Abstract
Background/Aim: Natural mofettes are gases resulting from post-volcanic emanations. This study aimed to examine the effect of mofette therapy on plasma oxidative stress and antioxidant parameters in rats after experimental induction of myocardial ischemia, as well as on structural changes in myocardial tissue. Materials and Methods: White Wistar-Bratislava rats were divided into three groups. In groups 2 and 3, myocardial ischemia was induced by isoproterenol. Rats in group 3 were additionally exposed to high levels mofettes. Oxidative stress and antioxidant parameters were determined in plasma. The structural changes of the myocardium were observed in paraffin embedded slices contrasted using Goldner’s trichrome staining. Results: A statistically significant change in serum oxidative stress biomarkers, including nitric oxide, malondialdehyde, total oxidant status, as well as in the tested antioxidant molecules and total antioxidant capacity were observed in group 3 compared to group 2. Also, rats of group 3 showed an obvious improvement in inflammatory infiltration and repair of necrotic areas through collagen proliferation (proliferation of fibrous connective tissue) compared to group 2. Conclusion: Mofette had a beneficial effect on the balance between oxidative stress and antioxidant status following experimentally induced myocardial ischemia.
Keywords: Moffete, oxidative stress, antioxidant plasma capacity, myocardial ischemia, histology
Natural mofettes are gases resulting from natural post-volcanic emanations (1) that are found in certain areas of Romania, e.g. in the Harghita volcanic massif, from which carbon dioxide, present in a 95-98% proportion, is used as a therapeutic factor (2,3). Along with carbon dioxide, mofette gas contains small amounts of other volcanic emission gases: ammonium, sulfur, helium and radon (4,5), which induce pulverization of CO2 molecules, increasing the penetration power of CO2 (1-3). Mofettes also contain positive and negative air ions, 2,000-15,000 ions/cm3, and have a radioactivity of 0.3 μCi/l, without cancer risk, and a radon concentration lower than 1% (4).
In Romania, there are natural mofettes in the spa resorts Covasna, Băile Tuşnad, Balvanyos, Balnaş, Harghita Băi, Buziaş, Borşa, Slănic Moldova, Sangeorz Băi (2,3,6,7). Mofettes, as natural phenomena, are extremely rare in Europe and the rest of the world; except those present in Romania, they can be found only in Hungary, in Grotta di Cani near Naples, in Marienberg and Andernach in Germany, in Tiskory Lanz in Czechia, in Jawa island and in the Yellowstone Park in the USA (8).
Given their therapeutic efficacy, in many medical rehabilitation centers, artificial mofettes supplied from external sources have been developed (1-3). Mofette gas is indicated for certain diseases, such as peripheral arterial disease stage 1, 2 and 3, arteritis, venopathy, leg ulcers, chronic stable ischemic heart disease, stable hypertension stage 1 and 2, dermatological, gynecological diseases, chronic degenerative rheumatism (2,3). However, the major indication of mofette is in secondary prevention of cardiovascular disease and cardiovascular disease rehabilitation (6,7).
The exclusive use of the properties of carbon dioxide is possible by eliminating the mechanical factor, hydrostatic pressure, the thermal factor and gas bubble micromassage that are added to the carbonated mineral bath (2,3). Natural mofette is considered to induce an increase in blood flow through penetration of CO2 into the skin and muscles, which has a direct action on the smooth muscles of blood vessels, particularly on very small vessels and arterioles, an action estimated to be superior to that of peripheral vasodilatory drugs (1-3).
Research performed on the natural mofette found in Covasna spa resort, Romania, showed some of its mechanisms of action, i.e. the effect of carbon dioxide inhaled by patients during mofette therapy (carbon dioxide concentration in the air 1.5-2 volumes %, demonstrated by capnography); following inhalation of carbon dioxide, an increase in cerebral blood flow, up to 75%, was found (2,3,6,7). During therapy, CO2 inhaled and dissolved in plasma, and absorbed into the skin, has many effects on the body; thus, at the level of peripheral circulation, it induces skin hyperemia by direct action on metarterioles, with an increase in skin temperature by 1.5-4 degrees C and a 44% increase in skeletal muscle blood flow (1-3).
There are also effects on cardiac hemodynamics by causing arterial vasodilation, a decrease in the pre-ejection period, an increase in the ejection period, and a reduction in blood pressure with consequences on peripheral oxygen use, which explains the improvement of cardiac performance parameters as well as the exercise capacity in coronary patients and patients with myocardial infarction sequelae (1-3,6).
The aim of this study was to observe the effect of treatment with mofette on plasma oxidative stress and on antioxidant parameters in rats after experimental induction of myocardial ischemia. The effect was assessed by the quantitative evaluation of these parameters and by the optical microscopic examination of structural changes in myocardial tissue.
Materials and Methods
Experimental protocol. The study was approved by the Ethics Committee of “Iuliu Hațieganu” University of Medicine and Pharmacy Cluj-Napoca (approval no. 391/16.10.2018) and the Sanitary Veterinary Authority (approval no. 146/29.11.2018). All regulations of the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes were respected.
White Wistar-Bratislava rats with a weight of 250-300 g were obtained from the research Biobase of the University of Medicine and Pharmacy Cluj-Napoca and kept in polypropylene cages at constant temperature (24±2˚C), 50±15% humidity, under a light-dark regime. Measures were taken to reduce suffering of animals; during the experiment there were no food or water restrictions.
Experimental design. In this study, 25 rats were randomly assigned to the groups, as follows: group 1 included 7 rats, and groups 2 and 3 comprised 9 rats each. Two rats of groups 2 and 3 were euthanized at 4 days after induction of myocardial ischemia in order to detect possible microscopic changes in the myocardium. Adequate protocols were applied to each group (Table I).
Table I. Experimental groups, protocol description.
Myocardial ischemia was induced by a single dose of isoproterenol (45 mg/BW, s.c.) (9). In group 2, myocardial ischemia was induced. In group 3, after induction of myocardial ischemia, the animals were taken to the mofette in Baile Tusnad daily, for 2 weeks, to be exposed to high levels of carbon dioxide, dry gas. During the first 2 days, rats were exposed for 5 min/day, over the next 2 days were exposed for 10 min/day, and starting from day 5, the animals were exposed for 15 min/day. No animal death occurred during the experiment. The mofette in Baile Tusnad is a natural mofette that contains carbon dioxide in a 90-95% proportion.
After the two weeks of treatment, 1 ml blood was collected from the retrobulbar sinus at the internal angle of the eye of each animal, using heparinized capillaries, at 8 a.m. Blood collection was performed under general anesthesia with a mixture of ketamine 10% and xylazine 2%, in a dose of 8 mg/kg body weight.
Euthanasia was performed by intramuscular injection of anesthetic. After euthanasia, heart tissue samples were taken for histological examination.Oxidative stress parameters, including nitric oxide (NOx), malondialdehyde (MDA), total oxidant status (TOS), as well as antioxidant plasma status, catalase (CAT) and total antioxidant capacity (TAC), were assessed in the collected blood according to the method of Bulboaca et al. (10).
Histopathological technique. Right after euthanizaton, 4 mm thick longitudinal slices were taken from the heart, including both the atria and the ventricles. Immediately after collection, the slices were fixed by immersion in Stieve mixture for 24 h.
After fixation, the samples were histologically processed by paraffin embedding, and 5 μm thick sections were cut. The sections were contrasted using Goldner’s trichrome staining. The histological preparations were examined with the optical microscope (Olympus BX41), photographs were taken (Olympus E 330) and digitally processed (Adobe Photoshop CS2).
Data analysis. Statistical analysis was performed with Statistix 10 software and presented as mean±SD. For statistical comparisons, the Mann-Whitney test was used, and p<0.05 was considered statistically significant.
Results
Oxidative stress/antioxidant status parameters. The values of oxidative stress/antioxidant status parameters are presented in Table II. The comparison between groups is shown in Table III and Figures Figure 1 Figure 2 Figure 3 Figure 4 Figure 5
Table II. Oxidative stress parameters (mean±standard deviation).
Table III. Comparisons between groups (p-Values).
Figure 1. Nitric oxide levels in the three groups.
Figure 2. Malondialdehyde levels in the three groups.
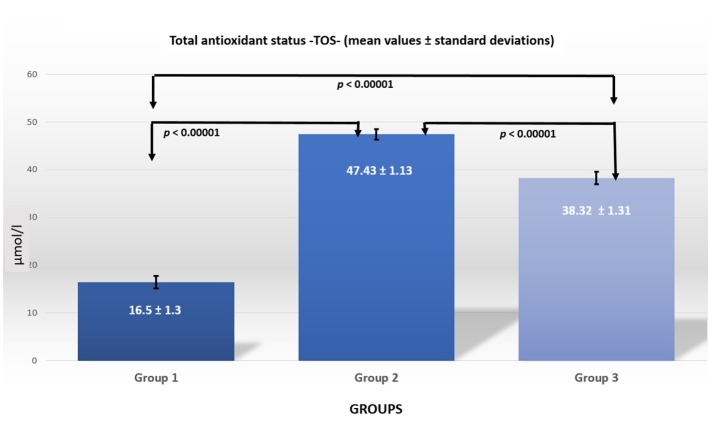
Figure 3. TOS levels in the three groups.
Figure 4. CAT levels in the three groups.
Figure 5. TAC levels in the three groups.
By comparing group 2 with group 1, a statistically significant difference (p<0.0001) was observed regarding the plasma levels of NOx, MDA, TOS, as well as significant differences were seen in the antioxidant status, including CAT and TAC. When comparing group 3 to group 1, there were statistically significant differences in all parameters (p<0.00001). A comparison between groups 2 and 3 showed that all parameters showed statistically significant changes, except for MDA levels.
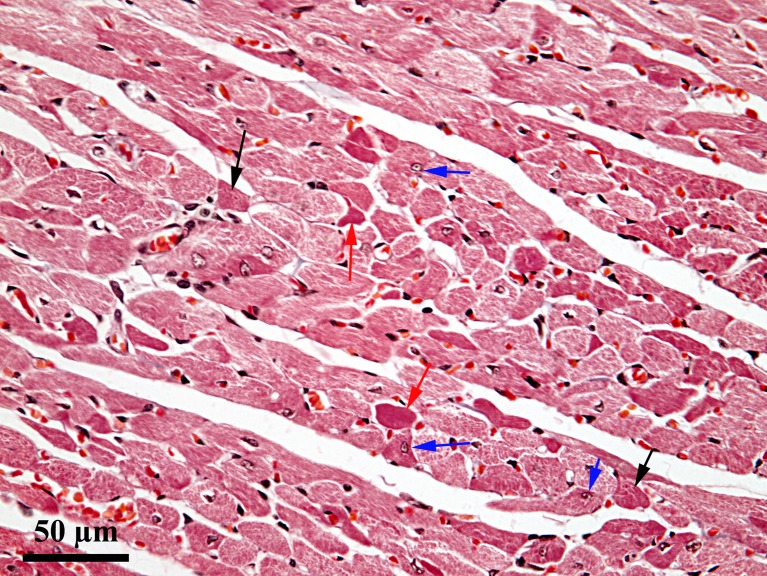
Histopathological assessment. At 4 days after induction of ischemia, in certain areas of the myocardium there were alterations, without significant differences between groups 2 and 3; in contrast, the extent and severity of these changes varied from one area of the heart to another. Thus, lesions ranging from reversible to irreversible were detected. Some cardiomyocytes exhibited cytoplasmic vacuolization, with eccentric displacement of the nucleus, while others showed hypercontraction bands. In some places, a small number of cells with different degrees of cytoplasmic homogenization were observed. There was a progressive disappearance of myofibrils in the cytoplasm, leading to cytoplasmic homogenization and cardiomyocyte necrosis (Figure 6).
Figure 6. Ischemic lesion on day 4 of the experiment; Goldner’s trichrome stain; black arrow: hypercontraction bands; red arrow: cellular necrosis; blue arrow: eccentric displacement of the nucleus.
In the connective tissue, discrete edema alternated with areas of inflammatory cells accompanied by fibroblast mobilization. These inflammatory infiltration areas were predominantly located around the small blood vessels in the myocardium, both subendocardially and subepicardially. In the apical area, relatively extensive areas with perivascular and intercellular edema accompanied by discrete cellular infiltrate were present (Figure 7).
Figure 7. Apical area - day 4 of the experiment; Goldner’s trichrome stain; black arrow: hypercontraction bands; red arrow: edema; blue arrow: discrete inflammatory infiltrate.
At the end of the experiment, in group 2 animals, no muscle cells with hypercontraction bands or cells with a cytoplasmic homogenization tendency were present on the section surface. In contrast, inflammatory infiltration, predominantly perivascular, was present, but the number of inflammatory cells was much smaller than at day 4 after induction of ischemia. Around the small or medium caliber blood vessels, along with the cellular infiltrate, mild perivascular edema was observed. Some limited areas were present where fibrosis with collagen proliferation was detected. These collagen proliferation areas were most likely the areas where necrosisoccured (Figure 8). Subendocardial and subepicardial fibrosis aspects were also present. In the apical area, fibrosis was more extensive than in other areas, without occupying large surfaces.
Figure 8. Group 2 myocardium – end of the experiment; Goldner’s trichrome stain; red arrow: perivascular edema; blue arrow: collagen proliferation.
In group 3 animals, the the pathological features described were more discrete than in group 2 both in terms of extension and severity. Thus, in the right ventricular wall, fibrosis areas were reduced, being predominantly perivascular, while in the interventricular septum, they were very discrete. In the left ventricular wall, connective tissue proliferation areas also had a perivascular arrangement and in some places, they tended to proliferate among cardiac cells (Figure 9). In the apical area, connective tissue proliferation areas were more abundant than in the rest of the myocardium, without occupying very extensive surfaces like in group 2 animals. Also, there was a greater connective consolidation of fibrosis areas in group 3 compared to group 2.
Figure 9. Apical area, group 3 – end of the experiment; Goldner’s trichrome stain; red arrow: intercellular edema; blue arrow: collagen proliferation.
Discussion
In this experimental study, oxidative balance was comparatively evaluated in a control group of rats versus a group of rats in which ischemic heart disease was induced and a group exposed to mofette treatment after experimental induction of ischemia, by measuring oxidative status and total antioxidant status after exposure to mofette.
There are no literature data regarding the effect of mofette therapy on cardiovascular rehabilitation in patients with ischemic heart disease (6,7). However, some studies have shown that using mofette along with other rehabilitation treatments such as carbonated mineral baths, climatotherapy, and kinesiotherapy in cardiovascular patients has beneficial effects (6,7).
Balneotherapeutic treatments, through the use of natural factors, are based on the physical properties, temperature and chemical content of mineral water and mofette. The balneotherapeutic treatments have indications for chronic cardiac diseases, but additional data regarding their efficacy, safety and possible side effects are required (11).
The biological mechanisms by which natural factors act and induce immunological and neuroendocrine responses are not yet completely understood, but according to some studies, they cause analgesic, anti-inflammatory, antioxidant, anabolic and chondroprotective effects, and regulate the neuroendocrine-immune system (12).
Also, it is considered that the hormonal effects of balneotherapy are determined by non-specific factors, such as heat, through the synthesis and release of heat shock proteins (12). There are studies regarding the local vasodilatatory effect of carbon dioxide therapy, with thermal (hot) water or dry gas, that was used for three weeks in patients with peripheral arterial disease (stage 2), through the reduced capacity of hemoglobin to bind oxygen, inducing oxygen release in cells (13). The effects of transcutaneous carbon dioxide treatment on heart rate variability parameters through its influence on the autonomic nervous system have also been studied (14).
There are clinical and experimental studies regarding the efficacy of carbonated mineral water and mofette in Baile Tusnad in chronic arterial occlusive disease, after stroke (15-17).
One of the most important factors that cause myocardial tissue lesions in ischemic heart disease is the increase in oxidative stress and the reduction in the protective effect of antioxidant mechanisms (18). The results of our study demonstrate that isoproterenol-induced myocardial ischemia induces significant changes on oxidative stress biomarkers (tested by the serum levels of NOx, MDA and TOS) as well as in the tested antioxidant molecules (CAT and TAC) (Tables II and III).
Following exposure of the rats to mofette, there were significant changes in the oxidative stress molecules tested, except for MDA (Tables II and III). The significant alterations in nitric oxide levels can be due to the increased NO synthesis induced by carbon dioxide in the mofette gas, causing significant reduction in total oxidative stress compared to the group with ischemic heart disease. Adding et al. have reported increased nitric oxide retention in the body under conditions of increased exogenous carbon dioxide administration by the respiratory route (19).
The effect of carbon dioxide on plasma nitric oxide concentration is independent of the activation/inhibition of the sympathetic nervous system and is not influenced by blood pH (19). In this way, two beneficial effects are combined, the vasodilatory effect exerted by carbon dioxide present in mofette gas and the vasodilatory effect of nitric oxide, which is maintained at a level that does not change TOS (this is significantly reduced after mofette exposure compared to controls). It is well known that nitric oxide is the main vasodilatory molecule, which is why it is used as a biomarker for endothelial dysfunction (20,21).
Similar therapeutic benefits were obtained by other authors, who used exogenous percutaneous administration of carbon dioxide and reported an improvement in blood flow (by Doppler examination), an increase in the vascular endothelial growth factor (VEGF) expression, as well as a rise in serum phosphorylated endothelial nitric oxide synthase (eNOS) levels (22). For this reason, we consider the increase in nitric oxide concentration in our study as an effect of the elevation of eNOS and not of inducible nitric oxide synthase (iNOS), which is activated under pathological conditions, leading to an increase in nitroxidative stress. An analysis of serum proteins by liquid chromatography and mass spectrometry has identified many other proteins whose synthesis is influenced by percutaneous carbon dioxide administration (22).
Carbon dioxide-rich springs have been used in Europe since ancient times for their beneficial effect on peripheral circulation, but the mechanisms that underlie these effects are not known. A possible mechanism could be the improvement of vasomotricity through the penetration of transcutaneously administered carbon dioxide into superficial microcirculation, an effect that might extend and exert general hemodynamic effects (23,24).
The beneficial vasodilatory effects of exogenously applied carbon dioxide are not changed by acute or chronic denervation of the treated tissue (24). The results that have been reported by Ito is of particular importance in patients with chronic lower limb ischemia, as well as those with cardiac ischemia, because these patients may undergo associated innervation disorders in time. Hartmann et al. have demonstrated a 300% increase in Doppler signal compared to the time prior to administration (through the vasodilatator effect of exogenously administered carbon dioxide), and an improvement of blood flow with a 10% increase in partial oxygen pressure in arterial blood (PO2) (23).
In this way, the authors demonstrated the vasodilatory action of exogenously administered carbon dioxide through the Bohr effect and its beneficial effect on vasomotricity. In another study, Hartmann et al. have reported positive vasomotor effects on peripheral blood circulation in patients with occlusive arterial disease of the lower limbs following repeated 30 min application of exogenous carbon dioxide treatments for 4 weeks (25).
In agreement with other studies (22), an increase in catalase (CAT) levels, part of the antioxidant system, under the action of exogenous percutaneous administration of carbon dioxide was demonstrated (Tables II and III). The elevation of serum antioxidant levels could be due to the increased energy metabolism in the tissues exposed to exogenous carbon dioxide, an increase due to its vasodilatory effect (22).
Similar positive effects have also been obtained for coronary circulation. Yamaguchi et al. showed that subcutaneous administration of high concentrations of carbon dioxide in rats can significantly ameliorate left ventricular dysfunction and reduce pulmonary hypertension (26). In the same study, a significant increase in eNOS was found, with an enhancement of nitric oxide synthesis and beneficial vasodilatory effects (26).
In another experimental study, Yamaguchi et al. have demonstrated that percutaneous carbon dioxide administration can improve cardiac function in experimental myocardial infarction induced in rats by ligation of a coronary artery (27). Because we wanted to investigate the effect of repeated mofette treatments, we only induced cardiac ischemia by s.c. administration of isoproterenol, in a single dose (9), allowing the survival of the animals.
The histological results obtained by us are comparable to those reported by other authors regarding the microscopic aspects consecutive to cardiac ischemia (28), with the difference that the inflammatory infiltrate observed by us is much more discrete. At 4 days after induction of cardiac ischemia, some lesions were reversible, while others were irreversible. The areas affected by irreversible lesions were the areas in which fibrosis was present at the end of the experiment. Between groups 2 and 3 there were differences regarding the surface of fibrosis areas, which were somewhat smaller in group 3. These differences can be probably attributed to the beneficial effect of mofette, which induced reversible alterations (ballooning, hypercontraction bands) and prevented necrosis of cells. Also, with respect to the degree of repair of necrotic areas by collagen synthesis, in group 3 these processes were in a more advanced stage of connective tissue consolidation because the density of collagen fibers was much higher and their diameter was much larger in group 3 than in group 2.
Study Limitations
The absence of previous clinical and experimental studies related to the efficacy of treatment of ischemic heart disease with natural mofettes in Baile Tusnad, as well as regarding their mechanism of action. However, based on our results, future integrated studies focusing on the treatment modality and timing as well as monitoring of adverse reactions should be conducted.
Conclusion
To our knowledge, this is the first study that demonstrates the beneficial effects of mofette on the balance between oxidative stress and antioxidant status in experimentally induced myocardial ischemia, an effect due to carbon dioxide present in mofette gas. The results showed the positive effects of exposure to natural mofette treatment in Baile Tusnad, through a reduction in oxidative stress, a significant improvement in the antioxidant status, an amelioration of the inflammatory infiltrate, and the repair of necrotic areas through collagen proliferation. However, a periodic analysis of the physical and chemical properties of natural mofettes is important. Experimental studies regarding its biological effects and mechanism of action, as well as further clinical studies regarding its therapeutic effect are also necessary.
Conflicts of Interest
The Authors report no conflicts of interest regarding this study.
Authors’ Contributions
Gabriela Dogaru (GD), Vasile Rus (VR), Constantin Munteanu (CM), Ioana Stanescu (IS) and Lorena Ciumarnean (LR) conceived the project, designed the experiments and jointly wrote the manuscript. Paul Mihai Boarescu (PMB) induced myocardial ischemia. VR performed the histological analysis. Adriana Bulboaca (AD) and Gyorgy Bodizs (GB) they determined the markers of oxidative stress. Adela Viviana Sitar Taut (AVST) and AD performed statistical analysis. All Authors contributed to the data analysis, drafting and revising the manuscript. The Authors are accountable for all aspects of this study.
References
- 1.Constantin M, Cinteza D. Editura Balneara, Bucuresti. 2011. Cercetarea factorilor naturali terapeutici; pp. 21–23. [Google Scholar]
- 2.Munteanu C. Editura Balneara, Bucuresti. 2013. Ape minerale terapeutice; pp. 35–40. [Google Scholar]
- 3.Cinteză D, Munteanu C, Munteanu D, Poenaru D. Editura Balneara, Bucuresti. 2012. Ape carbogazoase- rol terapeutic; pp. 5–38. [Google Scholar]
- 4.Néda T, Szakács A, Cosma C, Mócsy I. Radon concentration measurements in mofettes from Harghita and Covasna counties, Romania. J Environ Radioact. 2008;99(12):1819–1824. doi: 10.1016/j.jenvrad.2008.07.007. PMID: 18783856. DOI: 10.1016/j.jenvrad.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Hadnagy C, Benedek G. Information of action mechanism of mofettes in Covasna. Arch Phys Ther (Leipz) 1968;20(4):229–233. PMID: 5733024. [PubMed] [Google Scholar]
- 6.Suceveanu M, Suceveanu P, Pop D, Sitar Taut A, Zdrenghea D, Hâncu N. Role of mofette therapy in cardiovascular rehabilitation – the Covasna model. Balneo Res J. 2015;6(2):69–74. DOI: 10.12680/balneo.2015.1089. [Google Scholar]
- 7.Suceveanu M, Pop D, Suceveanu P, Sitar Tǎut AV, Zdrenghea D, Hâncu N. Effects of cardiovascular rehabilitation in patients admitted to the “dr Benedek Geza” hospital of rehabilitation in cardiovascular diseases, Covasna. Balneo Res J. 2015;6(1):53–59. DOI: 10.12680/balneo.2015.1086. [Google Scholar]
- 8.Covasna natural mofettes. 2019. Available from: https://www.itour.ro/despre-tratament-la-covasna-mofete-si-izvoare (last accessed 07-08-2019)
- 9.Boarescu PM, Chirilă I, Bulboacă AE, Bocsan IC, Pop RM, Gheban D, Bolboaca SD. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid Med Cell Longev. 2019;2019:7847142–7847142. doi: 10.1155/2019/7847142. PMID: 31205590. DOI: 10.1155/2019/7847142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulboacă AE, Porfire AS, Tefas LR, Boarescu PM, Bolboacă SD, Stănescu IC, Bulboacă AC, Dogaru G. Liposomal Curcumin is better than Curcumin to alleviate complication in experimental diabetes mellitus. Molecules. 2019;24:846–846. doi: 10.3390/molecules24050846. DOI: 10.3390/molecules24050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto S. Evaluation of the role of balneotherapy in rehabilitation medicine. J Nippon Med Sch. 2018;85(4):196–203. doi: 10.1272/jnms.JNMS.2018_85-30. PMID: 30259887. DOI: 10.1272/jnms.JNMS.2018_85-30. [DOI] [PubMed] [Google Scholar]
- 12.Gálvez I, Torres-Piles S, Ortega-Rincón E. Balneotherapy, immune system, and stress response: A Hormetic Strategy. Int J Mol Sci. 2018;19(6) pii:E1687–E1687. doi: 10.3390/ijms19061687. PMID: 29882782. DOI: 10.3390/ijms19061687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabry R, Dubost JJ, Schmidt J, Body J, Schaff G, Baguet JC. Thermal treatment in arterial diseases: an expensive placebo or an effective therapy. Therapie. 1995;50(2):113–122. PMID: 7631285. [PubMed] [Google Scholar]
- 14.Kreska Z, Németh B, Kiss I, Péter I, Ajtay Z, Hejjel L. Transcutaneous carbon dioxide treatment affects heart rate variability – a pilot study. In Vivo. 2018;32(5):1259–1264. doi: 10.21873/invivo.11374. PMID: 30150454. DOI: 10.21873/invivo.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogaru G, Radulescu A. Therapeutic effects of carbonated mineral waters in cardiovascular rehabilitation. Balneo Res J. 2015;6(1):36–49. DOI: 10.12680/balneo.2015.1083. [Google Scholar]
- 16.Dogaru G, Stănescu I, Pop D, Motricală M, Ákos M. Effects of carbonated mineral water treatment in Băile Tuşnad on chronic arterial occlusive disease – a case report. Balneo Res J. 2017;8(3):121–124. DOI: 10.12680/balneo.2017.146. [Google Scholar]
- 17.Dogaru G, Motricală M, Bulboacă A, Ciumărnean L, Stănescu I. The effect of carbonated mineral water and mofette treatment in Baile Tusnad after ischemic stroke – a case report. Balneo Res J. 2018;9(1):11–14. DOI: 10.12680/balneo.2018.163. [Google Scholar]
- 18.Sinning C, Westermann D, Clemmensen P. Oxidative stress in ischemia and reperfusion: current concepts, novel ideas and future perspectives. Biomark Med. 2017;11(11):11031–1040. doi: 10.2217/bmm-2017-0110. PMID: 29039206. DOI: 10.2217/bmm-2017-0110. [DOI] [PubMed] [Google Scholar]
- 19.Adding LC, Agvald P, Persson MG, Gustafsson LE. Regulation of pulmonary nitric oxide by carbon dioxide is intrinsic to the lung. Acta Physiol Scand. 1999;167(2):167–174. doi: 10.1046/j.1365-201x.1999.00585.x. PMID: 10571553. DOI: 10.1046/j.1365-201x.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 20.Walczak M, Suraj J, Kus K, Kij A, Zakrzewska A, Chlopicki S. Towards a comprehensive endothelial biomarkers profiling and endothelium-guided pharmacotherapy. Pharmacol Rep. 2015;67(4):771–777. doi: 10.1016/j.pharep.2015.06.008. PMID: 26321280. DOI: 10.1016/j.pharep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Gradinaru D, Borsa C, Ionescu C, Prada GI. Oxidized LDL and NO synthesis--Biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev. 2015;151:101–113. doi: 10.1016/j.mad.2015.03.003. PMID: 25804383. DOI: 10.1016/j.mad.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Izumi Y, Yamaguchi T, Yamazaki T, Yamashita N, Nakamura Y, Shiota M, Tanaka M, Sano S, Osada-Oka M, Shimada K, Wanibuchi H, Miura K, Yoshiyama M, Iwao H. Percutaneous carbon dioxide treatment using a gas mist generator enhances the collateral blood flow in the ischemic hindlimb. J Atheroscler Thromb. 2015;22(1):38–51. doi: 10.5551/jat.23770. PMID: 25132376. DOI: 10.5551/jat.23770. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann BR, Bassenge E, Pittler M. Effect of carbon dioxide-enriched water and fresh water on the cutaneous microcirculation and oxygen tension in the skin of the foot. J Atheroscler Thromb. 2015;22(1):38–51. doi: 10.1177/000331979704800406. DOI: 10.5551/jat.23770. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Moore JI, Koss MC. Topical application of CO2 increases skin blood flow. J Invest Dermatol. 1989;93(2):259–262. doi: 10.1111/1523-1747.ep12277584. PMID: 2502580. DOI: 10.1111/1523-1747.ep12277584. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann BR, Bassenge E, Hartmann M. Effects of serial percutaneous application of carbon dioxide in intermittent claudication: results of a controlled trial. Angiology. 1997;48(11):957–963. doi: 10.1177/000331979704801104. PMID: 9373047. DOI: 10.1177/000331979704801104. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Izumi Y, Yamazaki T, Nakamura Y, Sano S, Shiota M, Miura K, Iwao H, Yoshiyama M. Percutaneous carbon dioxide treatment using a gas mist generator attenuates the development of right ventricular dysfunction in monocrotaline-induced pulmonary hypertensive rats. Osaka City Med J. 2015;61(1):31–41. PMID: 26434103. [PubMed] [Google Scholar]
- 27.Yamaguchi T, Yamazaki T, Nakamura Y, Shiota M, Shimada K, Miura K, Iwao H, Yoshiyama M, Izumi Y. Percutaneous carbon dioxide mist treatment has protective effects in experimental myocardial infarction. J Pharmacol Sci. 2015;127(4):474–480. doi: 10.1016/j.jphs.2015.03.009. PMID: 25906762. DOI: 10.1016/j.jphs.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Vranyac-Tramoundanas A, Harrison JC, Sawant PM, Kerr DS, Sammut IA. Ischemic cardiomyopathy following seizure induction by domoic acid. Am J Pathol. 2011;179(1):141–154. doi: 10.1016/j.ajpath.2011.03.017. PMID: 21703399. DOI: 10.1016/j.ajpath.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]