Abstract
Background:
Peripartum cardiomyopathy (PPCM) is a serious complication of pregnancy associated with variable degrees of left ventricular (LV) recovery. The aim of this study was to test the hypothesis that global LV strain at presentation has prognostic value in patients with PPCM.
Methods:
One hundred patients with PPCM aged 30 ± 6 years were enrolled in the multicenter Investigation in Pregnancy Associated Cardiomyopathy study along with 21 normal female control subjects. Speckle-tracking global longitudinal strain (GLS) and global circumferential strain (GCS) analysis was performed. The predefined primary combined outcome variable was death, transplantation, LV assist device implantation, or evidence of persistent LV dysfunction (LV ejection fraction [LVEF] < 50%) at 1 year.
Results:
GLS measurement was feasible in 110 subjects: 89 of 90 patients with PPCM (99%) with echocardiographic data and all 21 control subjects. Of 84 patients (94%) with 1-year follow-up, 21 (25%) had unfavorable primary outcomes: four LV assist device placements, two deaths, and 15 patients with persistent LV dysfunction. GLS at presentation with a cutoff of 10.6% (absolute value) was specifically associated with the subsequent primary outcome with 75% sensitivity and 95% specificity. GCS at presentation with a cutoff of 10.1% was associated with the primary outcome with 78% sensitivity and 84% specificity. GLS and GCS remained significantly associated with outcomes after adjusting for LVEF (GLS odds ratio, 2.07; P < .001; GCS odds ratio, 1.37; P = .005). GLS was significantly additive to LVEF (C statistic = 0.76–0.91, net reclassification improvement = 1.32, P < .001).
Conclusions:
GLS and GCS in patients with PPCM at presentation were associated with subsequent clinical outcomes, including death, LV assist device implantation, and evidence of persistent LV dysfunction. Strain measures may add prognostic information over LVEF for risk stratification.
Keywords: Cardiomyopathy, Strain imaging, Heart failure, Pregnancy
Peripartum cardiomyopathy (PPCM) is a rare but important disease presenting in the later part of pregnancy or the first few months after childbirth.1–3 Race remains a risk factor for its development, but the causal mechanisms of PPCM are mostly uncertain, and the clinical course is heterogeneous.1 Although most patients with PPCM recover left ventricular (LV) function, a subset of patients have unfavorable clinical outcomes, with persistent LV dysfunction and heart failure being a major cause of maternal morbidity and mortality.2 Routine measures of LV size and LVejection fraction (LVEF) at presentation have been shown to have prognostic information in PPCM but are not specific.4 Strain imaging by speckle-tracking echocardiography can provide LV functional and prognostic information additive to LVEF in several clinical scenarios.5–8 Accordingly, the objective of this study was to test the hypothesis that global LV strain at presentation has prognostic value for predicting LV recovery and clinical outcomes in PPCM.
METHODS
Study Population
The study population was taken from 100 women (>18 years of age) with newly diagnosed PPCM enrolled in the prospective multicenter Investigations of Pregnancy Associated Cardiomyopathy study. The study included 30 North American centers and investigated the demographics, inflammatory pathogenesis, treatment, and clinical predictors of outcome with PPCM. Details of the study protocol were published elsewhere.4 Briefly, the diagnostic criteria of PPCM included LVEF ≤ 45% with or without heart failure symptoms in the last month of pregnancy or within 13 weeks after delivery. Exclusion criteria were valvular heart disease, coronary heart disease, other known cardiomyopathy, blood culture–positive septicemia, current drug and alcohol abuse, and chemotherapy or chest radiation therapy within 5 years. The predefined unfavorable outcomes of death, heart transplantation, LV assist device (LVAD) implantation, or lack of LV recovery (LVEF < 50%) at 1 year were combined as the primary outcome variable. To compare echocardiographic strain analysis in a control group of healthy young women of similar age using identical methods, we included 21 healthy young women (eight early postpartum and 13 nonpregnant volunteers). The study was approved by the institutional review board at each Investigations of Pregnancy Associated Cardiomyopathy participating center. All participating patients and control subjects gave written informed consent.
Echocardiography
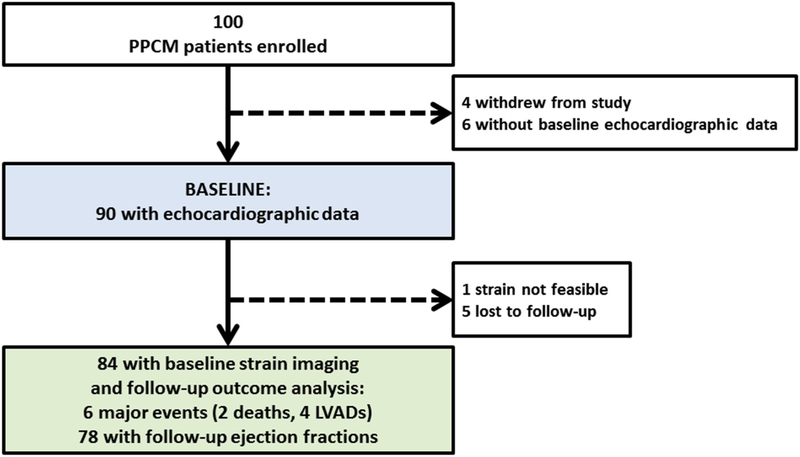
Transthoracic echocardiography was performed on patients at each site at the time of enrollment (baseline), and repeat echocardiography was offered at 6 months and 1 year postpartum. A flow chart of the study echocardiograms is presented in Figure 1. All echocardiographic examinations were analyzed in a core laboratory. Routine measures included LV volumes, LVEF, and left atrial volumes calculated using biplane Simpson’s methods using manual tracing of digital images. Left atrial volume was indexed to body surface area (left atrial volume index). All two-dimensional measurements were performed in accordance with the recommended guidelines of the American Society of Echocardiography.9,10 Speckle-tracking strain analyses were performed offline on routine Digital Imaging and Communications in Medicine data acquired at routine Digital Imaging and Communications in Medicine frame rates, typically at 30 Hz (Image Arena; TomTec Imaging Systems, Unterschleissheim, Germany). LV endocardium and epicardium were traced to set the regions of interest in images. Images were determined to be adequate for strain analysis by region of interest tracking on cine playback and inspection of time-strain curves for noise, as previously described.11,12 From the apical two-chamber, four-chamber, and long-axis views, the peak negative longitudinal strain values from each of the 18 LV segments over the entire cardiac cycle were averaged to calculate LV global longitudinal strain (GLS). From the parasternal short-axis view at the midventricular level, peak negative circumferential strain values from the six LV segments were averaged to calculate LV global circumferential strain (GCS; Figure 2).9 All echocardiographic parameters were analyzed by investigators blinded to all clinical and outcome data. Because strain values with a minus sign, where smaller values indicate better cardiac function, are not intuitive, especially for noncardiologists, we present longitudinal strain as absolute values in this study, as previously reported.13
Figure 1.
Study flowchart showing enrolled patients with PPCM and breakdown of echocardiographic and follow-up data available.
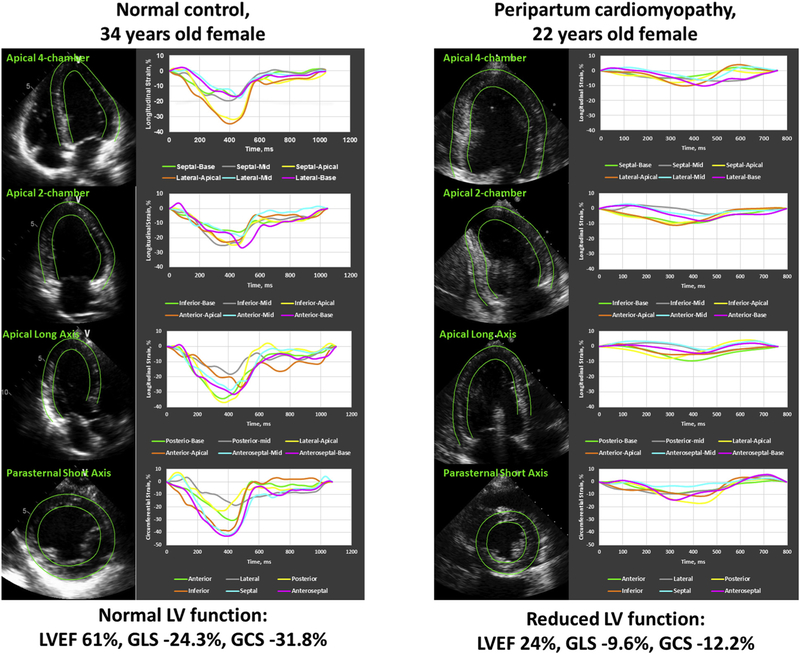
Figure 2.
Cardiac strain measurements. Echocardiographic strain imaging examples of a normal control subject (left) and patients with PPCM (right) from the study. Longitudinal strain curves from the LV apical four-chamber view (top row), apical two-chamber view (second row), and apical long-axis view (third row) and circumferential strain curves from the LV short-axis views (bottom row) are shown.
Reproducibility
Reproducibility of GLS and GCS analyses was expressed using intraobserver and interobserver variability intraclass correlation coefficients from investigators blinded to clinical data. For the analysis of intraobserver variability, 20 subjects were randomly picked and evaluated by the same observer >1 month after the first analysis. The repeated measurements were performed by two readers for interobserver visibility and used images from the same cardiac cycle for both intraobserver and interobserver variability.
Outcomes
Clinical outcome data were tracked for 1 year postpartum. LV recovery was defined as LVEF ≥ 50% at the last echocardiographic study within the 1-year follow-up period. The primary combined outcome variable was predefined as death, transplantation, LVAD implantation, or evidence of persistent LV dysfunction at 1 year (LVEF < 50%) over 1 year. As a secondary analysis of the prognostic ability of strain parameters for persistent severe LV dysfunction, we defined the secondary combined outcome variable as a composite of severe LV dysfunction, defined as LVEF ≤ 35%, death, heart transplantation, or LVAD placement during 1-year follow-up.
Statistical Analysis
Continuous variables are presented as mean ± SD or as median (interquartile range [IQR]) if the Shapiro-Wilk test showed a lack of normal distribution. Comparisons of means were analyzed using Student’s unpaired and paired t tests. Proportions were compared using χ2 or Fisher exact tests. Receiver operating characteristic curves were constructed, and areas under the curve (AUCs) were calculated for echocardiographic parameters’ ability to determine the cutoff values to discriminate between patients with and those without unfavorable outcomes. AUCs were compared using DeLong’s method. To explore the independent association of GLS and GCS with the primary outcome, multivariate logistic regression models were constructed using the clinically important covariates (i.e., age, race, New York Heart Association [NYHA] functional classification, and LVEF). Because there were only 21 events, models were constructed including only two variables to avoid overfitting. For subgroup comparisons by race, P values were adjusted for multiple comparisons using the Bonferroni correction test. Statistical analyses were performed using JMP version 13.1.0 (SAS Institute, Cary, North Carolina) and R (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P values < .05 were considered to indicate statistical significance.
RESULTS
Clinical Characteristics and Echocardiographic Findings
There were 111 subjects with echocardiographic data: 90 patients with PPCM with baseline echocardiographic data available and 21 normal female control subjects. As detailed in Figure 1, 100 patients with PPCM were enrolled, six did not comply with echocardiography, and four withdrew entirely from the study, leaving 90 patients with PPCM. Of these, strain analysis was feasible in 89 of 90 (99%), and five were lost to follow-up. No patient was enrolled in the last month of pregnancy, and the median time from delivery to first echocardiography was 22 days (range, 10–45 days). Baseline clinical characteristics and routine echocardiographic data in 90 patients with PPCM and 21 control subjects appear in Table 1. Patients with PPCM were younger (median age, 31 years [IQR, 25–34 years] vs 35 years [IQR, 31–41 years]; P = .001) and had a higher proportion of black race (27% vs 5%, P = .011) compared with normal control subjects. Heart rate was higher in patients with PPCM (median, 86 beats/min [IQR, 74–99 beats/min] vs 72 beats/min [IQR, 70–75 beats/min]; P < .001), while systolic and diastolic blood pressures were similar in both groups (Table 1). In the PPCM cohort, 9% had family histories of dilated cardiomyopathy, and 43% had histories of chronic or gestational hypertension. At the time of study entry, 87% of patients with PPMC were receiving β-blockers, 81% were receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, and 26% were receiving aldosterone blockers. Patients with PPCM at baseline had significant differences compared with control subjects, as expected, including dilated left ventricles (median LV end-diastolic diameter, 55 mm [IQR, 51–60 mm] vs 44 mm [IQR, 42–49 mm] [P < .001]; median LV end-systolic diameter, 47 mm [IQR, 41–52 mm] vs 31 mm [IQR, 29–33 mm] [P < .001]) and reduced LV systolic function (median LVEF, 35.6% [IQR, 41.1%–29.5%] vs 60.5% [IQR, 58.0%–61.6%]; P < .001). GLS was also significantly different than in control subjects (median, 11.4% [IQR, 9.2%–14.7%] vs 21.5% [IQR, 19.2%–24.5%]; P < .001; absolute values) and GCS (median, 11.8% [IQR, 8.6%–16.6%] vs28.3% [IQR, 25.1%–31.2%]; P < .001; absolute values).
Table 1.
Baseline clinical and echocardiographic characteristics in patients with PPCM and normal control subjects
| Variable | Patients with PPCM (n = 90) | Control subjects (n = 21) | P |
|---|---|---|---|
| Clinical variables | |||
| Age, y | 31 (25–34) | 35 (31–41) | .001 |
| Body surface area, m2 | 1.7 (1.6–2.0) | 1.7 (1.6–1.9) | .28 |
| BMI, kg/m2 | 27.5 (23.6–33.8) | 24.3 (20.7–28.6) | .024 |
| Race, black | 27 | 5 | .011 |
| Familial history of DCM | 9 | - | - |
| NYHA functional class, I/II/III/IV | 12/46/26/17 | - | - |
| Smoking | 34 | 19 | .16 |
| Diabetes | 9 | - | |
| Hypertension | 43 | - | |
| Heart rate, beats/min | 86 (74–99) | 72 (70–75) | <.001 |
| SBP, mm Hg | 109 (100–124) | 110 (101–118) | .94 |
| DBP, mm Hg | 68 (60–80) | 68 (64–75) | .77 |
| ACE inhibitor/ARB | 81 | - | |
| β-blocker | 87 | - | |
| Aldosterone blocker | 26 | - | |
| Diuretics | 71 | - | |
| Echocardiographic variables | |||
| Septal thickness, mm | 9 (7–10) | 9 (7–9) | .0034 |
| LV posterior wall thickness, mm | 9 (8–10) | 8 (7–9) | <.001 |
| LV diastolic diameter, mm | 55 (51–60) | 44 (42–49) | <.001 |
| LV systolic diameter, mm | 47 (41–52) | 31 (29–33) | <.001 |
| LV diastolic volume index, mL/m2 | 72.9 (59.0–85.7) | 43.5 (38.3–52.1) | <.001 |
| LV systolic volume index, mL/m2 | 46.3 (38.2–59.4) | 16.7 (15.2–20.6) | <.001 |
| LVEF, % | 35.6 (41.1–29.5) | 60.5 (58.0–61.6) | <.001 |
| LV mass index, g/m2 | 106.7 (88.8–124.8) | 61.0 (50.2–66.7) | <.001 |
| LA volume index, mL/m2 | 37.6 (31.4–46.2) | 24.7 (22.5–29.7) | <.001 |
| GLS, % | 11.4 (9.2–14.7) | 21.5 (19.2–24.5) | <.001 |
| GCS, % | 11.8 (8.6–16.6) | 28.3 (25.1–31.2) | <.001 |
ACE, Angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; LA, left atrial; NYHA, New York Heart Association; SBP, systolic blood pressure.
Data are expressed as percentages or as median (IQR).
Echocardiographic Parameters, LV Recovery, and Clinical Outcomes
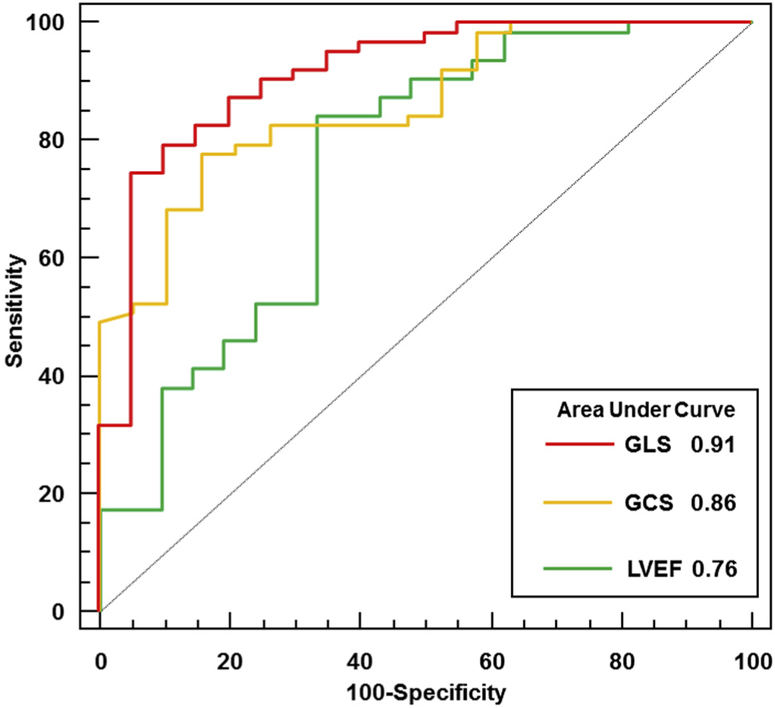
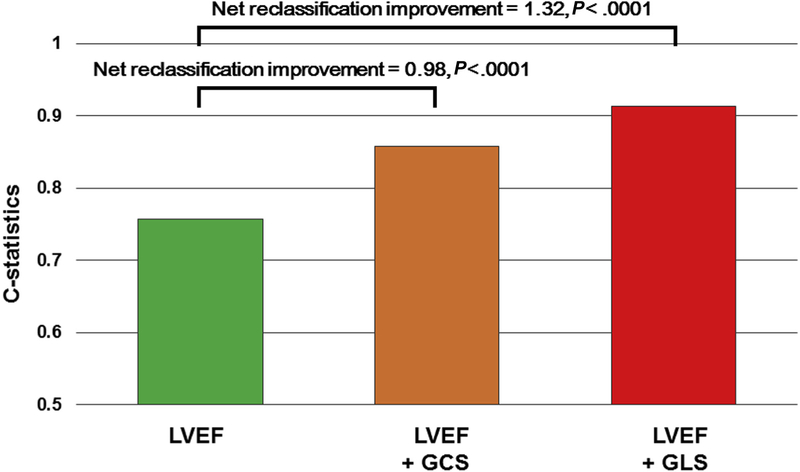
Five patients were lost to follow-up. Accordingly, there were 84 patients (93%) with baseline strain imaging and follow-up outcome data available. Among these patients, 63 (75%) showed LV functional recovery, defined as LVEF ≥ 50%. There were 21 patients (25%) with primary unfavorable clinical outcomes over 1-year follow-up. Two patients died, four required LVAD support, and 15 (19%) showed a lack of LV recovery (LVEF < 50%), with a median LVEF of 38% (IQR, 26%–45%), including five patients (7%) with persistent severe LV dysfunction (LVEF ≤ 35%). Receiver operating characteristic analyses showed that LVEF and GLS had significant associations with the primary outcomes. GLS at presentation with a cutoff value of 10.6% had sensitivity of 75% and specificity of 95% (AUC = 0.91). This was significantly greater than the AUC for LVEF (0.77, with a cutoff value of 30%), which had sensitivity of 84% and specificity of 67% (P = .048 vs LVEF). GCS at presentation with a cutoff value of10.1% was also associated with the primary combined outcome, with sensitivity of 78% and specificity of 84%. The AUC for GCS was 0.86, which was not statistically different from the AUC for LVEF (P = .21 vs LVEF; Figure 3). GLS and GCS remained significantly associated with clinical outcomes after adjusting for LVEF (GLS odds ratio, 2.07; P < .001; GCS odds ratio, 1.37; P = .005). GLS was significantly additive to LVEF (C statistic = 0.76–0.91, net reclassification improvement = 1.32, P < .001).
Figure 3.
Association between LV measurements and clinical outcomes. Receiver operating characteristic curves for association of the primary composite end point of lack of LV recovery, death, or need for mechanical circulatory support with GLS (red), GCS (orange), and LVEF (green).
There were nine patients whose LVEFs did not recover fully (≥50%) at 6 months but recovered afterward (late recovery group) and nine patients whose LVEFs were without recovery at 6 months and remained decreased afterward (persistently low LVEF group). There was no significant difference in baseline LVEF between the late recovery and persistently low LVEF groups (median, 32% [IQR, 27%–39%] vs 27% [IQR, 23%–30%]; P = .18). However, both baseline GLS and baseline GCS were significantly higher in the late recovery patients than the persistently low LVEF patients: for GLS, 11.6% (IQR, 10.7%–17.2%) versus 9.1% (IQR, 7.5%–12.4%; P = .002), and for GCS, 11.6% (IQR, 10.8%–17.9%] versus 6.7% (IQR, 3.6%–8.8%; P = .002). These results suggest that baseline strain values may also be predictive of patients with later LVEF recovery.
There were 11 patients (13%) who experienced more unfavorable outcomes in the secondary combined outcome analysis during 1-year of follow-up: two deaths, four LVAD placements, and five patients with persistent severe LV dysfunction, defined as LVEF ≤ 35%. Both GLS and GCS were significantly associated with more unfavorable outcomes by univariate analysis (GLS odds ratio, 1.79; 95% CI, 1.29–2.47; P < .001; GCS odds ratio, 1.50; 95% CI, 1.17–1.91; P < .001). The AUC for GLS (0.90; P = .73 vs LVEF) was slightly higher compared with those for GCS (0.88; P = .93 vs LVEF) and LVEF (0.87), but the difference was not statistically significant. The specificity and sensitivity of GLS were 91% and 88%, while those of GCS were 90% and 63%, respectively.
Changes in GLS and GCS and Racial Differences
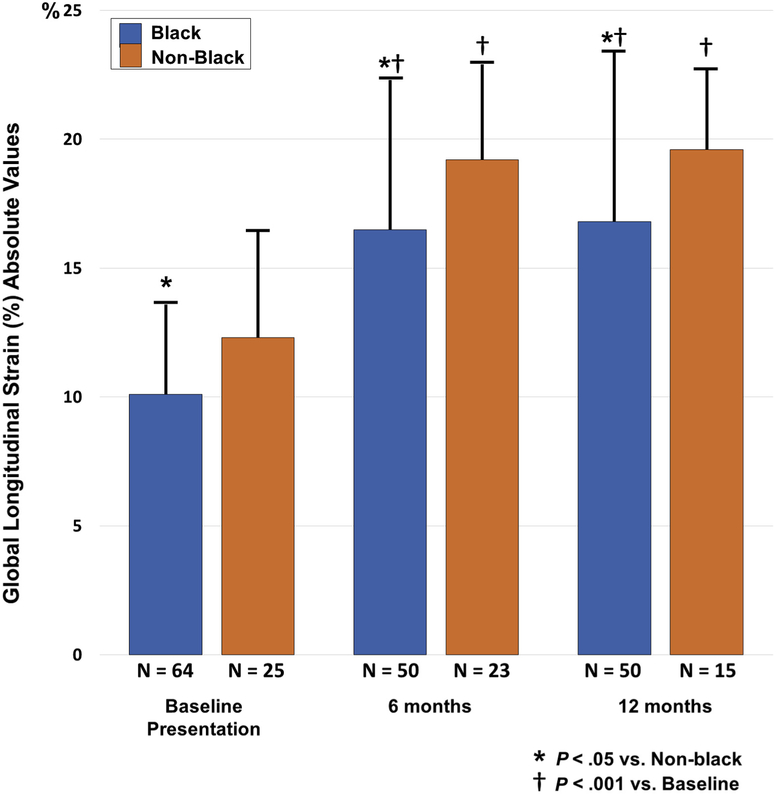
Race remains an important risk factor in PPCM, and McNamara et al.4 previously demonstrated that recovery of LVEF was different between patients of black and nonblack race. Accordingly, GLS and GCS were analyzed with respect to race. There was a subgroup of 73 patients who had 6-month echocardiograms available for serial strain analysis. LV function improved during follow-up, with median LVEF of 36% (IQR, 30%–42%), 53% (IQR, 46%–58%), and 56% (IQR, 51%–60%) and median GLS of 11.7% (IQR, 9.6%–15.1%),19.2% (IQR, 15.4%–21.2%), and 20.0% (IQR, 18.5%–21.7%) at baseline, 6 months, and 1 year, respectively. GLS significantly improved from baseline to 6 months and remained improved at 1 year (Figure 4). From baseline to 6 months and 1 year, GLS values were worse in blacks than in nonblacks (10.1 ± 3.5% vs 12.3 ± 4.1% [P = .02], 16.5 ± 5.8% vs 19.2 ± 3.7% [P = .03], and 16.8 ± 6.6% vs 19.6 ± 3.1% [P = .03]). GCS also improved, with changes in mean GCS at baseline, 6 months, and 1 year postpartum of 12.7% (IQR,8.9%–17.5%), 24.6% (IQR, 20.3%–28.1%), and 24.7% (IQR,21.9%–29.1%), respectively. GCS at 6 months was worse in blacks than in nonblacks (20.6 ± 8.9% vs 24.9 ± 5.3%, P = .01), while there were no significant racial differences in GCS at baseline and 1 year (10.7 ± 4.4% vs 13.0 ± 5.9% [P = .08] and 21.2 ± 9.7% vs 25.1 ± 5.8% [P = .06], respectively).
Figure 4.
Change in cardiac strain. Subgroup analysis of GLS in 73 patients with 6-month echocardiograms. Bar graphs show GLS at baseline presentation, 6 months, and 1 year in patients with PPCM grouped by race. Strain values are shown as absolute values. GLS was significantly lower in black patients compared with nonblack patients at each time point. Significant increases in GLS occurred from baseline presentation to 6 months and were sustained at 12 months, with GLS increases attenuated in black patients.
Independent and Incremental Value of Strain Parameters
In univariate logistic regression analyses performed on the 84 patients with 1-year follow-up data, both GLS and GCS at presentation were associated with unfavorable outcomes at 1 year postpartum. Both GLS and GCS remained significant after adjusting for each clinical or echocardiographic parameter, which were significantly different between patients with PPCM and normal control subjects (age, race, NYHA functional classification, heart rate, body mass index, LVEF, LV end-diastolic diameter ≥ 6.0 cm; Table 2). Consistent with the relatively small number of clinical events in this study, statistical models were restricted to include a relatively small number of independent variables in each. Both GLS and GCS remained significant even after adjustment for age, race, NYHA functional classification, or LVEF (Table 2). To test if these parameters have incremental value over baseline LVEF, the predictability of multivariate models adjusted with LVEF were compared with the predictability of LVEF alone. Adding GLS or GCS to LVEF significantly improved the prediction of primary clinical outcomes (GLS: C-statistic = 0.76–0.91, net reclassification improvement = 1.32, P < .001; GCS: C-statistic = 0.76–0.86, net reclassification improvement = 0.98, P < .001; Figure 5).
Table 2.
Logistic regression models for strain parameters and LV recovery
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Univariate | Adjustment for HR | Adjustment for age | ||||
| GLS | 1.97 (1.42–2.74) | <.001 | 1.97 (1.42–2.74) | <.001 | 1.99 (1.43–2.77) | <.001 |
| GCS | 1.44 (1.20–1.73) | <.001 | 1.44 (1.20–1.75) | <.001 | 1.44 (1.20–1.74) | <.001 |
| Adjustment for black race | Adjustment for NYHA functional class III/IV | Adjustment for LVEF | ||||
| GLS | 1.95 (1.41–2.7) | <.001 | 1.91 (1.36–2.67) | <.001 | 2.07 (1.4–3.05) | <.001 |
| GCS | 1.46 (1.20–1.77) | <.001 | 1.4 (1.15–1.69) | <.001 | 1.37 (1.1–1.71) | .005 |
| Adjustment for LVDd ≥ 6.0 cm | ||||||
| GLS | 1.81 (1.29–2.53) | <.001 | ||||
| GCS | 1.42 (1.16–1.74) | <.001 | ||||
HR, heart rate; LVDd, LV end-diastolic diameter; NYHA, New York Heart Association; OR, odds ratio.
Figure 5.
Incremental prognostic value of GLS and GCS. Net reclassification improvement analysis showed incorporating LVEF as a conventional echocardiographic parameter and additive prognostic value of GCS and GLS.
Reproducibility of Strain Analysis
The intraclass correlation coefficients for intraobserver variability were 0.98 (95% CI, 0.96–0.99) for GLS and 0.98 (95% CI, 0.95–0.99) for GCS. The intraclass correlation coefficients for interobserver variability were 0.98 (95% CI, 0.96–0.99) for GLS and 0.98 (95% CI, 0.96–0.99) for GCS. The coefficients of variation for intraobserver variability approximately 1 month apart were 6.16% for GLS and 7.96% for GCS. The coefficients of variation for interobserver variability approximately 1 month apart were 5.78% for GLS and 7.47% for GCS.
DISCUSSION
This is the first multicenter study of patients with PPCM to demonstrate the prognostic utility of echocardiographic strain imaging at the time of presentation. We previously reported from this PPCM cohort that the routine echocardiographic measures of larger LV end-diastolic dimensions and lower LVEF at presentation were associated with lower LVEF at 1-year follow-up.4 The present study extends these observations to demonstrate that measures of GLS and GCS were additive to routine LV functional measures and clinical factors, such as black race, for identifying patients at risk for unfavorable clinical outcomes. Specifically, patients with more impaired LV global strain at presentation had a higher incidence of lack of LV recovery, death, or LVAD implantation.
Factors proposed to be related to the pathogenesis of PPCM have included oxidative stress and inflammation due to PI3KAkt signaling14; 6-kD prolactin (full-length prolactin is 23 kD), which inhibits angiogenesis and induces endothelial apoptosis15; soluble Fms-like tyrosine kinase-1 from placenta possibly related to cardiac toxicity16; and mutations of TTN.17 It is unclear how these potential mechanisms affect GLS, but strain imaging measures appear to be useful indicators of disease severity in patients with PPCM.
Elkayam et al.2 previously reported that 63% of patients with PPCM recovered LV function, and 29% of patients with PPCM had residual or heart failure symptoms.2 Several clinical prognostic factors associated with unfavorable clinical outcomes, including worse NYHA functional class, multiparity, older age, black race, PPCM-concomitant eclampsia, and delayed diagnosis as PPCM.18,19 Full recovery of LVEF before subsequent pregnancies was associated with better clinical outcomes.20 Biomarkers have also been used for prognosis, including increased troponin T,21 N-terminal pro–brain natriuretic peptide, oxidized low-density lipoprotein, inter-feron-γ,22 and microRNA-164a,15 and endothelial microparticles23 relate with the pathophysiology of PPCM.
Previous studies have reported echocardiographic strain data in normal pregnant women.24–26 Savu et al.26 documented serial changes in LV function and myocardial deformation in each trimester and 3 to 6 months postpartum in normal women. They reported LV enlargement, increased stroke volume, and a mild decrease in GLS in the third trimester. They subsequently observed that LV size, stroke volume, and GLS would normalize over time postpartum.26 There have been previous studies of patients with PPCM. Briasoulis et al.27 studied GLS in 47 patients with PPCM with reduced LVEFs. They reported that reduced GLS was associated with increased heart failure hospitalization (hazard ratio, 1.19; P = .034). However, they did not find reduced GLS to be an independent predictor of all-cause death, hospitalization, or lack of LVEF recovery. We acknowledge that this result differs from our observations, perhaps because of their smaller sample size. Right ventricular (RV) function determined by fractional area change at baseline was associated with subsequent LV recovery and clinical outcomes, which we previously reported in these patients with PPCM.28 In our present study, 21 of 84 patients (25%) at 1-year follow-up had persistent LV dysfunction or an unfavorable clinical event, such as LVAD or death, even though all patients received standard heart failure therapy. Our results suggest that GLS > 10.6% at presentation was additive to LVEF for its prognostic value. The precise mechanism accounting for LV strain parameters to add prognostic value to LVEF remains unknown. We speculate that perhaps wall deformation properties, such as inflammation and edema in PPCM, relate to strain measures. Alternative imaging approaches for PPCM prediction are also under investigation. Schelbert et al.29 recently reported in a subgroup analysis that myocardial damage and fibrosis was an uncommon finding by cardiac magnetic resonance imaging late gadolinium enhancement in patients with PPCM but may be a marker for residual myocardial dysfunction. Only one patient (1%) enrolled in this study was treated with bromocriptine; accordingly, no observations regarding strain imaging and this potential treatment for PPCM may be reported.30
The clinical severity of heart failure at entry has been reported to be an important predictor for unfavorable events.31,32 Clinical outcomes were also associated with race in our series. Black women with PPCM had more unfavorable outcomes than nonblack women. GLS at entry, 6 months, and 1 year showed comparatively more impaired LV function in black than nonblack women with PPCM. Of interest, patients with PPCM of black race had comparatively worse GLS than nonblack patients at entry. Patients with PPCM of black race also showed less improvement in GLS over 1 year of follow-up. These results are supportive of previous observations of racial differences in PPCM.1,33,34 Although depressed LVEF is one of the criteria for the diagnosis of PPCM diagnosis by definition, GLS remained a significant predictor for unfavorable events in our study. This result was supported by a previous meta-analysis in which GLS played a major role in predicting heart failure prognosis.35 In patients with acute and chronic systolic heart failure, GLS has been shown to have additive prognostic information to LVEF with respect to risk stratification.7,13 Furthermore, the addition of GLS or GCS has improved incremental prognostic value to routine echocardiographic parameter in patients with acute and chronic heart failure.13,36
Study Limitations
Although our study was a multicenter study of a rare disease, the overall sample size was relatively small. Further validation in future larger studies would be of interest. It is a limitation that there were intervals with incomplete echocardiographic data and where strain imaging was not feasible. However, there was a high yield of high-quality images in this patient population of young women, with LV strain data being feasible in 99%. It may be considered a limitation that RV strain was not part of this present study. We observed previously that RV function was associated with clinical outcomes by routine measures in patients with PPCM, but RV strain was not predictive.28 It may be considered a limitation that the control group consisted of healthy postpartum women and healthy normal women of similar age. However, we observed no significant clinical or echocardiographic differences between normal postpartum and normal control women. It is a limitation that the cutoffs for strain parameters were derived from the present study cohort and will need to be validated prospectively in a future study. We acknowledge that major unfavorable clinical events, such as death or LVAD implantation, represent a different spectrum of disease severity than persistent LV dysfunction defined by LVEF, and it was a limitation that there was a small number of these events. The relationship of the pathophysiology of PPCM including biomarkers or molecular biology to strain imaging remains unknown, and future study is warranted.
CONCLUSION
Impaired speckle-tracking strain parameters at presentation in patients with PPCM was predictive of a subsequent lack of LV recovery or unfavorable clinical outcomes, such as LVAD implantation or death. GLS and GCS may add prognostic information over LVEF for risk stratification of patients with PPCM.
HIGHLIGHTS.
Ninety PPCM patients and 21 control subjects were studied.
GLS and GCS were assessed.
GLS and GCS at presentation were associated with subsequent clinical outcomes.
GLS and GCS were of additive prognostic value to ejection fraction at presentation.
Acknowledgments
This investigation was supported by the National Heart, Lung, and Blood Institute through contract HL102429. Dr. Gorcsan receives research grant support from GE, Hitachi, EBR Systems, and V-Wave.
Abbreviations
- AUC
Area under the curve
- GCS
Global circumferential strain
- GLS
Global longitudinal strain
- IQR
Interquartile range
- LV
Left ventricular
- LVAD
Left ventricular assist device
- LVEF
Left ventricular ejection fraction
- NYHA
New York Heart Association
- PPCM
Peripartum cardiomyopathy
- RV
Right ventricular
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 2007;100:302–4. [DOI] [PubMed] [Google Scholar]
- 2.Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 2001;344:1567–71. [DOI] [PubMed] [Google Scholar]
- 3.Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000; 283:1183–8. [DOI] [PubMed] [Google Scholar]
- 4.McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015;66:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol 2012;60:2074–81. [DOI] [PubMed] [Google Scholar]
- 6.Joyce E, Hoogslag GE, Leong DP, Debonnaire P, Katsanos S, Boden H, et al. Association between left ventricular global longitudinal strain and adverse left ventricular dilatation after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging 2014;7:74–81. [DOI] [PubMed] [Google Scholar]
- 7.Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015;8:1351–9. [DOI] [PubMed] [Google Scholar]
- 8.Reant P, Mirabel M, Lloyd G, Peyrou J, Lopez Ayala JM, Dickie S, et al. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 2016;102:741–7. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 11.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J III. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006;113:960–8. [DOI] [PubMed] [Google Scholar]
- 12.Cannesson M, Tanabe M, Suffoletto MS, Schwartzman D, Gorcsan J III. Velocity vector imaging to quantify ventricular dyssynchrony and predict response to cardiac resynchronization therapy. Am J Cardiol 2006;98:949–53. [DOI] [PubMed] [Google Scholar]
- 13.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 2018;71:1947–57. [DOI] [PubMed] [Google Scholar]
- 14.Ricke-Hoch M, Bultmann I, Stapel B, Condorelli G, Rinas U, Sliwa K, et al. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc Res 2014;101:587–96. [DOI] [PubMed] [Google Scholar]
- 15.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 2013;123:2143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blauwet LA, Cooper LT. Diagnosis and management of peripartum cardiomyopathy. Heart 2011;97:1970–81. [DOI] [PubMed] [Google Scholar]
- 19.Lindley KJ, Conner SN, Cahill AG, Novak E, Mann DL. Impact of preeclampsia on clinical and functional outcomes in women with peripartum cardiomyopathy. Circ Heart Fail 2017;10:e003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilfiker-Kleiner D, Haghikia A, Masuko D, Nonhoff J, Held D, Libhaber E, et al. Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail 2017;19:1723–8. [DOI] [PubMed] [Google Scholar]
- 21.Hu CL, Li YB, Zou YG, Zhang JM, Chen JB, Liu J, et al. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart 2007;93:488–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forster O, Hilfiker-Kleiner D, Ansari AA, Sundstrom JB, Libhaber E, Tshani W, et al. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail 2008;10:861–8. [DOI] [PubMed] [Google Scholar]
- 23.Walenta K, Schwarz V, Schirmer SH, Kindermann I, Friedrich EB, Solomayer EF, et al. Circulating microparticles as indicators of peripartum cardiomyopathy. Eur Heart J 2012;33:1469–79. [DOI] [PubMed] [Google Scholar]
- 24.Cong J, Fan T, Yang X, Squires JW, Cheng G, Zhang L, et al. Structural and functional changes in maternal left ventricle during pregnancy: a three-dimensional speckle-tracking echocardiography study. Cardiovasc Ultra-sound 2015;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan SG, Melikian N, Mushemi-Blake S, Dennes W, Jouhra F, Monaghan M, et al. Physiological reduction in left ventricular contractile function in healthy postpartum women: potential overlap with peripartum cardiomyopathy. PLoS ONE 2016;11:e0147074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savu O, Jurcut R, Giusca S, van Mieghem T, Gussi I, Popescu BA, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 2012;5:289–97. [DOI] [PubMed] [Google Scholar]
- 27.Briasoulis A, Mocanu M, Marinescu K, Qaqi O, Palla M, Telila T, et al. Longitudinal systolic strain profiles and outcomes in peripartum cardiomyopathy. Echocardiography 2016;33:1354–60. [DOI] [PubMed] [Google Scholar]
- 28.Blauwet LA, Delgado-Montero A, Ryo K, Marek JJ, Alharethi R, Mather PJ, et al. Right ventricular function in peripartum cardiomyopathy at presentation is associated with subsequent left ventricular recovery and clinical outcomes. Circ Heart Fail 2016;9:e002756. [DOI] [PubMed] [Google Scholar]
- 29.Schelbert EB, Elkayam U, Cooper LT, Givertz MM, Alexis JD, Briller J, et al. Myocardial damage detected by late gadolinium enhancement cardiac magnetic resonance is uncommon in peripartum cardiomyopathy. J Am Heart Assoc 2017;6:e005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, Franke A, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J 2017;38: 2671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart 2000;83:505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opasich C, Rapezzi C, Lucci D, Gorini M, Pozzar F, Zanelli E, et al. Precipitating factors and decision-making processes of short-term worsening heart failure despite “optimal” treatment (from the IN-CHF Registry). Am J Cardiol 2001;88:382–7. [DOI] [PubMed] [Google Scholar]
- 33.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J 2006;152:509–13. [DOI] [PubMed] [Google Scholar]
- 34.Kao DP, Hsich E, Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail 2013;1:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014;100:1673–80. [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Montero A, Tayal B, Goda A, Ryo K, Marek JJ, Sugahara M, et al. Additive prognostic value of echocardiographic global longitudinal and global circumferential strain to electrocardiographic criteria in patients with heart failure undergoing cardiac resynchronization therapy. Circ Cardiovasc Imaging 2016;9:e004241. [DOI] [PubMed] [Google Scholar]