subgroup.20
Ischemic stroke events occurred in 235 patients (2.7%) in the dapagliflozin group and in 231 patients (2.7%) in the placebo group. No difference was noted between the two treatment arms (HR 1.01; 95% CI 0.84 to 1.21). Fewer patients in the dapagliflozin group experienced a myocardial infarction (4.6% in the dapagliflozin arm versus 5.1% in the placebo arm), nevertheless the between group difference did not reach statistical significance (HR 0.80; 95% CI 0.77 to 1.01). In a subgroup analysis in patients with history of myocardial infarction (n = 3584 patients) treatment with dapagliflozin reduced the risk both for MACE (HR 0.84; 95% CI 0.72 to 0.99; P=0.039) as well as for the composite outcome of cardiovascular death or hospitalization for heart failure (HR 0.81; 95% CI 0.65 to 1.00; P=0.046), compared with placebo.21 All-cause mortality was similar between patients treated with dapagliflozin or placebo (HR 0.93; 95% CI 0.82 to 1.04).
Treatment with dapagliflozin was also associated with a clinically meaningful reduction in the rate of renal events. The cardiorenal secondary composite of ≥ 40% decrease in eGFR to < 60 mL/min/1.73 m2, new end-stage renal disease or death from renal or cardiovascular cause occurred less often in patients treated with dapagliflozin (370 patients out of a total of 8582) than in the placebo group (480 patients out of a total of 8578) (HR 0.76; 95% CI 0.67 to 0.87).22 Similarly, dapagliflozin resulted in a 47% lower risk of development of the renal-specific outcome, which included all the aforementioned individual components except for cardiovascular death (HR 0.53; 95% CI 0.43 to 0.66). The favorable effect was evident both among patients with established cardiovascular disease (HR 0.55; 95% CI 0.41 to 0.75; P=0.0001) and with multiple cardiovascular risk factors (HR 0.51; 95% CI 0.37 to 0.69; P<0.0001) as well as in patients with baseline eGFR ≥90 mL/min/1.73 m2 (HR 0.50; 95% CI 0.34 to 0.73; P=0.0003) or eGFR levels between 60 to 90 mL/min/1.73 m2 (HR 0.54; 95% CI 0.40 to 0.73; P<0.0001). A similar trend, albeit marginally non significant, was observed in patients with eGFR <60 mL/min per 1.73m2 (HR 0.60; 95% CI 0.35 to 1.02; P=0.059). Additionally, in subgroup analyses of the individual components of the composite renal outcomes, treatment with dapagliflozin reduced the risk of a sustained eGFR decrease of ≥ 40% to an eGFR of less than 60 mL/min/1.73 m2 (HR 0.54; 95% CI 0.43 to 0.67; P<0.0001) and the risk of end-stage renal disease (HR 0.31, 95% CI 0.13 to 0.79, P=0.013) in the overall population. Finally, although treatment with dapagliflozin resulted in a greater decrease in eGFR levels in the first year after randomization, by 3 and 4 years dapagliflozin preserved kidney function decline compared to placebo.22
Evidence from Phase 2 and Phase 3 Clinical Trials
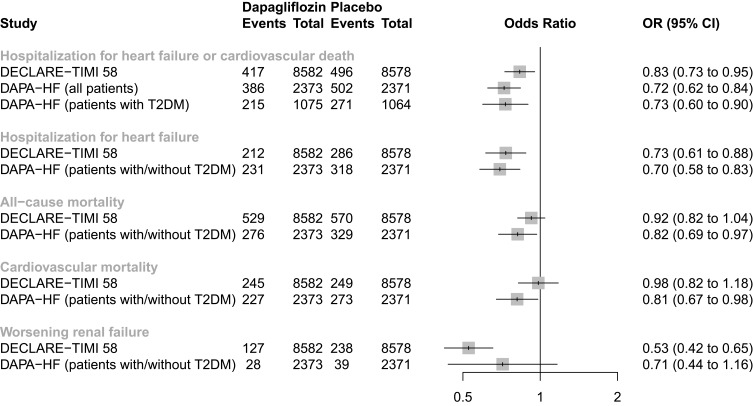
In addition to the advantages of reduced cardiovascular or renal adverse events observed in the DECLARE-TIMI 58 trial, several other clinical trials evaluating the therapeutic effect of dapagliflozin have also demonstrated its efficacy and safety. In a recently published phase 3, long-term cardiovascular outcome trial (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure, DAPA-HF), 4744 patients with chronic heart failure with reduced ejection fraction with or without T2DM were randomized to dapagliflozin or placebo, in addition to standard therapy. Consistent with the findings of the DECLARE-TIMI 58 trial, treatment with dapagliflozin over a median of about 18 months reduced the primary composite outcome of worsening heart failure (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) or cardiovascular death, both in patients without (HR 0.73; 95% CI 0.60 to 0.88) and with T2DM (HR 0.75; 95% CI 0.63 to 0.90).17 Figure 1 depicts the effect of dapagliflozin on major cardiovascular endpoints, as assessed in the two long-term cardiovascular outcome trials (DECLARE-TIMI 58 and DAPA-HF trial). Additionally, in the 12-week randomized DEFINE-HF (Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction) trial, use of dapagliflozin in 263 patients with heart failure with reduced ejection fraction, with or without T2DM, resulted in fewer heart failure-related symptoms and improved quality of life, compared with placebo.23 However, dapagliflozin was not associated with a reduction in the primary outcome of change in the mean N-terminal pro b-type natriuretic peptide (NT-proBNP) levels.
Figure 1.
Cardiovascular outcome trials assessing the effect of dapagliflozin on major cardiovascular outcomes.
Abbreviations: CI, confidence interval; OR, odds ratio.
The efficacy and safety of dapagliflozin in patients with T2DM and renal impairment (eGFR ≥45 to <60 mL/min/1.73m2, chronic kidney disease stage 3A) has also been investigated in the recently published double-blind, 24-week DERIVE trial;24 treatment with dapagliflozin resulted in greater reductions of HbA1c, body weight, and systolic blood pressure compared with placebo, without increasing the risk of adverse events. In line with these findings are the results of a pooled analysis of 11 Phase 3 clinical trials (n = 4404 patients) across several degrees of renal function.25 Compared with placebo, dapagliflozin reduced blood pressure, body weight and albuminuria regardless of baseline eGFR levels (eGFR ≥ 45 to < 60 mL/min/1.73 m2, eGFR ≥ 60 to < 90 mL/min/1.73 m2, eGFR ≥ 90 mL/min/1.73 m2). These findings suggest that the renoprotective effects of dapagliflozin are probably independent of glucosuria, and support the drug’s safety for treating patients with T2DM and mild renal impairment. The cardiorenal safety profile of dapagliflozin, its mechanism of action and its therapeutic role in patients with cardiovascular disease will be further elucidated by the results of several planned or ongoing clinical trials in patients with heart failure or chronic kidney disease, with or without T2DM (Table 2).
Table 2.
Ongoing Clinical Trials Assessing Dapagliflozin in Patients with Heart Failure or Chronic Kidney Disease
| Study Name | Treatment Arms | Duration | Population | Primary Outcome |
|---|---|---|---|---|
| DETERMINE-reduced - Dapagliflozin Effect on Exercise Capacity Using a 6 min Walk Test in Patients With Heart Failure With Reduced Ejection Fraction; NCT0387723740 | Dapagliflozin, placebo | 16 weeks | 300 patients with HFrEF | Change from baseline in 6 min walking distance |
| Effect of Dapagliflozin Plus Low Dose Pioglitazone on Hospitalization Rate in Patients With HF and HFpEF; NCT0379451841 | Dapagliflozin plus pioglitazone, placebo | 3 years | 648 patients with T2DM and HFpEF | Time to first hospitalization for heart failure after starting intervention |
| Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure. (DELIVER); NCT0361921342 | Dapagliflozin, placebo | 33 months | 4700 patients with or without T2DM and HFpEF | Time to the first occurrence of any of the components of the composite outcome: CV death, hospitalization for HF, or urgent HF visit |
| DETERMINE-preserved -Dapagliflozin Effect on Exercise Capacity Using a 6 min Walk Test in Patients With Heart Failure With Preserved Ejection Fraction; NCT0387722443 | Dapagliflozin, placebo | 16 weeks | 400 patients with HFpEF | Change from baseline in 6 min walking distance |
| Dapagliflozin in PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF); NCT0303023544 | Dapagliflozin, placebo | 12 weeks | 320 patients with HFpEF | Change from baseline in NTproBNP |
| Impact of Dapagliflozin on DIAstolic Dysfunction in Type 2 Diabetic Patients; NCT0275139845 | Dapagliflozin, placebo | 24 weeks | 60 patients with T2DM and diastolic dysfunction | Subclinical diastolic dysfunction assessed by supine bicycle diastolic stress echocardiography |
| A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (Dapa-CKD); NCT0303615046 | Dapagliflozin, placebo | 4 years | 4000 patients with or without T2DM and CKD | Time to the first occurrence of any of the components of the composite outcome: ≥50% sustained decline in eGFR or reaching ESRD or CV death or renal death |
Abbreviations: CKD, chronic kidney disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; mo, months; NTproBNP, N-terminal pro b-type natriuretic peptide; T2DM, type 2 diabetes mellitus.
Several meta-analyses have also investigated the effect of dapagliflozin in a wide array of outcomes. A meta-analysis of 21 Phase 2b and Phase 3 trials from the dapagliflozin clinical development program summarized the cardiovascular safety profile of dapagliflozin in patients with T2DM.26 Based on data from 9339 patients, there was no difference compared to the control group in the risk of MACE both in the overall population (HR 0.77; 95% CI 0.54 to 1.10) as well as in the subgroup of patients with a history of cardiovascular disease (HR 0.80; 95% CI 0.53 to 1.22), or in elderly patients aged ≥65 years with a history of cardiovascular disease and hypertension (HR 0.92; 95% CI 0.51 to 1.64). Interestingly, among patients with established cardiovascular disease, treatment with dapagliflozin also resulted in a significantly lower rate of hospitalization for heart failure (HR 0.37; 95% CI 0.16 to 0.89) compared with the control group. Additionally, in a meta-analysis evaluating the effect of SGLT-2 inhibitors on the incidence of stroke based on data from 75,540 patients with T2DM, there was no difference among patients treated with dapagliflozin compared to placebo (Risk Ratio [RR] 0.99; 95% CI 0.9 to 1.09).27 In another meta-analysis of 58 trials assessing the efficacy and safety of SGLT-2 inhibitors in 16,407 patients with T2DM, dapagliflozin decreased HbA1c and body weight by 0.59% and 1.9 kg respectively compared with placebo, and was also associated with a reduction both of the systolic (–3.20 mmHg; 95% CI –4.20 to –2.21) and the diastolic blood pressure (–1.74 mmHg, 95% CI –2.35 to –1.13).28 Finally, the favorable effect of dapagliflozin on the risk for hospitalization for heart failure or progression of renal disease is in line with the cardiorenal protection conferred by SGLT-2 inhibitors as a drug class. In a meta-analyis of three cardiovascular outcome trials in 34,322 patients with T2DM with or without established ASCVD, SGLT-2 inhibitors reduced the risk for a MACE-3 composite outcome (HR 0.89, 95% CI 0.83 to 0.96), cardiovascular death or hospitalization for heart failure (HR 0.77, 95% CI 0.71 to 0.84) as well as progression of renal disease (HR 0.55, 95% CI 0.48 to 0.64).29
Real-Word Data
Real-world evidence from large observational studies evaluating the effect of treatment with dapagliflozin on cardiovascular endpoints are in line with findings from randomised clinical trials. In an observational study assessing a real-world population similar to the one recruited in the DECLARE-TIMI 58 trial, initiation of dapagliflozin resulted in a 21% reduction in the composite outcome of hospitalization for heart failure or cardiovascular mortality (HR 0.79; 95% CI 0.69 to 0.92), and had a neutral effect on the risk of MACE (HR 0.90; 95% CI 0.79 to 1.03) compared with other glucose-lowering drugs.30 Treatment with dapagliflozin also resulted in a lower risk of both individual components of hospitalization for heart failure and cardiovascular mortality (HRs 0.79; 95% CI 0.67 to 0.93 and 0.75; 95% CI 0.57 to 0.97 respectively). In accordance with the findings of DECLARE-TIMI 58, there was no difference in the risk of myocardial infarction or stroke compared to other glucose-lowering agents (HRs 0.91; 95% CI 0.74 to 1.11 and 1.06; 95% CI 0.87 to 1.30 respectively). Moreover, in the CVD-REAL Nordic multinational observational study, real-world data from national registries were analyzed to assess the cardiovascular morbidity and mortality among new users of SGLT-2 inhibitors or other antidiabetic agents.31 In a population of 91,320 T2DM patients with a broad cardiovascular risk profile who were treated mainly with dapagliflozin (94% treated with dapagliflozin vs. 5% with empagliflozin and 1% with canagliflozin), new use of an SGLT-2 inhibitor significantly reduced the risk of cardiovascular mortality (HR 0.53; 95% CI 0.40 to 0.71), MACE (HR 0.78; 95% CI 0.69 to 0.87) or hospital events for heart failure (HR 0.70; 95% CI 0.61 to 0.81) compared with other glucose-lowering agents.
Non-Cardiovascular Safety Assessment
In the DECLARE-TIMI 58 trial, treatment with dapagliflozin was well tolerated and resulted to a lower rate of major hypoglycemia or serious adverse events compared with placebo (0.68; 95% CI 0.49 to 0.95 and HR 0.91; 95% CI 0.87 to 0.96 respectively). Furthermore, there was no difference in the risk of amputations, fractures, urinary tract infections or volume depletion associated adverse events.16 The safety profile of dapagliflozin was also corroborated in a pooled analysis of safety data from Phase IIb/III trials. Based on data from 13 placebo-controlled trials in 4655 patients with T2DM and treatment duration of up to 24 weeks, there was no difference between dapagliflozin and placebo in the incidence of overall adverse events, including hypoglycemia, urinary tract infections and volume depletion events.32 Genital infections were the most common adverse events associated with treatment with dapagliflozin (5.5% of patients treated with dapagliflozin compared to 0.6% of patients treated with placebo), while caution may be also warranted for diabetic ketoacidosis. Finally, there was no difference in the incidence of fractures or lower limb amputations between dapagliflozin and placebo/control groups.
Role of Dapagliflozin in Clinical Practice
Dapagliflozin, a selective SGLT-2 inhibitor, is currently licensed for the treatment of adults with T2DM inadequately controlled with diet and exercise, either as add-on therapy to other hypoglycemic agents or as monotherapy when metformin is contraindicated or not tolerated.33 Initiation of treatment is allowed only in patients with an eGFR above 60 mL/min/1.73 m2, nevertheless in patients already treated with dapagliflozin it can be used up to an eGFR of 45 mL/min/1.73 m2.
Recently released guidelines for the management of T2DM suggest that clinicians should consider the presence or absence of cardiovascular disease, heart failure or chronic kidney disease when making decisions about optimal treatment.34 Based on the results from CVOTs, the use of empagliflozin, canagliflozin or glucagon-like peptide 1 receptor agonists (GLP-1 RAs) with proven cardiovascular benefit is preferred in the presence of ASCVD or heart failure, while other agents, such as thiazolidinediones, should be avoided. Publication of the DECLARE-TIMI 58 results has led to an update of treatment guidelines.35 Dapagliflozin was no different than placebo in the incidence of the composite outcome of cardiovascular death, myocardial infarction or ischemic stroke in patients with or without established ASCVD. Nevertheless it led to a 27% reduction in the hospitalization for heart failure that was consistent in patients both with or without history of heart failure, hence could be considered for primary or secondary prevention of heart failure. In fact, in patients with heart failure and reduced ejection fraction, dapagliflozin reduced the relative risk of hospitalization for heart failure by 36% and was also associated with a clinically meaningful reduction in the rate of cardiovascular death or all-cause mortality by 45% and by 41% respectively.20 Based on these beneficial findings, dapagliflozin has recently been approved in the United States to reduce the risk of hospitalization for heart failure in patients with type 2 diabetes and cardiovascular risk factors.36
Additionally, in line with the findings for empagliflozin and canagliflozin, treatment with dapagliflozin is also associated with clinically important renoprotective effects in patients with T2DM, leading to a 24% reduction in the risk of the primary composite renal outcome and to a 47% reduction in the risk of a secondary renal-specific composite outcome in the DECLARE-TIMI 58 trial, both in patients with established ASCVD as well as in patients with multiple cardiovascular risk factors. Reflecting these therapeutic advantages, the recently released guidelines issued in collaboration by the European Society of Cardiology and the European Association for the Study of Diabetes recommend the use of SGLT-2 inhibitors in patients with T2DM and high cardiovascular risk to reduce the risk of hospitalization for heart failure or progression of diabetic kidney disease.37 Finally, contrary to respective ambiguous findings for canagliflozin,15 treatment with dapagliflozin was safe regarding the risk of amputation and fracture events.
Differences in the results among CVOTs of individual SGLT-2 inhibitors should be reviewed in the context of differences in the study design and patients recruited, which may affect the interpretation and translation to clinical practice. The EMPA-REG Outcome trial included solely patients with T2DM and established CV disease, while in the CANVAS program 66% of participants had a history of CV disease and the majority of patients in the DECLARE-TIMI trial had only risk factors for developing ASCVD (59.4%). Additionally, compared with the EMPA-REG and the CANVAS trial which recruited patients with baseline eGFR levels up to 30 mL/min/1.73 m2, patients recruited in the DECLARE-TIMI 58 trial had a creatinine clearance of at least 60 mL/min. These differences in the inclusion criteria and the baseline characteristics of patients included may partially explain the differences observed in the effect estimates between the EMPA-REG Outcome trial, the CANVAS program and the DECLARE-TIMI 58 trial.
Several molecular mechanisms have been suggested to explain the cardiovascular protective effect observed with SGLT2- inhibitors. Inhibition of reabsorption of glucose and sodium at the proximal tubule enhances glucosuria and natriuresis, hence reducing plasma volume, cardiac preload, afterload, blood pressure and arterial stiffness, and improves subendocardial blood flow.38,39 Additionally, natriuresis at the proximal tubule increases sodium concentration in the macula densa and activates the tubuloglomerular feedback, hence causing vasoconstriction of the afferent arteriole and vasodilation of the efferent arteriole by inhibition of renin release by juxtaglomerular cells. Although these changes are associated with a decline of eGFR following initiation of SGLT-2 inhibitors, the long-term reduction of the intraglomerular pressure lowers albuminuria and glomerular fibrosis, hence resulting in an amelioration of kidney function decline.38,39 Ongoing trials designed to explore the effect of dapagliflozin on the risk of heart failure and cardiovascular death in patients with heart failure (with or without diabetes) are expected to clarify the underlying mechanisms explaining the improvement of cardiovascular and renal outcomes with dapagliflozin.
Conclusions
The management of patients with T2DM may be complicated by the presence of ASCVD or chronic kidney disease. Guidelines for the management of type 2 diabetes suggest the use of SGLT-2 inhibitors or GLP-1 receptor agonists that can reduce the risk of cardiovascular events and delay the progression of renal disease as second line therapy after metformin. Data both from the dedicated cardiovascular outcomes trials and real world studies suggest that dapagliflozin is safe and reduces the rate of hospitalization for decompensated heart failure whilst also ameliorating renal adverse events. Dapagliflozin is a valuable addition to the therapeutic armamentarium for the management of patients with T2DM and multiple cardiovascular risk factors.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report that they do not have any financial or other relationships, which might lead to a conflict of interest regarding this paper.
References
- 1.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664 [DOI] [PubMed] [Google Scholar]
- 3.McGuire DK, Gore MO, Masoudi FA. Diabetes and heart failure in patients with coronary disease: separating markers from mediators. Diabetes Care. 2010;33(9):2120–2122. doi: 10.2337/dc10-1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 7.Matthews DR, Tsapas A. Four decades of uncertainty: landmark trials in glycaemic control and cardiovascular outcome in type 2 diabetes. Diabetes Vasc Dis Res. 2008;5(3):216–218. doi: 10.3132/dvdr.2008.036 [DOI] [PubMed] [Google Scholar]
- 8.Tsapas A, Matthews DR. N of 1 trials in diabetes: making individual therapeutic decisions. Diabetologia. 2008;51(6):921–925. doi: 10.1007/s00125-008-0983-2 [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes. JAMA. 2016;316(3):313. doi: 10.1001/jama.2016.9400 [DOI] [PubMed] [Google Scholar]
- 10.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes. JAMA. 2018;319(15):1580. doi: 10.1001/jama.2018.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium–glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75(12):1272–1277. doi: 10.1038/ki.2009.87 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Ropero A, Badimon JJ, Santos-Gallego CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicol. 2018;14(12):1287–1302. doi: 10.1080/17425255.2018.1551877 [DOI] [PubMed] [Google Scholar]
- 13.Madaan T, Akhtar M, Najmi AK. Sodium glucose CoTransporter 2 (SGLT2) inhibitors: current status and future perspective. Eur J Pharm Sci. 2016;93:244–252. doi: 10.1016/j.ejps.2016.08.025 [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 15.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 16.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2014;30(3):204–221. doi: 10.1002/dmrr.2479 [DOI] [PubMed] [Google Scholar]
- 19.Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the dapagliflozin effect on cardiovascular events (DECLARE)–TIMI 58 Trial. Am Heart J. 2018;200:83–89. doi: 10.1016/j.ahj.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 20.Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139(22):2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130 [DOI] [PubMed] [Google Scholar]
- 21.Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516–2527. doi: 10.1161/CIRCULATIONAHA.119.039996 [DOI] [PubMed] [Google Scholar]
- 22.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9 [DOI] [PubMed] [Google Scholar]
- 23.Nassif ME, Windsor S, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140(18):1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929 [DOI] [PubMed] [Google Scholar]
- 24.Fioretto P, Del Prato S, Buse JB, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE Study. Diabetes Obes Metab. 2018;20(11):2532–2540. doi: 10.1111/dom.13413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrykiv S, Sjöström CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12(5):751–759. doi: 10.2215/CJN.10180916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonesson C, Johansson PA, Johnsson E, Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol. 2016;15(1):37. doi: 10.1186/s12933-016-0356-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo M, Ding J, Li J, et al. SGLT2 inhibitors and risk of stroke in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(8):1977–1982. doi: 10.1111/dom.13295 [DOI] [PubMed] [Google Scholar]
- 28.Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–274. doi: 10.7326/0003-4819-159-4-201308200-00007 [DOI] [PubMed] [Google Scholar]
- 29.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 30.Norhammar A, Bodegård J, Nyström T, Thuresson M, Nathanson D, Eriksson JW. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21(5):1136–1145. doi: 10.1111/dom.13627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–717. doi: 10.1016/S2213-8587(17)30258-9 [DOI] [PubMed] [Google Scholar]
- 32.Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20(3):620–628. doi: 10.1111/dom.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forxiga, INN-dapagliflozin - European Medicines Agency. Available from: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf. Accessed April26, 2019.
- 34.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019 [web annotation]. Diabetes Care. 2019;42(Suppl.1):S90–S102. [DOI] [PubMed] [Google Scholar]
- 36.Farxiga approved in the US to reduce the risk of hospitalisation for heart failure in patients with type-2 diabetes. Available from: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2019/farxiga-approved-in-the-us-to-reduce-the-risk-of-hospitalisation-for-heart-failure-in-patients-with-type-2-diabetes-21102019.html. Accessed October22, 2019.
- 37.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019. doi: 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 38.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure. Circulation. 2017;136(17):1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in Diabetes. J Am Coll Cardiol. 2018;72(15):1845–1855. doi: 10.1016/j.jacc.2018.06.040 [DOI] [PubMed] [Google Scholar]
- 40.DETERMINE-reduced - dapagliflozin effect on exercise capacity using a 6-minute walk test in patients with heart failure with reduced ejection fraction. Available from: https://clinicaltrials.gov/ct2/show/NCT03877237. Accessed May8, 2019.
- 41.Effect of dapagliflozin plus low dose pioglitazone on hospitalization rate in patients with HF and HFpEF. Available from: https://clinicaltrials.gov/ct2/show/NCT03794518. Accessed May8, 2019.
- 42.Dapagliflozin evaluation to improve the lives of patients with preserved ejection fraction heart failure. Available from: https://clinicaltrials.gov/ct2/show/NCT03619213. Accessed May8, 2019.
- 43.DETERMINE-preserved - Dapagliflozin effect on exercise capacity using a 6-minute walk test in patients with heart failure with preserved ejection fraction. Available from: https://clinicaltrials.gov/ct2/show/NCT03877224. Accessed May8, 2019.
- 44.Dapagliflozin in preserved ejection fraction heart failure. Available from: https://clinicaltrials.gov/ct2/show/NCT03030235. Accessed May8, 2019.
- 45.Impact of dapagliflozin on diastolic dysfunction in type 2 diabetic patients. Available from: https://clinicaltrials.gov/ct2/show/NCT02751398. Accessed May8, 2019.
- 46.A study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease. Available from: https://clinicaltrials.gov/ct2/show/NCT03036150. Accessed May8, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Forxiga, INN-dapagliflozin - European Medicines Agency. Available from: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf. Accessed April26, 2019.