Abstract
Opisthorchiasis affects millions of people in Southeast Asia, and has been strongly associated with bile duct cancer. Current strategic control approaches such as chemotherapy and health education are not sustainable, and a prophylactic vaccine would be a major advance in the prevention of the disease. Tetraspanins are transmembrane proteins previously described as potential vaccine candidates for other helminth infections, and are also found in the membranes of the tegument and extracellular vesicles of O. viverrini. Here, we investigated the potential of a recombinant protein encoding for the large extracellular loop of O. viverrini tetraspanin-2 (rOv-LEL-TSP-2) in a hamster vaccination model. Hamsters were vaccinated with 50 and 100 μg of rOv-LEL-TSP-2 produced from Pichia pastoris yeast combined with alum CpG adjuvant via the intraperitoneal route. The number of worms recovered from hamsters vaccinated with rOv-LEL-TSP-2 was significantly reduced compared to adjuvant control groups. Fecal egg output was also significantly reduced in vaccinated animals, and the average length of worms recovered from vaccinated animals was significantly shorter than that of the control group. Vaccinated animals showed significantly increased levels of anti-rOv-TSP-2 IgG in the sera after three immunizations, as well as increased levels of several T helper type 1 cytokines in the spleen including IFN-γ and IL-6 but not the Th2/regulatory cytokines IL-4 or IL-10. These results suggest that rOv-TSP-2 could be a potential vaccine against opisthorchiasis and warrants further exploration.
Keywords: Opisthorchis viverrini, Tetraspanin, vaccination, Pichia pastosis
1. Introduction
Opisthorchiasis caused by the liver fluke Opisthorchis viverrini is an important fish-borne infectious disease that is endemic in several countries in Southeast Asia (Sithithaworn et al. 2012). Infection rate remains high in areas where people continue to eat raw or fermented fish that harbor metacercariae, the stage of the parasite that is infective to humans and other mammals (Chavengkun et al. 2016). Adult flukes reside in the biliary tract of the definitive hosts where they cause bile duct inflammation that can develop into fibrosis and even cholangiocarcinoma (CCA) - a fatal bile-duct cancer (Khuntikeo et al. 2018; Sripa et al. 2012a). Health education and mass drug treatment with praziquantel are currently the main control strategies against fluke infection (Khuntikeo et al. 2016). Unfortunately, high infection rates still persist in endemic areas due to rapid reinfection (Saengsawang et al. 2016). Ideally, a prophylactic vaccine combined with effective drug treatment and community education might be a more effective approach.
Tetraspanins (TSPs) are transmembrane proteins containing four transmembrane domains interspersed by a small extracellular loop (SEL) and a large extracellular loop (LEL). In platyhelminths, TSPs are distributed throughout the tegumental membranes, and have also been found in secreted extracellular vesicles (EVs) (Chaiyadet et al. 2015; Cwiklinski et al. 2015; Nowacki et al. 2015; Sotillo et al. 2016; Zhu et al. 2016). TSPs from O. viverrini (Ov-TSPs) have been found in both EVs and the tegument of adult flukes (Chaiyadet et al. 2017a; Chaiyadet et al. 2015; Piratae et al. 2012), and are essential for maintaining the integrity of the adult fluke tegument (Chaiyadet et al. 2017b; Piratae et al. 2012).
Despite substantial effort and many publications focusing on antigen discovery and efficacy assessment in animal models, there is still no vaccine available for any human helminth infection. Previous studies describing experimental vaccines against opisthorchiasis in animal models revealed partial protection with crude somatic antigen (Jittimanee et al. 2012) and irradiated larval stage (Papatpremsiri et al. 2016). Several vaccine candidate molecules have been identified from transcriptomics (Laha et al. 2007) and proteomics (Mulvenna et al. 2010) studies of the O. viverrini secretome, however Ov-TSP is the only subunit vaccine tested thus far (Chaiyadet et al. 2019). Noteworthy, a TSP from S. mansoni is a promising vaccine candidate against schistosomiasis (Tran et al. 2006) and has completed a phase 1 clinical trial (Tebeje et al. 2016). Moreover, we showed that antibodies to an O. viverrini TSP were able to block the uptake of fluke EVs by human cholangiocytes (Chaiyadet et al. 2015), the cells that line the bile ducts and are intimately exposed to O. viverrini during chronic human infections.
In this study, we have produced the large extracellular loop of O. viverrini tetraspanin-2 (rOv-LEL-TSP-2) as a recombinant protein in the yeast Pichia pastoris and evaluated its immunogenicity and efficacy in the hamster challenge model of opisthorchiasis.
2. Materials and methods
2.1. Production and purification of recombinant Ov-LEL-TSP-2
cDNA sequence corresponding to the LEL of Ov-tsp-2 was obtained by PCR as previously described (Chaiyadet et al. 2017a) using specific primers that included EcoR I and Not I restriction enzyme sites (indicated with bold and underlined type). The primers used were Ov-TSP2-EC2F (5’ACGCGAATTCCGCGATAAGATCCCCGG-3’) and Ov-TSP2-EC2R (5’ACGCGCGGCCGCCTGGATGAACTCTTCGAC-3’). The resulting amplicon was cloned into the plasmid pPICZαA resulting in pPICZαOv-lel-tsp-2, and the recombinant protein was produced in P. pastoris X33 strain following the manufacturer’s instructions with slight modifications. Briefly, pPICZα-Ov-lel-tsp-2 plasmid was linearized with Sac I restriction enzyme and transfected into P. pastoris X33 using electroporation (MicroPulser Electroporator, BioRad, USA). Selected transfected colonies containing pPICZα-Ov-lel-tsp-2 were inoculated in YPD culture medium containing Zeocin (1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) dextrose, 100 μg/ml Zeocin) and grown overnight at 28 °C on a shaking incubator. The culture was inoculated into BMGY (1% (w/v) yeast extract, 2% (w/v) peptone, 1.34% (w/v) yeast nitrogen base, 4 × 10−5 % (w/v) d-biotin and 1% (v/v) glycerol) containing 100 mM potassium phosphate, pH 6.0 and incubated for 24 h at 28°C with shaking. To induce expression of rOv-LEL-TSP-2, the cell pellet was resuspended in 200 ml of BMMY medium (100 mM potassium phosphate, pH 6.0, 1% (w/v) yeast extract, 2% (w/v) peptone, 1.34% (w/v) yeast nitrogen base, 4 × 10−5 % (w/v) d-biotin and 1% (v/v) methanol), incubated with shaking at 28°C for 72 h at 250 rpm and 100% methanol was added to a final concentration of 1% every 24 hours. The supernatant containing rOv-TSP-2 was collected and concentrated by Amicon ultra-15 centrifuge filter unit, 3 kDa MWCO (Merck, Germany). Lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM Imidazole, pH 8.0) was added to the supernatant prior to purification with a nickel–nitrilotriacetic acid (Ni-NTA) affinity column (Thermo Fisher Scientific, USA). The purified protein was dialyzed against 20 mM HEPES using an Amicon ultra-15 centrifuge filter unit, 3 kDa MWCO. The concentration of the purified rOv-LEL-TSP-2 protein was measured by NanoDrop 2000c spectrophotometer (Thermo Scientific, USA).
2.2. Immunoblot analysis of the recombinant Ov-LEL-TSP-2 protein
Purified rOv-LEL-TSP-2 protein was separated on a 15% SDS–PAGE gel and transferred onto a nitrocellulose membrane (Mini Trans-Blot Cell, Bio-Rad). The membrane was blocked with 5% skim in PBS containing 0.1% Tween-20 (PBST) for 2 hours, at room temperature and slight shaking. The membrane was, then, washed 3 times with PBST and incubated with rabbit anti-Ov-LEL-TSP-2 antiserum as previously described (Chaiyadet et al. 2017a) (diluted at 1:2,000 in PBST) overnight at 4°C. Finally, the probed membrane was washed 3 times with PBST and incubated with goat anti-rabbit HRP-conjugated secondary antibody (1:1,000 in PBST) at room temperature for 2 hours. The colorimetric signal was developed using LuminataTM Forte Western HRP Substrate (Millipore, USA).
2.3. Preparation of O. viverrini metacercariae
O. viverrini metacercariae were collected as previously described (Laha et al. 2007). Briefly, naturally infected cyprinid fishes were homogenized in a blender with 0.25% pepsin, 1.5% HCl in 0.85% NaCl solution. The mixture then was incubated in a shaking water bath at 37°C for 1 hour for digestion. The digested solution was filtered through a series of sieves (1,000, 300, and 106 μm meshes). The debris obtained by filtering on 106 μm mesh was washed and repeatedly sedimented with 0.85% NaCl in a sedimentation jar until the supernatant became clear. Sediments were examined for metacercariae under a stereomicroscope. O. viverrini metacercariae were collected and stored in sterile 0.85% NaCl at 4°C until used.
2.4. Experimental animals and vaccination protocol
Forty male Syrian golden hamsters 8 weeks-old were used for the vaccination experiment. Hamsters were maintained in the animal house care unit at the Faculty of Medicine, Khon Kaen University. Animal experiments were approved by the Animal Ethics Committee of Khon Kaen University (ACUC KKU 10/2559). Animals were divided into four groups (10 hamsters per group): 1) immunized with 20 mM HEPES buffer (Sigma-Aldrich, Germany); 2) immunized with colloidal suspension of aluminium hydroxide gel (Invivogen, USA) and CpG ODN 1826 (10 μg) (Invivogen, USA) (Alum-CpG); 3) immunized with 50 μg of rOv-LEL-TSP-2 diluted in 20 mM HEPES plus Alum-CpG; 4) immunized with 100 μg of rOv-LEL-TSP-2 in HEPES plus Alum-CpG. Immunizations were performed three times at 2-week intervals by the intraperitoneal route. Two weeks after the third immunization, each hamster was orally infected with 50 O. viverrini metacercariae, and eight weeks later all hamsters were sacrificed. At this time, livers were collected and the number of adult worms counted. Spleen cells were collected for cytokine determination and blood samples were taken at day 0 (pre-immunization) and at 6 weeks post-immunization via retrobulbar sinus; and at necropsy (termination) by cardiac puncture.
2.5. Worm recovery
Collected livers were washed with 0.85 % NaCl solution and adult worms were recovered from the bile ducts. The number of worms were counted and the percentage of worm reduction was calculated using the equation: % worm reduction = A - B/ A x 100, where A represents the mean parasite recovery from the adjuvant control group, and B represents the mean parasite recovery from the 50 μg and 100 μg rOv-LEL-TSP-2 immunized groups.
2.6. Measurement of worm length
All intact worms recovered from each experimental group were washed three times with sterile normal saline solution (NSS) then dehydrated and fixed in pre-warmed 10% formalin. Images of the worms were captured and worm length was measured using NIS-Element software (Nikon SMZ 745T, Japan).
2.7. Fecal egg counts
Feces from individual hamsters were collected weekly from the fourth to the seventh week post-challenge. Number of eggs per gram (EPG) of feces was investigated using a modified formalin-ether acetate technique (Elkins et al. 1986). Briefly, stool samples were suspended in NSS then filtered through 2 layers of gauze. The flow-through was centrifuged at 2,000 g for 5 min, the supernatants decanted, and 7 ml of 10% formalin and 3 ml of ethyl acetate were added to the pellet. The samples were shaken vigorously and centrifuged at 2,000 g for 5 min. The sediment was collected and suspended in 10% formalin. To determine the EPG, the total number of drops of sediment were counted and, two drops were examined. EPG was calculated as follows: EPG = TV/vw, where T = the number of eggs counted, V = total volume of sediment in drops, v = the number of drops examined, w = the weight of the fecal sample.
2.8. Indirect ELISA to assess IgG responses
Specific IgG against rOv-LEL-TSP-2 in the serum of hamsters was analyzed by indirect ELISA. Briefly, 96-well ELISA plates (NUNC-F96, Fisher Scientific, USA) were coated with 100 μl of rOv-LEL-TSP-2 (1 μg/ml) in coating buffer (15 mM Na2CO3, 35mM NaHCO3, pH 9.6) and incubated at 4°C overnight. Coated plates were washed three times with PBS plus 0.05% Tween-20 (PBST), and non-specific binding was blocked with 200 μl/well of 5% skim milk in coating buffer for 2 h at 37°C. Plates were then washed three times with PBST. One hundred μl of the hamster sera (1:200 dilution in PBST/2% skim milk) were added and incubated at 37°C for 2 h. Plates were washed with PBST, probed with 100 μl of anti-hamster IgG-HRP (Thermo Fisher scientific, USA) (diluted 1:3,000 in PBST) for 2 h at 37°C and washed with PBST. Finally, 50 μl of TMB (Thermo Fisher Scientific, USA) was added to each well, plates were incubated for 20 minutes, and development was stopped by addition of 50 μl/well of 0.5M H2SO4. The colorimetric reaction was read at a wavelength of 450 nm on a Spectra Max microplate reader (Molecular Devices, USA).
2.9. Hamster splenocyte isolation
Splenocytes from hamsters were isolated as described previously with some modifications (Fantini et al. 2007). Briefly, the spleens from the experimental hamster groups were removed and washed twice with sterile PBS and RPMI containing penicillin (200 U/ml) and streptomycin (200 μg/ml). Spleen cells were extracted by cutting and squeezing the spleens between two glass slides in complete RMPI medium. Splenocytes in RPMI were obtained by filtering through a 70 μm cell strainer (VWR international, LLC, PA, USA). Cell suspensions were centrifuged at 400 g for 6 min at 4°C. Supernatants were discarded and 3 ml of ACK cell lysis buffer (0.15M NH4Cl, 1mM KHCO3, 0.1mM EDTA, pH 7.2) was added to each sample to lyse the erythrocytes and incubated at room temperature for 10 min. Then, 30 ml of PBS was added to neutralize the ACK buffer. Erythrocyte lysis and ACK buffer neutralization were repeated once more by adding 3 ml ACK lysis buffer at 37°C for 90 sec. Finally, 30 ml of PBS was added. Cells were collected by centrifugation at 400 g for 8 min at 4°C and resuspended in 5-10 ml RPMI complete media. The splenocytes were counted and seeded at 1×106 cells/well in 96-well plates and cultured in RPMI complete media at 37°C, 5% CO2 atmosphere. Cells were harvested at 72 h of cultivation. In addition, hamster splenocytes were stimulated with 10 μg/ml of rOv-LEL-TSP-2 at 37°C, 5% CO2 atmosphere. Cells were harvested after 72 h of culture.
2.10. Hamster splenocyte RNA extraction and cDNA synthesis
Total RNA from isolated hamster splenocytes was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer’s recommendations. Briefly, splenocytes were homogenized with 200 μl of Trizol reagent on ice and incubated for 5 min. Then, 40 μl of chloroform was added and the homogenates were shaken vigorously for 15 seconds before incubation at room temperature for 3 min. The homogenates were then centrifuged at 12,000 g for 15 min at 4°C. The RNA in the aqueous phase was transferred into a new tube and precipitated by addition of 100 μl isopropanol. After centrifugation, the RNA pellet was washed with 75% ethanol and air dried for 10 min. Then, RNA was resuspended in 25 μl of RNase-free water. Quality and concentration of the RNA was estimated using a NanoDrop 2000c spectrophotometer (Thermo scientific, US).
cDNA construction was carried out by converting the total RNA to single-stranded cDNA using a Thermo Scientific RevertAid First Strand cDNA Synthesis Kit (Thermo scientific, US) following the manufacturer’s recommendations. Briefly, the reaction was performed by mixing 1 μg of total RNA, 1 μl of random hexamer primer, 4 μl of 5X Reaction Buffer, 1μl of RiboLock RNase Inhibitor, 2μl of 10 mM dNTP Mix, 1μl of RevertAid M-MuLV RT and nuclease-free water to a final volume of 20 μl. Tubes were incubated for 5 min at 25°C followed by 60 min at 42°C, and the reaction was terminated by heating at 70°C for 5 min. cDNAs were kept at −20°C until use.
2.11. Quantitative real-time PCR
Primers for PCR amplification of IFN-γ, IL-4, IL-10, TGF-β from hamster spleen cells were designed as described previously (Jittimanee et al. 2012), while primers for IL-6 were designed based on the hamster nucleotide sequences deposited in the GenBank database (AB028635.1; Ov-IL-6F: 5՛- CTCCGCAAGAGACTTCCATC -3՛: Ov-IL-6R: 5՛- ACCAAACCTCCGACTTGTTG-3՛). Actin mRNA (OvAE1657, GenBank EL620339.1 (Laha et al. 2007), OVactin RTF 5’- AGCCAACCGAGAGAAGATGA-3 ’ and OVactin RTR 5’- ACCTGACCATCAGGCAGTTG-3’) was used as the reference gene for normalization. Quantitative real-time PCR (qPCR) reactions were performed in triplicate using Maxima SYBR Green/ROX qPCR Master Mix (Thermo scientific, USA) on a LightCycler® 480 Real-Time PCR System (Roche, USA). A reaction was performed by mixing 12.5 μl of Maxima SYBR Green qPCR Master Mix (2X), 0.3 μl of each forward and reverse primer, 2 μl of the cDNA and nuclease-free water to a final volume of 25 μl. Conditions for the 45 cycles of quantitative amplification were 94°C for 3 min for initiation, 94°C for 15s for denaturation, 48°C for 30 s (for IL-4), 52°C for 30 s (IFN-γ) or 60°C for 30 s (for the rest of the cytokines) for annealing and 72°C for 30 s for extension. Melting curve analyses were performed for one cycle at 95°C for 15 s, 65°C for 15 s and continuous at 95°C for denaturation, annealing and melting, respectively. The expression level of a given cytokine was quantified from the standard curve. The levels of IFN-γ, IL-4, IL-6, IL-10, and TGF-β gene transcripts were evaluated between different groups of hamsters using the 2−ΔCt method (Livak and Schmittgen 2001).
2.12. Statistical analyses
All data are presented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). Data were analyzed by Student's unpaired t-test. P-values ≤0.05 were considered as statistically significant.
3. Results
3.1. Purified rOv-LEL-TSP-2 protein expressed in Pichia pastoris
The rOv-LEL-TSP-2 protein was expressed in P. pastoris (X33) strain as a secreted protein with a molecular weight of 12 kDa, including approximately 9 kDa of the Ov-TSP2 LEL sequence fused to the 3 kDa c-myc epitope and polyhistidine tag of pPICZαA (Supplemental Fig. 1). A western blot using anti-rOv-LEL-TSP-2 antibodies confirmed the identity of the protein (Supplemental Fig. 1). The yield of purified protein was 20 mg of rOv-LEL-TSP-2 protein from 200 ml culture media, which corresponded to 100 mg protein per liter of culture media.
3.2. Worm burden and EPG
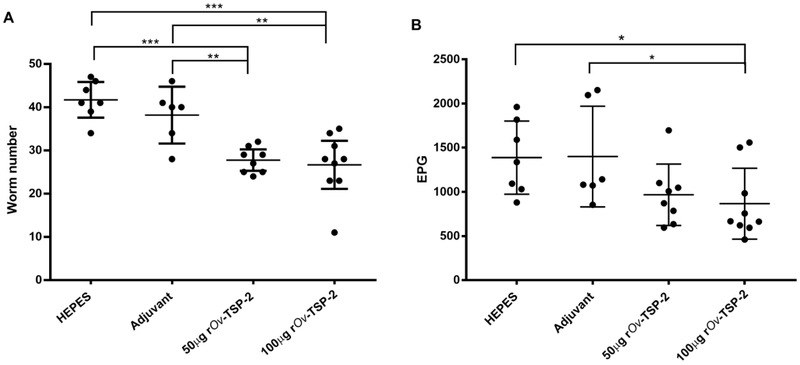
Vehicle and adjuvant control groups had a mean worm recovery of 41.71 ± 4.46 and 38.17 ± 6.27, respectively, while hamsters immunized with 50 μg and 100 μg of rOv-LEL-TSP-2 had a significantly lower average worm burden of 27.75 ± 2.96 and 26.67 ± 7.2, respectively (Fig. 1A). These equated to 36% and 31.1% reductions for the 100 μg rOv-TSP-2 group and 33.5 % and 27.3% for the 50 μg rOv-TSP-2 group compared to the vehicle control and adjuvant control groups, respectively. Fecal egg count of the 100 μg rOv-TSP-2 group was significantly reduced (37.5% and 38% reductions) compared to the vehicle and adjuvant control groups, respectively (Fig. 1B).
Fig. 1. Vaccination results in significant reductions in adult flukes and fecal egg output.
(A) Number of worms recovered from the liver of each hamster group infected with 50 O. viverrini metacercariae after vaccination. (B) Eggs per gram of feces (EPG) of vaccinated hamsters. Each dot represents the total worm number or EPG of an individual hamster. Mean and SD bar is shown. * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001.
3.3. Vaccination affects the size of recovered adult worms
The average length of the worms in both vaccinated groups was significantly shorter than in the control groups (P < 0.001) (Fig. 2A). The mean lengths of the worms in the vehicle and the adjuvant control groups was not significantly different. Mean ± SD of worm length in the vehicle control group, adjuvant control group, 50 μg of rOv-TSP-2 group and 100 μg of rOv-TSP-2 group were 4.65 ± 0.74 mm, 4.63 ± 0.3 mm, 3.9 ± 0.43 mm and 3.7 ± 0.36 mm, respectively (Fig. 2B).
Fig. 2. Stunting of adult flukes recovered from vaccinated hamsters.
(A) The average length of all intact worms from each group was measured. Each dot represents the length of a single worm. **** P ≤ 0.0001. (B) Representative image of adult worms recovered from vaccinated and control groups.
3.4. Antibody responses
The specific IgG levels against rOv-LEL-TSP-2 in the sera of immunized hamsters from either the 50 μg or 100 μg rOv-TSP2 group were significantly higher than the vehicle and adjuvant control groups (P<0.001) (Fig. 3). Furthermore, higher (although not statistically significant) absorbance values (OD450) were observed in the 100 μg rOv-TSP2 group compared to the 50 μg rOv-TSP-2 group. IgG levels were not significantly different between preimmunization and post- immunization animals from the vehicle and adjuvant control groups. Anti-rOv-TSP-2 IgG levels in the sera of infected hamsters were significantly higher in all experimental groups post-vaccination but pre-challenge compared to the sera from naive animals (Fig. 3). After parasite challenge, comparable IgG responses were detected in all groups, including the unvaccinated animals.
Fig. 3. Serum IgG levels in vaccinated hamsters.
Total serum IgG levels against rOv-LEL-TSP-2 were determined by indirect ELISA at pre-immunization, post-immunization and post-challenge infection. Results represent the mean absorbance measured at 450 nm. Each bar represents mean±SD of each group. *** P ≤ 0.001 and ****P≤ 0.0001.
3.5. Cytokine expression in splenocytes of immunized hamsters
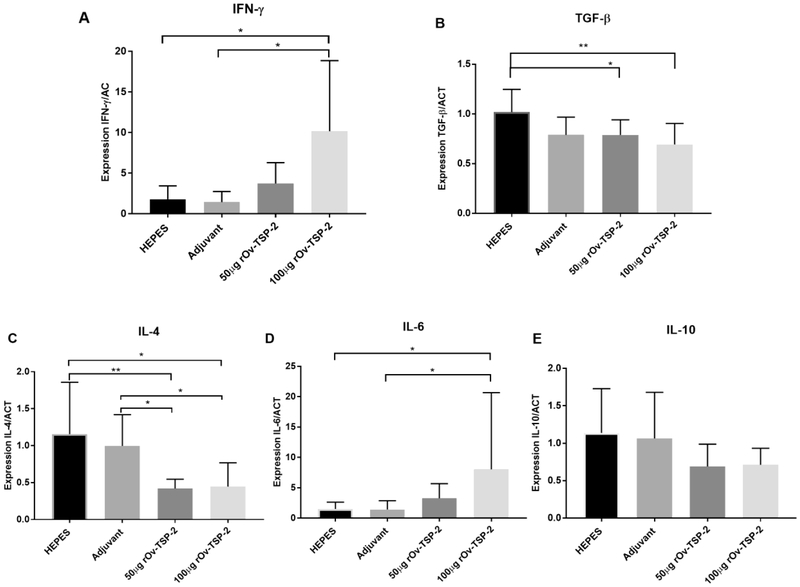
To investigate the role of cytokines in the immune responses of hamster to rOv-TSP-2 vaccination, the expression of genes encoding IFN-γ, IL- 4, IL- 6, IL- 10 and TGF-β was measured in splenocytes stimulated with rOv-TSP-2 using quantitative real time qRT-PCR (Fig. 4). The expression levels of the Th2 cytokine IL-4 in hamsters vaccinated with rOv-TSP-2 was significantly decreased in comparison to the adjuvant control group (P<0.05). In contrast, expression levels of the inflamamtory Th1 cytokines IL-6 and IFN-γ were significantly higher in hamsters vaccinated with 100 μg rOv-TSP-2 compared to the vehicle and adjuvant control groups (P<0.05). The expression levels of the regulatory cytokines IL-10 and TGF-β were not significantly different between the adjuvant and vaccine experimental groups (Fig. 4)
Fig. 4. Vaccination results in increased antigen-specific expression of IFN-γ and IL- 6 in splenocytes.
Expression levels of IFN-γ, IL- 4, IL- 6, IL- 10, and TGF-β genes in splenocytes isolated from hamsters stimulated ex vivo with rOv-LEL-TSP-2 protein. Cytokine expression was determined by real time RT-PCR and expressed as ratio of copy number of each cytokine to copy number of the control gene. Each bar represents mean ± SD in one group of animals. * P ≤ 0.05 and ** P ≤ 0.01.
4. Discussion
We show here that a soluble form of rOv-LEL-TSP-2 produced in P. pastoris drives T and B cell responses in vaccinated hamsters and stimulates protective responses against O. viverrini challenge infection in hamsters. The immunization protocol was selected based on numerous previous publications in mice (Tran et al. 2006) and hamsters (Chaiyadet et al. 2019) designed to test subunit vaccines for fluke infections. Hamsters were used because they are susceptible to O. viverrini infection; flukes develop to adult stage and produce eggs within a prepatent period that is similar to the true definitive host (Boonmars et al. 2009). Mice and rats are not suitable hosts because worms will not mature to adulthood. Reagents to profile the immune response of hamsters infected with O. viverrini are available but clearly there are fewer tools available than there are for mice. TSPs are transmembrane proteins that are highly expressed on the tegument and EV surfaces of flukes and their potential as vaccine candidates against several helminth infections such as S. mansoni (Tran et al. 2006), Schistosoma japonicum (Chen et al. 2016), filariasis (Dakshinamoorthy et al. 2013) and hydatid tapeworm (Dang et al. 2012) has been shown.
Three TSPs have been identified in the tegument of O. viverrini (Chaiyadet et al. 2017a; Piratae et al. 2012) and they are enriched in the membranes of O. viverrini exosome-like EVs (Chaiyadet et al. 2015). Recently, the vaccine efficacy of the LEL domains of Ov-TSP-2 and Ov-TSP-3 recombinantly produced in E. coli were tested in the hamster model. Those results showed that worm burden was reduced by 34% and 30% by rOv-TSP-2 and rOv-TSP-3, respectively (Chaiyadet et al. 2019). In our study here we have used a eukaryotic system (P. pastoris) to express the recombinant LEL domain of Ov-TSP-2 due to the capability of this system to perform post-translational modifications and ability to secrete high levels of heterologous protein (Wang et al. 2016). The yield of recombinant protein obtained in our study (20 mg of the purified protein from 200 ml of culture medium) was comparable to the yield of 2.5 g of rSm-TSP-2 harvested from 20 L of culture medium (Curti et al. 2013), and is much greater than the yield obtained in E. coli (4 mg of the purified protein from 200 ml of culture medium). This suggests that P. pastoris is a suitable expression system for producing large quantities of Ov-TSP-2 recombinant protein for vaccine production.
Despite vaccinated animals making a robust antibody response to vaccination, challenge infection did not result in a significant boost in titers. Moreover, IgG responses to rOv-TSP-2 in the sera of hamsters after parasite challenge were comparable between vaccinated and control animals, despite the vaccinated animals having significantly fewer and stunted worms. A possible explanation for this observation is that vaccination induced IgG secreting memory B cells specific to rOv-TSP-2 protein which acted upon newly excysted juvenile flukes as they migrate up the higher order bile ducts, interrupting EV-host cell communication (Chaiyadet et al., 2015) and retarding fluke development. The process to produce specific antibodies from memory B cells occurs in germinal centers with high-affinity and high antigen-binding capacity (Siegrist 2018). Therefore, the efficacy of the IgG antibodies to kill the worms in the vaccinated groups would be higher than in the control group, where IgG antibody responses would not be detected until adult flukes had established in the bile ducts and become less susceptible to antibody-mediated attack. Moreover, vaccination-induced T helper cell responses may have contributed to protection during the early phase of the infection as juvenile flukes were migrating and maturing, and we detected elevated antigen-specific Th1 cytokine responses (IFN-γ and IL-6) in vaccinated animals.
IL-6 has been implicated in development of chronic periductal fibrosis and associated with maintenance of a chronic inflammatory milieu that could lead to tumorigenesis in chronic opisthorchiasis (Sripa et al. 2012b). However, other studies revealed that IL-6 enhances antibody production by promoting B cell helper capabilities of CD4+ T cells through increased IL-21 production (Dienz et al. 2009), and this cytokine was important in supporting B-cell responses and antibody production in Plasmodium infection (Sebina et al. 2017). Therefore, high levels of IL-6 in the rOv-TSP-2 vaccinated hamsters might be associated with the stimulation of CD4+ T cell and B cell activation to produce antibodies that kill flukes.
In summary, we show that immunization of hamsters with rOv-TSP-2 expressed in yeast stimulates the expression of IFN-γ and IL-6 in the spleen, and production of antibodies that confer protection against fluke challenge infection in hamsters. rOv-LEL-TSP-2 might be considered as a vaccine candidate against opisthorchiasis. It is important to note that subunit vaccines for helminth infections are likely to be multi-valent and require distinct antigens from different developmental stages and/or distinct molecular pathways. To that end, a chimeric form of Sm-TSP-2 LEL from S. mansoni fused to a fragment of a hookworm protease resulted in soluble chimera expression and enhanced efficacy against S. mansoni challenge in mice compared to the LEL alone (Pearson et al. 2012). A similar approach based on chimeras of Ov-TSP-2 fused to other EV surface proteins and/or domains is worthy of further investigation in the search for the most efficacious subunit vaccines for opisthorchiasis.
Supplementary Material
Acknowledgments
This research was supported by Faculty of Medicine, Khon Kaen University, a project grant from the National Health and Medical Research Council of Australia (NHMRC), grant identification number APP1085309, and the National Cancer Institute, National Institute of Health, grant number 2R01CA164719-06A1. AL is supported by a senior principal research fellowship APP1117504.
Footnotes
Conflict of interest
The authors confirm that they have no conflicts of interest in relation to this work.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Boonmars T, Boonjaraspinyo S, Kaewsamut B (2009) Animal models for Opisthorchis viverrini infection. Parasitology research 104(3):701–3 doi: 10.1007/s00436-008-1268-x [DOI] [PubMed] [Google Scholar]
- Chaiyadet S, Krueajampa W, Hipkaeo W et al. (2017a) Suppression of mRNAs encoding CD63 family tetraspanins from the carcinogenic liver fluke Opisthorchis viverrini results in distinct tegument phenotypes. Sci Rep 7(1):14342 doi: 10.1038/s41598-017-13527-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Smout M, Laha T, Sripa B, Loukas A, Sotillo J (2017b) Proteomic characterization of the internalization of Opisthorchis viverrini excretory/secretory products in human cells. Parasitol Int 66(4):494–502 doi: 10.1016/j.parint.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Sotillo J, Krueajampa W et al. (2019) Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS neglected tropical diseases 13(5):e0007450 doi: 10.1371/journal.pntd.0007450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Sotillo J, Smout M et al. (2015) Carcinogenic Liver Fluke Secretes Extracellular Vesicles That Promote Cholangiocytes to Adopt a Tumorigenic Phenotype. J Infect Dis 212(10):1636–45 doi: 10.1093/infdis/jiv291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavengkun W, Kompor P, Norkaew J et al. (2016) Raw Fish Consuming Behavior Related to Liver Fluke Infection among Populations at Risk of Cholangiocarcinoma in Nakhon Ratchasima Province, Thailand. Asian Pacific journal of cancer prevention : APJCP 17(6):2761–5 [PubMed] [Google Scholar]
- Chen L, Chen Y, Zhang D et al. (2016) Protection and immunological study on two tetraspanin-derived vaccine candidates against schistosomiasis japonicum. Parasite Immunol 38(10):589–98 doi: 10.1111/pim.12338 [DOI] [PubMed] [Google Scholar]
- Curti E, Kwityn C, Zhan B et al. (2013) Expression at a 20L scale and purification of the extracellular domain of the Schistosoma mansoni TSP-2 recombinant protein: a vaccine candidate for human intestinal schistosomiasis. Hum Vaccin Immunother 9(11):2342–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiklinski K, de la Torre-Escudero E, Trelis M et al. (2015) The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol Cell Proteomics 14(12):3258–73 doi: 10.1074/mcp.M115.053934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Munirathinam G, Stoicescu K, Reddy MV, Kalyanasundaram R (2013) Large extracellular loop of tetraspanin as a potential vaccine candidate for filariasis. PLoS One 8(10):e77394 doi: 10.1371/journal.pone.0077394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Z, Yagi K, Oku Y et al. (2012) A pilot study on developing mucosal vaccine against alveolar echinococcosis (AE) using recombinant tetraspanin 3: Vaccine efficacy and immunology. PLoS neglected tropical diseases 6(3):e1570 doi: 10.1371/journal.pntd.0001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP et al. (2009) The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med 206(1):69–78 doi: 10.1084/jem.20081571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins DB, Haswell-Elkins M, Anderson RM (1986) The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans R Soc Trop Med Hyg 80(5):774–92 [DOI] [PubMed] [Google Scholar]
- Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C (2007) In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc 2(7):1789–94 doi: 10.1038/nprot.2007.258 [DOI] [PubMed] [Google Scholar]
- Jittimanee J, Sermswan RW, Kaewraemruaen C et al. (2012) Protective immunization of hamsters against Opisthorchis viverrini infection is associated with the reduction of TGF-beta expression. Acta tropica 122(2):189–95 doi: 10.1016/j.actatropica.2012.01.010 [DOI] [PubMed] [Google Scholar]
- Khuntikeo N, Sithithaworn P, Loilom W et al. (2016) Changing patterns of prevalence in Opisthorchis viverrini sensu lato infection in children and adolescents in northeast Thailand. Acta tropica 164:469–472 doi: 10.1016/j.actatropica.2016.10.017 [DOI] [PubMed] [Google Scholar]
- Khuntikeo N, Titapun A, Loilome W et al. (2018) Current Perspectives on Opisthorchiasis Control and Cholangiocarcinoma Detection in Southeast Asia. Front Med (Lausanne) 5:117 doi: 10.3389/fmed.2018.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha T, Pinlaor P, Mulvenna J et al. (2007) Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics 8:189 doi: 10.1186/1471-2164-8-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–8 doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mulvenna J, Sripa B, Brindley PJ et al. (2010) The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics 10(5):1063–78 doi: 10.1002/pmic.200900393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki FC, Swain MT, Klychnikov OI et al. (2015) Protein and small non-coding RNA-enriched extracellular vesicles are released by the pathogenic blood fluke Schistosoma mansoni. J Extracell Vesicles 4:28665 doi: 10.3402/jev.v4.28665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatpremsiri A, Junpue P, Loukas A et al. (2016) Immunization and challenge shown by hamsters infected with Opisthorchis viverrini following exposure to gamma-irradiated metacercariae of this carcinogenic liver fluke. Journal of helminthology 90(1):39–47 doi: 10.1017/S0022149X14000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MS, Pickering DA, McSorley HJ et al. (2012) Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS neglected tropical diseases 6(3):e1564 doi: 10.1371/journal.pntd.0001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piratae S, Tesana S, Jones MK et al. (2012) Molecular characterization of a tetraspanin from the human liver fluke, Opisthorchis viverrini. PLoS neglected tropical diseases 6(12):e1939 doi: 10.1371/journal.pntd.0001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengsawang P, Promthet S, Bradshaw P (2016) Reinfection by Opisthorchis viverrini after treatment with praziquantel. Asian Pac J Cancer Prev 17(2):857–62 [DOI] [PubMed] [Google Scholar]
- Sebina I, Fogg LG, James KR et al. (2017) IL-6 promotes CD4(+) T-cell and B-cell activation during Plasmodium infection. Parasite Immunol 39(10) doi: 10.1111/pim.12455 [DOI] [PubMed] [Google Scholar]
- Siegrist C-A (2018) Vaccine Immunology. p 16–34.e729902992 [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD et al. (2012) The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int 61(1):10–6 doi: 10.1016/j.parint.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo J, Pearson M, Potriquet J et al. (2016) Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol 46(1):1–5 doi: 10.1016/j.ijpara.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J et al. (2012a) The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol 28(10):395–407 doi: 10.1016/j.pt.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Thinkhamrop B, Mairiang E et al. (2012b) Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS neglected tropical diseases 6(5):e1654 doi: 10.1371/journal.pntd.0001654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebeje BM, Harvie M, You H, Loukas A, McManus DP (2016) Schistosomiasis vaccines: where do we stand? Parasit Vectors 9(1):528 doi: 10.1186/s13071-016-1799-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MH, Pearson MS, Bethony JM et al. (2006) Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med 12(7):835–40 doi: 10.1038/nm1430 [DOI] [PubMed] [Google Scholar]
- Wang M, Jiang S, Wang Y (2016) Recent advances in the production of recombinant subunit vaccines in Pichia pastoris. Bioengineered 7(3):155–65 doi: 10.1080/21655979.2016.1191707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Liu J, Dao J et al. (2016) Molecular characterization of S. japonicum exosome-like vesicles reveals their regulatory roles in parasite-host interactions. Sci Rep 6:25885 doi: 10.1038/srep25885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.