Abstract
Background/Aim: Establishment of mouse xenograft models is necessary for oncological research and depends on the characteristics of the cell lines and the immune system of the host. In this study, we describe the development of mouse xenograft models using human gastric cancer (GC) cell lines. Materials and Methods: MKN1 stably-expressing luciferase (MKN1-Luc), N87, KATO III, MKN45 stably-expressing luciferase (MKN45-Luc), NUGC4, and OCUM-1 human GC cell lines were injected intraperitoneally into mice to establish peritoneal metastasis models. MKN45-Luc were injected into subcutaneously implanted spleen, and MKN1-Luc and MKN45-Luc were injected directly into the portal veins of mice for the establishment of hepatic metastasis models. Results: Peritoneal metastasis was formed after implantation of MKN1-Luc, N87, KATO III, MKN45-Luc, and NUGC4 in nude mice, but not formed in OCUM-1 even in NOD/SCID mice. After intrasplenic injection of MKN45-Luc, we found no hepatic metastasis formation. We identified hepatic metastasis formation after direct injection of MKN45-Luc and MKN1-Luc into the portal veins of NOD/SCID mice. Conclusion: Peritoneal and hepatic metastasis mouse xenograft models were successfully established using several human GC cell lines.
Keywords: Gastric cancer, peritoneal metastasis, hepatic metastasis, xenograft
Gastric cancer (GC) is the fourth leading cause of cancer-related death worldwide (1). Perioperative adjuvant therapy has improved prognosis to a certain degree (2-4). However, it is still less than satisfactory in its therapeutic efficacy, because some patients with advanced GC often experience recurrence even after curative gastrectomy. To develop a therapeutic strategy, elucidation of the mechanisms of metastasis formation and identification of therapeutic targets are urgently required.
As previously reported, complex processes such as invasion into the circulation, survival in the circulation, adhesion, migration, invasion and proliferation in other organs are necessary for metastasis formation (5). Various molecules are involved in these processes (5-10). Analysis of certain molecules and development of therapeutic agents are undertaken using in vitro experiments. However, it is necessary to establish xenograft models for further molecular biological analysis and validation via in vivo experiments. Nonetheless, it is not always possible to develop xenograft models, because xenotransplantation results in the major histocompatibility complex (MHC) class II molecules of xenotransplanted cells and antigen-presenting cells phagocytosing the graft due to antigen presentation, thereby activating CD4-positive T cells. These activated CD4-positive cells also activate cell-mediated immunity and antibody-mediated immunity, which exclude implanted grafts (11,12).
Herein we attempted to establish mouse xenograft models using several human GC cell lines. We hope that our experience will be useful for other researchers conducting in vivo studies.
Materials and Methods
Cell lines and cell culture. MKN1 cells stably expressing luciferase (MKN1-Luc), MKN45 cells stably expressing luciferase (MKN45-Luc), NUGC4, and OCUM-1 were obtained from the Japanese Collection of Research Bio Resources Cell Bank (JCRB) (Osaka, Japan). N87 and KATO III cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were incubated at 37˚C with 5% CO2 in the recommended medium supplemented with 10% fetal bovine serum. The characteristics of the gastric cancer cell lines are listed in Table I.
Table I. Characteristics of gastric cancer cell lines.
RPMI: Roswell Park Memorial Institute medium; FBS: fetal bovine serum; DMEM: Dulbecco’s Modified Eagle medium; ALP: alkaline phosphatase; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; EGF: epidermal growth factor.
Mice. All animal experiments conformed to ARRIVE (Animal Research: Reporting of In Vivo Experiments) guideline and were approved by the Animal Research Committee of Nagoya University (IRB No. 29329) (13). Four-week-old male nude mice (BULB/cSlc-nu/nu) were obtained from Chubu Kagaku Shizai (Nagoya, Japan). Four-week-old male NOD/SCID mice (nod/shi-SCID) were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Mice were housed and adapted to the breeding environment for two weeks before the experiment.
Mouse subcutaneous xenograft model. A total of 1×106 of MKN1, KATO III, and MKN45 cells were suspended in 100 μl of phosphate buffered saline (PBS) and subcutaneously injected into the bilateral flanks of nude mice.
Mouse peritoneal metastasis models. MKN1-Luc, N87, KATO III, MKN45-Luc, NUGC4, and OCUM-1 cells were injected into nude mice intraperitoneally with 1 ml PBS. A total of 1.0×106 OCUM-1 cells were injected into NOD/SCID mice intraperitoneally with 1 ml PBS. After each observation period, these mice were sacrificed and peritoneal metastasis formation was observed under direct viewing.
Mouse hepatic metastasis models. Under general anesthesia, we mobilized and excised the spleens of nude mouse, which were then subcutaneously implanted. A total of 0.5×106 MKN45-Luc cells were suspended in 100 μl of PBS and injected into the subcutaneously implanted spleens. For direct injection into the portal vein, nude mice and NOD/SCID mice were placed under general anesthesia and laparotomized. Then, 1.0×106 MKN45-Luc and 0.5×106 MKN1-Luc cells were suspended in 100 μl of PBS and directly injected into the portal veins of mice using a 35-gauge NanoNeedle (NIPPON Genetics, Tokyo, Japan). After injection of the cell suspensions, we oppressed the puncture site of the portal vein using SURGICEL (Johnson & Johnson, NJ, USA) for a few minutes. Twelve weeks after injection, these mice were killed and hepatic metastasis formation was observed under direct viewing.
In vivo imaging. The In Vivo Imaging System (IVIS) Lumina (Xenogen, Alameda, CA, USA) was employed every four weeks after injection to non-invasively measure the volumes of peritoneal and liver metastases. D-Luciferin (150 mg/kg) (Summit Pharmaceuticals International, Tokyo, Japan) was administered intraperitoneally and luciferase activity was measured using the IVIS at 15 min after injection of D-luciferin. We used Living Image Ver. 2.6 (Xenogen) software to acquire and analyze the data. Magnetic resonance imaging (MRS 3000, MR solutions, Guildford, UK) was employed twelve weeks after injection as an alternative approach to detecting the formation of hepatic metastasis.
Results
Subcutaneous tumor formation. Subcutaneous tumors were formed after subcutaneous injection of MKN1-Luc, KATO III, and MKN45-Luc cells. Previous reports have shown that N87, NUGC4 and OCUM-1 can form subcutaneous tumors in nude mice (14-18).
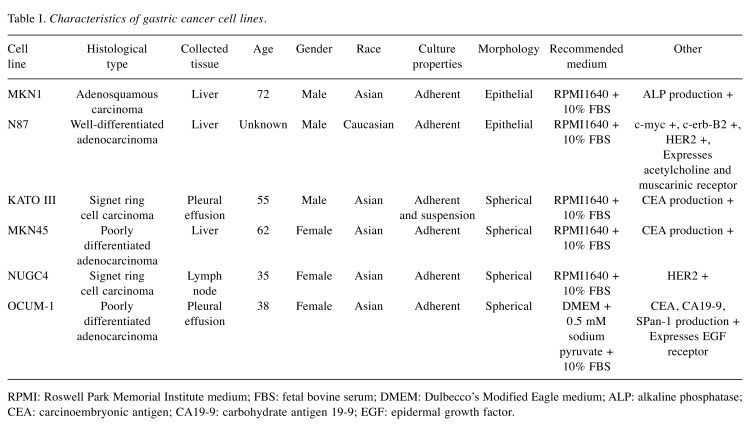
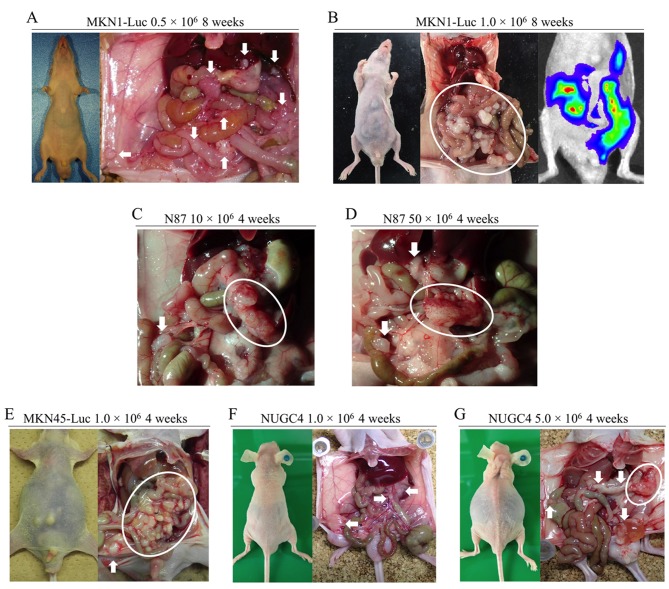
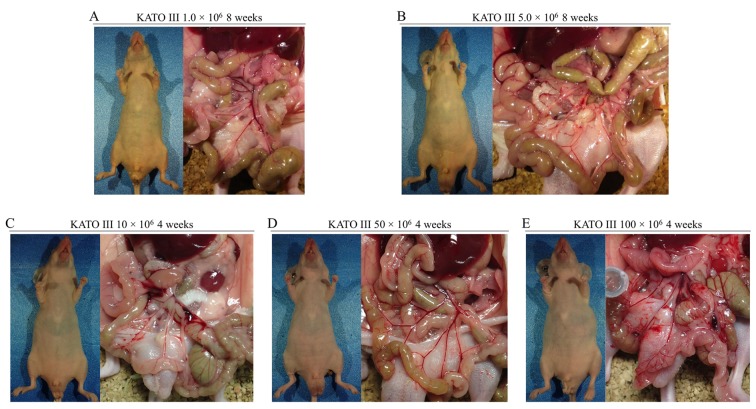
Peritoneal metastasis formation. Peritoneal metastasis formation was noted eight weeks after implantation of 0.5×106 and 1.0×106 MKN1-Luc cells in nude mice (Figure 1A and B). IVIS images of mice injected with 1.0×106 MKN1-Luc cells are shown in Figure 1B. Four weeks after implantation of 10×106 and 50×106 N87 cells, peritoneal metastasis was observed in nude mice (Figure 1C and D). Four weeks after implantation of 1.0×106 MKN45-Luc and 1.0×106 and 5.0×106 NUGC4 cells, hemorrhagic ascites and peritoneal metastasis was also evident in nude mice (Figure 1E, F, G). Peritoneal metastasis formation was not detected eight weeks after implantation of 1.0×106 and 5.0×106 KATO III cells and four weeks after implantation of 10×106, 50×106, and 100×106 KATO III cells in nude mice (Figure 2A-E). However, nine weeks after implantation of 100×106 KATO III cells in nude mice, peritoneal metastasis was detected (data not shown). We could not identify peritoneal metastasis at four weeks after intraperitoneal injection of 1.0×106 or 5.0×106 OCUM-1 cells in nude mice, or 1.0×106 OCUM-1 cells in NOD/SCID mice(Figure 3A-C).
Figure 1. Peritoneal metastasis formation in nude mice. (A) Eight weeks after injection with 0.5×106 MKN1-Luc cells. (B) Eight weeks after injection with 1.0×106 MKN1-Luc cells. (C) Four weeks after injection with 10×106 N87 cells. (D) Four weeks after injection with 50×106 N87 cells. (E) Four weeks after injection with 1.0×106 MKN45-Luc cells. (F) Four weeks after injection with 1.0×106 NUGC4 cells. (G) Four weeks after injection with 5.0×106 NUGC4 cells.
Figure 2. Peritoneal metastasis formation in nude mice after intraperitoneal injection of KATO III cells. No peritoneal metastasis was found (A) eight weeks after injection with 1.0×106 cells, (B) eight weeks after injection with 5.0×106 cells, (C) four weeks after injection with 10×106 cells, (D) four weeks after injection with 50×106 cells, or (E) four weeks after injection with 100×106 cells.
Figure 3. Peritoneal metastasis formation after intraperitoneal injection of OCUM-1 cells. No peritoneal metastasis was found four weeks after injection of (A) 1.0×106 and (B) 5.0×106 OCUM-1 cells in nude mice. No peritoneal metastasis was found (C) four weeks after injection of 1.0×106 OCUM-1 cells in NOD/SCID mice.
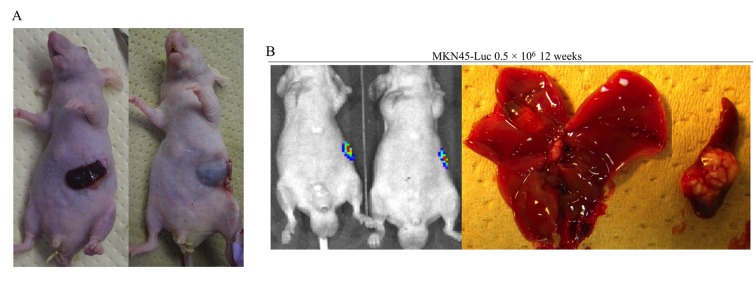
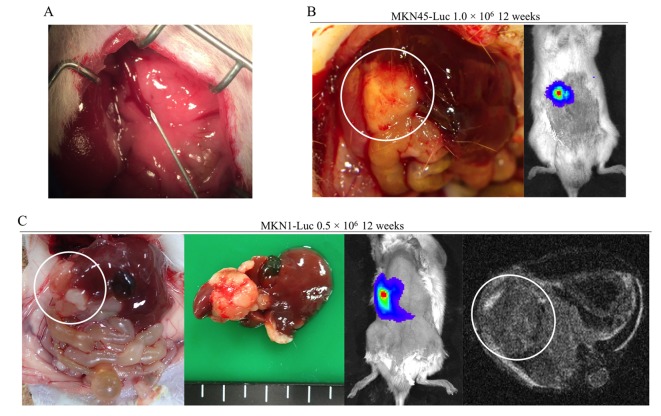
Hepatic metastasis formation. Subcutaneously implanted spleen data are shown in Figure 4A. Hepatic metastasis was not identified in nude mice, but splenic tumors were found twelve weeks after injection of 0.5×106 MKN45-Luc cells into subcutaneously implanted spleen (Figure 4B). The procedure for direct injection into the portal veins of mice is shown in Figure 5A. In our early experiences, most of the mice died immediately after treatment if they underwent thoracotomy. Although another major cause of treatment-related death is hemorrhage from the portal vein after injection, it can be prevented by usage of a 35-gauge needle and astriction with an oxidized cellulose cotton. We did not find hepatic metastasis formation in nude mice at all. However, we identified hepatic metastasis formation twelve weeks after injection of 1.0×106 MKN45-Luc cells in the portal veins of NOD/SCID mice (Figure 5B). Hepatic metastasis was also detected twelve weeks after injection of 0.5×106 MKN1-Luc cells in the portal veins of NOD/SCID mice (Figure 5C). IVIS and MRI images of mice twelve weeks after injection are also shown in Figure 5C.
Figure 4. Hepatic metastasis formation after intrasplenic injection in nude mice. (A) Subcutaneously implanted spleen. (B) Twelve weeks after injection with 0.5×106 MKN45-Luc cells.
Figure 5. Hepatic metastasis formation after direct injection of the portal vein in NOD/SCID mice. (A) Direct injection procedure into the portal veins of mice. (B) Twelve weeks after injection of 1.0×106 MKN45-Luc cells into the portal vein. (C) Twelve weeks after injection of 0.5×106 MKN1- Luc cells into portal vein.
Discussion
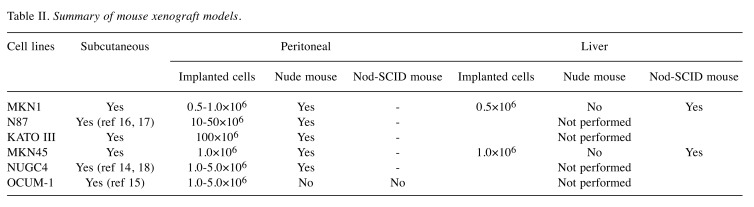
Before conducting phase I studies, we need to validate the effects of novel pharmacological agents and treatment methods through the use of animal models (19). Experiments using mice are the first step in laboratory animal studies before the use of large-sized animals such as dogs and monkeys. Peritoneal metastasis and hepatic metastasis are the main reasons for gastric cancer-related death (20). In this study, we attempted to establish peritoneal and hepatic metastasis mouse xenograft models using several gastric cancer cell lines that can form subcutaneous tumors in nude mouse to generate tools, for examining the mechanisms of metastasis formation and for developing novel therapeutic strategies. A summary of this study is shown in Table II.
Table II. Summary of mouse xenograft models.
Generally, the host immune system rejects transplanted grafts in xenotransplantation (11,12). Thus, immunocompromised animals are needed to establish xenograft models. In 1962, the nude mouse was detected as the first immunocompromised mouse. The nude mouse has no mature T cells due to the lack of a thymus gland, resulting in poor immunity (21). A number of human cancer cell lines were engrafted successfully in nude mice (22-24). These mice have subsequently been used in cancer research and in the development of anticancer agents. However, the nude mouse has some immunocytes besides mature T cells, and many human cell lines cannot be engrafted successfully. Subsequently, the NOD/SCID mouse was developed as a immunocompromised mouse that lacks both T cells and B cells (25). The discovery of such mice has facilitated the establishment of xenograft models (26-28), although these animals do not absolutely lack immunity.
We observed peritoneal metastasis formation after injection of MKN1-Luc, N87, KATO III, MKN45-Luc, and NUGC4 cells. These cell lines can be disseminated in manageable nude mice and may be suitable for peritoneal metastasis xenograft models. However, OCUM-1 failed to form peritoneal metastases, even in the NOD/SCID mouse. This cell line has not been reported previously in peritoneal metastasis xenografts and is unsuitable for this practice.
We can generate hepatic metastasis mouse xenograft models by injecting cell suspensions directly into the liver. However, this procedure can only assess the survival and proliferation of cancer cells in hepatic tissue. Implantation by injection into the spleen is reportedly one of the ways to establish hepatic metastasis xenograft models (29-31). There have been several reports of successful establishment of hepatic metastasis models by subcapsular injection of murine cancer cell lines to the spleen (32-34). The procedure of intrasplenic injection is simple and convenient. However, this procedure does not reliably establish hepatic metastasis because cancer cells must migrate and invade into the splenic vein to reach the portal vein system. In our experience using human gastric cancer cell lines, we could not establish hepatic metastasis by intrasplenic injection of MKN45-Luc cells in nude mice. We conducted the procedure by directly injecting into the portal vein to ensure the cell suspension reached the portal vein system. However, it took some time to establish this procedure because of the difficulty of direct injection into the thin mouse portal vein, as well as the hemostasis after injection. In the early days of this study, hemostasis was not obtained after injection of the cell suspension into the portal vein and mice frequently died of blood loss. Finally, we were able to establish hepatic metastasis for a brief time in NOD/SCID mice after injection with 0.5×106 of MKN1-Luc and 1.0×106 of MKN45-Luc cells using our procedure.
Using immunosuppressed mice, human GC cell lines were successfully engrafted at different organs from the original organs from which the cell lines were derived. This enables us to use these cell lines properly according to the purpose of the animal experiment by applying several characteristics, such as cell differentiation and molecular expression. These findings contributed to our previous studies of the identification of molecules related to the formation of peritoneal and hepatic metastasis (35,36).
In conclusion, we developed a procedure to establish peritoneal and hepatic mouse xenograft models with several human GC cell lines. This information will be useful for the elucidation of the mechanism of metastasis formation and the development of novel treatment approaches for GC.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
Mitsuro Kanda and Yasuhiro Kodera make substantial contributions to conception and design. Shinichi Umeda and Haruyoshi Tanaka and Dai Shimizu make substantial contributions to acquisition of data. Chie Tanaka and Daisuke Kobayashi and Masamichi Hayashi and Suguru Yamada and Goro Nakayama and Masahiko Koike make substantial contributions to interpretation of data.
Acknowledgements
The Authors would like to thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. PMID: 21296855. DOI: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzen F, Noh SH. Adjuvant capecitabine and oxaliplatin for gastric cancer after d2 gastrectomy (classic): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. doi: 10.1016/S0140-6736(11)61873-4. PMID: 22226517. DOI: 10.1016/s0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 3.Kanaji S, Suzuki S, Matsuda Y, Hasegawa H, Yamamoto M, Yamashita K, Oshikiri T, Matsuda T, Nakamura T, Sumi Y, Kakeji Y. Recent updates in perioperative chemotherapy and recurrence pattern of gastric cancer. Ann Gastroenterol Surg. 2018;2(6):400–405. doi: 10.1002/ags3.12199. PMID: 30460342. DOI: 10.1002/ags3.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K. Adjuvant chemotherapy for gastric cancer with s-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi: 10.1056/NEJMoa072252. PMID: 17978289. DOI: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu D, Kanda M, Kodera Y. Emerging evidence of the molecular landscape specific for hematogenous metastasis from gastric cancer. World J Gastrointest Oncol. 2018;10(6):124–136. doi: 10.4251/wjgo.v10.i6.124. PMID: 29988904. DOI: 10.4251/wjgo.v10.i6.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Mattos-Arruda L, Bidard FC, Won HH, Cortes J, Ng CK, Peg V, Nuciforo P, Jungbluth AA, Weigelt B, Berger MF, Seoane J, Reis-Filho JS. Establishing the origin of metastatic deposits in the setting of multiple primary malignancies: The role of massively parallel sequencing. Mol Oncol. 2014;8(1):150–158. doi: 10.1016/j.molonc.2013.10.006. PMID: 24220311. DOI: 10.1016/j.molonc.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudler P. Challenges of deciphering gastric cancer heterogeneity. World J Gastroenterol. 2015;21(37):10510–10527. doi: 10.3748/wjg.v21.i37.10510. PMID: 26457012. DOI: 10.3748/wjg.v21.i37.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makohon-Moore AP, Zhang M, Reiter JG, Bozic I, Allen B, Kundu D, Chatterjee K, Wong F, Jiao Y, Kohutek ZA, Hong J, Attiyeh M, Javier B, Wood LD, Hruban RH, Nowak MA, Papadopoulos N, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49(3):358–366. doi: 10.1038/ng.3764. PMID: 28092682. DOI: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA, Elledge SJ, Jain RK. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357(6346):55–60. doi: 10.1126/science.aai8515. PMID: 28684519. DOI: 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu D, Kanda M, Kodera Y. Review of recent molecular landscape knowledge of gastric cancer. Histol Histopathol. 2018;33(1):11–26. doi: 10.14670/HH-11-898. PMID: 28447336. DOI: 10.14670/hh-11-898. [DOI] [PubMed] [Google Scholar]
- 11.Chapman HA. Endosomal proteolysis and mhc class ii function. Curr Opin Immunol. 1998;10(1):93–102. doi: 10.1016/s0952-7915(98)80038-1. PMID: 9523118. [DOI] [PubMed] [Google Scholar]
- 12.Pamer E, Cresswell P. Mechanisms of mhc class i--restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. PMID: 9597133. DOI: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 13.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The arrive guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20(4):256–260. doi: 10.1016/j.joca.2012.02.010. PMID: 22424462. DOI: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Hu N, Yin JF, Ji Z, Hong Y, Wu P, Bian B, Song Z, Li R, Liu Q, Wu F. Strengthening gastric cancer therapy by trastuzumab-conjugated nanoparticles with simultaneous encapsulation of anti-mir-21 and 5-fluorouridine. Cell Physiol Biochem. 2017;44(6):2158–2173. doi: 10.1159/000485955. PMID: 29241186. DOI: 10.1159/000485955. [DOI] [PubMed] [Google Scholar]
- 15.Kubo T. Establishment and characterization of a new gastric cancer cell line (ocum-1), derived from borrmann type IV tumor. Nihon Geka Gakkai Zasshi. 1991;92(10):1451–1460. PMID: 1961183. [PubMed] [Google Scholar]
- 16.Kubota T, Kuroda S, Kanaya N, Morihiro T, Aoyama K, Kakiuchi Y, Kikuchi S, Nishizaki M, Kagawa S, Tazawa H, Fujiwara T. Her2-targeted gold nanoparticles potentially overcome resistance to trastuzumab in gastric cancer. Nanomedicine. 2018;14(6):1919–1929. doi: 10.1016/j.nano.2018.05.019. PMID: 29885899. DOI: 10.1016/j.nano.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Kim H, Kang YR, Kim H, Kim JY, Lee YJ, Kim JM, Kim JS. Selection criteria for determination of optimal reconstruction method for cu-64 trastuzumab dosimetry on siemens inveon pet scanner. J Clin Med. 2019;8(4) doi: 10.3390/jcm8040512. PMID: 31014003. DOI: 10.3390/jcm8040512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takabayashi K, Kashiwagi K, Kawata T, Sato T, Matsuoka K, Hisamatsu T, Takaishi H, Hibi T, Ogata H, Yahagi N, Kitagawa Y, Shigematsu N, Kanai T. Continuous low-dose irradiation by i-125 seeds induces apoptosis of gastric cancer cells regardless of histological origin. Cancer Biol Ther. 2014;15(1):81–88. doi: 10.4161/cbt.26610. PMID: 24149371. DOI: 10.4161/cbt.26610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. PMID: 24141714. DOI: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 20.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, Ono H, Tanabe S, Kaminishi M. Gastric cancer treated in 2002 in japan: 2009 annual report of the jgca nationwide registry. Gastric Cancer. 2013;16(1):1–27. doi: 10.1007/s10120-012-0163-4. PMID: 22729699. DOI: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flanagan SP. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8(3):295–309. doi: 10.1017/s0016672300010168. PMID: 5980117. [DOI] [PubMed] [Google Scholar]
- 22.Beattie GM, Knowles AF, Jensen FC, Baird SM, Kaplan NO. Induction of sarcomas in athymic mice. Proc Natl Acad Sci U S A. 1982;79(9):3033–3036. doi: 10.1073/pnas.79.9.3033. PMID: 6283553. DOI: 10.1073/pnas.79.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faguet GB, Agee JF, DiPiro JT. Blood kinetics, tissue distribution, and radioimaging of anti-common chronic lymphatic leukemia antigen (CCLLA) monoclonal antibody cll2 in mice transplanted with cclla-bearing human leukemia cells. Blood. 1990;75(9):1853–1861. PMID: 2331525. [PubMed] [Google Scholar]
- 24.Ovejera AA, Houchens DP, Catane R, Sheridan MA, Muggia FM. Efficacy of 6-diazo-5-oxo-l-norleucine and n-[n-gamma-glutamyl-6-diazo-5-oxo-norleucinyl]-6-diazo-5-oxo-norleucine against experimental tumors in conventional and nude mice. Cancer Res. 1979;39(8):3220–3224. PMID: 572261. [PubMed] [Google Scholar]
- 25.Gerling IC, Serreze DV, Christianson SW, Leiter EH. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in nod/lt mice. Diabetes. 1992;41(12):1672–1676. doi: 10.2337/diab.41.12.1672. PMID: 1446808. [DOI] [PubMed] [Google Scholar]
- 26.Korbelik M, Sun J. Cancer treatment by photodynamic therapy combined with adoptive immunotherapy using genetically altered natural killer cell line. Int J Cancer. 2001;93(2):269–274. doi: 10.1002/ijc.1326. PMID: 11410876. DOI: 10.1002/ijc.1326. [DOI] [PubMed] [Google Scholar]
- 27.Mancini R, Giarnieri E, De Vitis C, Malanga D, Roscilli G, Noto A, Marra E, Laudanna C, Zoppoli P, De Luca P, Affuso A, Ruco L, Di Napoli A, Mesiti G, Aurisicchio L, Ricci A, Mariotta S, Pisani L, Andreetti C, Viglietto G, Rendina EA, Giovagnoli MR, Ciliberto G. Spheres derived from lung adenocarcinoma pleural effusions: Molecular characterization and tumor engraftment. PLoS One. 2011;6(7):e21320–e21320. doi: 10.1371/journal.pone.0021320. PMID: 21789168. DOI: 10.1371/journal.pone.0021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stany MP, Vathipadiekal V, Ozbun L, Stone RL, Mok SC, Xue H, Kagami T, Wang Y, McAlpine JN, Bowtell D, Gout PW, Miller DM, Gilks CB, Huntsman DG, Ellard SL, Wang YZ, Vivas-Mejia P, Lopez-Berestein G, Sood AK, Birrer MJ. Identification of novel therapeutic targets in microdissected clear cell ovarian cancers. PLoS One. 2011;6(7):e21121. doi: 10.1371/journal.pone.0021121. PMID: 21754983. DOI: 10.1371/journal.pone.0021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopper L, Van Hanh T, Lapis K. Experimental model for liver metastasis formation using lewis lung tumor. J Cancer Res Clin Oncol. 1982;103(1):31–38. doi: 10.1007/BF00410303. PMID: 7076715. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Liang Y, Yang G, Lan Y, Han J, Wang J, Yin D, Song R, Zheng T, Zhang S, Pan S, Liu X, Zhu M, Liu Y, Cui Y, Meng F, Zhang B, Liang S, Guo H, Liu Y, Hassan MK, Liu L. Tetraspanin 1 promotes epithelial-to-mesenchymal transition and metastasis of cholangiocarcinoma via pi3k/akt signaling. J Exp Clin Cancer Res. 2018;37(1):300. doi: 10.1186/s13046-018-0969-y. PMID: 30514341. DOI: 10.1186/s13046-018-0969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JW, Li LX, Wu WZ, Pan TJ, Yang ZS, Yang YK. Anti-tumor effects of paeoniflorin on epithelial-to-mesenchymal transition in human colorectal cancer cells. Med Sci Monit. 2018;24:6405–6413. doi: 10.12659/MSM.912227. PMID: 30208371. DOI: 10.12659/msm.912227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraoka K, Kimura T, Logg CR, Kasahara N. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin Cancer Res. 2006;12(23):7108–7116. doi: 10.1158/1078-0432.CCR-06-1452. PMID: 17145835. DOI: 10.1158/1078-0432.ccr-06-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotta Y, Kasuya H, Bustos I, Naoe Y, Ichinose T, Tanaka M, Kodera Y. Curative effect of hf10 on liver and peritoneal metastasis mediated by host antitumor immunity. Oncolytic Virother. 2017;6:31–38. doi: 10.2147/OV.S127179. PMID: 28331843. DOI: 10.2147/ov.s127179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlik TM, Nakamura H, Mullen JT, Kasuya H, Yoon SS, Chandrasekhar S, Chiocca EA, Tanabe KK. Prodrug bioactivation and oncolysis of diffuse liver metastases by a herpes simplex virus 1 mutant that expresses the cyp2b1 transgene. Cancer. 2002;95(5):1171–1181. doi: 10.1002/cncr.10776. PMID: 12209705. DOI: 10.1002/cncr.10776. [DOI] [PubMed] [Google Scholar]
- 35.Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, Takami H, Niwa Y, Yamada S, Fujii T, Sugimoto H, Kodera Y. Synaptotagmin xiii expression and peritoneal metastasis in gastric cancer. Br J Surg. 2018;105(10):1349–1358. doi: 10.1002/bjs.10876. PMID: 29741294. DOI: 10.1002/bjs.10876. [DOI] [PubMed] [Google Scholar]
- 36.Kanda M, Tanaka H, Shimizu D, Miwa T, Umeda S, Tanaka C, Kobayashi D, Hattori N, Suenaga M, Hayashi M, Iwata N, Yamada S, Fujiwara M, Kodera Y. Syt7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 2018;37(39):5355–5366. doi: 10.1038/s41388-018-0335-8. PMID: 29858600. DOI: 10.1038/s41388-018-0335-8. [DOI] [PubMed] [Google Scholar]