Abstract
Background/Aim: In this study, we assessed the safety and efficacy of combination therapy with S-1, oxaliplatin and leucovorin (SOL) in advanced esophageal squamous cell carcinoma (ESCC) patients. Patients and Methods: Ten unresectable or recurrence ESCC patients, who had been previously treated with more than two regimens were included in this study. The treatment schedule comprised S-1 40-60 mg and fixed dose of leucovorin 25 mg together orally twice a day for one week, followed by one-week of rest. Oxaliplatin 85 mg/m2 was given as an intravenous infusion on day one, repeated every two weeks. Results: Of the eight patients with measurable lesions, two patients with partial response (25%) and two with stable disease (25%) were observed. Disease control rate was 50%. Median progression-free survival and overall survival were 5.0 and 9.3 months, respectively. The main common adverse events were malaise (60%), decreased appetite (50%), peripheral sensory neuropathy (40%). Conclusion: SOL therapy showed promising antitumor activity with acceptable toxicity even for heavily pretreated ESCC.
Keywords: Oxaliplatin, SOL, ESCC
Esophageal cancer is the ninth most common cancer and the sixth leading cause of cancer-related deaths worldwide. Squamous cell carcinoma is the most common histological type of esophageal cancer, which exhibits a high-incidence in eastern Asia including China and Japan (1). Although chemotherapy or radiation therapy (CRT) followed by extensive surgery has improved prognosis, unresectable or recurrent cases have a poor prognosis with a considerable decline in health-related quality of life (HRQoL).
Several chemotherapy regimens are in clinical use for unresectable or metastatic ESCC. Cisplatin (CDDP) and 5-fluorouracil (5-FU) based regimens has been a standard treatment for ESCC. In recent years, triplet regimens, cisplatin and fluorouracil plus docetaxel (DCF) have been regarded as a promising therapy, especially in neoadjuvant settings (2,3). However, these regimens are nephrotoxic and emetogenic, therefore, sometimes too toxic for patients who have undergone multiple chemotherapy cycles. Patients with recurrent and metastatic esophageal cancer can easily become malnourished due to appetite loss and in some cases esophageal stricture (4). Furthermore, there are few second or later-line chemotherapy options, and response rate of these regimens are far from being satisfactory. New drug combination strategies should be considered for better prognosis of advanced ESCC.
S-1, an oral prodrug of 5-FU-containing regimens are increasingly used for ESCC (3,5). Previous reports have shown the combination of S-1 with other key chemotherapeutic agents, including oxaliplatin, enhances the anti-tumor activity (6). Oxaliplatin, a platinum derivative, which has less nephrotoxic and emetogenic effects than the traditionally used CDDP, has been widely used in a variety of regimens for colorectal, pancreatic and gastric cancer (7). However, the anti-tumor activity of oxaliplatin and its combination with S-1 has not been sufficiently investigated in ESCC. A previous report demonstrated that SOL (S-1, oxaliplatin plus leucovorin) showed a high objective response rate (66%) with acceptable toxic effects in advanced gastric cancer (8). In this study, we have investigated the efficiency and safety of SOL therapy in patients with advanced ESCC, who have been pretreated with various chemotherapeutic agents and/or CRT.
Patients and Methods
Patients. Between January 2017 and February 2018, 10 patients with histologically proven squamous cell carcinoma of the thoracic esophagus, who experienced recurrence after curative surgery or underwent R2 resection were included in this study. All patients were mainly treated at the Osaka University hospital. All patients had been previously treated with at least two regimens of chemotherapy. Patients with an Eastern Cooperative Oncology Group performance status of 0-1, and with no serious vital organ dysfunction (hematologic, liver, and renal) were selected based on the following values: platelet count ≥10×104/mm3, hemoglobin ≥8.0 g/dl, neutrophil count ≥1,500/mm3, serum bilirubin ≤1.5 mg/dl, serum aspartate aminotransferase and alanine aminotransferase ≤twice the upper limit of normal range, and creatinine ≤1.2 mg/dl. Reduced S-1 dose was used for patients with estimated Glomerular filtration rate (eGFR) <60. Pathological stage was evaluated according to the seventh edition of the Tumor Node Metastasis Classification of the International Union Against Cancer (UICC-TNM). This study was approved by medical review committee of Osaka University School of Medicine and informed consent was obtained from all patients.
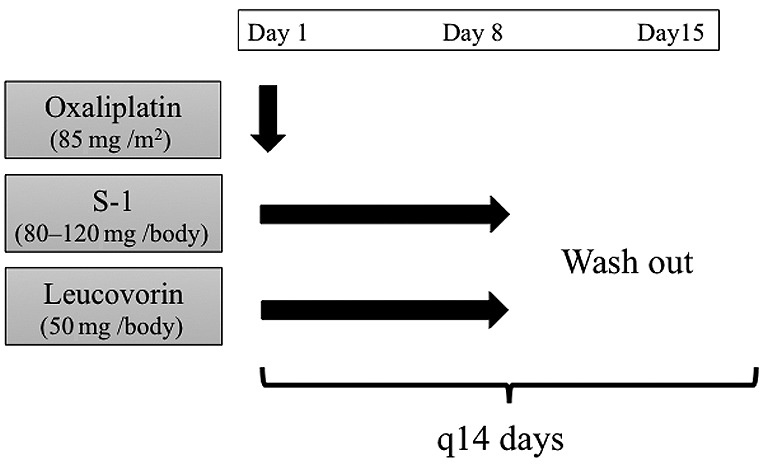
Study treatment. The SOL regimen comprised 40-60 mg of S-1 and a fixed dose of 25 mg of leucovorin, administered orally, twice a day for one week, followed by one-week of rest. Oxaliplatin, 85 mg/m2, was given as an intravenous infusion on day 1, and repeated every 2 weeks (Figure 1). Dose reduction was set as follows; 85 mg/m2, 65 mg/m2, and 50 mg/m2 for oxaliplatin, 60 mg, 50 mg, and 40 mg twice daily for S-1. If grade 3 or worse gastrointestinal disorders including nausea, diarrhea or stomatitis were observed, the dose of S-1 was reduced. If grade 3 or worse peripheral sensory neuropathy was observed, oxaliplatin was withheld until neuropathy improved to grade 2 or better, and the dose of oxaliplatin was reduced in the following treatment. If grade 3 or worse neutropenia or thrombocytopenia, or febrile neutropenia was recorded, the dose of all drugs except for leucovorin was reduced. Treatment was continued until disease progression, the development of serious adverse effect, or the patient refused further treatment.
Figure 1. Schedule of study treatment.
Outcomes. The primary end point was overall response rate (ORR). Secondary end points were overall survival (OS), progression-free survival (PFS) and safety. Tumor response was assessed using computed tomography (CT) scans of the chest and abdomen within six weeks before the start of the treatment and were repeated every 12 weeks until discontinuation of protocol treatment. Results were evaluated by RECIST v1.1 for response rate and disease control rate. Change in diameter of target lesions from baseline was assessed at the time of best response. The severity of adverse events during the treatment period was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Safety and laboratory data were assessed at baseline and at least once every two weeks after the start of treatment.
Statistical analysis. OS and PFS were calculated by the Kaplan–Meier method. OS was defined as the time from the first induction of SOL therapy to death from any cause, or the date of last contact in the case of continuing survival. PFS was defined as the time from the first induction of SOL therapy to disease progression or death from any cause.
Results
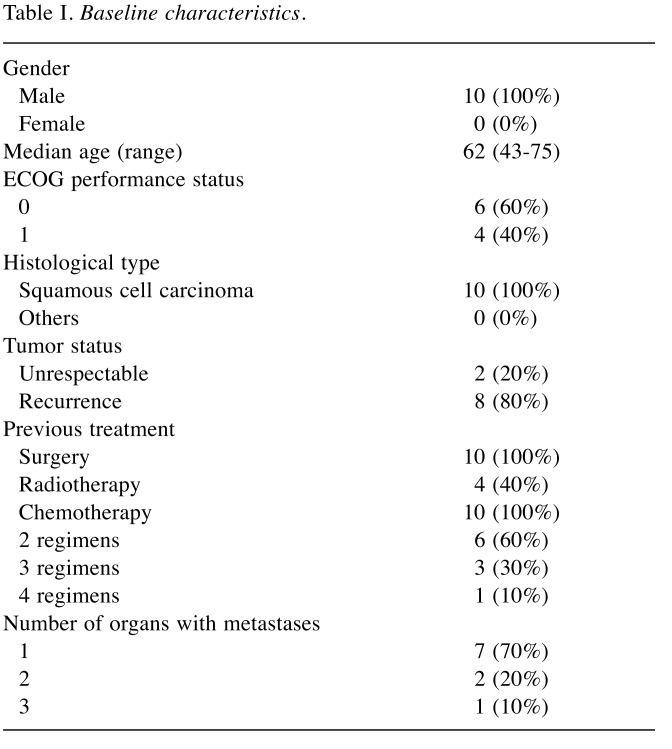
Patient characteristics. The baseline characteristics of patients are shown in Table I. Eight patients experienced recurrence after curative surgery and two patients underwent R2 resection. All patients were treated with at least two regimens of chemotherapy and were refractory or intolerant to fluoropyrimidine-based, platinum-based, and taxane-based chemotherapy. Four patients had received CRT before SOL therapy. The most commonly used regimens were paclitaxel treatment (8/10) and DCF with or without radiotherapy (8/10). Cisplatin plus 5-FU (FP) was administered in 4 patients. Of the eight patients who experienced recurrence after R0 resection, six patients had lymph node (mediastinal, cervical and abdominal lymph nodes) recurrence. Liver metastasis was observed in two patients, and lung, peritoneal, and adrenal gland metastasis was observed in one patient each.
Table I. Baseline characteristics.
Treatment and compliance. Patients received a median of 9.5 cycles (range=5.8-10.8 cycles) of SOL therapy. Seven patients (70%) required dose reduction of oxaliplatin according to adverse events including peripheral sensory neuropathy, decreased appetite and malaise. Seven patients (70%) required dose reduction of S-1 according to stomatitis, decreased appetite and malaise.
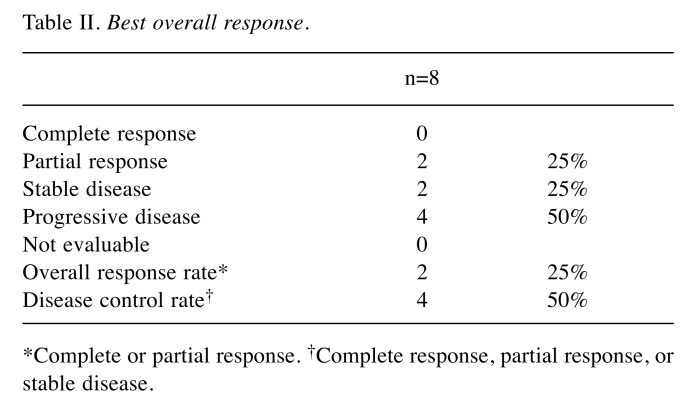
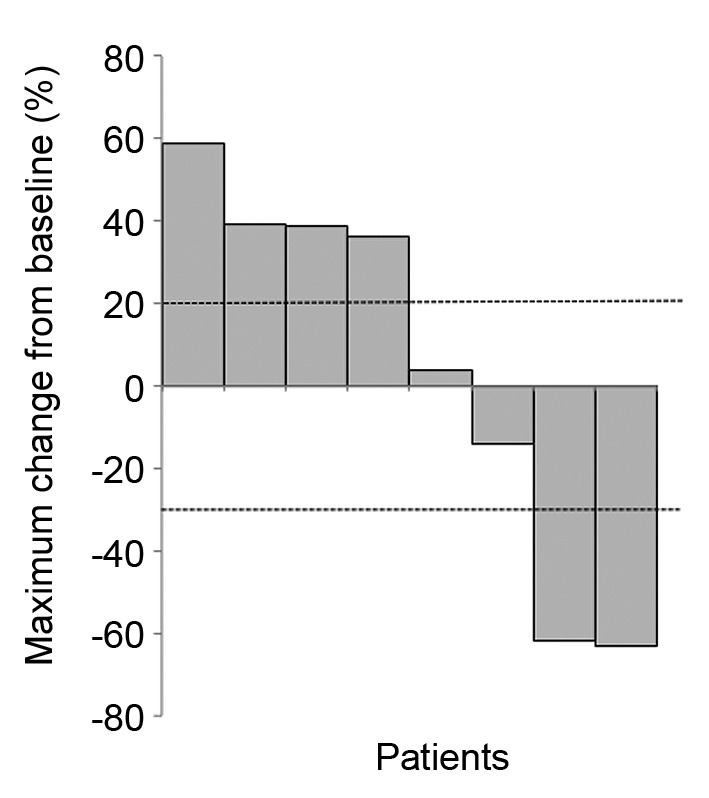
Tumor response. Eight of the ten patients had measurable disease and were evaluated by RECIST1.1 criteria. Partial response (PR) and stable disease (SD) were observed in 25% (n=2) and 25% (n=2), respectively. ORR was 25% and disease control rate was 50% (Table II). For these patients with measurable lesions, changes in the size of target lesions from baseline were calculated and shown in Figure 2. In four patients with lung metastasis, ORR was 25% [PR n=1/SD n=1/progressive disease (PD) n=2]. Two cases of liver metastasis demonstrated one PR and one PD.
Table II. Best overall response.
*Complete or partial response. †Complete response, partial response, or stable disease.
Figure 2. Change in size of target lesions from baseline, analyzed in patients with measurable disease (n=8).
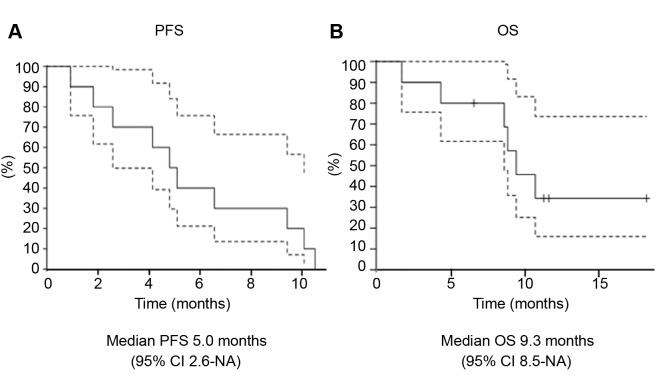
OS and RFS. At the time of analysis, median follow-up was 9.1 months (range=7.0-11.0 months). All patients experienced PD and six people died during the follow-up period. Median OS and PFS were 5.0 months (95%CI=2.6-NA) and 9.3 months (95%CI=8.5-NA), respectively (Figure 3).
Figure 3. Kaplan–Meier analysis of PFS (A) and OS (B). Dashed line indicates 95% confidence interval.
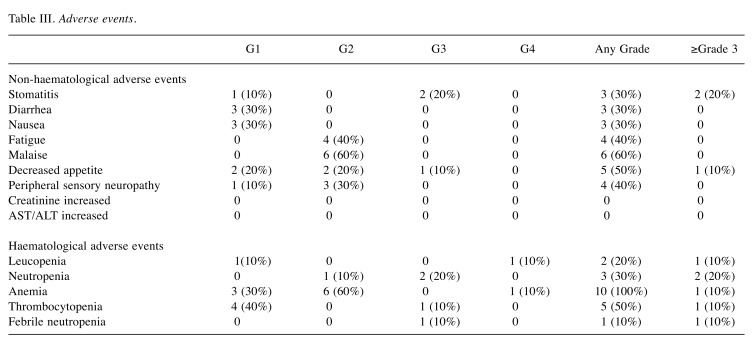
Toxicity. Frequently observed non-haematological toxicities included malaise (G1/2 60%; G3/40%), decreased appetite (G1/2 40%; G3/4 10%) and fatigue (G2 40%). Peripheral neuropathy was observed in 40% (G1/2 40%) of the patients, although a G3 event was not observed in this study (Table III). Bone marrow suppression was relatively mild, with 10% (n=1) of the patients experiencing G4 leucopenia, 20% (n=2) had G3 neutropenia and 10% (n=1) had G3 thrombocytopenia. Anemia (G1/2 90%; G3 10%) was frequently observed in this study.
Table III. Adverse events.
Discussion
Growing evidence has been accumulated regarding chemotherapy and CRT for ESCC. The standard FP regimen showed an ORR of 35% (9,10), while the recently developed triplet regimen, DCF, demonstrated an ORR of 60-75% (11-13). OS improvement from the DCF regimen is currently being investigated in a phase III trial. Taxanes are also frequently used in second line therapy, and paclitaxel has shown an ORR of 20-44% (14-16). However, most of the evidence so far has been limited to first- and second-line treatments, and later line chemotherapy for advanced ESCC has not been intensively explored.
In this retrospective study, we have demonstrated that SOL therapy is effective in heavily treated advanced ESCC patients with a manageable tolerability profile. Treatment of patients with metastatic and recurrent esophageal cancer needs to be carefully considered, because of deterioration of the general condition due to malnutrition or prolonged adverse events caused by previously administered chemotherapy (17). SOL can be administered on an outpatient basis every two weeks, and our results showed that this regimen is effective and well-tolerated in patients who have been previously administered two or more chemotherapy regimens.
FP regimen has been regarded as standard treatment for esophageal cancer; however, recent reports have demonstrated that oxaliplatin is as effective as cisplatin in patients with esophagogastric cancer (18). Combination of 5-FU, leucovorin and oxaliplatin (FLO) was shown to be less toxic than 5-FU/leucovorin/ cisplatin (FLP) in a phase III trial of gastric and gastroesophageal cancers (19). The combination of oxaliplatin, capecitabine and oral fluoropyrimidine has also been shown to be effective in advanced or metastatic esophageal cancer (20). Intensive infusion and hospitalization are not required for regimens including oxaliplatin, making this drug a useful chemotherapy option for gastrointestinal cancers. Although pre-clinical experimental models have shown that there is possibly cross-resistance of oxaliplatin and cisplatin (21), a previous phase II study of oxaliplatin, 5-FU and LV demonstrated efficacy in previously cisplatin-treated gastric cancer patients (22). Because all patients recruited in this study had received cisplatin-containing regimens before SOL therapy, use of this treatment in an early line setting might be beneficial. In fact, SOL regimen was used as first-line treatment for advanced gastric cancer and showed a promising ORR in a previous study (8).
Leucovorin facilitates the anti-tumor effect of fluorouracil through enhancing the inhibition of thymidylate synthase activity. Hironaka et al. demonstrated that objective response rate was similar between S1 plus leucovorin and S1 plus CDDP (43% vs. 46%, p=0.84), and that addition of SOL showed a much better objective response rate (66%) in advanced gastric cancer (8). Although most of the evidence obtained so far is in regard to adenocarcinoma of the esophagus, previous reports have suggested that oxaliplatin-containing regimens are also effective in ESCC (23). FOLFOX6 has been shown to be effective as a first-line treatment of ESCC. The overall response rate and disease control rate were 23.2% and 67.9 %, respectively. The median PFS was 4.4 months, and the median OS was 7.7 months (24). Safety and efficacy of SOL therapy in ESCC have been largely unknown, especially in later-line treatments. In this study, 2 (20%) showed PR and 2 (20%) showed SD to oxaliplatin-based SOL treatment, despite heavy pre-treatment with cisplatin-containing regimens. This might be because SOL regimen is well-tolerated and a relatively high dose intensity of oxaliplatin is maintained during a median treatment period of 5.0 months (3.0-8.8).
In regard to the toxicity, peripheral neuropathy was commonly observed in 40% of the patients, although a G3 event was not observed in this study. Because 8 to 10 patients had received paclitaxel or paclitaxel-containing regimens before SOL, the influence of prior treatment must be considered. A previous study in gastric cancer also showed a relatively high frequency of G1/2 neuropathy events and 9% of G3 events (8). Bone marrow suppression was relatively mild, with 10% (n=1) of the patients experiencing G4 leucopenia, 20% (n=2) had G3 neutropenia and 10% (n=1) had G3 thrombocytopenia. Anemia (G1/2 90%; G3 10%) was more commonly observed, although moderate anemia has already been observed at the start of treatment in most of the patients, due to the long history of prior chemotherapy. The frequency of neutropenia was relatively low compared with previous studies showing the effectiveness of SOL in gastric cancer, where 26% of patients in the SOL cohort showed G3/4 neutropenia (8).
The limitations of this study were the small sample size, a wide variety of prior treatment regimens including CRT and a relatively short follow-up duration. Nevertheless, the efficacy and safety data of the SOL regimen shown here might be useful information for heavily pretreated ESCC patients, who have few treatment options available.
Conflicts of Interest
N. Nishida: Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. T. Satoh: Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. D. Sakai: Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. All other Authors have declared no conflicts of interest regarding this study.
Authors’ Contributions
MY initiated this project. NN, MY, KO, KY, KT, DS, TM, TT, YK, TS, MM and YD designed the study protocol and wrote the manuscript. KO, NN and MY collected clinical information and performed statistical analysis.
Acknowledgements
Institutional endowments were received partially from Chugai Co., Ltd., Yakult Honsha Co., Ltd. and Ono Pharmaceutic Co., Ltd. These funding sources had no role in the main experimental equipment, supply expenses, study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Authors thank T. Ishikawa and Y. Shimatani for their technical assistance.
References
- 1.Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396. doi: 10.1016/S0140-6736(17)31462-9. PMID: 21112905. DOI: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, Tamura S, Kimura Y, Miyata H, Motoori M, Shiraishi O, Makino T, Satoh T, Mori M, Doki Y. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003) Ann Oncol. 2017;28:116–120. doi: 10.1093/annonc/mdw439. PMID: 27687307. DOI: 10.1093/annonc/mdw439. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Yoshida K, Suetsugu T, Imai T, Matsuhashi N, Yamaguchi K. Recent advancements in esophageal cancer treatment in Japan. Ann Gastroenterol Surg. 2018;2:253–265. doi: 10.1002/ags3.12174. DOI: 10.1002/ags3.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato K, Muro K, Ando N, Nishimaki T, Ohtsu A, Aogi K, Aoyama N, Nagai K, Kato H. A phase II study of nedaplatin and 5-fluorouracil in metastatic squamous cell carcinoma of the esophagus: The Japan Clinical Oncology Group (JCOG) Trial (JCOG 9905-DI) Esophagus. 2014;11:183–188. DOI: 10.1007/s10388-014-0427-7. [Google Scholar]
- 5.Tanaka Y, Yoshida K, Tanahashi T, Okumura N, Matsuhashi N, Yamaguchi K. Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma. Cancer Sci. 2016;107:764–772. doi: 10.1111/cas.12943. PMID: 27061001. DOI: 10.1111/cas.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. doi: 10.1093/annonc/mdu472. PMID: 25316259. DOI: 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 7.Veer E Ter, Mohammad NH, Van Valkenhoef G, Ngai LL, Mali RMA, Anderegg MC, Van Oijen MGH, Van Laarhoven HWM. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: A network meta-analysis. J Natl Cancer Inst. 2016;108:1–13. doi: 10.1093/jnci/djw166. PMID: 27576566. DOI: 10.1093/jnci/djw166. [DOI] [PubMed] [Google Scholar]
- 8.Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, Tsuji A, Nakajima TE, Gotoh M, Machida N, Bando H, Esaki T, Emi Y, Sekikawa T, Matsumoto S, Takeuchi M, Boku N, Baba H, Hyodo I. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:99–108. doi: 10.1016/S1470-2045(15)00410-6. PMID: 26640036. DOI: 10.1016/S1470-2045(15)00410-6. [DOI] [PubMed] [Google Scholar]
- 9.Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, Bedenne L, Namer M, De Besi P, Gay F, Collette L, Sahmoud T. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216–1220. doi: 10.1016/s0959-8049(97)00088-9. PMID: 9301445. [DOI] [PubMed] [Google Scholar]
- 10.Iizuka T, Kakegawa T, Ide H, Ando N, Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol. 1992;22:172–176. PMID: 1518165. [PubMed] [Google Scholar]
- 11.Honda M, Miura A, Izumi Y, Kato T, Ryotokuji T, Monma K, Fujiwara J, Egashira H, Nemoto T. Doxorubicin, cisplatin, and fluorouracil combination therapy for metastatic esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:641–645. doi: 10.1111/j.1442-2050.2010.01070.x. PMID: 20545978. DOI: 10.1111/j.1442-2050.2010.01070.x. [DOI] [PubMed] [Google Scholar]
- 12.Hironaka S, Tsubosa Y, Mizusawa J, Kii T, Kato K, Tsushima T, Chin K, Tomori A, Okuno T, Taniki T, Ura T, Matsushita H, Kojima T, Doki Y, Kusaba H, Fujitani K, Taira K, Seki S, Nakamura T, Kitagawa Y, Japan Esophageal Oncology Group/Japan Clinical Oncology Group. Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807) Cancer Sci. 2014;105:1189–1195. doi: 10.1111/cas.12486. PMID: 25041052. DOI: 10.1111/cas.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. PMID: 17075117. DOI: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 14.Shirakawa T, Kato K, Nagashima K, Nishikawa A, Sawada R, Takahashi N, Shoji H, Sasaki Y, Honma Y, Iwasa S, Takashima A, Okita N, Hamaguchi T, Yamada Y, Shimada Y. A retrospective study of docetaxel or paclitaxel in patients with advanced or recurrent esophageal squamous cell carcinoma who previously received fluoropyrimidine- and platinum-based chemotherapy. Cancer Chemother Pharmacol. 2014;74:1207–1215. doi: 10.1007/s00280-014-2597-3. PMID: 25267597. DOI: 10.1007/s00280-014-2597-3. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Tahara M, Hironaka S, Muro K, Takiuchi H, Hamamoto Y, Imamoto H, Amano N, Seriu T. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol. 2011;67:1265–1272. doi: 10.1007/s00280-010-1422-x. PMID: 20703479. DOI: 10.1007/s00280-010-1422-x. [DOI] [PubMed] [Google Scholar]
- 16.ter Veer E, Haj Mohammad N, van Valkenhoef G, Ngai LL, Mali RMA, van Oijen MGH, van Laarhoven HWM. Second- and third-line systemic therapy in patients with advanced esophagogastric cancer: a systematic review of the literature. Cancer Metastasis Rev. 2016;35:439–456. doi: 10.1007/s10555-016-9632-2. PMID: 27417221. DOI: 10.1007/s10555-016-9632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13:185–198. doi: 10.1038/nrclinonc.2015.200. PMID: 26573424. DOI: 10.1038/nrclinonc.2015.200. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. PMID: 18172173. DOI: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 19.Al-Batran S-E, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jäger E, Arbeitsgemeinschaft Internistische Onkologie Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. PMID: 18349393. DOI: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 20.Van Meerten E, Eskens FALM, Van Gameren EC, Doorn L, Van Der Gaast A. First-line treatment with oxaliplatin and capecitabine in patients with advanced or metastatic oesophageal cancer: A phase II study. Br J Cancer. 2007;96:1348–1352. doi: 10.1038/sj.bjc.6603750. PMID: 17437008. DOI: 10.1038/sj.bjc.6603750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855–1865. doi: 10.1016/s0006-2952(97)81490-6. PMID: 8951344. [DOI] [PubMed] [Google Scholar]
- 22.Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, Kim NK. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol. 2003;14:383–387. doi: 10.1093/annonc/mdg106. PMID: 31321276. DOI: 10.1093/annonc/mdg106. [DOI] [PubMed] [Google Scholar]
- 23.Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, Etienne PL, Boige V, Martel-Lafay I, Michel P, Llacer-Moscardo C, François E, Créhange G, Abdelghani M, Ben, Juzyna B, Bedenne L, Adenis A. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ ACCORD17): Final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–314. doi: 10.1016/S1470-2045(14)70028-2. PMID: 24556041. DOI: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Chang J, Yu H, Wu X, Wang H, Li W, Ji D, Peng W. A phase II study of oxaliplatin in combination with leucovorin and fluorouracil as first-line chemotherapy in patients with metastatic squamous cell carcinoma of esophagus. Cancer Chemother Pharmacol. 2013;71:905–911. doi: 10.1007/s00280-013-2081-5. PMID: 23370661. DOI: 10.1007/s00280-013-2081-5. [DOI] [PubMed] [Google Scholar]