Abstract
FtsEX is a member of a small subclass of ABC transporters that uses mechano-transmission to perform work in the periplasm. FtsEX controls periplasmic peptidoglycan (PG) hydrolase activities in many Gram negative and positive organisms to ensure the safe separation of daughter cells during division. FtsEX localizes to the Z ring and uses its ATPase activity to regulate its periplasmic effectors. In E. coli, FtsEX also participates in building the divisome and coordinates PG synthesis with PG hydrolysis. This review discusses studies that are beginning to elucidate the mechanisms of FtsEX’s various roles in cell division.
Keywords: FtsEX, ABC transporter, peptidoglycan hydrolyases, Z ring, divisome
The long road to FtsEX’s role in cell division
Filamentation temperature sensitive (fts) describes a class of mutants that grow with normal cell morphology at the permissive temperature but form long nonseptate filaments and eventually die at the restrictive temperature. Seven fts mutants, including ftsE, were first isolated using chemical mutagenesis in the late 1960s by Hirota et al. [1]. These authors noted that many of the mutant’s defects were suppressed by the addition of salts, sucrose, glycerol or glucose but how these compounds aided survival were and are still largely unknown [2]. Since “true” division proteins were expected to be essential in all growth conditions, doubts were raised about FtsE and FtsX since they could be bypassed by the addition of osmoprotectants [3–6]. Also, ftsE and ftsX are in an operon with ftsY, which is required for co-translational targeting and delivery of integral membrane proteins to the Sec translocon [7–10]. This finding added suspicion that FtsEX might have an indirect role in cell division and act with FtsY to insert division proteins into the membrane.
Meanwhile, various reports described mutations in ftsE and/or ftsX homologues in other bacteria such as Neisseria, Flavobacteria and Aeromonas that cause morphological defects suggesting their involvement in the cell separation step during division [11–13]. Importantly, in 2004 Schmidt et al. [4] determined that FtsE and FtsX were bona fide division proteins despite a lingering doubt about their essentiality. Their findings indicated that FtsE and FtsX form a complex that localizes to the division site with a role in the assembly and stability of the divisome. This was followed by reports that FtsE and FtsX interact with several bona fide division proteins [14, 15]. In 2007, Reddy reinvestigated the essentiality of FtsEX in different growth conditions and confirmed that the septation block conferred by ftsE or ftsX null alleles is suppressed by increasing the osmolarity of the media [5]. Suppression is also conferred by overproduction of, or by mutations in, various cell division genes [5]. Together these observations led to the conclusion that FtsEX is essential to ensure divisome “stability” at low osmolarity. However, until recently the various roles of FtsEX in division were not clear (see below).
Bacterial cell division primer
Bacterial cell division is orchestrated by a protein complex called the divisome or septal ring (SR) which includes a core of essential proteins and many other non-essential proteins (Fig. 1). The divisome coordinates constriction of all layers of the cell envelope resulting in the generation of two daughter cells (for reviews see [16–18]). In E. coli, the divisome core consists of the essential division proteins FtsA, FtsB, FtsE, FtsI, FtsK, FtsL, FtsN, FtsQ, FtsW, FtsX, FtsZ and ZipA (Fig. 1). Except for the cytoplasmic FtsZ, these proteins are inner membrane proteins or associate with the membrane (FtsA or FtsE through FtsX). Other divisome proteins can reside in any cell compartment (cytoplasm, inner or outer membranes [IM or OM] or periplasm) and are dispensable for viability under laboratory conditions. They have a role in improving the proficiency of the divisome and likely the fitness of the organism in the environment.
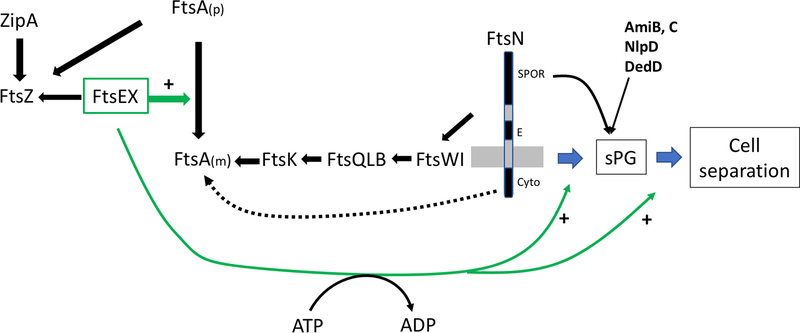
Fig. 1.
Roles of FtsEX in cell division. The Z ring consists of dynamic polymers of FtsZ attached to the membrane by ZipA and FtsA. Both proteins bind to the conserved C-terminal tail (CCTP) of FtsZ. FtsEX also interacts with the conserved CCTP of FtsZ and once at the Z ring, FtsEX, in a step that does not require ATP hydrolysis, acts on FtsA to promote formation of monomers which are active in recruiting the remaining divisome proteins that occurs in a sequential manner. The recruitment of FtsN, which triggers the initiation of sPG synthesis, involves interaction of the cyto domain of FtsN with FtsA and the interaction of the E domain of FtsN with the divisome in the periplasm. FtsN action leads to ATP hydrolysis by FtsEX and activation of FtsWI to synthesize sPG. It also leads to the recruitment of many other proteins including amidases (AmiB&C), an activator (NlpD of AmiC) and another stimulator of sPG (DedD). ATP hydrolysis by FtsEX also regulates EnvC, which is bound to the large periplasmic loop of FtsX, causing it to activate amidases in the periplasm leading to cell separation. Since FtsEX is required for activation of sPG synthesis and PG hydrolysis it couples these processes at the septum.
In the first step of the cell division process, FtsZ, a tubulin homolog and the most conserved divisomal protein, assembles into polymers attached to the inner membrane to form the Z ring [19]. Attachment depends upon two membrane anchors ZipA, a bitopic inner membrane protein, and FtsA, a member of the actin family associated with the membrane through an amphipathic alpha helix [20–24]. Other essential proteins (FtsEX) and non-essential proteins (ZapA, ZapC, ZapD), join the Z ring at this step [18, 25]. Many of these proteins (FtsA, ZipA, ZapD, FtsEX at least in E. coli) associate with the Z ring by binding directly to FtsZ’s conserved C-terminal peptide (CCTP) [26], which is linked to its tubulin-like polymerizing domain by a long intrinsically disordered linker (~50 amino acids in E. coli). The Z ring is a very dynamic structure [27] and functions as a scaffold to recruit the rest of the divisome machinery and then as a guide to direct the constriction process.
In the second step, the recruitment of the other essential division proteins occurs to complete the divisome. This recruitment happens in an ordered sequence in which the presence of one protein is required for the recruitment of the next (in the following sequence FtsK, FtsQ + FtsL + FtsB, FtsW + FtsI and finally FtsN)[17, 18]. Both ZipA and FtsA are required for this step, but only FtsA is directly involved in the recruitment (Fig. 1). FtsA is an atypical actin homolog in which the classic actin domain 1B is replaced by an unrelated domain 1C positioned on a different side of the molecule. Nonetheless, FtsA polymerizes into filaments similar to actin filaments [28]. The 1C domain is critical for polymerization but also has a role in the recruitment of the later cell division proteins [29, 30]. FtsA is very conserved among bacteria and has been shown to interact directly with other essential division proteins in many organisms [17]. It has been proposed that the two roles of the IC domain are mutually exclusive, meaning that polymerized FtsA is unable to recruit downstream proteins [30]. This proposal is based on the observation that numerous mutants of FtsA with a defect in the ability to self-interact gain the ability to bypass ZipA. These observations led to a model in which FtsA polymers have to be converted to monomers at the Z ring in order to recruit other cell division proteins and this is likely to be a key regulatory step in divisome assembly [30] (Fig.1). The recruitment of the last essential protein FtsN requires the subcomplexes FtsWI, FtsQLB and its interaction with FtsA [31–33]. FtsN arrival signals that the divisome is complete and triggers the constriction process [18, 34, 35]. Recent studies revealed that FtsEX acts on FtsA to start the recruitment process and is also involved in triggering the constriction process [36](see later and Fig. 1).
Most bacterial cells are surrounded by a layer of peptidoglycan (PG), also called the murein sacculus, which gives the cells their shape, protects them from osmotic lysis and serves as an anchor for other cell components such as the outer membrane of Gram negative organisms [37]. This PG layer is a continuous polymer made of linear glycan strands crosslinked by short peptides. It is constantly being remodeled by synthetic and degradative enzymes working together as complexes (elongasome or divisome) to allow the cells to grow or divide. One of the key roles of the divisome is to coordinate the invagination of all layers of the cell envelope (OM, PG and IM), while ensuring that their integrity is not compromised. This requires a precise coordination between the inward synthesis of new septal peptidoglycan (sPG) and its degradation by lytic enzymes to form the new poles [38].
The synthesis of sPG is carried out by FtsW, a PG glycosyltransferase of the newly characterized SEDS family, and FtsI (or PBP3 aka Penicillin Binding Protein 3) with D-D-transpeptidase activity [39, 40]. The activity of this FtsWI subcomplex is coordinated with the activity of PBP1b, a bifunctional (glycosyltransferase and transpeptidase activities) PG synthetase, which is not an essential division protein, but is recruited at the divisome during septation and reinforces the sPG made by FtsWI [40–42]. The localization and activity of FtsWI at the constriction site are under the control of another divisome sub-complex formed by FtsQ, FtsL and FtsB, which itself responds to signals from FtsN in the periplasm and FtsEX in the cytoplasm [33, 36, 43] (Fig.1).
The onset of sPG synthesis results in the recruitment of many additional proteins to the septum. Some of these proteins are involved in the remodeling of sPG or the invagination of the outer membrane such as the amidases (AmiB and AmiC) [44, 45] and the Tol-Pal complex [46], respectively. Among the proteins recruited after the start of constriction (Fig. 1), those with a SPOR domain depend in part on the presence of denuded PG strands generated by the action of amidases for their recruitment [47–49]. Among them, FtsN is the best known and its SPOR domain dependent recruitment to the septum has been shown to be self-enhancing, i.e. FtsN triggers PG synthesis leading to amidase activity and to more FtsN recruitment [49]. This self-enhancement is essential at FtsN’s physiological level, but can be bypassed if FtsN deleted for the SPOR domain is overexpressed [49, 50]. The roles of the other SPOR domain containing proteins (DedD, DamX, RlpA in E. coli) in division are still poorly understood, however, FtsN and DedD seem to have a redundant function in maintaining efficient PG synthesis at the septum once constriction is started [47, 51].
FtsE and FtsX are members of a unique subgroup of the ABC transporter superfamily
FtsEX is a member of a small subfamily of the large ABC transporter family. FtsE is the cytoplasmic ATP-binding component (NBD) and FtsX is the membrane component (TMD) [3, 7]. Since FtsX has only four transmembrane (TM) segments that lack charged amino acids residues usually found in ABC transporters concerns were raised early on about it being a true transporter [52, 53]. Also, it has an unusually large periplasmic loop (EC1) between TM1 and TM2, making it unlikely to form a substrate channel [54, 55]. Nonetheless, similar to ABC transporters it was suggested that ATP hydrolysis by FtsE in the cytoplasm could cause a conformational change that would be transmitted by the TM domains of FtsX to drive an event in the periplasm involving FtsX’s EC1 loop [54]. FtsEX is a member of a small subfamily (Type VII) of the ABC superfamily that includes MacB and LolCDE, neither of which are transporters but instead use mechanotransmission to perform work in the periplasm [53, 56]. MacB is the best characterized and its unique fold defines the Type VII subgroup of the ABC transporter superfamily [56]. Its structure provides a molecular mechanism for mechanotransmission (Fig. 2A).
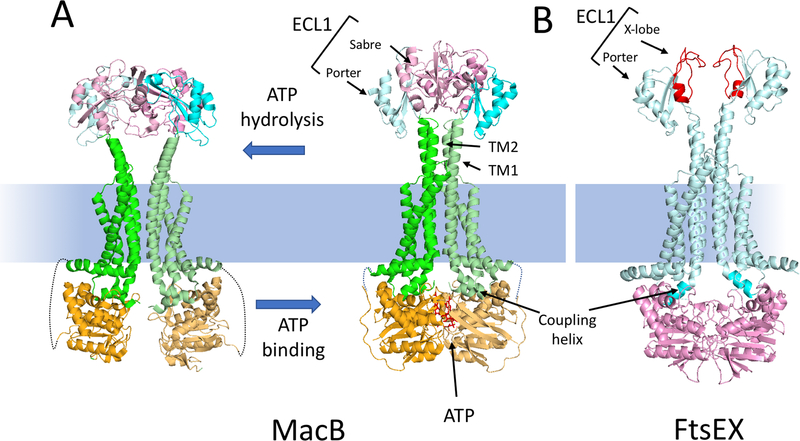
Fig. 2.
MacB as a model for the Type VII subfamily of ABC transporters and comparison to a model of FtsEX. (A) The structures of a MacB dimer in the nucleotide free form (5NIL) and the ATP bound form (5LJ7) are depicted. The NBD (light orange), the membrane component (green) and the large extracellular domain (ECL1) are depicted. ECL1 is split into two domains: porter (cyan) and sabre (pink). ATP binding brings the NBDs together causing the long TM1 and TM2 helices to form a tight helical bundle distal to the membrane causing conformational changes in the ECL1. The linkage between the NBD and the membrane component is not resolved in the structure and is indicated by a dotted line. (B) A model of the ATP form of FtsEX [59]; FtsE (pink) FtsX (cyan). The ECL1 of FtsX has a porter domain and a unique FtsX domain (called X-lobe [red]) instead of a sabre domain. This domain is responsible for interaction with the coiled coil region of its effector. The coupling helix of the TMD (FtsX) is inserted into a cleft of the NBD (FtsE).
Like FtsX, each MacB monomer has four transmembrane helices (TMs) and a large periplasmic loop (EC1) between TM1 and TM2 [56]. Helical extensions of TM1 and TM2 in the monomer form a four-helix bundle in the MacB dimer that elevates EC1 above the plane of the membrane. TM3 and TM4 are outside the helical bundle and are linked by a short periplasmic loop referred as the shoulder loop (EC2). In the cytoplasm, an amphipathic helix running parallel to the membrane surface connects the N terminus of TM1 to MacB’s NBD. The coupling helix, located on the cytoplasmic side of the membrane between TM2 and TM3, fits into a groove of the NBD domain and communicates conformational changes in the NBD to the TMD. [52, 53].
MacB has two subdomains in its large EC1 loop, one called Porter (for its structural similarity to a domain of AcrB) is directly connected to the raised helices between TM1 and TM2 and another called Sabre (for Small Alpha Beta Rich Extracytoplasmic) is mostly absent in FtsX [57, 58]. Instead FtsX has something we designated the X-lobe (Fig. 2B). The Porter domain is present in all Type VII ABC transporters known to date and is likely to be an intrinsic part of the mechanotransmission mechanism. Comparison of the MacB structures with and without ATP reveals long-range conformational changes in the TM helices, stalk helices and the EC1 periplasmic domain [56]. ATP binding induces dimerization of the NBD domains causing the coupling helices to push upwards on the TM2s helices (Fig. 2A). This push into the rigid periplasmic helical bundle on top of TM1 and TM2 causes significant changes in the EC1 loop [56].
In the following we discuss the evidence that FtsEX serves as a regulator that transmits signals between its binding partners on each side of the inner membrane to synchronize different aspects of the division process. Despite the striking resemblance to MacB, the nature of the conformational changes and the role of the ATP hydrolysis in these processes remain poorly understood.
Role of FtsEX in the divisome assembly
Schmidt et al. (2004)[4] established a direct role for FtsEX in cell division with several key findings. They showed that division inhibition is a primary consequence of depleting FtsEX since filamentation occurred before any effect on growth. Also, depletion of either FtsE or FtsX or of both, did not affect Z ring formation but prevented localization of the later division proteins (FtsK, FtsQ, FtsI or FtsN) (Fig.1). Importantly, they showed that fully functional GFP fusions to FtsE or FtsX localize to the septum, FtsE localization requires FtsX, and FtsX can still localize, albeit weakly, when expressed without FtsE. This dependence of FtsE localization on FtsX was confirmed in subsequent reports [15, 59]. By analogy to MacB, Du et al. [59] generated a model of FtsEX (Fig. 2B) allowing them to predict important residues in FtsX’s coupling helix and the corresponding groove of FtsE. Mutating these residues eliminates the ability of FtsEX to rescue a FtsEX depletion strain and prevents FtsE from localizing to the membrane and the septum as expected.
Since FtsX localizes weakly to the Z ring without FtsE, it was possible that FtsX was the main localization determinant for the FtsEX complex [4]. However, this idea was challenged by more recent observations. Based upon co-immunoprecipitation experiments, Corbin et al. [15] concluded that FtsE interacts with FtsZ independently of FtsX and the conserved C-terminal tail of FtsZ. Also, FtsEX’s recruitment to the Z ring appeared to be independent of FtsA and in some conditions also independent of ZipA, so it was postulated that FtsE is the main determinant once it is associated with FtsX at the membrane.
The apparent discrepancy in the dependency of FtsEX for localization led Du et al. to carefully reinvestigate the dependency [59]. They showed that FtsEX depends only on FtsZ for localization to the Z ring. They observed that mild overexpression of FtsEX blocks division by displacing FtsA from the Z ring and even higher overexpression leads to Z ring collapse indicating ZipA is also displaced. Since both FtsA and ZipA bind to the CCTP of FtsZ, it suggested that FtsEX also binds the CCTP. Interaction of FtsE with FtsZ’s conserved CCTP makes geometric sense too since FtsZ polymers, anchored by FtsA and ZipA, are 16 nm from the membrane, too far to contact the globular domain of FtsE [60]. In contrast, FtsZ’s CCTP, which is at the end of a long linker, could reach FtsE bound to FtsX. The CCTP appears to bind to FtsE61−77 which is on the surface of FtsE that is predicted to be close to the membrane [59].
Despite the above findings, overexpression of FtsN or FtsP, as well as mutants of FtsA impaired in self-interaction suppress the loss of FtsEX [4, 5, 25, 36]. Such suppressed cells grow and divide with only a mild chaining phenotype. Under these conditions, the late division proteins are recruited bypassing the need for FtsEX [4]. A recent study demonstrated that most of these suppressive conditions require and promote the ability of FtsN to interact with FtsA [25]. This strengthened interaction results in premature recruitment of FtsN to the septum which usually arrives last. FtsN then back recruits the FtsWI and FtsQLB sub-complexes, which both interact with FtsN and are responsible for sPG synthesis.
The FtsA-FtsX interaction is essential for FtsA to recruit the late divisome proteins to the Z ring. Mutations in ftsA (ftsAR63H or ftsAG366D) or ftsX (ftsXΔ4−69) that affect their ability to interact have no effect on the localization of FtsEX to the Z ring but prevent the recruitment of the other divisome proteins [36, 59]. Mutations or conditions that bypass the need for this interaction and allow formation of a complete divisome either impair FtsA’s self-interaction and/or promote FtsA’s interaction with FtsN. Both situations have been shown to also bypass ZipA, which in addition to anchoring FtsZ polymers, is necessary for FtsA to recruit the divisome proteins. FtsEX is suggested to antagonize FtsA polymerization directly, hence enhancing the ability of FtsA to build the divisome [18, 30, 36] whereas ZipA is thought to act indirectly by stabilizing the Z ring (Fig.1).
Deletion of ftsE and ftsX causes cell division defects due to effects on cell separation in many organisms [11, 12, 57, 61–63]. FtsE and/or FtsX have been shown to localize at the septum in a wide range of bacteria, (from such genera as Caulobacter, Streptoccoccus, Mycobacterium). In some cases, their dependence for localization (at least FtsZ) has been examined, but in general not much is known about their septal localization or their role in building the divisome in these organisms.
FtsE’s ATPase activity is not essential for FtsEX localization or to build the divisome but is required for activation of constriction
Overall the localization of FtsE and FtsX to the Z ring is co-dependent as FtsE must bind to FtsX at the membrane before it binds to the CCTP of FtsZ. Once FtsEX is at the Z ring, FtsX interacts with FtsA to promote FtsA’s ability to recruit the late cell division proteins (Fig. 1). The Weiss lab [54] asked if the ATPase function of FtsE was necessary to build the divisome by introducing mutations in the conserved Walker A and Walker B motifs of FtsE. These mutations did not affect FtsEX’s ability to localize and promote the recruitment of the other cell division proteins but the assembled divisome was inactive. More recently is was shown that FtsEX ATPase mutants block cell division with a phenotype mimicking inhibitors of FtsI activity [36]. Suppressors of this block include mutants of FtsA and of FtsX that disrupt the FtsX-FtsA interaction (in conditions which still allows the bypass of this interaction to build the divisome) indicating that this interaction is essential for FtsEX to modulate sPG synthesis [36, 59].
Even if the mechanism is not well understood, FtsN appears to be the main trigger for starting constriction and this activity requires its ability to interact with FtsQLB and FtsWI subcomplexes in the periplasm [33, 43]. The interaction of FtsN with FtsA has also been shown to be important under physiological conditions, suggesting that part of this activation mechanism is also happening in the cytoplasm [33, 43, 50, 64]. The suppressors of an FtsEX ATPase mutant identified by Du et al. [36], strongly indicate FtsEX ATPase in the cytoplasm contributes to the start of sPG synthesis through an interaction with FtsA, independent of its role in promoting the recruitment of divisome proteins by FtsA (Fig. 1). In their study, Du et al. [36] identified a motif in FtsA that is required to mediate its interaction with FtsX which is conserved in many Gram negative bacteria but is absent in Gram positive species. This led the authors to wonder if the roles of FtsEX in the regulation of divisome assembly and the start of sPG have evolved in those Gram negative bacteria on top of its more universally conserved role in controlling PG hydrolysis (see below and Fig. 1). They speculated that a thinner PG layer may warrant a coupling between PG synthesis and hydrolysis during septation to avoid lysis due to perturbations to sPG synthesis caused by rapid changes in osmolarity.
More work needs to be done in other organisms to determine whether these roles of FtsEX in building the divisome and coupling PG synthesis with PG hydrolysis are conserved. Also, investigation of the mechanism of how FtsEX ATPase mutants inhibit activation of constriction may help elucidate divisome activation.
FtsEX is a conserved regulator of PG Hydrolysis
Septal PG needs to be hydrolyzed to separate daughter cells during the constriction process. In E. coli, this is largely achieved by three amidases, AmiA, AmiB and AmiC [65]. Deletion of all three amidases results in the formation of extremely long chains of cells indicative of a failure in cell separation. PG hydrolases that are required for septation appear to be autoinhibited (probably to prevent catastrophic PG damage that could lead to cell lysis) and need to be activated at the constriction site. Bernhardt’s group found that the activities of the amidases are controlled by two periplasmic factors (EnvC and NlpD) that are recruited to the divisome but lack PG hydrolytic activity despite carrying a LytM like domain [44]. Instead, EnvC activates AmiA and AmiB and AmiC is stimulated by NlpD. Cells defective in both EnvC and NlpD form long chains of cells that resemble the triple amidase mutant.
In a search for factors involved in cell wall assembly Bernhardt’s laboratory found that FtsEX recruits EnvC to regulate the activity of AmiA and AmiB [66] (Fig. 2). They found that FtsEX− cells, like EnvC− cells become dependent on NlpD for cell separation. In a separate study published at the same time, Winkler’s laboratory showed that FtsX from S. pneumoniae interacts directly with PcsB, a PG hydrolase with a CHAP domain, which is essential for cell separation during division [63, 67]. They found that FtsX depletion phenocopies PcsB depletion and that FtsEX is also essential for cell division. They demonstrated that FtsEX recruits PcsB to the divisome and activates it. These two publications uncovered the most conserved and key role of the FtsEX complex in cell division: to regulate the activity of PG hydrolases necessary for daughter cell separation. Many recent studies have extended the conserved role of FtsEX as a regulator of PG hydrolysis in organisms such as Caulobacter cresentus [61, 68], Bacillus anthracis [69], Mycobacterium tuberculosis [57], Bacillus subtilis [70, 71] and Fusobacterium nucleatum [72]. In most bacteria the role of FtsEX in cell wall hydrolysis is at the septum but in B. subtilis it is involved in cell elongation.
The interaction between FtsX and its periplasmic partners has been characterized for FtsX-EnvC of E. coli (Fig. 3), FtsX-RipC of M. tuberculosis and especially FtsX-PcsB of S. pneumoniae. FtsX’s partners (EnvC, PcsB and RipC) all have an N-terminal coiled-coil domain that is essential for −interaction with the large periplasmic loop (EC1) of FtsX and localization to the division site [57, 58, 63, 66]. Analysis of the structure of the EC1 loop of FtsX from M. tuberculosis in addition to a biochemical study of the FtsX-RipC pair reveals that the site of interaction between FtsX and its partner is conserved and is a hydrophobic cleft formed by a unique fold of FtsX (X-lobe, Fig. 2B). It has been proposed that ATP hydrolysis by FtsE affects the interaction of the EC1 loop of FtsX with the coiled coil domain of its partner which overcomes the self-inhibited state of the hydrolase [57, 62, 63, 66] (Fig. 3).
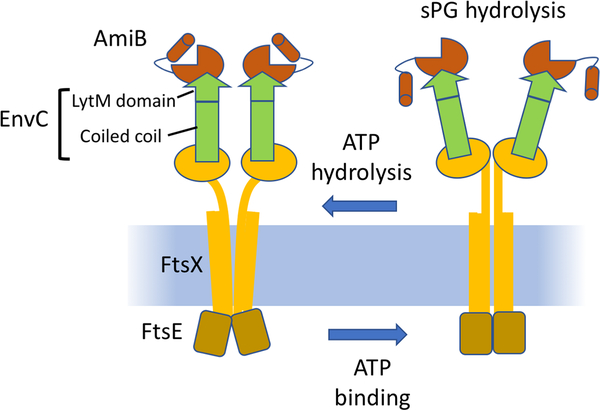
Fig. 3.
Model for the activation of amidases by FtsEX. EnvC binds to the large extracellular loop of FtsX independent of ATP. This binding occurs through the coiled coil domain of EnvC. Note, the stoichiometry between FtsX and EnvC is not known, but is drawn here as 1:1. As sPG is synthesized amidases are recruited and activated by ATP hydrolysis of FtsEX. ATP binding leads to conformational changes in FtsEX which impact EnvC leading to activation of AmiB (and AmiA). These amidases are autoinhibited and this must be reversed by the conformational changes in FtsX which are transmitted to EnvC. ATP hydrolysis by FtsEX resets the system.
FtsEX mutants impaired for the ATPase activity cannot suppress the extreme chaining phenotype of E. coli cells depleted for both NlpD and FtsEX [66]. Also, the ATPase function of FtsEX is essential for cell division in E. coli and S. pneumoniae [54, 62]. Because of these results, it has been suggested that the ATPase is critical for FtsEX’s ability to control PG hydrolysis in vivo (Fig. 1 and 3). However, in vitro, FtsEX is not required for EnvC to activate the amidase activity of AmiA and AmiB. Also, the PG cleavage activity of RipC is stimulated upon binding to FtsX (without FtsE), meaning that binding alone may be sufficient for activation.
The activity of the FtsEX-CwlO pair in B. subtilis is involved in cell wall expansion during cell elongation and does not have a role in cell division [70, 71]. This observation suggests that the role of FtsEX in controlling PG hydrolysis is not restricted to cell division and that additional factors other than FtsEX exist for sPG splitting during cell division. Also, involvement of FtsEX in other cell morphogenetic processes suggests an additional set of regulatory partners other than components of the divisome. It appears that FtsEX-CwlO is regulated by a set of proteins (MreB isologues) that are not part of the divisome but are part of the elongasome [70, 71].
How does FtsEX couple PG synthesis with PG hydrolysis during septation?
FtsEX requires a functional ATPase to control two activities at the Z ring: PG synthesis and PG hydrolysis in the periplasm (Fig. 1). Both activities, at least in E. coli, also require FtsX to be able to interact with FtsA. This is pretty much what is known about the coupling of these two roles of FtsEX.
The recent discovery that the nonessential division protein DedD is synthetic lethal with FtsN mutants impaired for their SPOR domain function suggests a speculative mechanism [51]. In these conditions, DedD appears to be essential for the activation of sPG synthesis. Both DedD and FtsN are SPOR domain proteins which bind denuded PG strands created by the activity of amidases. FtsN localization is not entirely dependent on its SPOR domain as it can weakly localize to the divisome through its interactions with FtsA and possibly FtsQLB and FtsWI [33, 50, 64]. However, its SPOR domain is necessary for the enhanced localization to the Z ring once constriction starts and is required for FtsN to function at its physiological level (Fig. 1). Localization of DedD to the divisome also depends largely upon its SPOR domain. Without FtsEX’s ATPase, AmiA and AmiB are not activated so the denuded PG strands necessary for FtsN and DedD localization are not generated. This impaired recruitment of FtsN and DedD could delay sPG synthesis and this may be how PG hydrolysis is linked to PG synthesis. In support of this theory are the observations that FtsEX−, EnvC− or mutants of FtsN with a nonfunctional SPOR domain display mild chaining similar to DedD− cells. In addition, they are all synthetic lethal with a common set of mutations such as deletion of PBP1b or min, suggesting that they all could be involved in the same pathway. Since both FtsX and FtsN interact with FtsA, these interactions could also be part of a feedback loop in which FtsN binding to FtsA could cause FtsX to promote ATP hydrolysis by FtsE, hence controlling amidase activity. This hypothesis should be investigated and it will be of interest to determine whether the coupling of sPG synthesis and hydrolysis by FtsEX happens in other organisms encoding cell division proteins containing a SPOR domain.
Conclusions and perspectives
Recent studies have highlighted three roles for FtsEX in the cell division process. One, FtsEX has a clear role in the building of the divisome as it is involved in the recruitment of many essential proteins to the Z ring through its regulation of FtsA. Second, FtsEX also participates in the activation and regulation of sPG synthesis. Third, FtsEX is responsible for the recruitment and activation of hydrolases which split sPG to form new cell poles. Importantly, FtsE’s ATPase function appears to be essential in the latter two roles and its homology to the Type VII subfamily of ABC transporters suggests that it acts on its periplasmic effectors using a mechanotransmission mechanism that sense signals in the cytoplasm or cytoplasmic membrane to produce action in the periplasm. The ever increasing number of reports identifying FtsX and an interacting protein involved in regulation of PG hydrolysis in a wide range of bacterial species substantiate a role for FtsEX in the control of PG hydrolysis at the septum (or elongation in B. subtilis).
Much work still needs to be done to understand how the ATPase cycle is triggered and how it is responsible for the control of PG hydrolysis. Questions include what triggers FtsE’s ATPase in the cytoplasm, what is the function of the FtsA-FtsX interaction, what are the effects on FtsX in response to FtsE ATPase that activate its periplasmic effector and what is the stoichiometry between FtsX and its effectors? It is interesting to note that in some organisms FtsEX is not essential or only partially essential (Ec or Mt’s FtsEX are essential in low osmolarity media but cells are less affected in higher osmolarity conditions). This likely reflects a redundancy in the mechanisms for recruitment of the downstream divisome proteins as well as activating PG hydrolysis at the septum. It has been suggested that in E. coli, FtsEX might be important since it is exposed to environments with changing osmolarity [38]. Abrupt osmolarity changes may cause delays or perturbations during constriction potentially increasing the risk of lysis. Bacteria capable of living in niches with widely varying osmolarity may have evolved a tighter coupling of PG hydrolysis and synthesis at the septum to enhance envelope integrity during septation. However, in E. coli FtsEX appears to have also acquired a role in assuring that a complete divisome is built in media with low osmolarity [4]. It may be noteworthy that FtsE interacts with FtsZ which hydrolyses GTP to drive Z ring dynamics which is linked to PG synthesis activities [73]. One interesting idea is that the ATPase activity of FtsEX is coupled with Z ring dynamics through FtsZ GTPase activity, thus coordinating Z ring constriction with sPG synthesis and separation of the cells by PG hydrolases. Understanding how FtsEX can couple sPG synthesis and sPG hydrolysis during constriction will elucidate a poorly understood part of cell division -- how is the start of constriction triggered once the divisome is assembled?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hirota Y, Ryter A, Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harbor symposia on quantitative biology 1968;33:677–93. [DOI] [PubMed] [Google Scholar]
- [2].Ricard M, Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. Journal of bacteriology 1973;116:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].de Leeuw E, Graham B, Phillips GJ, ten Hagen-Jongman CM, Oudega B, Luirink J. Molecular characterization of Escherichia coli FtsE and FtsX. Molecular microbiology 1999;31:983–93. [DOI] [PubMed] [Google Scholar]
- [4].Schmidt KL, Peterson ND, Kustusch RJ, Wissel MC, Graham B, Phillips GJ, et al. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. Journal of bacteriology 2004;186:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reddy M Role of FtsEX in cell division of Escherichia coli: viability of ftsEX mutants is dependent on functional SufI or high osmotic strength. Journal of bacteriology 2007;189:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taschner PE, Huls PG, Pas E, Woldringh CL. Division behavior and shape changes in isogenic ftsZ, ftsQ, ftsA, pbpB, and ftsE cell division mutants of Escherichia coli during temperature shift experiments. Journal of bacteriology 1988;170:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gibbs TW, Gill DR, Salmond GP. Localised mutagenesis of the ftsYEX operon: conditionally lethal missense substitutions in the FtsE cell division protein of Escherichia coli are similar to those found in the cystic fibrosis transmembrane conductance regulator protein (CFTR) of human patients. Molecular & general genetics : MGG 1992;234:121–8. [DOI] [PubMed] [Google Scholar]
- [8].Crickmore N, Salmond GP. Genetic and physical clarification of the Escherichia coli genetic map in the 76.5-minute essential gene cluster containing heat shock and cell division genes. Journal of bacteriology 1992;174:7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gill DR, Hatfull GF, Salmond GP. A new cell division operon in Escherichia coli. Molecular & general genetics : MGG 1986;205:134–45. [DOI] [PubMed] [Google Scholar]
- [10].Salmond GP, Plakidou S. Genetic analysis of essential genes in the ftsE region of the Escherichia coli genetic map and identification of a new cell division gene, ftsS. Molecular & general genetics : MGG 1984;197:304–8. [DOI] [PubMed] [Google Scholar]
- [11].Bernatchez S, Francis FM, Salimnia H, Beveridge TJ, Li H, Dillon JA. Genomic, transcriptional and phenotypic analysis of ftsE and ftsX of Neisseria gonorrhoeae. DNA research : an international journal for rapid publication of reports on genes and genomes 2000;7:75–81. [DOI] [PubMed] [Google Scholar]
- [12].Kempf MJ, McBride MJ. Transposon insertions in the Flavobacterium johnsoniae ftsX gene disrupt gliding motility and cell division. Journal of bacteriology 2000;182:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Merino S, Altarriba M, Gavin R, Izquierdo L, Tomas JM. The cell division genes (ftsE and X) of Aeromonas hydrophila and their relationship with opsonophagocytosis. FEMS microbiology letters 2001;198:183–8. [DOI] [PubMed] [Google Scholar]
- [14].Karimova G, Dautin N, Ladant D. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. Journal of bacteriology 2005;187:2233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Corbin BD, Wang Y, Beuria TK, Margolin W. Interaction between cell division proteins FtsE and FtsZ. Journal of bacteriology 2007;189:3026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tsang MJ, Bernhardt TG. Guiding divisome assembly and controlling its activity. Current opinion in microbiology 2015;24:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lutkenhaus J, Du S. E. coli Cell Cycle Machinery. Subcell Biochem 2017;84:27–65. [DOI] [PubMed] [Google Scholar]
- [18].Du S, Lutkenhaus J. Assembly and activation of the Escherichia coli divisome. Molecular microbiology 2017;105:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature 1991;354:161–4. [DOI] [PubMed] [Google Scholar]
- [20].Pichoff S, Lutkenhaus J. Identification of a region of FtsA required for interaction with FtsZ. Molecular microbiology 2007;64:1129–38. [DOI] [PubMed] [Google Scholar]
- [21].Pichoff S, Lutkenhaus J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Molecular microbiology 2005;55:1722–34. [DOI] [PubMed] [Google Scholar]
- [22].Pichoff S, Lutkenhaus J. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. The EMBO journal 2002;21:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hale CA, de Boer PA. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. Journal of bacteriology 1999;181:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hale CA, de Boer PA. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 1997;88:175–85. [DOI] [PubMed] [Google Scholar]
- [25].Pichoff S, Du S, Lutkenhaus J. Disruption of divisome assembly rescued by FtsN-FtsA interaction in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 2018;115:E6855–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ortiz C, Natale P, Cueto L, Vicente M. The keepers of the ring: regulators of FtsZ assembly. FEMS microbiology reviews 2016;40:57–67. [DOI] [PubMed] [Google Scholar]
- [27].Stricker J, Maddox P, Salmon ED, Erickson HP. Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proceedings of the National Academy of Sciences of the United States of America 2002;99:3171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Szwedziak P, Wang Q, Freund SM, Lowe J. FtsA forms actin-like protofilaments. The EMBO journal 2012;31:2249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rico AI, Garcia-Ovalle M, Mingorance J, Vicente M. Role of two essential domains of Escherichia coli FtsA in localization and progression of the division ring. Molecular microbiology 2004;53:1359–71. [DOI] [PubMed] [Google Scholar]
- [30].Pichoff S, Shen B, Sullivan B, Lutkenhaus J. FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA’s self-interaction competes with its ability to recruit downstream division proteins. Molecular microbiology 2012;83:151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Busiek KK, Eraso JM, Wang Y, Margolin W. The early divisome protein FtsA interacts directly through its 1c subdomain with the cytoplasmic domain of the late divisome protein FtsN. Journal of bacteriology 2012;194:1989–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Addinall SG, Cao C, Lutkenhaus J. FtsN, a late recruit to the septum in Escherichia coli. Molecular microbiology 1997;25:303–9. [DOI] [PubMed] [Google Scholar]
- [33].Liu B, Persons L, Lee L, de Boer PA. Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated cell constriction in Escherichia coli. Molecular microbiology 2015;95:945–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].den Blaauwen T, Luirink J. Checks and Balances in Bacterial Cell Division. mBio 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weiss DS. Last but not least: new insights into how FtsN triggers constriction during Escherichia coli cell division. Molecular microbiology 2015;95:903–9. [DOI] [PubMed] [Google Scholar]
- [36].Du S, Pichoff S, Lutkenhaus J. FtsEX acts on FtsA to regulate divisome assembly and activity. Proceedings of the National Academy of Sciences of the United States of America 2016;113:E5052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Egan AJ, Biboy J, Van’t Veer I, Breukink E, Vollmer W. Activities and regulation of peptidoglycan synthases. Philos Trans R Soc Lond B Biol Sci 2015;370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Egan AJF. Bacterial outer membrane constriction. Molecular microbiology 2018;107:676–87. [DOI] [PubMed] [Google Scholar]
- [39].Meeske AJ, Riley EP, Robins WP, Uehara T, Mekalanos JJ, Kahne D, et al. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 2016;537:634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Taguchi A, Welsh MA, Marmont LS, Lee W, Sjodt M, Kruse AC, et al. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat Microbiol 2019;4:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rohs PDA, Buss J, Sim SI, Squyres GR, Srisuknimit V, Smith M, et al. A central role for PBP2 in the activation of peptidoglycan polymerization by the bacterial cell elongation machinery. PLoS genetics 2018;14:e1007726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pazos M, Peters K, Casanova M, Palacios P, VanNieuwenhze M, Breukink E, et al. Z-ring membrane anchors associate with cell wall synthases to initiate bacterial cell division. Nature communications 2018;9:5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsang MJ, Bernhardt TG. A role for the FtsQLB complex in cytokinetic ring activation revealed by an ftsL allele that accelerates division. Molecular microbiology 2015;95:925–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peters NT, Dinh T, Bernhardt TG. A fail-safe mechanism in the septal ring assembly pathway generated by the sequential recruitment of cell separation amidases and their activators. Journal of bacteriology 2011;193:4973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bernhardt TG, de Boer PA. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Molecular microbiology 2003;48:1171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gerding MA, Ogata Y, Pecora ND, Niki H, de Boer PA. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Molecular microbiology 2007;63:1008–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yahashiri A, Jorgenson MA, Weiss DS. The SPOR Domain, a Widely Conserved Peptidoglycan Binding Domain That Targets Proteins to the Site of Cell Division. Journal of bacteriology 2017;199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moll A, Thanbichler M. FtsN-like proteins are conserved components of the cell division machinery in proteobacteria. Molecular microbiology 2009;72:1037–53. [DOI] [PubMed] [Google Scholar]
- [49].Gerding MA, Liu B, Bendezu FO, Hale CA, Bernhardt TG, de Boer PA. Self-enhanced accumulation of FtsN at Division Sites and Roles for Other Proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. Journal of bacteriology 2009;191:7383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Busiek KK, Margolin W. A role for FtsA in SPOR-independent localization of the essential Escherichia coli cell division protein FtsN. Molecular microbiology 2014;92:1212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu B, Hale CA, Persons L, Phillips-Mason PJ, de Boer PAJ. Roles of the DedD Protein in Escherichia coli Cell Constriction. Journal of bacteriology 2019;201:e00698–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wilkens S Structure and mechanism of ABC transporters. F1000prime reports 2015;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Greene NP, Kaplan E, Crow A, Koronakis V. Antibiotic Resistance Mediated by the MacB ABC Transporter Family: A Structural and Functional Perspective. Frontiers in microbiology 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Arends SJ, Kustusch RJ, Weiss DS. ATP-binding site lesions in FtsE impair cell division. Journal of bacteriology 2009;191:3772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Daley DO, Rapp M, Granseth E, Melen K, Drew D, von Heijne G. Global topology analysis of the Escherichia coli inner membrane proteome. Science 2005;308:1321–3. [DOI] [PubMed] [Google Scholar]
- [56].Crow A, Greene NP, Kaplan E, Koronakis V. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proceedings of the National Academy of Sciences of the United States of America 2017;114:12572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mavrici D, Marakalala MJ, Holton JM, Prigozhin DM, Gee CL, Zhang YJ, et al. Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proceedings of the National Academy of Sciences of the United States of America 2014;111:8037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rued BE, Alcorlo M, Edmonds KA, Martinez-Caballero S, Straume D, Fu Y, et al. Structure of the Large Extracellular Loop of FtsX and Its Interaction with the Essential Peptidoglycan Hydrolase PcsB in Streptococcus pneumoniae. mBio 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Du S, Henke W, Pichoff S, Lutkenhaus J. How FtsEX localizes to the Z ring and interacts with FtsA to regulate cell division. Molecular microbiology 2019. (). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Szwedziak P, Wang Q, Bharat TA, Tsim M, Lowe J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. Elife 2014;3:e04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Meier EL, Daitch AK, Yao Q, Bhargava A, Jensen GJ, Goley ED. FtsEX-mediated regulation of the final stages of cell division reveals morphogenetic plasticity in Caulobacter crescentus. PLoS genetics 2017;13:e1006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sham LT, Jensen KR, Bruce KE, Winkler ME. Involvement of FtsE ATPase and FtsX extracellular loops 1 and 2 in FtsEX-PcsB complex function in cell division of Streptococcus pneumoniae D39. mBio 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sham LT, Barendt SM, Kopecky KE, Winkler ME. Essential PcsB putative peptidoglycan hydrolase interacts with the essential FtsXSpn cell division protein in Streptococcus pneumoniae D39. Proceedings of the National Academy of Sciences of the United States of America 2011;108:E1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pichoff S, Du S, Lutkenhaus J. The bypass of ZipA by overexpression of FtsN requires a previously unknown conserved FtsN motif essential for FtsA-FtsN interaction supporting a model in which FtsA monomers recruit late cell division proteins to the Z ring. Molecular microbiology 2015;95:971–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Heidrich C, Ursinus A, Berger J, Schwarz H, Holtje JV. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. Journal of bacteriology 2002;184:6093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yang DC, Peters NT, Parzych KR, Uehara T, Markovski M, Bernhardt TG. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proceedings of the National Academy of Sciences of the United States of America 2011;108:E1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ng WL, Kazmierczak KM, Winkler ME. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Molecular microbiology 2004;53:1161–75. [DOI] [PubMed] [Google Scholar]
- [68].Zielinska A, Billini M, Moll A, Kremer K, Briegel A, Izquierdo Martinez A, et al. LytM factors affect the recruitment of autolysins to the cell division site in Caulobacter crescentus. Molecular microbiology 2017;106:419–38. [DOI] [PubMed] [Google Scholar]
- [69].Margulieux KR, Liebov BK, Tirumala V, Singh A, Bushweller JH, Nakamoto RK, et al. Bacillus anthracis Peptidoglycan Integrity Is Disrupted by the Chemokine CXCL10 through the FtsE/X Complex. Frontiers in microbiology 2017;8:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dominguez-Cuevas P, Porcelli I, Daniel RA, Errington J. Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis. Molecular microbiology 2013;89:1084–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Meisner J, Montero Llopis P, Sham LT, Garner E, Bernhardt TG, Rudner DZ. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Molecular microbiology 2013;89:1069–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wu C, Al Mamun AAM, Luong TT, Hu B, Gu J, Lee JH, et al. Forward Genetic Dissection of Biofilm Development by Fusobacterium nucleatum: Novel Functions of Cell Division Proteins FtsX and EnvC. mBio 2018;9:e00360–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yang X, Lyu Z, Miguel A, McQuillen R, Huang KC, Xiao J. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science 2017;355:744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]