Abstract
Studies of membrane protein structure and function often rely on reconstituting the protein into lipid bilayers through the formation of liposomes. Many measurements conducted in proteoliposomes, e.g. transport rates, single-molecule dynamics, monomer-oligomer equilibrium, require some understanding of the occupancy statistics of the liposome population for correct interpretation of the results. In homogenous liposomes, this is easy to calculate as the act of protein incorporation can be described by the Poisson distribution. However, in reality, liposomes are heterogeneous, which alters the statistics of occupancy in several ways. Here, we determine the liposome occupancy distribution for membrane protein reconstitution while considering liposome size heterogeneity. We calculate the protein occupancy for a homogenous population of liposomes with radius r = 200 nm, representing an idealization of vesicles extruded through 400 nm pores and compare it to the right-skewed distribution of 400 nm 2:1 POPE:POPG vesicles. As is the case for E. coli polar lipids, this synthetic composition yields a sub-population of small liposomes, 25–30 nm in radius with a long tail of larger vesicles. Previously published microscopy data of the co-localization of the CLC-ec1 Cl−/H+ transporter with liposomes, and vesicle occupancy measurements using functional transport assays, shows agreement with the heterogeneous 2:1 POPE:POPG population. Next, distributions of 100 nm and 30 nm extruded 2:1 POPE:POPG liposomes are measured by cryo-electron microscopy, demonstrating that extrusion through smaller pores does not shift the peak, but reduces polydispersity arising from large liposomes. Single-molecule photobleaching analysis of CLC-ec1-Cy5 shows the 30 nm extruded population increases the ‘Poisson-dilution’ range, reducing the probability of vesicles with more than one protein at higher protein/lipid densities. These results demonstrate that the occupancy distributions of membrane proteins into vesicles can be accurately predicted in heterogeneous populations with experimental knowledge of the liposome size distribution.
Keywords: Liposome size distribution, Poisson, membrane protein, reconstitution, single-molecule photobleaching, CLC-ec1
1. Introduction
Membrane protein reconstitution involves the incorporation of purified membrane-embedded proteins into lipid bilayers for functional and structural interrogation (5). It provides a means to study the protein in the context of the native solvent structure, which is a distinctly different environment than the detergent micelle state from which the protein is usually purified. There are several methods of reconstituting membrane proteins (6), each involving the mixing of a known amount of protein with a known amount of lipids, followed by a procedure to allow for the faithful incorporation of the protein into the bilayer while preserving the protein structure. Since most membrane proteins do not spontaneously insert into lipid bilayers (8), this requires a gentle approach of exchanging the protein from the stable detergent solubilized state to a lipid bilayer solvated condition. A common method to do this is to mix a small amount of protein stabilized in a detergent micelle, with lipids that have been solubilized in a detergent with a high critical micelle concentration. From this mixed-micelle condition, the detergent is then slowly removed by dialysis, resulting in the formation of bilayers with the protein incorporated within, that then assemble into self-contained liposomes (9).
One of the benefits of the compartmentalized structure of liposomes is that it enables the establishment of gradients across the membrane, which allows interrogation of the protein’s function (10, 11). One limitation of the dialysis method often used to prepare liposomes, is that it typically generates small, unilamellar vesicles (12) that incorporate the protein according to the state in the mixed micelle condition. Since liposomes do not spontaneously exchange with one another on a reasonable time scale, this means that the protein state that is examined in the resultant proteoliposome population may not reflect equilibrium. This presents an issue for considering stoichiometry of the protein, particularly at ‘Poisson-dilutions’ where most of the liposomes are unoccupied or have a single protein species. However, this is the range of dilution that is most desirable when considering protein function, as the membrane transport behavior reflects a single-molecule and so unitary transport rates can be estimated (4). Still, when investigating the function of the native assembly, it is advantageous to capture the equilibrium state of the protein in the membrane into single vesicles for further analysis of function and dynamics.
One way of doing this is by taking the small unilamellar liposomes from the dialysis procedure and fusing them into larger membranes. It has been observed that repeated cycles of freezing and thawing can lead to the growth of large multilamellar, or multivesicular membranes with increased trapped volume (13, 14). This occurs due to the formation of ice during freezing which break the membrane structure, allowing for annealing with other membranes. For E. coli polar lipids, phase contrast optical microscopy showed that the freeze-thaw process forms large multilamellar vesicles with outer diameters of 10 μm (15), much larger than a typical biological cell membrane. In these large membranes, it now becomes possible for protein species to encounter one another and sample the thermodynamic consequences of the system. From here, smaller liposomes can be formed by fractionating the membrane by extrusion through polycarbonate filters with defined pore sizes to produce reproducible distributions of liposomes. During this process, the large membrane is pushed through the pores, anywhere from 30 nm to 1 μm, forming a membrane bubble that breaks off into a vesicle that is approximate in diameter to the extrusion pore size (2). The probability of protein incorporation into that vesicle is a random process that depends on the density of protein in the overall membrane, and the number of vesicles in the resultant liposome population (1, 4, 7).
It is advantageous to understand how protein is incorporated into the liposome population, for the interpretation of functional and structural studies as described above. If the liposome size distribution is uniform and there is a single protein species of interest, then this can be calculated using the formula for the Poisson distribution (equation 1). However, realistically, extruded liposomes lead to non-uniform distributions except for the smallest pore sizes. Liposome size distributions can be directly measured by imaging liposome samples, using freeze-fracture or cryo-electron microscopy (EM) approaches (12). Alternatively, numerical distributions can be obtained by dynamic light scattering spectroscopy, which exhibits good agreement with EM studies (16). These studies have revealed that extrusion of freeze-thawed multilamellar membranes through polycarbonate filters leads to vesicle populations with mean diameters comparable to the pore size of the filter (17). However, there is significant polydispersity when using pore sizes > 200 nm, often yielding bimodal distributions. In particular, 400 nm extruded liposomes obtained from freeze-thawed E. coli polar lipids or 2:1 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE):1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (POPG) membranes, are much smaller than expected, with a peak population near 25–30 nm and a long rightward tail of larger vesicles (3, 4). The heterogeneity in liposome sizes has an important impact on the reconstitution statistics of membrane proteins, which must be known for proper interpretation of experimental data, in particular for knowing the ‘Poisson-dilution’ range, and the expected oligomeric assemblies during equilibrium reactions.
In this study, we examine the effect of liposome heterogeneity on the statistics of membrane protein reconstitution into liposomes. First, we outline the theory behind calculating the liposome occupancy probability distribution for heterogeneous liposome populations. We focus on the 2:1 POPE:POPG lipid composition, a synthetic mimic of the E. coli membrane and a commonly used lipid composition in reconstitution studies. By comparing simulations with experimental data, we show that the heterogeneous profile of the 400 nm extruded population, agrees with previously published data on fractional occupancy by co-localization microscopy, and fractional volume measured by proteoliposome efflux assays. Next, we measure the size distributions of 2:1 POPE:POPG extruded with 30 nm and 100 nm pores, and demonstrate that the single-molecule photobleaching distributions of the CLC-ec1 Cl−/H+ antiporter labelled with Cy5 agrees with simulations based on these liposome distributions. Finally, we show how extrusion through 30 nm filters increases the ‘Poisson-dilution’ limit and expands the dynamic range of discriminating between expected monomer and dimer reconstitutions distributions. These results are expected to be useful in future experiments examining CLC-ec1 dimerization equilibrium, studies of other membrane protein oligomers in membranes, and the general study of membrane protein dynamics and function by single-molecule microscopy approaches.
2. METHODS
2.1. MATLAB simulations
MATLAB was used to simulate the random process of multi-species subunit capture into a heterogeneous liposome population, as described previously (1, 18). For each liposome sub-population with radius r, a matrix was created with size Nliposomes(r), and each Nprotein(r) sub-species was randomly assigned to the liposome population. Unless otherwise noted, the following parameters were used: area per lipid, Alipid = 0.6 nm2 (19); probability of subunit labeling, PCy5 = 0.7; probability of non-specific subunit labeling, Pns = 0.1; mole-fraction recovery, yield = 0.5; liposome size distribution, Pradius(ri); liposomes accessibility factor, bias = 4 (excludes 2.5–22.5 nm bins from Pradius(ri)), to model the exclusion of ~(5 nm × 10 nm) CLC-ec1 dimers from smaller liposomes. MATLAB files for the simulation program, and the analytical solution of the heterogeneous Poisson distribution are included as supplementary information.
2.2. Cryo-electron microscopy imaging of liposomes
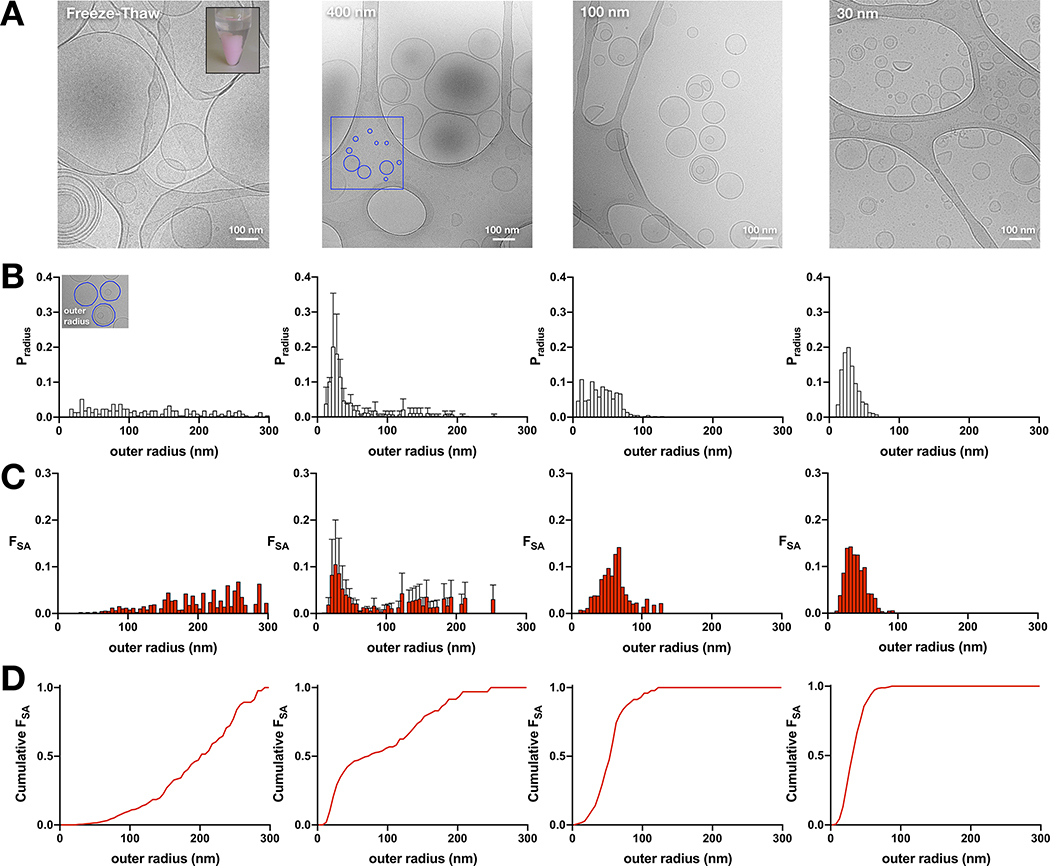
A 2:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine: 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (POPE:POPG (Avanti Polar Lipids Inc., Alabaster AL)) in chloroform was dried under a N2 gas line whilst the vial was slowly spun by hand to ensure an even distribution. The sample was then resuspended in 1 mL of pentane before being dried again. Dialysis buffer (300 mM KCl, 20 mM citric acid, pH 4.5 using NaOH) was added to the lipids to a final concentration of 20 mg/mL. Next, 35 mM of 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate (CHAPS (Anatrace, Maumee OH)) detergent was added and the solution was sonicated in a cylindrical bath sonicator for 30–60 minutes, until the cloudy solution had become transparent. The sample was dialyzed in Slide-A-Lyzer cassettes (Thermo Scientific, Waltham MA), 10 kDa MWCO, for 2 days at 4°C in dialysis buffer which was changed every 10–12 hours. Once dialysis was complete, the empty liposomes were freeze-thawed seven times by incubating in a dry ice/ethanol bath for 5 min, followed by a room temperature water bath for 15 min, before being stored at room temperature until they were required for imaging. Note, thawing occurs above the phase transition temperature, previously reported to be ~19°C (15). This procedure results in large multilamellar vesicles that are turbid and settle at the bottom of the tube (Figure 4A). On the day of imaging, liposomes were extruded 21-times through filters with pore sizes of 30, 100 and 400 nm then applied to glow-discharged Lacey carbon suport films (ELECTRON MICROSCOPY SCIENCES, HATFIELD PA)in 3 μL drops, blotted, and plunged into liquid ethane using a Vitrobot system (FEI). Grids were imaged at 300 kV in a JEOL 3200 fs microscope with K2 Summit direct electron detector camera (Gatan). A magnification of 30,000 was used for the 30 nm and 100 nm samples, and a magnification of 15,000 used for the 400 nm sample. For analysis, the polygon selection tool in imageJ (20) was used to determine the radius of each liposome in nanometers in order to produce radius distributions. Note, all liposomes were analyzed include those lying on the carbon support. For each extrusion pore size, two distributions were obtained: an outer radius distribution and a total radius distribution. The outer radius amused all liposomes were unilamellar and so only recorded the radius of the outmost liposome in multilamellar liposomes. On the other hand, the total radius was the sum of all radii in multilamellar liposomes meaning the distribution was shifted slightly to the right with some liposomes having much larger radii.
Figure 4. Size distributions of the outer radii of extruded liposomes composed of 2:1 POPE:POPG lipids.
(A) Cryo-electron microscopy images of 2:1 POPE:POPG liposomes after seven cycles of freeze-thaw, or extrusion through 400 nm, 100 nm, or 30 nm filters. The inset in the freeze-thaw image shows the resultant large, multilamellar vesicles that are visible by eye and settle to the bottom of the tube (pink color is due to the addition of 0.1% PE-RhB). The red box in the 400 nm image highlights smaller vesicles identified on the carbon grid. (B) Probability distributions of the liposome outer radii, Pradius, for the corresponding samples. (C) Probability distributions of the fractional surface area, FSA, for the corresponding samples. (D) Cumulative sum of FSA. The 30 nm and 100 nm distributions are presented in Table 1 while the 400 nm distribution was presented previously (3).
2.3. Protein purification and reconstitution
The methods of protein purification were carried out as described previously (1, 3). Purified C85A/H234C CLC-ec1 with a C-terminal hexahistidine tag in 150 mM NaCl, 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS), pH 7.0 and 5 mM n-Decyl-β-D-Maltopyranoside, DM, (Anatrace, Maumee OH) was labelled with Cy5-malemide (Lumiprobe, Hunt Valley MD) for 10 minutes followed by quenching with 100 mM cysteine. Free dye was removed by binding the protein to a cobalt affinity column, with successive washes. The labelled protein was eluted with 400 mM imidazole, and then the eluate was run on a ~2.5 mL Sephadex G-50 (GE Lifesciences) size exclusion column to separate the imidazole and allow for quantification of the protein labeling yield by measuring the absorbances at 280 nm and 655 nm. The average labeling yield was PCy5 = 0.65 ± 0.01 (mean ± std, n = 3). The fluorescently labelled protein in DM micelles was added to 20 mg/mL 2:1 POPE:POPG solubilized in 300 mM KCl, 20 mM citrate, pH 4.5 adjusted with NaOH and 35 mM CHAPS. The samples were dialyzed in 10 kDa MWCO cassettes, against a 2000-fold excess volume of the same buffer without the detergent, in the dark, at 4°C, with 3–4 buffer changes overall, with changes every 8–12 hours. The last round of dialysis was carried out at room temperature.
Calculating the reconstitution density is carried out as follows. CLC-ec1 C85A/H234C including the hexahistidine tag has a molecular weight of 51,997 g/mole, MWPOPE = 717.996 g/mole and MWPOPG = 770.989 g/mole. Therefore, a proteoliposome sample reconstituted at 1 μg/mg in 2:1 POPE/POPG lipids corresponds to a mole fraction of:
Assuming a 50% mole fraction recovery as was previously observed (1), then our observed mole fraction is χobserved = 0.7 × 10−5 subunits/lipid.
2.4. Single-molecule TIRF microscopy of fluorescently labelled proteoliposomes
Co-localization microscopy
Studies were carried out as previously described (1). Briefly, the primary amine on POPE was conjugated to Alexa Fluor 488 Carboxylic Acid, 2,3,5,6-Tetrafluorophenyl (TFP) ester (Thermo Fisher Scientific) during the POPE/POPG/CHAPS stage, at room temperature and pH 8.0. After 4 hours of incubation, excess free dye was quenched with Tris, and then the lipids were used in the reconstitution procedure as usual, with free dye removed during dialysis. After freeze-thaw and extrusion, liposomes were applied to the slide, and first imaged with the longer wavelength 637 nm laser to detect the Cy5 labelled protein. Next, we imaged the liposomes in the same field using the 488 nm laser. Multicolor tetraSpeck 0.1 μm beads (Thermo Fisher Scientific) or co-labelled liposomes were loaded onto a separate lane for imaging in both channels, in order to create an independent mapping file to determine co-localization using a custom-built MATLAB software (21).
Photobleaching analysis
Photobleaching analysis was carried out as described previously (1). After completion of dialysis, multilamellar vesicles were obtained by seven cycles of freezing (dry-ice/ethanol bath for 5 min or −80°C, 10 minutes) and thawing (room temperature water bath, 10–15 minutes) of the proteoliposome samples. Sodium azide (0.02% w/v) was added to each sample which was then incubated at room temperature for 3–4 days before imaging. Immediately prior to imaging, proteoliposomes were sequentially extruded using 0.4, 0.1, and 0.03 micron nucleopore filters (GE Lifesciences, Maidstone UK) using a LiposoFast Basic extruder (Avestin Inc., Ottawa, Canada). Imaging of fluorescent spots and analysis of steps taken before complete photobleaching was performed as described before (1). Note that the photobleaching studies are always carried out independently from the co-localization studies.
2.5. Analysis of membrane fusion by freeze-thaw using FRET
Membrane fusion during freeze-thaw was quantified by measuring the increase in Förster Resonance Energy Transfer (FRET) signal between liposome populations that were distinctly labelled with donor or acceptor fluorophores. 2:1 POPE/POPG vesicles were prepared with either 0.02% donor 18:1 PE-NBD (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl (donor)), 0.1% acceptor 18:1 PE-RhB (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)) or both (Avanti Polar Lipids Inc.) as described previously. Samples were dialyzed in individual containers to prevent cross-contamination. Fluorescence emission spectra was measured using a Fluorolog 3–22 (Horiba, Kyoto, Japan) for 0.2 mL samples in a quartz cuvette, at room temperature (~22°C). The excitation wavelength was set to 463 nm, while the emission was collected between 475–670 nm, with 2 nm silt-widths on either end. The FRET signal was quantified as FRET = fA/(fA+fD), using the peak fluorescence intensity (f) in the acceptor emission range, and normalized between the NBD donor (set to 0) and NBD/RhB positive control (set to 1).
3. Theory
In preparing a population of liposomes, one starts from the freeze-thawed multilamellar membrane state and then extrudes the sample through filters with precisely defined pore sizes. This action causes segments of membranes to be pinched off to form intact, mainly unilamellar vesicles. In this procedure, the protein residing within the membrane is randomly captured into vesicles following a Poisson process. This means that liposome occupancy is expected to follow a Poisson distribution, however in reality, experimental samples are heterogenous, which complicates the reconstitution statistics. Heterogeneity arises from diversity in the protein species that are incorporated into the membrane, as well as polydispersity in the surface area of liposomes that form the compartments. In this study, we will focus on outlining the impact of liposome heterogeneity on the resultant membrane protein occupancy distribution.
3.1. Liposome occupancy distribution with homogenous compartments
First consider the Poisson distribution for a homogenous system with a single protein species and one size of vesicle with radius r. The distribution is calculated as,
| (1) |
where n is the protein occupancy value, Fn is the fraction of vesicles with occupancy n, and λ is the Poisson parameter,
| (2) |
equal to the average occupancy and the variance. In reconstitution experiments, an experimentalist explicitly sets the number of protein subunits, Nsubunits = Nprotein, and number of lipids, Nlipids, in a sample for a defined density, or mole fraction of protein per lipid, χ,
| (3) |
Here, we have taken the molar ratio approximation for the mole fraction, which applies to dilute systems where the number of lipid molecules exceeds the number of protein particles in the system. It is advantageous to use as Nprotein and Nlipid values are experimentally set during reconstitution, and has been used when studying reactions involving detergent micelle solubilized membrane proteins (22). The approximation begins to fail at molar ratios greater than 10−2 subunits/lipid, where the error in the approximation is > 1% (Supplementary Figure 1). Still, for dilute reconstitution densities less than 1 protein molecule per hundred lipids, which is applicable to most experimental reconstitution conditions, the molar ratio and mole fraction can be used interchangeably.
For many lipids, the surface area of a lipid in the bilayer, Alipid, has been experimentally determined (23), and so the total bilayer surface area, SAtotal, can be calculated as:
| (4) |
With this, Nliposomes can be determined,
| (5) |
Substituting (3) and (5) into (2) yields,
| (6) |
3.2. Liposome occupancy distribution with heterogeneous compartment
For a heterogeneous population of liposomes with a single protein species, the reconstitution distribution can still be solved for analytically as long as the probability distribution of liposomes sizes, Pradius(ri), is known. Here, we consider that each sub-population of liposomes with radius ri, reduces to an independent homogeneous Poisson process with a different parameter that depends on the radius of the sub-population:
| (7) |
Note, the number of liposomes depends on the probability of liposomes of that size occurring in the population. Also, the numbers of protein particles allocated to those liposomes depends on the fraction of the total membrane area in that sub-population (Figure 1). The occupancy distribution for each sub-population is therefore,
| (8) |
and the resultant occupancy statistics across all liposomes is:
| (9) |
Figure 1. The effect of liposome heterogeneity on membrane protein capture statistics.
A ‘toy’ distribution of liposomes vs. radius, ri. In this example, Nliposozes = 31, with each liposome depicted to-scale as a representation of the surface area, . The inset shows the probability distribution for the same population of liposomes. CLC-ec1 monomers (Nsubunits = 31) are overlayed onto the total membrane area, representing the random assignment of subunit occupancy in vesicles. CLC-ec1 has been enlarged ~100x for visualization in the large membrane. In this example, the total surface area is 4.1 × 10−6 nm2, with 1.4 × 107 lipids in the bilayer (Alipid = 0.6 nm2), amounting to χreconstituted = 2.3 × 10−6 subunits/lipid, or ~0.2 μg/mg. The cartoon illustrates how size heterogeneity promotes over-filling of larger vesicles and under-occupancy of small vesicles, at ‘Poisson-dilution’ densities.
Where the summation over i reflects all liposome sub-populations. The radius dependent Poisson parameter is equivalent to the homogenous form derived in equation (6). This is shown as follows:
| (10) |
Where Nliposome,total is the total number of liposomes in the population. To calculate this, we need to again consider the total bilayer surface area set in the experiments:
| (11) |
Equating this to (4) allows us to solve the total number of liposomes in the sample in a heterogeneous population:
| (12) |
Thus, the number of liposomes in each sub-population of radius ri is calculated as,
| (13) |
The number of protein particles that are available to partition into each sub-population of liposomes, is proportional to the fraction of membrane surface area occupied by Nliposomes(ri).
| (14) |
Where the fractional surface area is equal to:
| (15) |
Therefore,
| (16) |
Substituting (16) and (13) into (7) reduces to equation (6), but now with a variable radius, ri:
| (17) |
Where the average Poisson parameter over the entire liposome population is:
| (18) |
3.3. Numerical fraction of unoccupied vesicles
The fraction of unoccupied vesicles, F0,num, can be calculated by letting n = 0:
| (19) |
For the homogenous distribution, this reduces to a single exponential decay:
| (20) |
3.4. Fractional volume of unoccupied vesicles
The fraction of unoccupied vesicle volume can also be calculated, F0,vol. First, consider the volume trapped inside of the sub-population of liposomes with radius ri, Vliposomes(ri):
| (21) |
The total volume encapsulated in all liposomes is:
| (22) |
Therefore, the fraction of unoccupied volume is:
| (23) |
3.5. Simulations of membrane protein reconstitution
While the analytical solutions described above allow for predictions of single species incorporation into heterogeneous liposome populations, the calculations become more complicated once multiple protein species are introduced. This requires enumeration of the distribution of different species, treating each as an independent Poisson process that occurs in the same overlapping liposome population. The overall statistics depend on many joint probabilities of co-occupancy of various protein species, of which there are many combinations, making the calculation daunting. A simpler solution is offered by statistical simulation in MATLAB that carries out the random assignment of protein species into matrices representing different liposome sub-populations. Figure 1 shows a schematic of the simulation, which highlights effect of polydispersity in liposome sizes on the protein occupancy distribution. The details of the simulation method have been outlined previously and we refer the reader to these papers for further details on the simulation (1, 3). The key parameters in the simulation are all experimentally determined, including the area per lipid, Alipid = 0.6 nm2 (19); probability of subunit labeling, Pfluor; probability of non-specific subunit labeling, Pns measured in a cysteine-inaccessible construct; mole-fraction recovery, yield; liposome size distribution, Pradius(ri); and liposomes accessibility factor, bias = 4 (excludes 2.5–22.5 nm bins from Pradius(ri)), to model the exclusion of ~5 nm × 10 nm CLC-ec1 dimers from smaller liposomes. MATLAB files for the simulation program, and the analytical solution of the heterogeneous Poisson distribution are included as Supplementary Information. Note that the simulation results converge to the analytical results when a single protein species is considered (Figure 2).
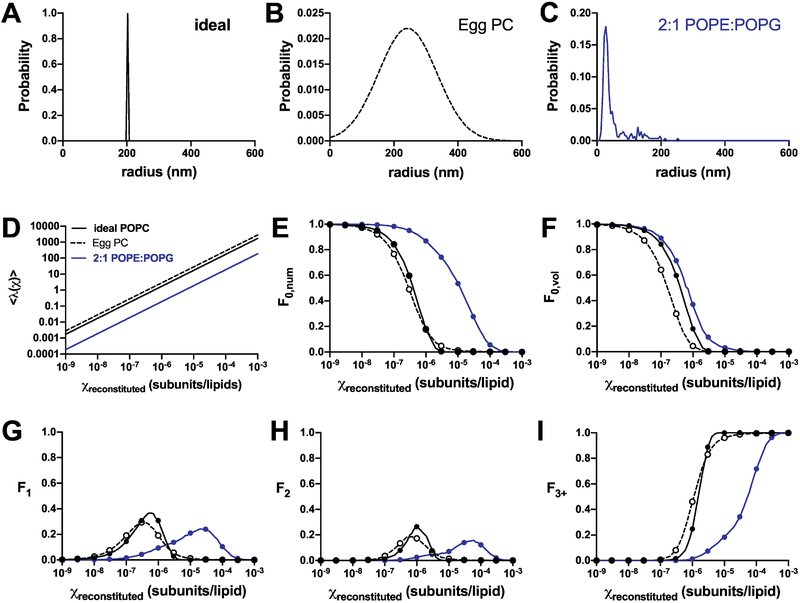
Figure 2. Reconstitution statistics for 400 nm extruded liposome populations.
Calculations of the statistics of independent protein subunits reconstituted into liposome populations with varying heterogeneity. (A) Model of ideally extruded, homogenous population with radius = 200 nm (black, solid). (B) Egg PC liposomes (2) following a gaussian distribution with μ = 243 nm and σ = 91 nm (black, dashed). (C) 2:1 POPE:POPG liposomes (Chadda et al., 2018), demonstrating smaller than expected sizes and a rightward tail (blue, solid). The color coding throughout the figure corresponds to results based on these three distributions. (D) Nliposomes as a function of the reconstitution density (constant Nprotein = 106). (E) Fraction of unoccupied vesicles, F0,num. (F) Fractional volume of unoccupied vesicles, F0,vol, corresponding to liposomal efflux studies. Fraction of vesicles containing (G) one, (H) two, or (I) more than three subunits. Both analytical solutions (circles) and statistical simulations (lines) are shown, indicating convergence of results. Note, the simulations are stochastic and not expected to be smooth.
4. Results
4.1. The effect of liposome heterogeneity on occupancy statistics
For single-component membranes, it is often assumed that the liposome population obtained from freeze-thaw and extrusion generates a uniform population with diameters matching the size of the extrusion pore (Figure 2A). Indeed, cryo-electron microscopy studies of 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) liposomes extruded through 50 nm pores show a distribution with a mean of 27 nm (24). Egg PC vesicles extruded through 400 nm pores was reported as “relatively homogeneous” with a distribution centered at 243 ± 91 nm (Figure 2B), as measured by freeze-fracture electron microscopy (2). On the other hand, lipid mixtures of E. coli polar lipid (EPL) extracts (4), or the synthetic mimic 2:1 POPE:POPG (3) yield a heterogenous distribution with a peak at 27.5 nm and a rightward tail of larger liposomes (Figure 2C). To examine how this affects the reconstitution statistics, we calculated the liposome occupancy distributions for the Poisson process of incorporating single protein subunits into liposomes, based on three examples of 400 nm extruded liposome size distributions: the idealized homogenous population, and experimental distributions of Egg PC or 2:1 POPE:POPG. Figure 2D shows the average Poisson parameter for each population, demonstrating how the ideal approximation provides a rough estimate for the occupancy statistics of the broad Egg PC distribution. This occurs because the number of liposomes is similar between these two populations (Supplementary Figure 2), arising from the symmetry of the normal distribution used to estimate the Egg PC data. For example, at χ = 10−6 subunits/lipid, and Nprotein = 106 subunits, Nliposomes,total is calculated as 6 × 105 and 4 × 105 for the respective populations. The overall liposome number is bounded by the total membrane area, and in the case of the broad Egg PC distribution, the smaller and larger vesicle areas add up to similar values as if the liposomes all had the mean radii. On the other hand, the 2:1 POPE:POPG sample is expected to have more liposomes, 5 × 106, since a significant proportion of the population are smaller in size. Along these lines, the fraction of occupied vesicles is similar for the ideal and Egg PC conditions (Figure 2E), while the the 2:1 POPE:POPG curve is shifted to the right, requiring much higher protein densities to saturate the liposomes. Examination of the fractional volume from unoccupied vesicles shows how these statistics are different than the numerical filling of liposomes (Figure 2F). The fractional volume is often measured during functional measurements of ion efflux from vesicles, and has been used to inform on membrane protein reconstitution statistics and protein stoichiometry (4, 7, 25). Here, the intra-liposomal volumes in the Egg PC population become accessible at lower densities compared to the other two distributions, due to the presence of larger liposomes that have a higher probability of becoming initially occupied (Supplementary Figure 3). Examining the protein occupancy statistics (F1, F2, F3+) demonstrates how heterogeneity in the 2:1 POPE:POPG distribution leads to a spreading of single and double occupancy states over a wider range of densities (Figure 2G,H), but the increased number of liposomes reduces the probability of saturation with more than three protein subunits (Figure 2I). Note that simplified approximations for the 2:1 POPE:POPG population, such as an ideal distribution at the mode, i.e. peak value, or a Gaussian distribution defined by the population mean and standard deviation, do not serve as accurate estimates due to the significant right-skewed characteristics of the experimental distribution (Supplementary Figure 4). For all cases in Figure 2, we have calculated the analytical solution (dots) in parallel to simulating the reconstitution process (lines) and there is complete agreement between the two methods since we are only considering a single, monomeric species of protein.
4.2. Testing the 400 nm 2:1 POPE:POPG liposome size distribution
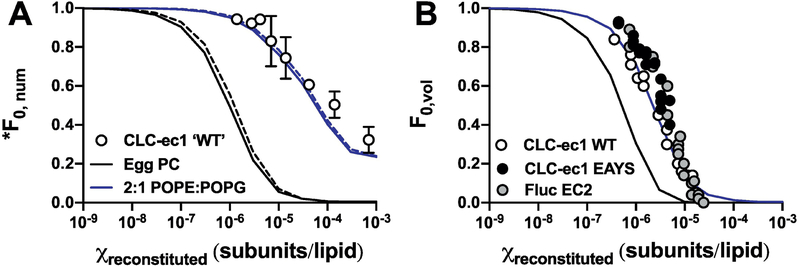
The previous findings that EPL and 2:1 POPE:POPG lipid bilayers extruded through 400 nm pores yield significant number of liposomes with radius ~ 25 nm is unexpected. To test whether this population agrees with experimental observations, we compared co-localization microscopy data of ‘WT’ CLC-ec1-Cy5 (i.e. C85A/H234C background) with AlexaFluor-488 labelled 400 nm extruded 2:1 POPE:POPG vesicles to the expected fraction of Cy5 unoccupied vesicles, *F0,num, simulated for the 400 nm extruded Egg PC or 2:1 POPE:POPG liposome distributions (Figure 3A). Note the fluorescent protein occupancy, *F0,num, differs from the total protein occupancy, F0,num, in that it only considers liposomes that are occupied by fluorescently labeled protein, and does not account for unlabeled, i.e. protein that is invisible by fluorescence microscopy. The experimental co-localization data agrees with the 2:1 POPE:POPG distribution, with 50% of the vesicles occupied at χ0.5 ~ 9 × 10−5 subunits/lipid in contrast to the Egg PC distribution that predicts a higher probability of filling at lower densities, with χ0.5 ~ 2 × 10−6 subunits/lipid. Next, we compared the occupancy data from functional measurements of liposomal efflux of the CLC-ec1 Cl−/H+ antiporter (4) and Fluc F− channel reported in the literature (7). In these “ion-dump” assays, liposomes are loaded with a high concentration of the transport ion, and then exchanged into a low ion concentration. Since the movement of the ion across the membrane is electrogenic for either protein, efflux is initiated by addition of valinomycin, which sets the membrane potential to zero allowing for the transported ion to flow down its concentration gradient. Vesicles that do not contain active protein are still ion-loaded, and addition of a membrane disrupting detergent releases the remaining amount of trapped ion. From this, a fractional volume of empty vesicles, F0,vol, can be experimentally measured. We examined three studies where F0,vol was reported as a titration of protein density and extrapolated the data: WT CLC-ec1, E148A/Y445S CLC-ec1 and the EC2 Fluc F− channel (4, 7). The data were compared to simulations of protein reconstitution into 400 nm extruded vesicles from Egg PC or 2:1 POPE:POPG membranes (Figure 3B). Each set of experimental data overlays with one another, independent of the protein construct. Furthermore, the data agrees with the 2:1 POPE:POPG liposome population, with χ0.5 ~ 5 × 10−6 subunits/lipid compared to χ0.5 ~ 7 × 10−7 subunits/lipid expected from an Egg PC distribution. Altogether, both methods of measuring liposome occupancy agrees with the presence of smaller liposomes indicated by the 2:1 POPE:POPG distribution, and not the idealized or Egg PC distributions.
Figure 3. A comparison of experiments and simulations for quantifying vesicle occupancy.
(A) The fraction of vesicles that do not contain Cy5 labeled CLC-ec1, *F0,num, measured by co-localization TIRF microscopy of ‘WT’, i.e. C85A/H234C CLC-ec1-Cy5 reconstituted in 2:1 POPE:POPG vesicles doped with POPE-AF488 (circles, mean ± s.e.m, n = 3 ((1) and new data)). Simulations of *F0,num based on 400 nm extruded 2:1 POPE:POPG liposomes (blue) or 400 nm extruded Egg PC liposomes (black), with KD = 2 × 10−8 subunits/lipids, yield = 0.5, and liposomes with r < 25 nm inaccessible to dimers. Solid lines show the total protein occupancy F0,num (i.e. Pfluor = 1.0 & Pns = 0) while the dotted lines represent the fluorescent protein occupancy, *F0,num (i.e. Pfluor = 0.7 & Pns = 0.1). (B) Fractional volume of inactive vesicles, F0,vol, measured in efflux assays using ion-sensitive electrodes. Experimental data extracted from the literature for WT CLC-ec1 (4), CLC-ec1 E148A/Y445S and Fluc EC2 (5) are shown as circles as circles. The simulation parameters are the same as in (A) and considers the total protein occupancy (Pfluor = 1.0 & Pns = 0).
4.3. Liposome size distributions as a function of extrusion pore size
Considering the 400 nm 2:1 POPE:POPG liposomes are much smaller than expected, we went on to examine what happens when these membranes are extruded through smaller pores. Figure 4A shows cryo-electron microscopy images of liposomes after freeze/thaw and following extrusion by 400 nm, 100 nm and 30 nm pores. Images of the freeze-thawed samples show the presences of smaller vesicles amongst multilamellar vesicles (MLVs). Since the thickness of the vitreous ice is much smaller than the reported 10 μm diameter of these vesicles (15), there is likely a size-exclusion effect biasing the observation of smaller vesicles in these preparations. Note that we can visualize the process of fusion in this lipid composition, because the large MLVs are visible by eye and will settle into one contained pellet at the bottom of the tube (inset, Figure 4A). Yet, these results indicate that small vesicles also exist and so we investigated whether they are capable of mixing with the larger membranes during the fusion procedure by measuring Förster Resonance Energy Transfer (FRET) within bilayers. 2:1 POPE:POPG vesicles were prepared including 0.02% PE-NBD, a FRET donor, and/or 0.1% PE-RhB, a FRET acceptor. NBD liposomes mixed with RhB liposomes show an increase in background FRET due to the proximity of fluorophores in different vesicles. However, upon freeze-thaw, the magnitude of the FRET signal converges to the value obtained for samples that were co-prepared with NBD/RhB, indicating ideal mixing between all membranes (Supplementary Figure 5).
The probability distributions of the outer radii of each vesicle (Figure 4B) and the fractional surface area (Figure 4C) are reported for each population. The mean ± standard deviation of outer radii of the freeze-thawed samples is (and 48% as MLVs). As discussed previously, this freeze-thawed distribution is not quantitative since larger liposomes are excluded from the vitreous ice. The average values of the extruded distributions decrease with pore size, with (13% MLVs), (17% MLVs) and (10% MLVs). Note that this analysis included counting liposomes that resided on the carbon grid, outside of the vitreous ice, for a complete representation of all vesicles in the population. The three extruded distributions of 2:1 POPE:POPG vesicles shows that the populations are not overwhelmingly different, and are all peaked around 25–30 nm. The major difference is the reduction of the larger liposome tail, resulting in increasingly homogenous populations of vesicles upon reducing the size of the extrusion pore. Analysis of the cumulative FSA as a function of increasing liposome radius, illustrates the reduction in heterogeneity in the liposomes with smaller extrusion sizes (Figure 4D). We also considered the amount of membrane in the MLVs by calculating the total radius distributions (Supplementary Figure 6). Here, the area from all bilayers within a MLV are added together and counted as a single liposome with larger radius. Since the fraction of multilamellar vesicles is small, this results in a nearly undetectable rightward shift of each distributions. The data for all of the liposome size distributions are reported in Tables 1 & 2.
Table 1.
2:1 POPE:POPG extruded vesicle distributions – outer radii.
| 30 nm | 100 nm | ||||
|---|---|---|---|---|---|
| r (nm) | SA (nm2) | P radius | FSA | Pradius | FSA |
| 0–5 | 7.85E+01 | 0 | 0 | 0 | 0 |
| 5–10 | 7.07E+02 | 0.001 | 0.000 | 0.047 | 0.001 |
| 10–15 | 1.96E+03 | 0.037 | 0.005 | 0.109 | 0.008 |
| 15–20 | 3.85E+03 | 0.137 | 0.039 | 0.047 | 0.007 |
| 20–25 | 6.36E+03 | 0.186 | 0.087 | 0.050 | 0.012 |
| 25–30 | 9.50E+03 | 0.200 | 0.140 | 0.103 | 0.036 |
| 30–35 | 1.33E+04 | 0.147 | 0.142 | 0.080 | 0.039 |
| 35–40 | 1.77E+04 | 0.097 | 0.126 | 0.059 | 0.038 |
| 40–45 | 2.27E+04 | 0.075 | 0.126 | 0.088 | 0.074 |
| 45–50 | 2.84E+04 | 0.044 | 0.092 | 0.079 | 0.083 |
| 50–55 | 3.46E+04 | 0.039 | 0.099 | 0.076 | 0.097 |
| 55–60 | 4.15E+04 | 0.014 | 0.044 | 0.053 | 0.081 |
| 60–65 | 4.91E+04 | 0.012 | 0.042 | 0.070 | 0.127 |
| 65–70 | 5.73E+04 | 0.008 | 0.033 | 0.068 | 0.142 |
| 70–75 | 6.61E+04 | 0.002 | 0.011 | 0.024 | 0.057 |
| 75–80 | 7.55E+04 | 0.001 | 0.004 | 0.015 | 0.041 |
| 80–85 | 8.55E+04 | 0 | 0 | 0.009 | 0.028 |
| 85–90 | 9.62E+04 | 0.001 | 0.006 | 0.006 | 0.021 |
| 90–95 | 1.08E+05 | 0.001 | 0.006 | 0.006 | 0.023 |
| 95–100 | 1.19E+05 | 0 | 0 | 0 | 0 |
| 100–105 | 1.32E+05 | 0 | 0 | 0.003 | 0.014 |
| 105–110 | 1.45E+05 | 0 | 0 | 0.006 | 0.031 |
| 110–115 | 1.59E+05 | 0 | 0 | 0 | 0 |
| 115–120 | 1.73E+05 | 0 | 0 | 0.003 | 0.019 |
| 120–125 | 1.89E+05 | 0 | 0 | 0 | 0 |
| 125–130 | 2.04E+05 | 0 | 0 | 0.003 | 0.022 |
| 130–135 | 2.21E+05 | 0 | 0 | 0 | 0 |
| 135–140 | 2.38E+05 | 0 | 0 | 0 | 0 |
| 140–145 | 2.55E+05 | 0 | 0 | 0 | 0 |
| 145–150 | 2.73E+05 | 0 | 0 | 0 | 0 |
| 150–155 | 2.92E+05 | 0 | 0 | 0 | 0 |
| 155–160 | 3.12E+05 | 0 | 0 | 0 | 0 |
| 160–165 | 3.32E+05 | 0 | 0 | 0 | 0 |
| 165–170 | 3.53E+05 | 0 | 0 | 0 | 0 |
| 170–175 | 3.74E+05 | 0 | 0 | 0 | 0 |
| 175–180 | 3.96E+05 | 0 | 0 | 0 | 0 |
| 180–185 | 4.19E+05 | 0 | 0 | 0 | 0 |
| 185–190 | 4.42E+05 | 0 | 0 | 0 | 0 |
| 190–195 | 4.66E+05 | 0 | 0 | 0 | 0 |
| 195–200 | 4.90E+05 | 0 | 0 | 0 | 0 |
| 200–205 | 5.15E+05 | 0 | 0 | 0 | 0 |
| 205–210 | 5.41E+05 | 0 | 0 | 0 | 0 |
| 210–215 | 5.67E+05 | 0 | 0 | 0 | 0 |
| 215–220 | 5.94E+05 | 0 | 0 | 0 | 0 |
| 220–225 | 6.22E+05 | 0 | 0 | 0 | 0 |
| 225–230 | 6.50E+05 | 0 | 0 | 0 | 0 |
| 230–235 | 6.79E+05 | 0 | 0 | 0 | 0 |
| 235–240 | 7.09E+05 | 0 | 0 | 0 | 0 |
| 240–245 | 7.39E+05 | 0 | 0 | 0 | 0 |
| 245–250 | 7.70E+05 | 0 | 0 | 0 | 0 |
| 250–255 | 8.01E+05 | 0 | 0 | 0 | 0 |
| 255–260 | 8.33E+05 | 0 | 0 | 0 | 0 |
| 260–265 | 8.66E+05 | 0 | 0 | 0 | 0 |
| 265–270 | 8.99E+05 | 0 | 0 | 0 | 0 |
| 270–275 | 9.33E+05 | 0 | 0 | 0 | 0 |
| 275–280 | 9.68E+05 | 0 | 0 | 0 | 0 |
| 280–285 | 1.00E+06 | 0 | 0 | 0 | 0 |
| 285–290 | 1.04E+06 | 0 | 0 | 0 | 0 |
| 290–295 | 1.08E+06 | 0 | 0 | 0 | 0 |
| 295–300 | 1.11E+06 | 0 | 0 | 0 | 0 |
Table 2.
2:1 POPE:POPG extruded vesicle distributions – total radii.
| 30 nm | 100 nm | 400 nm | |||||
|---|---|---|---|---|---|---|---|
| r (nm) | SA (nm2) | Pradius | FSA | Pradius | FSA | Pradius | FSA |
| 0–5 | 7.85E+01 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5–10 | 7.07E+02 | 0.001 | 0.000 | 0.047 | 0.001 | 0 | 0 |
| 10–15 | 1.96E+03 | 0.036 | 0.004 | 0.109 | 0.005 | 0.038 | 0.001 |
| 15–20 | 3.85E+03 | 0.136 | 0.031 | 0.047 | 0.005 | 0.101 | 0.018 |
| 20–25 | 6.36E+03 | 0.181 | 0.069 | 0.050 | 0.008 | 0.197 | 0.077 |
| 25–30 | 9.50E+03 | 0.195 | 0.110 | 0.103 | 0.025 | 0.181 | 0.099 |
| 30–35 | 1.33E+04 | 0.135 | 0.107 | 0.079 | 0.027 | 0.114 | 0.081 |
| 35–40 | 1.77E+04 | 0.089 | 0.094 | 0.050 | 0.022 | 0.046 | 0.050 |
| 40–45 | 2.27E+04 | 0.067 | 0.090 | 0.079 | 0.046 | 0.035 | 0.034 |
| 45–50 | 2.84E+04 | 0.038 | 0.064 | 0.062 | 0.044 | 0.032 | 0.030 |
| 50–55 | 3.46E+04 | 0.038 | 0.078 | 0.070 | 0.062 | 0.019 | 0.017 |
| 55–60 | 4.15E+04 | 0.016 | 0.040 | 0.044 | 0.047 | 0.017 | 0.015 |
| 60–65 | 4.91E+04 | 0.021 | 0.061 | 0.053 | 0.066 | 0.004 | 0.005 |
| 65–70 | 5.73E+04 | 0.016 | 0.053 | 0.050 | 0.073 | 0.008 | 0.007 |
| 70–75 | 6.61E+04 | 0.009 | 0.037 | 0.021 | 0.035 | 0.010 | 0.019 |
| 75–80 | 7.55E+04 | 0.002 | 0.010 | 0.029 | 0.056 | 0.011 | 0.008 |
| 80–85 | 8.55E+04 | 0.003 | 0.016 | 0.015 | 0.032 | 0.011 | 0.014 |
| 85–90 | 9.62E+04 | 0.002 | 0.013 | 0.009 | 0.022 | 0.006 | 0.020 |
| 90–95 | 1.08E+05 | 0.003 | 0.020 | 0.018 | 0.048 | 0.005 | 0.016 |
| 95–100 | 1.19E+05 | 0.002 | 0.017 | 0.006 | 0.018 | 0.007 | 0.009 |
| 100–105 | 1.32E+05 | 0.002 | 0.012 | 0.009 | 0.030 | 0.011 | 0.022 |
| 105–110 | 1.45E+05 | 0.002 | 0.013 | 0.009 | 0.033 | 0.007 | 0.011 |
| 110–115 | 1.59E+05 | 0.002 | 0.022 | 0.009 | 0.036 | 0.001 | 0.009 |
| 115–120 | 1.73E+05 | 0 | 0 | 0.009 | 0.039 | 0.007 | 0.013 |
| 120–125 | 1.89E+05 | 0.001 | 0.009 | 0.003 | 0.014 | 0.025 | 0.048 |
| 125–130 | 2.04E+05 | 0.002 | 0.028 | 0.006 | 0.031 | 0.007 | 0.015 |
| 130–135 | 2.21E+05 | 0 | 0 | 0.003 | 0.017 | 0.007 | 0.016 |
| 135–140 | 2.38E+05 | 0 | 0 | 0.006 | 0.035 | 0.014 | 0.035 |
| 140–145 | 2.55E+05 | 0 | 0 | 0 | 0 | 0.011 | 0.028 |
| 145–150 | 2.73E+05 | 0 | 0 | 0 | 0 | 0.011 | 0.030 |
| 150–155 | 2.92E+05 | 0 | 0 | 0 | 0 | 0.001 | 0.016 |
| 155–160 | 3.12E+05 | 0 | 0 | 0 | 0 | 0.007 | 0.023 |
| 160–165 | 3.32E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 165–170 | 3.53E+05 | 0 | 0 | 0 | 0 | 0.007 | 0.026 |
| 170–175 | 3.74E+05 | 0 | 0 | 0 | 0 | 0.004 | 0.014 |
| 175–180 | 3.96E+05 | 0 | 0 | 0.003 | 0.030 | 0 | 0 |
| 180–185 | 4.19E+05 | 0 | 0 | 0 | 0 | 0.007 | 0.030 |
| 185–190 | 4.42E+05 | 0 | 0 | 0 | 0 | 0.004 | 0.016 |
| 190–195 | 4.66E+05 | 0 | 0 | 0 | 0 | 0.007 | 0.034 |
| 195–200 | 4.90E+05 | 0 | 0 | 0 | 0 | 0.004 | 0.018 |
| 200–205 | 5.15E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 205–210 | 5.41E+05 | 0 | 0 | 0 | 0 | 0.004 | 0.020 |
| 210–215 | 5.67E+05 | 0 | 0 | 0.003 | 0.042 | 0.001 | 0.031 |
| 215–220 | 5.94E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 220–225 | 6.22E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 225–230 | 6.50E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 230–235 | 6.79E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 235–240 | 7.09E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 240–245 | 7.39E+05 | 0 | 0 | 0.003 | 0.055 | 0 | 0 |
| 245–250 | 7.70E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 250–255 | 8.01E+05 | 0 | 0 | 0 | 0 | 0.004 | 0.029 |
| 255–260 | 8.33E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 260–265 | 8.66E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 265–270 | 8.99E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 270–275 | 9.33E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 275–280 | 9.68E+05 | 0 | 0 | 0 | 0 | 0 | 0 |
| 280–285 | 1.00E+06 | 0 | 0 | 0 | 0 | 0 | 0 |
| 285–290 | 1.04E+06 | 0 | 0 | 0 | 0 | 0 | 0 |
| 290–295 | 1.08E+06 | 0 | 0 | 0 | 0 | 0 | 0 |
| 295–300 | 1.11E+06 | 0 | 0 | 0 | 0 | 0 | 0 |
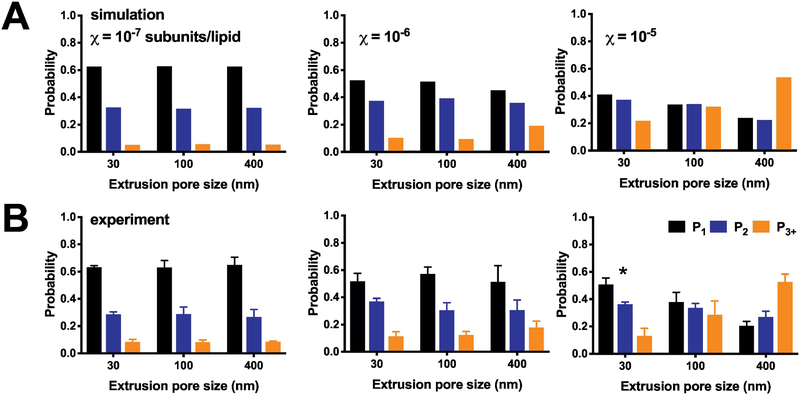
4.4. Photobleaching distributions of protein occupancy
To examine how the reduction of liposome heterogeneity affects the reconstitution statistics, we carried out experiments of single-molecule photobleaching analysis of CLC-ec1-Cy5 in 2:1 POPE/POPG liposomes extruded through the three different pore sizes (Figure 5). At low densities, χreconstituted = 10−6 & 10−7 subunits/lipid, there is no change in the photobleaching probability distributions across the different extrusions. This is because these densities lie below the ‘Poisson-dilution’ limit, where there is a near zero probability of a vesicle being occupied by more than one protein (Figure 2D). However, this also shows that the probability distribution, and monomer-dimer populations, are independent of the extrusion method. On the other hand, extrusion of the highest density sample, χreconstituted = 10−5 subunits/lipid, is dependent on the extrusion pore size. At this density, liposomes contain many protein particles, and so reducing the number of larger liposomes in the population allows the protein to distribute across more vesicles. A direct comparison of the simulations with this new experimental data (Supplementary Figure 7) shows the overall agreement and predictive power of the simulation, provided the liposome size distribution is experimentally measured.
Figure 5. Photobleaching probability distributions as a function of liposome size.
(A) Simulations of the expected (P1, P2, P3+) photobleaching distribution at densities of χ = 10−7, 10−6 and 10−5 subunit/lipid, using the 2:1 POPE:POPG liposome size distributions presented in Figure Simulations parameters include fluorophore labeling yields Pfluor = 0.7 & Pns = 0.1, reconstitution yield = 1, monomer-dimer equilibrium KD = 1 × 10−8 subunits/lipids, and liposomes with r < 25 nm inaccessible to dimers. Note that the simulation densities used here correspond to the expected observed mole fraction densities, assuming a recovery yield = 0.5. (B) Experimental photobleaching distributions of ‘WT’ CLC-ec1-Cy5 in 2:1 POPE:POPG liposomes as a function of protein density and extrusion pore size. Data shown as mean ± sem, n = 3–4. Experimental data is statistically compared to the simulated distributions by the chi-square test showing no significant discrepancies, except the 30 nm extruded 10−5 experiment, which is significantly different with p < 0.0164.
4.5. Extrusion through 30 nm pores expands the dynamic range of discriminating between monomer and dimer reconstitution statistics
Previously, we have shown that photobleaching analysis of subunit-capture statistics into liposomes can be used to inform on the populations of protein stoichiometry, from which information about equilibrium reactions in the membranes can be obtained (1). For instance, CLC-ec1 behaves in a monomer-dimer equilibrium that can be followed by changes in the photobleaching probability distribution as a function of the density. The power of this approach depends on the ability for protein species - monomers, dimers or other oligomeric forms - to be captured independently into different liposomes. Over-filling of liposomes at higher densities limits the ability to detect differences between protein populations limiting the dynamic range of the experiment. One possible solution is to use a smaller liposome population that will allow for the protein to distribute across more vesicles to allow for better resolution of the monomer vs. dimer statistics. Figure 6 shows the expected ideal monomer (Figure 6A) and ideal dimer (Figure 6B) occupancy statistics for the three distributions of 2:1 POPE:POPG liposomes. Here, ideal refers that the protein species are non-reactive, i.e. there is no affinity to form a complex, thus mapping out the monomer and dimer end-points. To calculate the fraction of dimer (FDimer) in the population, we previously used least-squares analysis to fit the experimental data between the monomer and dimer models (1) or experimental controls (3). The ability to resolve differences in FDimer depends on the scalar distance between the endpoints. Figure 6C shows the maximum distance, Rmax, between the monomer and dimer states, defined by the following experimental observables: P1, P2, P3+ and *F0,num. For example, when considering P1 and P2, then . In our previous analysis, we were able to estimate FDimer at densities < 10−5 subunits/lipid when considering P1, P2, and P3+, indicating that the cutoff for Rmax must be greater than 0.3. The analysis shows that P1 alone can be used to estimate FDimer at dilute densities, but resolution is increased by adding in P2. Using the smaller extrusion sizes, 100 nm and 30 nm, increase Rmax in the critical range of 10−7 < χ < 10−5 subunits/lipid densities. Adding in P3+ and *F0,num also increases the resolution, but the Rmax threshold is only achieved with the 30 nm liposome population for the χ = 10−5 density. This analysis shows the benefit of using 100 nm and 30 nm extruded populations to expand the dynamic range for studying monomer-dimer reactions in membranes.
Figure 6. The dynamic range of single-molecule fluorescence microscopy measurements of subunits occupancy as a function of liposome size.
Simulation of subunit capture of CLC-ec1 (fluorescent labeling yields: Pfluor = 0.7 & Pns = 0.1) as a function of reconstituted protein density χ subunits/lipid, assuming 100% recovery (yield = 1). *F0,num – fraction of unoccupied vesicles by co-localization microscopy. P1, P2, P3+ – single molecule photobleaching probabilities of fluorophore occupied vesicles. Ideal (A) monomers (KD = 10100 subunits/lipid) and (B) dimers (KD = 10−100 subunits/lipid), liposomes r < 25 nm as inaccessible to protein. Ideal refers to species that are non-reactive, i.e. there is no additional affinity for association. (C) The scalar distance between the two points defining the monomer and dimer states: where Pi corresponds to P1, P2, P3+ or *F0,num. The dotted line represents the Rmax cutoff for estimating the fraction of dimer from least-squares analysis.
5. Discussion
In this study, we examine how the liposome population, as a function of size and heterogeneity, affects the reconstitution statistics of membrane proteins into vesicles. Previously, we found that liposomes obtained by extruding freeze-thawed 2:1 POPE:POPG membranes through 400 nm filters generate a heterogeneous size distribution containing smaller liposomes with peak radii around 25 nm and a long tail of larger vesicles (3), in agreement with results reported for membranes derived from E. coli polar lipids (4). This heterogeneity spreads out the filling statistics, and protein occupancy across higher mole fraction densities. Methods of measuring membrane protein reconstitution statistics by proteoliposome efflux assays, co-localization microscopy, and single-molecule photobleaching analysis agree with simulations using the experimental 2:1 POPE:POPG distributions. Extrusion of these membranes through smaller 100 & 30 nm pores reduces the rightward tail, making the liposome population more homogeneous. Furthermore, single-molecule photobleaching analysis of protein reconstitution in these populations correspond to the expected change in the size distributions. These results demonstrate that knowledge of the experimental liposome size distribution is sufficient to predict the statistics of protein reconstitution into vesicles. This information is important in experimental design of single-molecule studies of membrane protein structure and function, or to extract information about assembly reactions, such as oligomerization (1). In turn, this paper provides key benchmarks for informing on how membrane proteins reconstitute into a synthetic mimic of the E. coli membrane, a standard lipid composition for functional assessment of membrane protein structure and function.
At first consideration, it is surprising that EPL and 2:1 POPE/POPG membranes extruded through 400 nm pores generate a population of 25–30 nm liposomes. However, this has been observed often in liposome populations extruded through filters with pore sizes 200 nm or larger. This effect appears to be independent of the liposome composition, with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) (16) and 1,2-dioleoyl-sn-glycero-3-phosphoglycerol (DOPG) (26) showing a similar effect. Smaller vesicles are also observed in the freeze-fracture micrographs of 400 nm egg PC liposomes, although the overall distribution is described as “relatively homogeneous” (2). In fact, the large standard deviation reported for this distribution indicates that the population contains high polydispersity. In addition, smaller vesicles are observed across different methods of liposome preparation, including direct hydration of dried lipids, or by solubilization in detergent followed by dialysis. It is possible that these populations are dependent on particular salt and buffer concentrations within the sample, as so it is always worthwhile to check the liposome size distributions when experimental conditions change.
There are several ways in which smaller liposomes may be introduced into a population. One possibility is that the these vesicles are carried along from dialysis and do not participate in fusion during the freeze-thaw procedure. However, our measurements of FRET show that all membranes undergo fusion, with ideal mixing for the 2:1 POPE:POPG condition (Supplementary Figure 2). Another option is that the smaller vesicles form during fusion, which could occur by freezing-induced fragmentation. During the freeze-thaw step, smaller liposomes can be derived due to the breakage of small membranes that reform into distinct vesicles. This has been demonstrated to occur form dioleoyl-phosphatidylcholine, DOPC membranes (27) as well as mixtures of DOPC and dioleoyl-phosphatidic acid, DOPA (28). We do observe smaller liposomes in cryo-EM images of our freeze-thawed samples (Figure 4), supporting this option. However, it is important to note that we are certainly biasing the observation by the act of freezing the sample in vitreous ice, as the ice is only 100–200 nm thick, and will selectively capture the smaller vesicles within the sample. Again, our FRET measurements indicate that the smaller vesicles participate in fusion, indicating that if fragmentation occurs, it does not prevent the mixing of membranes. Finally, these smaller vesicles may arise during the physical act of extrusion. This could occur if the elasticity of the membrane is altered, leading to the extrusion of fragments of membrane that are much smaller than expected. All of these possibilities may be contributing factors to why the liposome size distributions contain vesicles that are much smaller than expected in our condition of 2:1 POPE:POPG membranes.
Upon initial consideration, the observation of smaller vesicles in the freeze-thawed samples seems to contradict our assumption that freeze-thawed membranes serve as a model for “infinitely” large bilayers. However, it is unlikely that smaller vesicles in the freeze-thawed sample will affect the reconstitution statistics or the mole fraction density of the underlying equilibrium reactions. This is because of the square dependency of the membrane area relative to the liposome radius. Consider an extreme population where 99% of liposomes have a radius of 100 nm, and 1% of liposomes have a radius of 5 μm. Surprisingly, this means that 96% of the membrane area is contained in the large vesicle population, even though the overwhelming majority of liposomes are smaller. This also means that 96% of the protein particles will partition into the larger vesicles, and so the mole fraction for the reaction equilibrium remains unchanged. This is another example of larger vesicles acting as protein sinks. In this case, we take advantage of this and anchor the membrane protein density within the MLV membranes despite the presence of smaller vesicles within the overall liposome population.
With the 400 nm and 100 nm extruded liposomes containing a large component of smaller vesicles already, the 30 nm extruded liposome population offers several benefits for the study of membrane proteins in proteoliposomes. First, it generates a nearly homogeneous population of vesicles, which is desirable for quantitative measurements of transport function (4). However, these vesicles are small, and the membranes are highly curved which can have an influence on protein activity. Yet, it is also important to keep in mind that these smaller vesicles are present in the larger extruded liposome populations, so these effects are already included in the heterogeneous proteoliposome samples. Second, it increases the number of liposomes in the sample, and as a result, extends the ‘Poisson-dilution’ range with single or zero occupancy prevailing up to higher densities of 10−5 subunits/lipid. This can be useful for single-molecule studies of membrane proteins. By extruding samples through 30 nm filters, it is possible to examine singly-occupied vesicles at 10-fold higher density. This also offers an ability to increase the efficiency of collecting single-molecule microscopy data, for instance dynamic FRET trajectories mapping conformational changes (29), or single-vesicle functional assays (30). One can increase the protein density while maintaining a high probability that liposomes contain only a single-molecule. Third, it increases the dynamic range to discriminate between monomer and dimer populations in the freeze-thawed multilamellar membrane, since the chance of random co-capture of subunits is reduced. However, it is important to consider how curvature during extrusion may also impact the subunit-capture approach. In general, our results indicate that extrusion acts as a passive capture approach and does not affect the characteristics of the reaction in the MLV membrane. At low densities, we see no significant differences the photobleaching probability distributions of CLC-ec1-Cy5 as a function of extrusion pore size. However, at our highest density of χ = 10−5 subunits/lipid, we observe the 30 nm probability distribution is slightly shifted to contain more monomers. One possibility is that the samples also contain weaker higher-order assemblies that are not reflected in the models, and that these are dispersed during the 30 nm extrusion. In any case, our results indicate that at dilute densities, the 30 nm liposome population provides a suitable alternative that preserves information about the underlying monomer-dimer equilibrium reaction. Therefore, with proper consideration about the size and curvature, extrusion through 30 nm pores offers an option for obtaining a homogeneous distribution for 2:1 POPE:POPG liposomes, that may be useful for studying membrane protein structure, dynamics and function.
6. Conclusion
In conclusion, it is important to understand the statistics of membrane protein reconstitution into liposomes that are generally used for studies of membrane protein structure and function. While the commonly used composition of 2:1 POPE:POPG, a mimic of the E. coli polar lipids composition, yields an unexpectedly smaller population of liposomes, the heterogeneity in the liposome sizes does not affect the ability to calculate the expected liposome occupancy distribution. Single-molecule microscopy data describing the (i) co-localization fraction of CLC-ec1-Cy5 with AF488-labeled vesicles (ii) and single-molecule photobleaching distributions of CLC-ec1-Cy5, as well as (iii) functional studies of the inactive fraction of vesicles in transport assays of CLC-ec1 or the Fluc F- channel, all agree with the calculated liposome occupancy distribution based on the heterogeneous 400 nm 2:1 POPE:POPG liposome population. Therefore, complete description of liposome occupancy can be calculated for any system provided the liposome size distribution has been measured by cryo-EM or other methods. We recommend proper consideration of this when changing lipid composition in reconstitution studies, as unexpected changes in the size distribution can arise.
Supplementary Material
Highlights.
Analytical calculations and simulations of membrane protein occupancy in heterogeneous liposome populations
Measurement of 2:1 POPE:POPG liposome size distributions by cryo-electron microscopy as a function of extrusion pore size
Single-molecule photobleaching analysis of the CLC-ec1 Cl−/H+ antiporter as a function of extrusion pore size
Demonstration that 30 nm liposomes expand the ‘Poisson-dilution’ range for improvement on resolution of protein structure and function assays in liposomes
Acknowledgements
We acknowledge Jonathan Remis at the Structural Biology Facility at Northwestern University for help in imaging of liposome samples. The Structural Biology Facility is partially supported by the R.H. Lurie Comprehensive Cancer Center of Northwestern University.
Funding
This work was supported by the National Institute of General Medical Science, National Institutes of Health [grant numbers R01GM120260, R21GM126476].
Abbreviations
- CHAPS
3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate
- DM
n-Decyl-β-D-Maltopyranoside
- DOPA
1,2-dioleoyl-sn-glycero-3-phosphate
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- POPC
1-palmitoyl-2-oleoyl-glycero-3-phosphocholine
- POPE
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol)
- MOPS
3-(N-morpholino)propanesulfonic acid
- CLC-ec1
Cl−/H+ antiporter E. coli homologue 1
- Cy5
cyanine 5
- Cryo-EM
cryogenic electron microscopy
- E. coli
Escherichia coli
- MWCO
molecular weight cut-off
- EPL
E. coli polar lipids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chadda R, Krishnamani V, Mersch K, Wong J, Brimberry M, Chadda A, et al. The dimerization equilibrium of a ClC Cl(−)/H(+) antiporter in lipid bilayers. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986;858(1):161–8. [DOI] [PubMed] [Google Scholar]
- 3.Chadda R, Cliff L, Brimberry M, Robertson JL. A model-free method for measuring dimerization free energies of CLC-ec1 in lipid bilayers. J Gen Physiol. 2018;150(2):355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walden M, Accardi A, Wu F, Xu C, Williams C, Miller C. Uncoupling and turnover in a Cl−/H+ exchange transporter. J Gen Physiol. 2007;129(4):317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller C RE. Reconstitution of Membrane Transport Functions In: OB RD, editor. General Principles and Procedures The Receptors (A Comprehensive Treatise). 1. Boston, MA: Springer; 1979. p. 1–31. [Google Scholar]
- 6.Stockbridge RB, Tsai MF. Lipid reconstitution and recording of recombinant ion channels. Methods Enzymol. 2015;556:385–404. [DOI] [PubMed] [Google Scholar]
- 7.Stockbridge RB, Robertson JL, Kolmakova-Partensky L, Miller C. A family of fluoride-specific ion channels with dual-topology architecture. Elife. 2013;2:e01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cymer F, von Heijne G, White SH. Mechanisms of integral membrane protein insertion and folding. J Mol Biol. 2015;427(5):999–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagawa Y, Racker E. Partial Resolution of Enzymes Catalyzing Oxidative Phosphorylation .25. Reconstitution of Vesicles Catalyzing P-32i - Adenosine Triphosphate Exchange. J Biol Chem. 1971;246(17):5477–&. [Google Scholar]
- 10.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–52. [DOI] [PubMed] [Google Scholar]
- 11.Bangham AD. Model membranes. Chem Phys Lipids. 1972;8(4):386–92. [DOI] [PubMed] [Google Scholar]
- 12.Szoka F Jr., Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9:467–508. [DOI] [PubMed] [Google Scholar]
- 13.Mayer LD, Hope MJ, Cullis PR, Janoff AS. Solute Distributions and Trapping Efficiencies Observed in Freeze-Thawed Multilamellar Vesicles. Biochimica Et Biophysica Acta. 1985;817(1):193–6. [DOI] [PubMed] [Google Scholar]
- 14.Costa AP, Xu XM, Burgess DJ. Freeze-Anneal-Thaw Cycling of Unilamellar Liposomes: Effect on Encapsulation Efficiency. Pharm Res-Dordr. 2014;31(1):97–103. [DOI] [PubMed] [Google Scholar]
- 15.Pozo Navas B, Lohner K, Deutsch G, Sevcsik E, Riske KA, Dimova R, et al. Composition dependence of vesicle morphology and mixing properties in a bacterial model membrane system. Biochim Biophys Acta. 2005;1716(1):40–8. [DOI] [PubMed] [Google Scholar]
- 16.Hallett FR, Watton J, Krygsman P. Vesicle Sizing - Number Distributions by Dynamic Light-Scattering. Biophysical Journal. 1991;59(2):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson F, Hunt CA, Szoka FC, Vail WJ, Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979;557(1):9–23. [DOI] [PubMed] [Google Scholar]
- 18.Chadda R, Robertson JL. Measuring Membrane Protein Dimerization Equilibrium in Lipid Bilayers by Single-Molecule Fluorescence Microscopy. Methods Enzymol. 2016;581:53–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murzyn K, Rog T, Pasenkiewicz-Gierula M. Phosphatidylethanolamine-phosphatidylglycerol bilayer as a model of the inner bacterial membrane. Biophys J. 2005;88(2):1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman LJ, Gelles J. Multi-wavelength single-molecule fluorescence analysis of transcription mechanisms. Methods. 2015;86:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming KG. Standardizing the free energy change of transmembrane helix-helix interactions. J Mol Biol. 2002;323(3):563–71. [DOI] [PubMed] [Google Scholar]
- 23.Kucerka N, Kiselev MA, Balgavy P. Determination of bilayer thickness and lipid surface area in unilamellar dimyristoylphosphatidylcholine vesicles from small-angle neutron scattering curves: a comparison of evaluation methods. Eur Biophys J. 2004;33(4):328–34. [DOI] [PubMed] [Google Scholar]
- 24.Tonggu L WL. Cryo-EM sample preparation method for extremely low concentration liposomes. bioRxiv. 2018;494997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maduke M, Pheasant DJ, Miller C. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J Gen Physiol. 1999;114(5):713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ertel A, Marangoni AG, Marsh J, Hallett FR, Wood JM. Mechanical properties of vesicles. I. Coordinated analysis of osmotic swelling and lysis. Biophys J. 1993;64(2):426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald RC, Jones FD, Qiu RZ. Fragmentation into Small Vesicles of Dioleoylphosphatidylcholine Bilayers during Freezing and Thawing. Bba-Biomembranes. 1994;1191(2):362–70. [DOI] [PubMed] [Google Scholar]
- 28.Traikia M, Warschawski DE, Recouvreur M, Cartaud J, Devaux PF. Formation of unilamellar vesicles by repetitive freeze-thaw cycles: characterization by electron microscopy and P-31-nuclear magnetic resonance. Eur Biophys J Biophy. 2000;29(3):184–95. [DOI] [PubMed] [Google Scholar]
- 29.Akyuz N, Georgieva ER, Zhou Z, Stolzenberg S, Cuendet MA, Khelashvili G, et al. Transport domain unlocking sets the uptake rate of an aspartate transporter. Nature. 2015;518(7537):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veshaguri S, Christensen SM, Kemmer GC, Ghale G, Moller MP, Lohr C, et al. Direct observation of proton pumping by a eukaryotic P-type ATPase. Science. 2016;351(6280):1469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.