Abstract
Background/Aim: Epithelioid hemangio-endothelioma (EHE) of the liver is an uncommon vascular tumor with variable clinical courses ranging from stable disease to fatal outcome. EHE can mimic epithelioid angiosarcoma, which has a more aggressive behavior, especially in a small biopsy sample. EHEs are known to have the WWTR1-CAMTA1 fusion gene, and nuclear expression of CAMTA1 by immunohistochemistry (IHC) has been reported in about 90% of EHEs in multiple organs. Our study aimed to validate the diagnostic utility of CAMTA1 expression in EHEs, especially in the liver. Patients and Methods: IHC was performed using anti-CAMTA1 antibody in 34 tumors (24 hepatic EHEs and 10 angiosarcomas). In CAMTA1-negative EHEs, TFE3 IHC was performed. Results: Of the 24 hepatic EHEs, 22 (91.6%) showed nuclear staining for CAMTA1. One of two CAMTA1-negative cases showed TFE3 positivity. The other case was negative for TFE3. Meanwhile, all 10 angiosarcoma cases had no CAMTA1 expression. Conclusion: CAMTA1 is a highly sensitive and specific marker for diagnosis of hepatic EHE. It is helpful for differentiation of hepatic EHE and angiosarcoma, especially in small biopsy samples.
Keywords: CAMTA1, TFE3, liver, epithelioid hemangioendothelioma, cancer, angiosarcoma, prognosis
Epithelioid hemangioendothelioma is a rare vascular tumor that can develop in different organs. Described in 1982 by Weiss and Enzinger, epithelioid hemangioendothelioma (EHE) shows an intermediate clinical course between that of benign hemangioma and malignant angiosarcoma (1). Some patients maintain stable disease without any treatment, while others die immediately after diagnosis (2).
EHEs have chromosomal translocations involving 1p36.3 and 3q25, resulting in WWTR1 (WW domain–containing transcription regulator1) - CAMTA1 (calmodulin-binding transcription activator1) fusion genes (3). CAMTA1 immunohistochemical staining has limited expression in normal human brain, making CAMTA1 IHC useful for diagnosing EHEs (4,5). Nuclear expression of CAMTA1 by immunohistochemistry has been reported in about 90% of EHEs in multiple organs (6). Recently, the YAP1-TFE3 gene fusion has been reported in WWRT1-CAMTA1 negative cases of EHE (7).
The histological features of EHEs are relatively distinctive and include dendritic or epithelioid morphology with myxochondroid and sclerotic stroma. However, some hepatic epithelioid hemangioendotheliomas (EHEs) have necrosis or moderate to severe atypia and scaffolding growth, features that overlap with epithelioid angiosarcoma, which has a more aggressive clinical course than EHE (8). Definite diagnosis may be difficult in a small biopsy sample.
Our study aimed to validate the diagnostic utility of CAMTA1 expression for hepatic EHEs by comparison with angiosarcoma, a potential histologic mimicker. Clinicopatho-logical analysis of hepatic EHEs will also be discussed.
Patients and Methods
Patient selection. From June 2000 to December 2018, the pathology database of Samsung Medical Center, Seoul, Korea, was queried for all biopsy and resected specimens with results including ‘epithelioid hemangioendothelioma’ of the liver. Forty-four pathologic results were initially retrieved. After reviewing hematoxylin and eosin (H&E) stained slides and checking the residual formalin fixed paraffin embedded blocks, cases that were not EHE or that were not available for immunohistochemical staining due to insufficient remnant tissue were excluded. Some specimens were acquired from the same patient twice. Finally, 24 cases of hepatic EHE (6 cases with biopsy and subsequent resection, 18 cases with biopsy) were included in this study. For comparison, 10 cases of hepatic angiosarcomas were selected. This study was approved by the Institutional Review Board of Samsung Medical Center (No. 2019-04-031). Clinical data, including epidemiologic features, treatment methods, and clinical courses, were reviewed through electronic medical records.
Immunohistochemistry. Immunohistochemistry (IHC) was performed on representative sections from a total of 34 tumors (24 hepatic EHEs and 10 angiosarcomas) using a Ventana BenchMark XT immunostainer (Ventana Medical Systems, Tucson, AZ, USA). Formalin-fixed paraffin-embedded (FFPE) tissues were used with 4-μm thick tissue sections. After antigen retrieval with CC1 for 48 min, the sections were incubated with an anti-CAMTA1 antibody (1:500 dilution; NBP1-93620; Novus Biologicals, Littleton, CO, USA) at 37˚C for 36 min. Antigen-antibody chromogenic reactions were developed for 12 min and detected using an OptiView DAB IHC Detection kit (Ventana, Catalog no. 760-700). IHC for TFE3 was performed on EHE cases negative for CAMTA1 using a rabbit monoclonal antibody (1:20 dilution; clone MRQ-37; Cell Marque, Rocklin, CA, USA). Nuclear expression of CAMTA1 and TFE3 was evaluated.
Results
Clinicopathologic characteristics of hepatic EHEs. Clinicopathologic features of hepatic EHEs are summarized in Table I. Of 24 hepatic EHEs, 14 patients were male and 10 patients were female. The median age was 55.5 (range=22-78). Nineteen of 24 (79.2%) patients had no initial symptoms, and the main symptom was abdominal discomfort. Twenty-one patients (87.5%) presented with multiple masses. Six patients (25.0%) showed metastatic lesions at the time of initial diagnosis.
Table I. Clinical data of 24 hepatic epithelioid hemangioendotheliomas.
1Observation period is defined as a duration from initially diagnosed by histologic confirmation to surgical resection; 2Survival period is defined as a duration from initial histologic diagnosis to last follow up; 3Recurrence in a transplanted liver and multiple bone metastasis 13 months after operation; 4Local recurrence 5 years after resection. S: Segment; NA: not applicable; ND: not done; AD: alive with disease; NED: no evidence of disease.
A total of 10 patients underwent surgical treatment including liver transplantation (n=5). Most patients have had no recurrence after surgical treatment, and two patients have had recurrence. One patient had a bone metastasis 13 months after liver transplantation and died of the disease. The other patient had an intrahepatic recurrence treated by radiofrequency ablation and has remained alive for nine years.
Among 14 patients without surgical treatment, only one patient with distant metastasis had conventional chemotherapy, and another patient underwent radiotherapy of a bone metastasis. The 12 remaining patients did not have any treatment. Five patients with distant metastasis at time of diagnosis died of disease, and the nine remaining patients are alive with disease.
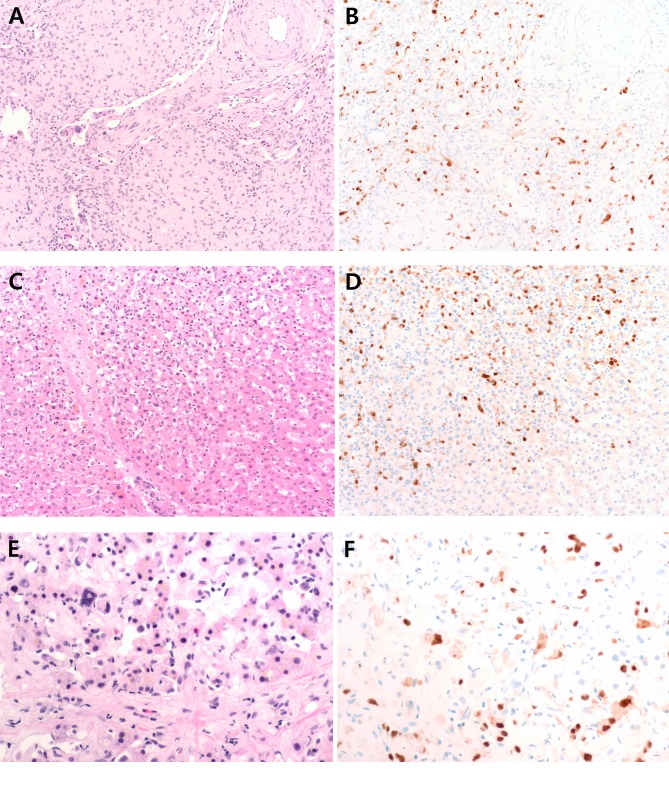
Histologic features of hepatic EHEs. Hepatic EHE shows nested or single infiltrative epithelioid tumor cells with frequent intracytoplasmic vacuoles in a background of myxoid and hyalinized stroma (Figure 1A). There are papillary projections and tufts into vessels. Focal areas of EHEs, especially at the tumor periphery, show large hyperchromatic atypical cells along vascular spaces without a typical stromal component, which can potentially mimic angiosarcoma (Figure 1C and E).
Figure 1. Representative figures of hepatic epithelioid hemangioendothelioma with CAMTA1 expression. (A-B) Hepatic epithelioid hemangioendothelioma shows infiltrative epithelioid tumor cells in a background of myxoid stroma. There are papillary projections and tufts into vessels. Tumor cells show nuclear staining for CAMTA1. (C-D) At the tumor periphery, tumor cells are located along the sinusoid without typical stroma component. Tumor cells in this area are also positive for CAMTA1. (E-F) Hepatic epithelioid hemangioendothelioma with bizarre pleomorphic nuclei along the sinusoidal space, which can potentially mimic angiosarcoma, has nuclear expression of CAMTA1.
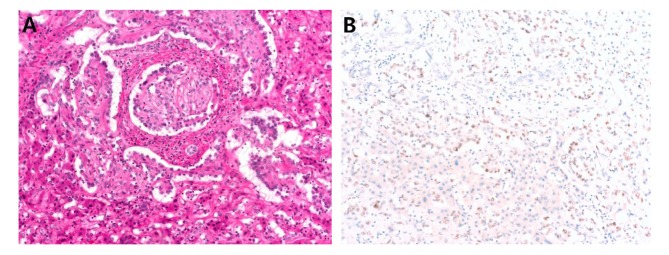
CAMTA-1 and TFE3 IHC results. Twenty two of 24 (91.6%) hepatic EHEs showed nuclear CAMTA1 expression (Figure 1) with moderate to strong intensity. One of two CAMTA1-negative cases showed TFE3 positivity (Figure 2). The other CAMTA1-negative case was also negative for TFE3, but showed a relatively typical histology of EHE. Meanwhile, all 10 angiosarcoma cases were negative for CAMTA1.
Figure 2. Representative figure of CAMTA1-negative hepatic epithelioid hemangioendothelioma. (A) There are papillary projections and tufts into vessels. (B) Tumor cells show nuclear expression of TFE3.
Discussion
In the present study, 91.6% of hepatic EHEs showed nuclear expression of CAMTA1, while all angiosarcomas were negative for CAMTA1 expression. One CAMTA1-negative case was positive for TFE3 (4.2%), which represents a known subset of EHE with a molecular gene rearrangement (YAP1-TFE3).
Previous studies of multi-organ EHEs by Shibuya et al. (9) and Doyle et al. (6) revealed that 87% and 86% of EHE, respectively, were positive for CAMTA1 IHC. The frequency of CAMTA1 positivity in these studies is similar to that in our study. In the study by Doyle et al., TFE3 positivity was observed in six of eight CAMTA1 negative EHEs, and two EHEs were negative for both CAMTA1 and TFE3. In our study, one of two CAMTA1 negative cases expressed TFE3, and another case was negative for both CAMTA1 and TFE3.
Diagnosis of vascular tumors including hepatic EHEs and angiosarcomas can be challenging in small liver biopsy samples. Hepatic EHEs can show necrosis and marked nuclear atypia or scaffolding growth, which can mimic angiosarcomas (10). If a biopsy was obtained in a low-grade area of an angiosarcoma or if the tumor has epithelioid features, it may be misdiagnosed as EHE. Usual IHC markers for diagnosis of hepatic EHEs are CD31, CD34, and factor Ⅷ, which are also expressed in angiosarcomas. There is limited utility of p53 and Ki-67 labelling in the differential diagnosis of these two entities. In these circumstances, CAMTA1 IHC can be useful for differential diagnosis. About 90% of hepatic EHEs express CAMTA1, while all angiosarcomas do not. Additionally, TFE3 can be helpful in CAMTA1 negative cases. Considering the more aggressive clinical behavior of angiosarcomas compared to EHEs, pathologic confirmation is important for determining treatment and predicting prognosis.
In conclusion, CAMTA1 immunohistochemical staining is highly sensitive and specific for diagnosis of hepatic EHE. Especially in small liver biopsy specimens, CAMTA1 expression is helpful for differential diagnosis of hepatic EHE with histologic features overlapping those of angiosarcoma.
Conflicts of Interest
None of the Authors have any conflicts of interest to declare regarding this study.
Authors’ Contributions
Conception and design: HJ, SYH; Acquisition of data: HJ, HK, YJ, CKP, SYH; Analysis and interpretation of data: HJ, SYH; Drafting the article: HJ, SYH; Revising and final approval of the article to be published: HJ, HK, YJ, CKP, SYH; All authors read and approved the final manuscript.
Acknowledgements
This study was funded by the Samsung Medical Center intramural Grant (#SMO1161731) and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2017R1C1B5017890).
References
- 1.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. PMID: 7093931. DOI: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Ishak KG, Sesterhenn IA, Goodman ZD, Rabin L, Stromeyer FW. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839–852. doi: 10.1016/s0046-8177(84)80145-8. PMID: 6088383. DOI: 10.1016/s0046-8177(84)80145-8. [DOI] [PubMed] [Google Scholar]
- 3.Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangio-endothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. PMID: 21584898. DOI: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huentelman MJ, Papassotiropoulos A, Craig DW, Hoerndli FJ, Pearson JV, Huynh KD, Corneveaux J, Hanggi J, Mondadori CR, Buchmann A, Reiman EM, Henke K, de Quervain DJ, Stephan DA. Calmodulin-binding transcription activator 1 (CAMTA1) alleles predispose human episodic memory performance. Hum Mol Genet. 2007;16:1469–1477. doi: 10.1093/hmg/ddm097. PMID: 17470457. DOI: 10.1093/hmg/ddm097. [DOI] [PubMed] [Google Scholar]
- 5.Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, Flanagan J, Luo Y, Fenwick K, Natrajan R, Mitsopoulos C, Zvelebil M, Hoch BL, Weiss SW, Debiec-Rychter M, Sciot R, West RB, Lazar AJ, Ashworth A, Reis-Filho JS, Lord CJ, Gerstein MB, Rubin MA, Rubin BP. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3:98ra82–98ra82. doi: 10.1126/scitranslmed.3002409. PMID: 21885404. DOI: 10.1126/scitranslmed.3002409. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LA, Fletcher CD, Hornick JL. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am J Surg Pathol. 2016;40:94–102. doi: 10.1097/PAS.0000000000000511. PMID: 26414223. DOI: 10.1097/PAS.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 7.Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, Chen HW, Pathan N, Krausz T, Dickson BC, Weinreb I, Rubin MA, Hameed M, Fletcher CD. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangio-endothelioma. Genes Chromosomes Cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. PMID: 23737213. DOI: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32:924–927. doi: 10.1097/pas.0b013e31815bf8e6. PMID: 18551749. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya R, Matsuyama A, Shiba E, Harada H, Yabuki K, Hisaoka M. CAMTA1 is a useful immunohistochemical marker for diagnosing epithelioid haemangioendothelioma. Histopathology. 2015;67:827–835. doi: 10.1111/his.12713. PMID: 25879300. DOI: 10.1111/his.12713. [DOI] [PubMed] [Google Scholar]
- 10.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver. Cancer. 1999;85:562–582. doi: 10.1002/(sici)1097-0142(19990201)85:3<562::aid-cncr7>3.0.co;2-t. PMID: 10091730. DOI: 10.1002/(sici)1097-0142(19990201)85:3<562::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]