Abstract
Background: Surgical stress and anesthesia affect the patient’s immune system. Analysis of the lymphocyte response after breast-conserving surgery was conducted to investigate the differences between effects after general and local anesthesia. Materials and Methods: Fifty-six patients with breast cancer were enrolled for BCS through local or general anesthesia. Total leukocytes, total lymphocytes, lymphocyte-subsets including CD3+, CD19+, CD4+, CD8+, CD16+CD56+ and CD4+/CD8+ ratio was examined at baseline and on postoperative days 1, 2 and 3. Results: Baseline data showed no statistical difference between the two groups. Within-group ANOVA test showed significant differences for total leukocyte count (p<0.001), total lymphocyte count (p=0.009) and proportion of natural-killer cells (p=0.01) in the control group. Between-group analysis showed lower median values of total lymphocytes in the awake surgery group on postoperative days 1, 2 and 3 (p=0.001, p=0.02 and p=0.01, respectively) when compared to the control group. Patients who underwent surgery under general anesthesia had higher total lymphocyte counts on postoperative day 2 (p=0.04). Conclusion: In this randomized study, breast-conserving surgery plus local anesthesia had a lower impact on postoperative lymphocyte response when compared to the same procedure performed under general anesthesia.
Keywords: Breast cancer, anaesthesia, local anaesthesia, immune function, immunological, surgery, conservative surgery, lymphocytes, leukocytes, white blood cell, cancer, awake surgery
Lymphocytes are fundamental types of white blood cells. Cells of the lymphatic system play a crucial role in the immune system due to their regulatory function through regulatory cytokines and due to cytotoxic activity against tumors and infections (1,2). As underlined in the literature, surgical stress and general anesthesia may reduce the numbers of circulating lymphocytes (1-6). It is a widely held view that impairment of immune function can predispose to infectious complications such as surgical site infections (SSI) (5-8). Moreover, reduced cytotoxic activity of peripheral-blood lymphocytes can increase the probability of tumor progression and metastasis (4,5,9-13).
According to several studies, use of a minimally invasive approach in thoracic surgery (1,14,15) and abdominal surgery (16-18) demonstrated that immune function may be better preserved. However, there is lack of high-level evidence about the protective role of minimally invasive techniques in early lymphocyte response and it is conceivable that, regardless of the surgical approach, any type of surgical procedure may have an impact on the early postoperative immune response. Surgical trauma and adverse effect of drugs administered during general anesthesia may interfere with the immune system (19-22).
In 2011, we started an investigational clinical program in the Breast Surgery Department performing breast-conserving surgery (BCS) and sentinel lymph node biopsy (SLNB) under local anesthesia alone in awake patients (awake breast surgery).
The hypothesis that was tested is that awake breast surgery may minimize the impact of surgery on early postoperative lymphocyte response by avoiding adverse effects of general anesthesia.
Materials and Methods
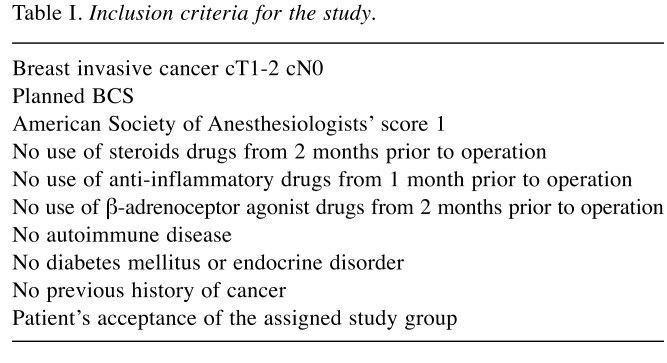
Study design and randomization procedure. This study was designed as monocentric, prospective, randomized, two-arm, blinded (awake group versus a control general-anesthesia group). The Institutional Review Board of Policlinico Tor Vergata Foundation waived the need for a formal approval because of the clinical nature of the study with no evidence of detrimental effect or clinical risk for the patients. Main eligibility criteria were the diagnosis of invasive malignant breast disease and planned BCS (other eligibility criteria are listed in Table I). Prior to enrolment, each patient was fully informed about the pros and cons of each type of surgical approach by a surgeon. All patients signed written-informed consent for participation in the study. Therefore, 80 consecutive patients were recruited for this study from December 2011 to June 2016 in accordance with the main eligibility criteria. Trial size was determined by the number of patients necessary to yield, by a nonparametric two-sided test with a power of 0.8 and with an α error of 0.05, a 1.5-fold difference in the median total leukocyte count. In order to avoid selection bias, the allocation of patients (awake group versus control group) was performed using software during study design by the Statistical Department. Surgeons performing breast surgeries were not informed about the type of anesthesia until the time of operation and it was communicated to them by telephone.
Table I. Inclusion criteria for the study.
The impact of anesthesia on postoperative lymphocyte response was assessed using the lymphocyte count at baseline and postoperative days 1, 2 and 3, which was chosen as the primary endpoint. This interval was chosen because in a previous pilot investigation, we found out the most significant change from baseline value to occur within this time frame.
Venous blood sampling time points and preoperative set up. Before the start of daily scheduled surgeries (at 7: 30 a.m.), venous-blood samples of patients were collected via peripheral vein of antecubital arm; blood samples were acquired at the same time on postoperative days 1, 2 and 3. Only after blood sampling were the patients sent for lymphoscintigraphy with 99mTc albumin nano colloid for preoperative sentinel node identification and wire-guided localization if needed. Total lymphocytes count, and changes in proportion of specific lymphocyte subsets [including CD19+, CD3+, CD4+, CD8+, CD4+/CD8+ ratio and natural-killer (NK) lymphocytes] and total leukocytes were evaluated in the three samples at the Hematology Department of our Institution for immediate, real-time assay without need for storage. Samples were processed with a cell counter (Coulter Beckmann, MedLab, Cupertino, CA, USA). FACS Calibur (BD Biosciences, Franklin Lakes, NJ, USA) three-color flow cytometry was used to obtain lymphocyte subsets by incubating blood samples for 30 minutes with monoclonal antibodies at 4˚C. The percentage of subsets was calculated through differential gating after triple-color staining.
Anesthesia and surgical techniques-postoperative management. Surgical planning was evaluated before communication of group assignment so as not to be influenced by the anesthesia to be administered. All patients were placed in supine decubitus position with arm open at 90˚on the side of operation. The operative techniques consisted of two different incisions. For the awake group, surgery was carried out after injection of local anesthesia (ropivacaine 7.5% plus lidocaine 2%). In the control group, patients were provided with volatile aesthetic agents or endovascular drugs or both according to the anesthesiologist’s preference and supraglottic devices were used for airway management. Quadrantectomy was performed on the breast and frozen sections were prepared for extemporaneous specimen microscopic evaluation of the margins. Evaluation of SLNs identified with 99mTc albumin nano colloid by gamma detection system through frozen section was performed. When margins were involved or macrometastasis was found, general anesthesia was administered for performing mastectomy or axillary node dissection, respectively; these patients were excluded from the analysis. At the end of the surgery 15-Fr. drainage was placed and removed when serous fluid loss was less than 30 ml/24 hours. Intravenous cefotaxime at 2 g twice a day until postoperative day 2 was administered as antibiotic prophylaxis. During the surgical procedure, fluid infusion at 1.5 ml/kg/h of normal saline and Ringer’s solution were used in both groups of patients. In the awake group, fluid infusion was stopped 2 hours after surgery and oral intake immediately was allowed. In the control group, fluid infusion continued postoperatively for 12 hours. The evaluation of creatinine level and urinary output demonstrated no significant difference in fluid balance between the groups. The use of nsaid nonsteroidal anti-inflammatory drugs and steroid drugs was forbidden after surgery, and postoperative analgesia was guaranteed through an elastomeric device with tramadol (200 mg/24 hours at a rate of 2 ml/hour).
Statistical analysis. Median and interquartile range (IQR) were calculated for the collected data. Two-way ANOVA test was used for repeated measures at baseline and postoperative days 1, 2 and 3, respectively. Variables were subsequently analyzed by means of the Mann–Whitney U-test at each time between groups. All statistical analyses and randomization were carried out using the SPSS platform (V.23; IBM, Armonk, NY, USA).
Results
Baseline data and surgical results. After enrollment, out of 80 patients, eight withdrew consent, five of them preferring general anesthesia, and another three preferring local anesthesia.
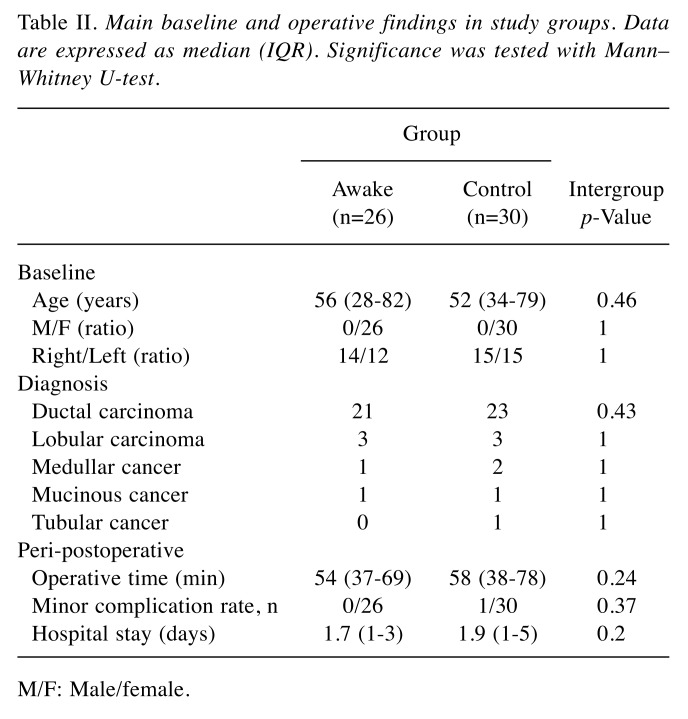
During the surgical procedure, 16 patients were excluded because they underwent under general anesthesia in order to complete the mastectomy (n=7) or lymphadenectomy (n=9). Table II summarizes demographics, surgical procedure, preoperative and perioperative data, and analysis of these showed no statistical differences between groups. Out of the 56 patients, the majority of cases were ductal carcinoma (44, 79%), and five patients had positive SLNB after definitive staining, and they were assigned to axillary lymphadenectomy. No difference was found in median operative time, nor in intraoperative complication or technical difficulty. No patients in the awake group needed change in anesthesia strategy. The hospital stay was slightly longer in the control group (Table II).
Table II. Main baseline and operative findings in study groups. Data are expressed as median (IQR). Significance was tested with Mann– Whitney U-test.
M/F: Male/female.
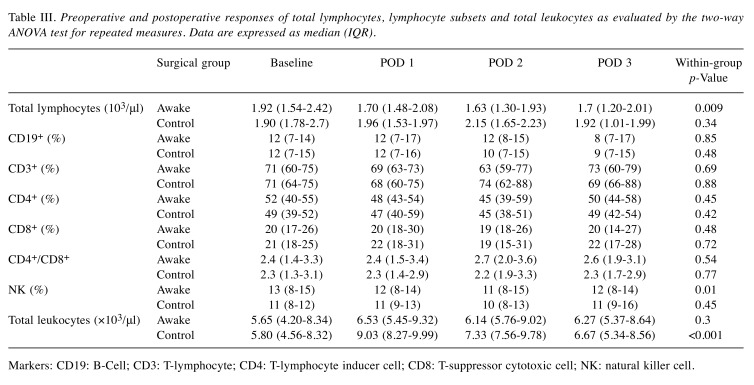
Total lymphocyte counts and lymphocyte subsets. Table III displays the summary of the results of the blood tests on the day of surgery and at the three postoperative timepoints. Before the procedure, the total lymphocyte count, proportions of lymphocyte subpopulations and total white blood cell did not differ between the two groups. Within-group ANOVA test showed significant differences for total leukocyte and total lymphocyte counts (p<0.001 and p=0.009, respectively) in the control group. Analyzing the differences between groups, the awake group had a lower median total leukocyte count compared with the control group on postoperative day 1 (p=0.001) day 2 (p=0.02) and day 3 (p=0.01) higher total lymphocyte count on postoperative day 2 was found (p=0.04) in the control group. No difference was found in the remaining lymphocyte subset.
Table III. Preoperative and postoperative responses of total lymphocytes, lymphocyte subsets and total leukocytes as evaluated by the two-way ANOVA test for repeated measures. Data are expressed as median (IQR).
Markers: CD19: B-Cell; CD3: T-lymphocyte; CD4: T-lymphocyte inducer cell; CD8: T-suppressor cytotoxic cell; NK: natural killer cell.
Discussion
The main role of the immune system is to preserve and defend body integrity against pathogens and diseases. Lymphocyte functions enhance resistance to postoperative SSI and postoperative infections in general (3-5). Moreover, lymphocytes and NK cells represent a first line of defense against intravascular spread of tumor through non-specific and specific immune response (4,8,10-12,14). In addition, impairment in immune function and lymphocyte activity was associated with several adverse effects and an increased risk of tumor development and grown (3-5,8-12). In our study, different types of anesthesia are associated with different postoperative pattern of lymphocyte responses according to quantity and subset. The results of the awake group showed a lesser lymphocyte response then the equivalent breast procedure performed under general anesthesia. Our data suggest a different model of response between the two groups, specifically after a peak in the postoperative day 1, total leukocyte counts decreased postoperatively (postoperative day 2 and day 3) in the control group while remaining relatively steady in patients of the awake group. Even if various other factors may have played a role in producing these results, randomization and the lack of significant difference in patients’ baseline data indicate that the method of anesthesia performed appears to be linked to postoperative changes in white blood cells. In literature (22,23) it was demonstrated how a combination of local and epidural analgesia may play a protective role in late (<72 h) postoperative suppression of lymphocytes, but this effect was not found when the analysis was focused on the early postoperative period. Yokoyama et al. showed that local and epidural anesthesia are linked with a transient reduction of NK cell activity considered unrelated to the pain level and the disease treated (5). The same authors linked the depression of NK cell activity with plasma cortisol increase. Furthermore, infusion of epinephrine improved NK cell activity (24) and the administration of β-adrenoceptor antagonist during the procedure reduced NK cell activity (25), according to the authors as a direct consequence of adrenergic-specific receptors expressed by lymphocytes in proximity of sympathetic nerve endings (26).
During general anesthesia, drugs administered may also condition the lymphocyte response (14,18). Opioids may depress immunity (18-21) even if part of the decrease can be linked to non-opioid aesthetics and propofol. Pirttikangas et al. found a higher percentage of CD4+ cells following anesthesia with propofol in comparison to conventional combined anesthesia (20).
Several studies stated that ventilation, and in particular thoracic one-lung ventilation, can elicit an oxidative cascade in the ventilated and non-ventilated lung, which can stimulate eventually the secretion of proinflammatory chemokines (27-33). The paracrine secretion of these mediators may elicit systemic spread through the vessel architecture of the lung. This spread can play a role in the modulation of postoperative immune response. According to our study, total lymphocytes and total leukocytes counts remained relatively stable in the postoperative day 1, 2 and 3 only in the awake group.
The minimally invasive approach showed great advantage in thoracic and general surgery (21,22,34-36) alone or within enhanced recovery after surgery (ERAS) protocols, multimodal fast-track surgery programs, which they permit a reduced length of stay, total complications, and total costs across all types of surgeries. In gastrointestinal surgery, the ERAS protocol Ied to a faster return of gastrointestinal function. Application of the same protocols in breast surgery might allow length of stay and opioid use to be reduced, with an even faster prompt resumption of common activities of daily life and freeing-up of hospital beds (32-33). The breast cancer surgery ACOSOG Z0011 (Alliance) randomized clinical trial demonstrated the non inferiority of SLNB alone compared to axillary node dissection in patients with SLNB metastases treated with BCS, increasing the number of procedures that can be completed under local anesthesia alone (37-39).
Our study showed some limitations. The small cohort of our study was calculated a priori to ensure a statistically acceptable power to detect intergroup differences in results, yet all the preoperative and operative data showed no statistical difference. Another limitation of our study might lie in the analysis of quantitative assessment of total lymphocytes and subsets without any information on actually lymphocyte activities, inflammation biomarkers and endocrine functions.
In conclusion, further studies are needed to assess the benefit and the potential of this minimally invasive surgery protocol. Our current findings may reveal a practical relevance once demonstrated in larger studies in that postoperative lymphocyte depletion, as observed in the general anesthesia group, is associated with an increased risk of postoperative infection, such as SSI, and tumor progression.
Conflicts of Interest
The Authors declare no conflicts of interest in regard to this study.
Authors’ Contributions
Study conception and design: Federico Tacconi, Vincenzo Ambrogi; Acquisition of data: Anemona Lucia, Buonomo Chiara, Dauri Mario; Analysis of data: Schillaci Orazio, Chiaravalloti Agostino; Interpretation of data: Gianluca Vanni, Meucci Rosaria, Perretta Tommaso; Drafting of article: Materazzo Marco, Buonomo Oreste Claudio; Critical revision: Vanni Gianluca, Petrella Giuseppe; Critical revision of literature: Granai Alessandra Vittoria, Maurizio Rho, Sara Ingallinella.
Acknowledgements
This study was found with the non-conditional contribution of the Italian Ministry of Health.
References
- 1.Kutza J, Gratz I, Afshar M, Murasko DM. The effects of general anesthesia and surgery on basal and interferon stimulated Natural killer cell activity of humans. Anesth Analg. 1997;85:918–923. doi: 10.1097/00000539-199710000-00037. PMID: 9322480. [DOI] [PubMed] [Google Scholar]
- 2.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. PMID: 10927999. DOI: 10.1093/ bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Koltun WA, Bloomer MM, Tilberg AF, Seaton JF, Ilahi O, Rung G, Gifford RM, Kauffman GL Jr. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am J Surg. 1996;171(1):68–72. doi: 10.1016/S0002-9610(99)80076-2. PMID: 8554154. DOI: 10.1016/S0002-9610 (99)80076-2. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Itano Y, Katayama H, Morimatsu H, Takeda Y, Takahashi T, Nagano O, Morita K. The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesth Analg. 2005;101(5):1521–1527. doi: 10.1213/01.ANE.0000184287.15086.1E. PMID: 16244024. DOI: 10.121 3/01.ANE.0000184287.15086.1E. [DOI] [PubMed] [Google Scholar]
- 5.Ahlers O, Nachtigall I, Lenze J, Goldmann A, Schulte E, Höhne C, Fritz G, Keh D. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101(6):781–787. doi: 10.1093/bja/aen287. PMID: 18922851. DOI: 10.1093/ bja/aen287. [DOI] [PubMed] [Google Scholar]
- 6.Tonnesen E, Hohndorf K, Lerbjerg G, Christensen NJ, Huttel S, Andersen K. Immunological and hormonal response to lung surgery during one-lung ventilation. Eur J Anaesth. 1993;10(3):189–195. PMID: 8495681. [PubMed] [Google Scholar]
- 7.Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980;284(5757):622–625. doi: 10.1038/284622a0. PMID: 7366733. DOI: 10.1038/ 284622a0. [DOI] [PubMed] [Google Scholar]
- 8.Herberman RB, Ortaldo JR. Natural killer cells: Their role in defenses against disease. Science. 1981;241(4516):24–30. doi: 10.1126/science.7025208. PMID: 7025208. DOI: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- 9.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. PMID: 11117 911. DOI: 10.1016/S0140-6736 (00)03231-1. [DOI] [PubMed] [Google Scholar]
- 10.Colacchio TA, Yeager MP, Hildebrandt LW. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167(1):174–179. doi: 10.1016/0002-9610(94)90070-1. PMID: 8311130. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G, Perussia B. Human natural killer cells: Biologic and pathologic aspects. Lab Invest. 1984;50(5):489–513. PMID: 6201674. [PubMed] [Google Scholar]
- 12.Whitson BA, D’Cunha J, Andrade RS, Kelly RF, Groth SS, Wu B, Miller JS, Kratzke RA, Maddaus MA. Thoracoscopic versus thoracotomy approaches to lobectomy: Differential impairment of cellular immunity. Ann Thorac Surg. 2008;86(6):1735–44. doi: 10.1016/j.athoracsur.2008.07.001. PMID: 19021967. DOI: 10.1016/j.athoracsur. 2008. 07.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Gleason N, Blanco I, Asi ZK, Whelan RL. Higher colon cancer tumor proliferative index and lower tumor cell death rate in mice undergoing laparotomy vs. insufflation. Surg Endosc. 2002;16(1):36–39. doi: 10.1007/s004640080199. PMID: 11961601. DOI: 10.1007/s0 04640080199. [DOI] [PubMed] [Google Scholar]
- 14.Vanni G, Tacconi F, Sellitri F, Ambrogi V, Mineo TC, Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg. 2010;90(3):973–978. doi: 10.1016/j.athoracsur.2010.04.070. PMID: 20732526. DOI: 10.1016/j.athoracsur. 2010.04.070. [DOI] [PubMed] [Google Scholar]
- 15.Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs. laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc. 1999;13(3):233–235. doi: 10.1007/s004649900952. PMID: 10064753. [DOI] [PubMed] [Google Scholar]
- 16.Lee SW, Feingold DL, Carter JJ, Zhai C, Stapleton G, Gleason N, Whelan RL. Peritoneal macrophage and blood monocyte functions after open and laparoscopic-assisted cecectomy in rats. Surg Endosc. 2003;17(12):1996–2002. doi: 10.1007/s00464-003-8154-5. PMID: 14569448. DOI: 10.1007/s00464-003-8154-5. [DOI] [PubMed] [Google Scholar]
- 17.Tubaro E, Borelli G, Croce C, Cavallo G, Santiangeli C. Effect of morphine on resistance to infection. J Infect Dis. 1983;148(4):656–666. doi: 10.1093/infdis/148.4.656. PMID: 6355311. DOI: 10.1093/infdis/ 148.4.656. [DOI] [PubMed] [Google Scholar]
- 18.Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, Guyre PM. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83(3):500–558. doi: 10.1097/00000542-199509000-00008. PMID: 7661350. [DOI] [PubMed] [Google Scholar]
- 19.Pirttikangas CO, Perttilä J, Salo M, Vainio O, Liukko-Sipi S. Propofol infusion anaesthesia and immune response in minor surgery. Anaesthesia. 1994;49(1):13–16. doi: 10.1111/j.1365-2044.1994.tb03304.x. PMID: 8311204. DOI: 10.1007/s00464-003-8154-5. [DOI] [PubMed] [Google Scholar]
- 20.Pompeo E, Mineo TC. Awake operative videothoracoscopic pulmonary resections. Thorac Surg Clin. 2008;18(3):311–320. doi: 10.1016/j.thorsurg.2008.04.006. PMID: 18831509. DOI: 10.1016/j.thorsurg.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Pompeo E, Tacconi F, Mineo D, Mineo TC. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg. 2007;133(3):786–790. doi: 10.1016/j.jtcvs.2006.11.001. PMID: 17320585. DOI: 10.1016/j.jtcvs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, Bessler H. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97(3):822–827. doi: 10.1213/01.ANE.0000078586.82810.3B. PMID: 12933409. [DOI] [PubMed] [Google Scholar]
- 23.Kappel M, Tvede N, Galbo H, Haahr PM, Kjaer M, Linstow M, Klarlund K, Pedersen BK. Evidence that the effect of physical exercise on NK cell activity is mediated by epinephrine. J Appl Physiol. 1991;70(6):2530–2534. doi: 10.1152/jappl.1991.70.6.2530. PMID: 1885446. DOI: 10.1152/jappl.1991.70.6.2530. [DOI] [PubMed] [Google Scholar]
- 24.Bachen EA, Manuck SB, Cohen S, Muldoon MF, Raible R, Herbert TB, Rabin BS. Adrenergic blockade ameliorates cellular immune responses to mental stress in humans. Psychosom Med. 1995;57(4):366–372. doi: 10.1097/00006842-199507000-00008. PMID: 7480566. [DOI] [PubMed] [Google Scholar]
- 25.Livnat S, Felten SY, Carlson SL, Bellinger DL, Felten DL. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985;10(1):5–30. doi: 10.1016/0165-5728(85)90031-1. PMID: 3902888. [DOI] [PubMed] [Google Scholar]
- 26.Kozian A, Schilling T, Fredén F, Maripuu E, Röcken C, Strang C, Hachenberg T, Hedenstierna G. One-lung ventilation induces hyperperfusion and alveolar damage in the ventilated lung: an experimental study. Br J Anaesth. 2008;100(4):549–559. doi: 10.1093/bja/aen021. PMID: 18308740. DOI: 10.1093/bja/aen021. [DOI] [PubMed] [Google Scholar]
- 27.Mineo TC, Sellitri F, Vanni G, Gallina FT, Ambrogi V. Immunological and inflammatory impact of non-intubated lung metastasectomy. Int J Mol Sci. 2017;18(7):E1466. doi: 10.3390/ijms18071466. PMID: 28686211. DOI: 10.3390/ijms18071466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng YJ, Chan KC, Chien CT, Sun WZ, Lin CJ. Oxidative stress during 1-lung ventilation. J Thorac Cardiovasc Surg. 2006;132(3):513–518. doi: 10.1016/j.jtcvs.2006.03.060. PMID: 16935103. DOI: 10.1016/ j.jtcvs.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Elia S, Guggino G, Mineo D, Vanni G, Gatti A, Mineo TC. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: A safe outpatient procedure. Eur J Cardiothorac Surg. 2005;28(2):312–317. doi: 10.1016/j.ejcts.2005.03.046. PMID: 15949944. DOI: 10.1016/j.ejcts.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 30.Calì Cassi L, Biffoli F, Francesconi D, Petrella G, Buonomo O. Anesthesia and analgesia in breast surgery: The benefits of peripheral nerve block. Eur Rev Med Pharmacol Sci. 2017;21(6):1341–1345. PMID: 28387892. [PubMed] [Google Scholar]
- 31.Pompeo E, Rogliani P, Tacconi F, Dauri M, Saltini C, Novelli G, Mineo TC. Awake Thoracic Surgery Research Group: Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg. 2017;143(1):47–54. doi: 10.1016/j.jtcvs.2011.09.050. PMID: 28686211. DOI: 10.3390/ijms18071466. [DOI] [PubMed] [Google Scholar]
- 32.Ackerman RS, Hirschi M, Alford B, Evans T, Kiluk JV, Patel SY. Enhanced REVENUE after surgery? A cost-standardized enhanced recovery pathway for mastectomy decreases length of stay. World J Surg. 2019;43(3):839–845. doi: 10.1007/s00268-018-4850-0. PMID: 30456482. DOI: 10.1007/s00268-018-4850-0. [DOI] [PubMed] [Google Scholar]
- 33.Lau CS, Chamberlain RS. Enhanced Recovery After Surgery Programs improve patient outcomes and recovery: A meta-analysis. World J Surg. 2017;41(4):899–913. doi: 10.1007/s00268-016-3807-4. PMID: 27822725. DOI: 10.1007/s00268-016-3807-4. [DOI] [PubMed] [Google Scholar]
- 34.Pompeo E, Mineo D, Rogliani P, Sabato AF, Mineo TC. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg. 2004;78(5):1761–1768. doi: 10.1016/j.athoracsur.2004.05.083. PMID: 15511470. DOI: 10.1016/j.athoracsur.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 35.Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg. 2007;32(1):13–19. doi: 10.1016/j.ejcts.2007.04.004. PMID: 17467287. DOI: 10.1016/j.ejcts.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Papadima A, Boutsikou M, Lagoudianakis EE, Kataki A, Konstadoulakis M, Georgiou L, Katergiannakis V, Manouras A. Lymphocyte apoptosis after major abdominal surgery is not influenced by anesthetic technique: a comparative study of general anesthesia versus combined general and epidural analgesia. J Clin Anesth. 2009;21(6):414–421. doi: 10.1016/j.jclinane.2008.10.015. PMID: 198332 74. DOI: 10.1016/j.jclinane.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Orsaria P, Chiaravalloti A, Fiorentini A, Pistolese C, Vanni G, Granai AV, Varvaras D, Danieli R, Schillaci O, Petrella G, Buonomo OC. PET probe-guided surgery in patients with breast cancer: Proposal for a methodological approach. In Vivo. 2017;31(1):101–110. doi: 10.21873/invivo.11031. PMID: 28064227. DOI: 10.21873/invivo.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orsaria P, Caredda E, Genova F, Materazzo M, Capuano I, Vanni G, Granai AV, DE Majo A, Portarena I, Sileri P, Petrella G, Palombi L, Buonomo OC. Additional nodal disease prediction in breast cancer with sentinel lymph node metastasis based on clinicopathological features. Anticancer Res. 2018;38(4):2109–2117. doi: 10.21873/anticanres.12451. PMID: 29599329. DOI: 10.21873/anticanres. 12451. [DOI] [PubMed] [Google Scholar]
- 39.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis . The ACOSOG Z0011 (Alliance) Randomized Clinical Trial JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. PMID: 28898379. DOI: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]