Abstract
Background/Aim: To date, no cell-based assay that focuses on the prime cause of acne initiation through activation of toll-like receptor2 and 4 and interleukin-8 (IL-8) production exists. Herein, we present an assay that evaluates acne by determining TLR2 and 4 expression and activation. Materials and Methods: Viability of keratinocytes was determined by the MTT assay. IL-8 was evaluated by ELISA. Immunocytochemistry was performed for determining receptor expression. Results: Lipoteichoic acid (LTA), peptidoglycan (PGN) and lipopolysaccharide (LPS) induced IL-8 production. Pre-treatment of cells with TLR2 and TLR4 inhibitors, before stimulation, reduced IL-8 production. Zinc gluconate was used for verification. Zinc can significantly suppress IL-8 production in the system. Treatment of cells with LTA+PGN or LPS resulted in increased TLR2 and TLR4 expression on the cell surface. This effect was prevented by zinc treatment. Conclusion: The measurement of IL-8 and TLR2 and TLR4 levels can be used for the evaluation of anti-acne treatment.
Keywords: Acne vulgaris, host defense, keratinocytes, IL-8, TLR2, TLR4
Acne vulgaris is a chronic disease. It is common among adolescents and can have a huge impact on physical appearance and health both short-term and long-term as a result of the adverse effects of the medication (1). The adverse effects are induced by non-anti-acne actions such as antibiotics and steroids (2). It is known that inflammation derived from the activation of innate immunity plays a primary role in the induction of acne lesions rather than infection or an allergic reaction (3). Clinically, there are multiple types of acne lesions. However, the induction of all types of lesions is primarily initiated by colonization of Propionibacterium acnes, a Gram-positive anaerobic bacterium that plays a key role in maintaining and influencing the inflammatory phase of acne (4). The major cell wall components of P. acnes, lipoteichoic acid (LTA) and peptidoglycan (PGN) were found to stimulate immune cells to release mediators of inflammation (5). Apart from LTA and PGN, lipopolysaccharide (LPS) –a component of gram-negative bacteria– is also an immunostimulatory factor (6).
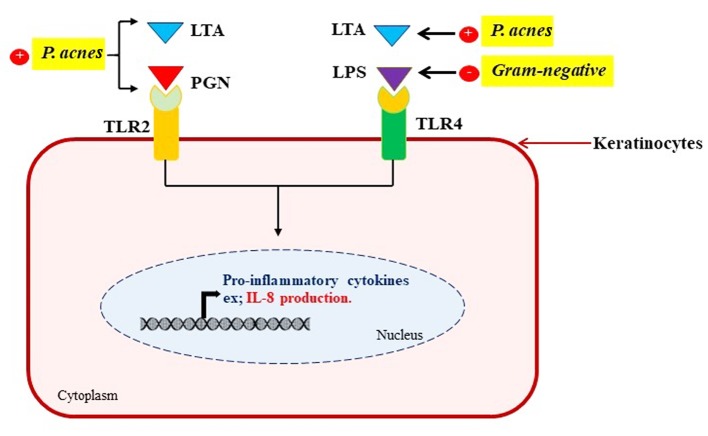
The innate immune system of the skin epidermis has several defense components and mechanisms involving keratinocytes, neutrophils, mast cells and macrophages (7). The key step of acne formation is the interaction of bacterial components such as LPS, LTA or PGN with toll-like receptors (TLRs) in epidermal cells (8). TLR4 primarily mediates cellular signaling induced by Gram-negative bacteria (9), while TLR2 is required for pro-inflammatory signaling to components of Gram-positive bacteria (10). It is known that TLR2 and TLR4 are predominantly expressed on the cell surface of the infundibular keratinocytes. The activation of these TLRs results in signaling cascades that produce interleukin 8 (IL-8) (11). As a consequence, IL-8 acts as a pro-inflammatory cytokine and a powerful chemoattractant (12). This cytokine plays a major role in inflammatory events by its activity as chemokine and as an activator of crucial neutrophil functions (11).
So far, the anti-acne therapies mostly target bacteria. Based on the knowledge regarding the early activation of acne reaction through TLR2 and TLR4 leading to the IL-8 production, we aimed at developing a method for evaluating anti-acne substances and specifically the cell signaling and the inflammatory response. The method includes evaluation of the effect of compounds on TLR2- and TLR4-mediated IL-8 production and on the suppression of TLR2 and TLR4 expression.
Materials and Methods
Cell culture. Human keratinocyte cell line HaCaT was purchased from the Cell Lines Service (CLS) GmbH (Eppelheim, Germany). HaCaT cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Merck, DA, Germany), 2 mM L-glutamine and 100 units/ml penicillin/streptomycin (Gibco). The cells were incubated at 37˚C in a CO2 incubator (5% CO2 (v/v), 95% humidity in air). Cells were subcultured by trypsinization (Thermofisher Scientific, Waltham, MA, USA).
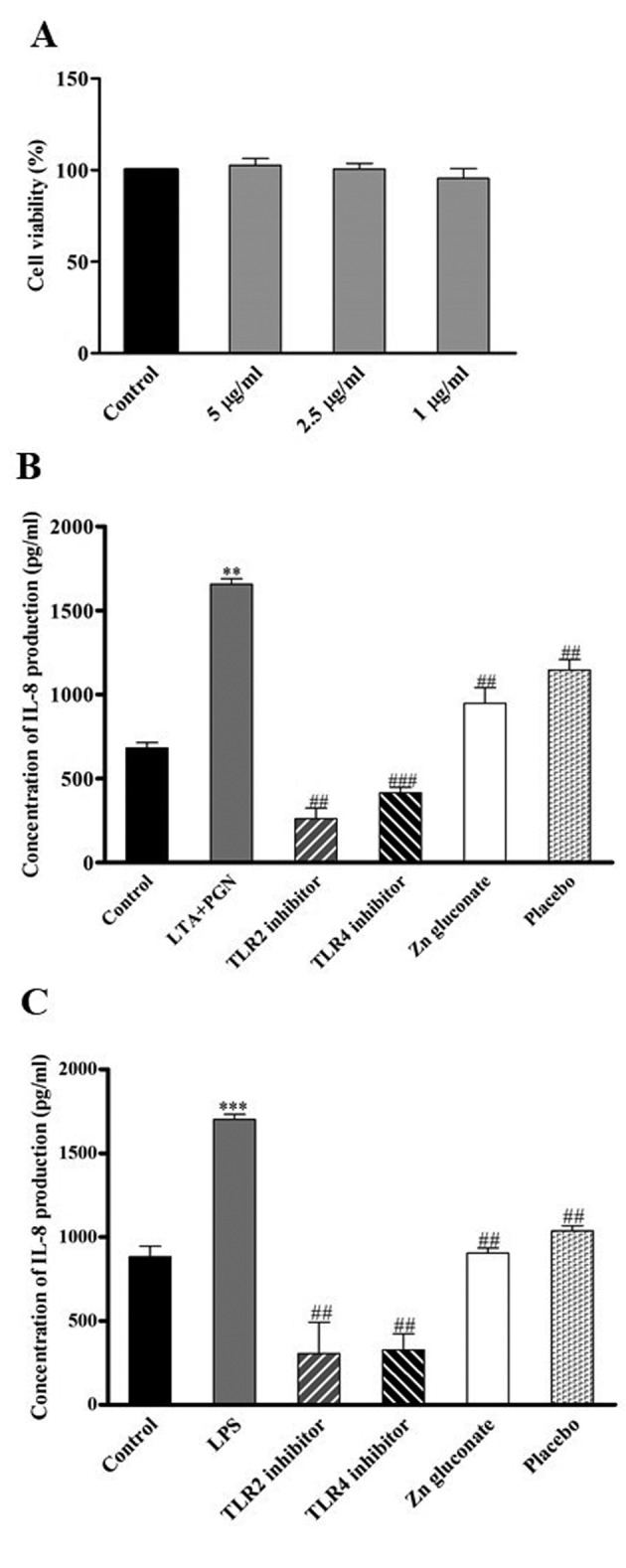
Cell viability assay. Cell viability was investigated by the MTT assay. Briefly, HaCaT cells were seeded into 96-well plates at a density of 1×104 cells per well. The cells were treated with Zinc gluconate at various concentrations (5, 2.5 and 1 μg/ml) for 24 h. Then, cells were incubated with 100 μl of MTT solution for 3 h at 37˚C. After incubation, supernatants were removed and 100 μl of DMSO was added in each well. The intensity was measured at wavelength of 570 nm by microplate reader (Anthros, Durham, NC, USA). The percentage of cell viability were calculated by comparison with untreated cells (control group).
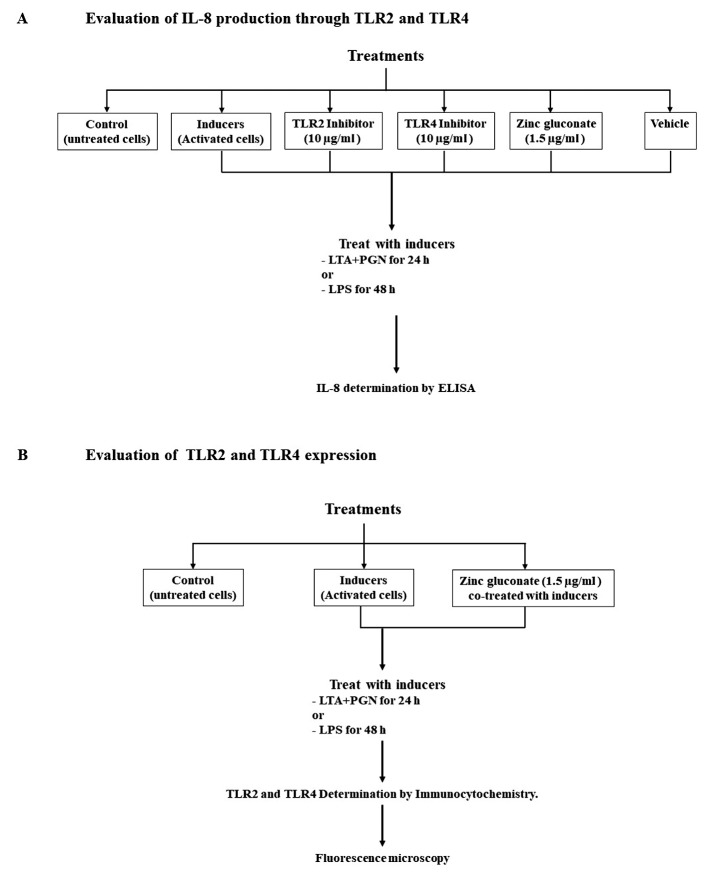
Determination of IL-8 secretion by ELISA Kit. Signaling through the TLR2 and TLR4 results in increased production and secretion of IL-8 (Figure 1). Therefore to study the secretion of IL-8, cells sub-cultured and seeded into 96-well plates at density of 8×103 cells per well were co-treated with inducers of TLRs [combination of LTA and PGN (10 μg/ml)] and TLR2 inhibitor (10 μg/ml), TLR4 inhibitor (10 μg/ml), or zinc gluconate (1.5 μg/ml) for 24 h. Also, cells were treated with LPS (100 ng/ml) in the presence of TLR2 inhibitor, TLR4 inhibitor, or zinc gluconate for 48 h as shown in Figure 2A. After incubation, the supernatants were kept and IL-8 levels were determined by using a sandwich ELISA system (R&D systems, Minneapolis, MN, USA). Addition, untreated cells were also used as control.
Figure 1. Schematic of the mechanism of IL-8 production via TLR2 and TLR4 activation.
Figure 2. Design of the analysis assays. IL-8 induction assay (A), TLR2 and TLR4 expression assay (B).
Determination of TLR2 and TLR4. Cells were subcultured and seeded into 96-well plates at a density of 8×103 cells per well. Then cells were treated with inducers (combination of LTA and PGN or LPS) in the presence of zinc gluconate as described in Figure 2B. Following treatment, cells were washed with 1X PBS, fixed with 4% (w/v) paraformaldehyde for 15 min and permeabilized with 0.5% (v/v) Triton-X for 5 min. Then, cells were incubated with 10% (w/v) FBS for 1 h, washed and incubated with specific antibody against TLR2 or TLR4 (InvivoGen, San Diego, CA, USA) overnight at 4˚C. Following washing cells were incubated with FITC and Texas Red (Abcam, Cambridge, UK) conjugated goat Human IgA H&L or rabbit Anti-Rat IgG H&L secondary antibody, respectively. Nuclei were stained with Hoechst 33,342 for 1 h at room temperature in the dark. Stained cells were washed, mounted with 50% glycerol, and visualized and captured using fluorescence microscopy (Nikon model ECLIPSE Ts2-FL, Tokyo, Japan).
Statistical analysis. The data were obtained from at least three independent experiments and are presented as the mean±standard deviation (SD). Statistical differences between two groups was determined by One-way analysis of variance (ANOVA) with a Tukey’s test to compare the multiple groups at a significance level of p<0.05, 0.01 and 0.001. Minitab version 16.0 was used for all statistical analyses.
Results
IL-8 production. HaCat keratinocyte cells at passages of 5-15 were stimulated by bacterial components and TLR2- and TLR4-mediated IL-8 production was measured (Figure 1). Following subculture and overnight incubation, the cells were treated with 5, 2.5 and 1 μg/ml of zinc gluconate for 24 h to determine its non-toxic doses. Cell viability was evaluated by MTT assay. As shown in Figure 3A, treatment with zinc gluconate at the indicated concentrations did not result in cytotoxicity. The monolayer of keratinocytes was exposed to LPS or LTA+PGN as shown in Figure 2, and IL-8 secretion was assayed in the supernatants by ELISA-immunoassay. As shown in Figure 3B and C, the levels of IL-8 protein in culture supernatants of untreated cells was very low. However, in the presence of LPS (100 ng/ml) or LTA+PGN (10 μg/ml), the secreted levels of IL-8 were significantly increased. Treatment of the cells with TLR2 as well as TLR4 inhibitors dramatically suppressed production of IL-8, indicating that IL-8 production in keratinocytes is mediated via TLR2 and TLR4 activation. Zinc gluconate was also found to significantly suppress IL-8 production.
Figure 3. HaCaT cells were treated with 5, 2.5 or 1 μM of zinc gluconate for 24 h. Cell viability was investigated using the MTT assay. Data represent the mean±SD (n=3) (A). IL-8 secretion, assayed by ELISA, by HaCaT cells was induced following incubation with 10 μg/ml LTA+PGN for 24 h (B) or 100 ng/ml LPS for 48 h (C). Co-treatment with 1.5 μg/ml zinc gluconate or solvent (ratio of 1:1,000). Data are presented as the mean±SD (n=3). **p<0.01 vs. non-treated control. ###p<0.001 and ##p<0.01 vs. LTA+PGN or LPS-treated cells.
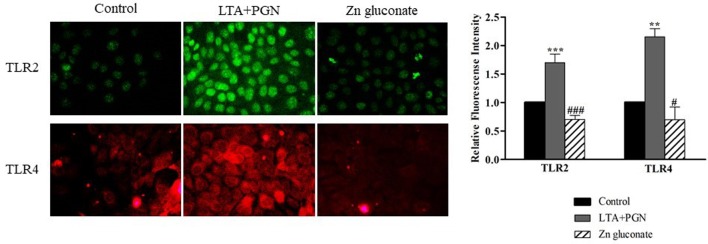
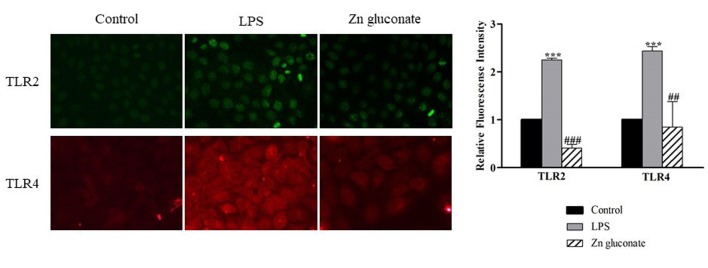
Evaluation of TLR2 and TLR4 expression on the surface of keratinocytes. Cell surface expression of TLR2 and TLR4 was examined in HaCaT cells using immunocytochemistry and fluorescence intensity analysis. LTA+PGN-treated cells (Figure 4) and LPS (Figure 5) showed higher intensity of fluorescence at the cell surface compared to control cells. Zinc gluconate suppressed the expression of the receptors at the cell surface.
Figure 4. TLR2 and TLR4 expression levels were detected by polyclonal antibody specific for human TLR2 and TLR4, respectively and visualized by a fluorescence microscopy. Cells were activated by 10 μg/ml LTA+PGN for 24 h. Data are presented as the mean±SD (n=3). ***p<0.001 and **p<0.01 vs. non-treated control. ###p<0.001 and #p<0.05 vs. LTA+PGN.
Figure 5. TLR2 and TLR4 expression levels were detected by polyclonal antibody specific for human TLR2 and TLR4, respectively and visualized by a fluorescence microscopy. Cells were activated by 100 ng/ml LPS for 48 h. Data are presented as the mean±SD (n=3). ***p<0.001 vs. nontreated control. ###p<0.001 and ##p<0.01 vs. LPS-treated cells.
Discussion
Acne vulgaris is a common disease that affects millions of people. It is a disease that has a significant impact on the psychological and physical well-being of patients (13). The pathogenesis of acne is multifactorial, including micro-biological, hormonal and immunological mechanisms (14). The epidermis is an important site of interactions between microbes and the host and keratinocytes are one of the skin components that participate in host defense. They initiate and regulate the cutaneous inflammation and immune responses by producing cytokines (15). Microbes are the main organisms that lead to acne lesions. Gram-positive and anaerobic P. acnes have been implicated in acne inflammation and pathogenesis. LTA and PGN are the main components of P. acnes that trigger TLR2 activation and the release of the neutrophil chemoattractant cytokine IL-8 (16). On the other hand, LTA triggers TLR4 activation. In addition, LPS, a component of Gram-negative bacteria, also induces TLR4 activation (17). Therefore, the combination of LTA+PGN was chosen to simulate the effect of P. acnes. In order to select the appropriate concentrations of these compounds, viability of the treated cells should not be significantly different from untreated cells (control) (8). Zinc gluconate was initially screened using the MTT assay. The results showed that none of the concentrations of Zinc gluconate used had an effect on viability of HaCaT cells.
When skin is attacked by microbes, keratinocytes being at the first line of defense are activated and produce IL-8, a major cytokine which plays a role as a chemotactic factor to recruit immune cells (18). Therefore, IL-8 levels were determined by a sandwich ELISA. LPS and LTA+PGN-treated cells were found to produce and secrete increased levels of IL-8. Zinc gluconate-treatment decreased secretion of IL-8. A previous study has also reported that the induction of IL-8 depends on TLR family (19). Specifically, TLR2 and TLR4 that recognize bacterial cell-wall components, such as PGN, LTA and LPS. TLRs are also induced by endogenous ligands generated at sites of tissue inflammation that represent damage signals to the host (20). Interestingly, zinc gluconate treatment resulted in decreased expression of TLR2 and TLR4 at the cell surface when compared with the induced groups. These results indicated that zinc gluconate decreased IL-8 secretion via inhibition of TLR2 and TLR4 expression (Figure 1). As previously mentioned, the analyses performed in this study (Figure 2) are appropriate for estimating the efficacy of drugs against acne vulgaris. These analyses are simple and relatively short.
Conflicts of Interest
The Authors declare that there is no conflict of interest in regard to this research.
Authors’ Contributions
PS and PC designed, analyzed, and concluded the experiments, TT and OP performed the experiments and drafted the manuscript. PL reviewed and concluded the experiments. PC concluded and revised the manuscript. All Authors approved the final version of the manuscript.
Acknowledgements
This research was funded by the Chulalongkorn University CU_GRS_61_08_33_01.
References
- 1.Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25. doi: 10.2147/AHMT.S55832. PMID: 26955297. DOI: 10.2147/AHMT.S55832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuki T, Yoshida H, Akazawa Y, Komiya A, Sugiyama Y, Inoue S. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J Immunol. 2011;187(6):3230–3237. doi: 10.4049/jimmunol.1100058. PMID: 21841130. DOI: 10.4049/jimmunol.1100058. [DOI] [PubMed] [Google Scholar]
- 3.Pivarcsi A, Bodai L, Réthi B, Kenderessy-Szabó A, Koreck A, Széll M, Beer Z, Bata-Csörgoo Z, Magócsi M, Rajnavölgyi E, Dobozy A, Kemény L. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15(6):721–730. doi: 10.1093/intimm/dxg068. PMID: 12750356. DOI: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, Brightbill HD, Holland D, Cunliffe WJ, Akira S, Sieling PA, Godowski PJ, Modlin RL. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169(3):1535–1541. doi: 10.4049/jimmunol.169.3.1535. PMID: 12133981. DOI: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo HS, Michalek SM, Nahm MH. Lipoteichoic acid is important in innate immune responses to gram-positive bacteria. Infect Immun. 2008;76(1):206–213. doi: 10.1128/IAI.01140-07. PMID: 17954723. DOI: 10.1128/IAI.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill AM, Gallo RL. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6:1–16. doi: 10.1186/s40168-018-0558-5. PMID: 30285861. DOI: 10.1186/s40168-018-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelsen KS, Aicher A, Mohaupt M, Hartung T, Dimmeler S, Kirschning CJ, Schumann RR. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276(28):25680–25686. doi: 10.1074/jbc.M011615200. PMID: 11316801 DOI: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 8.Phiboonchaiyanan PP, Petpiroon N, Sritularak B, Chanvorachote P. Phoyunnanin E induces apoptosis of non-small cell lung cancer cells via p53 activation and down-regulation of surviving. Anticancer Res. 2018;38(11):6281–6290. doi: 10.21873/anticanres.12984. PMID: 30396948. DOI: 10.21873/anticanres.12984. [DOI] [PubMed] [Google Scholar]
- 9.Molteni M, Gemma S, Rossetti C. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediat Inflamm. 2016;2016:1–6. doi: 10.1155/2016/6978936. PMID: 27293318. DOI: 10.1155/2016/6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nascimento LO, Massari P, Wetzler LM. The Role of TLR2 in Infection and Immunity. Front Immunol. 2012;3:1–17. doi: 10.3389/fimmu.2012.00079. PMID: 22566960. DOI: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamadzadeh M, Müller M, Hultsch T, Enk A, Saloga J, Knop J. Enhanced expression of IL-8 in normal human keratinocytes and human keratinocyte cell line HaCaT in vitro after stimulation with contact sensitizers, tolerogens and irritants. Exp Dermatol. 1994;3(6):298–303. doi: 10.1111/j.1600-0625.1994.tb00292.x. PMID: 7749573. DOI: 10.1111/j.1600-0625.1994.tb00292. [DOI] [PubMed] [Google Scholar]
- 12.Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, Chung KF. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118(3):641–648. doi: 10.1016/j.jaci.2006.05.013. PMID: 16950283. DOI: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Behnam B, Taheri R, Ghorbani R, Allameh P. Psychological impairments in the patients with acne. Indian J Dermatol. 2013;58(1):26–29. doi: 10.4103/0019-5154.105281. PMID: 23372208. DOI: 10.4103/0019-5154.105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovecková Y, Havlíková I. A microbiological approach to acne vulgaris. Biomed Papers. 2002;146(2):29–32. doi: 10.5507/bp.2002.005. PMID: 12572891. DOI: 10.5507/bp.2002.005. [DOI] [PubMed] [Google Scholar]
- 15.Bourke CD, Prendergast CT, Sanin DE, Oulton TE, Hall RJ, Mountford AP. Epidermal keratinocytes initiate wound healing and pro-inflammatory immune responses following percutaneous schistosome infection. Int J Parasitol. 2015;45(4):215–224. doi: 10.1016/j.ijpara.2014.11.002. PMID: 25575749. DOI: 10.1016/j.ijpara.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dammermann W, Wollenberg L, Bentzien F, Lohse A, Lüth S. Toll like receptor 2 agonists lipoteichoic acid and peptidoglycan are able to enhance antigen specific IFNγ release in whole blood during recall antigen responses. J Immunol Methods. 2013;396(1-2):107–115. doi: 10.1016/j.jim.2013.08.004. PMID: 23954282. DOI: 10.1016/j.jim.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. PMID: 18304834. DOI: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Jia S, Xie P, Zhong A, Galiano RD, Mustoe TA, Hong SJ. The expression of proinflammatory genes in epidermal keratinocytes is regulated by hydration status. J Invest Dermatol. 2014;134(4):1044–1055. doi: 10.1038/jid.2013.425. PMID: 24226202. DOI: 10.1038/jid.2013.425. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112(3):428–436. doi: 10.1111/j.1365-2567.2004.01898.x. PMID: 15196211. DOI: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. PMID: 10549626. DOI: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]