Abstract
Household air pollution emitted from solid-fuel cookstoves used for domestic cooking is a leading risk factor for morbidity and premature mortality globally. There have been attempts to design and distribute lower emission cookstoves, yet it is unclear if they meaningfully improve health. Using a crossover design, we assessed differences in central aortic hemodynamics and arterial stiffness following controlled exposures to air pollution emitted from five different cookstove technologies compared to a filtered air control.
Forty-eight young, healthy participants were assigned to six 2-hour controlled treatments of pollution from five different cookstoves and a filtered air control. Each treatment had a target concentration for fine particulate matter: filtered air control = 0 μg/m3, liquefied petroleum gas = 10 μg/m3, gasifier = 35 μg/m3, fan rocket = 100 μg/m3, rocket elbow = 250 μg/m3, three stone fire = 500 μg/m3. Pulse wave velocity (PWV), central augmentation index (AIx), and central pulse pressure (CPP) were measured before and at three time points after each treatment (0, 3, and 24 hours). Linear mixed models were used to assess differences in the outcomes for each cookstove treatment compared to control.
PWV and CPP were marginally higher 24 hours after all cookstove treatments compared to control. For example, PWV was 0.15 m/s higher (95% confidence interval: −0.02, 0.31) and CPP was 0.6 mmHg higher (95% confidence interval: −0.8, 2.1) 24 hours after the three stone fire treatment compared to control. The magnitude of the differences compared to control was similar across all cookstove treatments. PWV and CPP had no consistent trends at the other post-treatment time points (0 and 3 hours). No consistent trends were observed for AIx at any post-treatment time point.
Our findings suggest higher levels of PWV and CPP within 24 hours after 2-hour controlled treatments of pollution from five different cookstove technologies. The similar magnitude of the differences following each cookstove treatment compared to control may indicate that acute exposures from even the cleanest cookstove technologies can adversely impact these subclinical markers of cardiovascular health, although differences were small and may not be clinically meaningful.
Keywords: air pollution, biomass burning, central hemodynamics, arterial stiffness, epidemiology
1. Introduction
Household air pollution resulting from combustion of solid fuels for domestic cooking is a leading environmental risk factor for global morbidity and mortality. Fine particulate matter (PM2.5; airborne particles less than 2.5 μm in aerodynamic diameter) exposures from solid cooking fuels resulted in an estimated 60 million disability adjusted life-years in 2017, including 1.6 million premature deaths (Stanaway et al. 2018). Improved, cleaner-burning cookstove technologies have been developed and distributed in an attempt to lower exposures to household air pollution, but it is still unclear if these new cookstoves are resulting in improved health outcomes (Bruce et al. 2015; Quansah et al. 2017). Further research is necessary to understand if currently available improved cookstove technologies are capable of reducing exposures enough to result in improved health outcomes compared to traditional cookstoves.
It is estimated that 40% of the 1.6 million premature deaths that resulted from household air pollution exposure in 2017 occurred due to cardiovascular outcomes such as ischemic heart disease and stroke (Stanaway et al. 2018). Current research suggests that household air pollution from cookstove use can adversely impact blood pressure, endothelial function, and heart rate variability, as well as alter circulating biomarkers related to inflammation, coagulation, and oxidative stress (Fatmi and Coggon 2016; McCracken et al. 2012). However, conclusive epidemiologic evidence is still lacking due to limitations in study designs (e.g. limited internal validity in observational studies and lack of quantitative exposure assessment in many field studies) and a narrow scope of the outcomes assessed; further research is needed to understand how household air pollution impacts cardiovascular health (McCracken et al. 2012).
Measures of arterial stiffness and central hemodynamics provide information on cardiovascular disease risk beyond that of traditional measures such as peripheral blood pressure (Vlachopoulos et al. 2010a; Vlachopoulos et al. 2010b). Carotid-femoral pulse wave velocity (PWV) is the gold standard for assessing aortic arterial stiffness and is strongly associated with cardiovascular disease risk and mortality (Townsend et al. 2015; Vlachopoulos et al. 2010b). Central augmentation index (AIx) is a measure of pulse wave reflection and an indirect measure of peripheral vascular stiffening (Tomiyama and Yamashina 2010). AIx and central pulse pressure (CPP) are measures of central hemodynamics and overall cardiovascular performance, and similar to PWV, both are strongly associated with risk of adverse cardiovascular events and mortality (Vlachopoulos et al. 2010a). For PWV, AIx, and CPP, higher values are associated with increased risk of adverse cardiovascular outcomes (Vlachopoulos et al. 2010a; Vlachopoulos et al. 2010b). Studying the associations between household air pollution and these outcomes will give us a better understanding of the cardiovascular health impacts of cookstove use.
There is evidence that particulate and gaseous air pollution from ambient sources can impact measures of arterial stiffness and central hemodynamics (Zanoli et al. 2017). Currently only one study has evaluated the association between these outcomes and household air pollution; this field study reported associations between increased levels of household air pollution and higher central blood pressure, CPP, and AIx in a population of 205 women in rural China (Baumgartner et al. 2018). However, the authors reported that a semi-gasifier cookstove intervention did not improve hemodynamic outcomes compared to participants who did not receive the intervention (Clark et al. 2019). In a controlled human exposure study with a crossover design called the SToVES Study (Subclinical Tests on Volunteers Exposed to Smoke), we assessed PWV, AIx, and CPP following 2-hour exposures to air pollution emitted from five cookstove technologies compared to filtered air. Our aim was to investigate the impact of exposure to different levels of cookstove air pollution on acute cardiovascular and respiratory health outcomes (other outcomes reported separately).
2. Methods
2.1. Study design
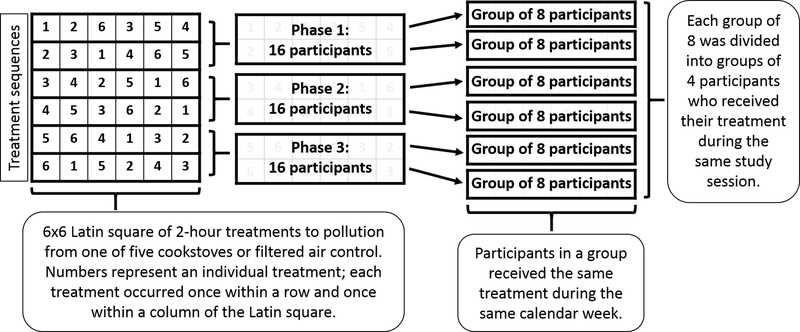
A description of the study design and methods has been published previously (Fedak et al. 2019). The crossover study design consisted of a Latin-square with six 2-hour controlled treatments of pollution emitted from five cookstove technologies (referred to as “cookstove treatments”) and a filtered air control (Figure 1). Each treatment had a target level of PM2.5. The study was divided into three rounds that lasted 3 to 4 months in duration depending on holidays and academic schedules. The 16 participants in each round were divided into two groups of eight, primarily based on participant schedules and availability. Each group of eight participants was assigned to a unique sequence of the treatments with at least 2 weeks between treatments to minimize a carryover effect of the previous treatment. The 2-week washout period between treatments was chosen to be consistent with previous studies that had washout periods of 1 to 3 weeks (Barregard et al. 2008; Bonlokke et al. 2014; Riddervold et al. 2012; Sehlstedt et al. 2010; Stockfelt et al. 2013). Each group of eight participants who shared a unique treatment sequence received their assigned treatments during the same calendar week, with four participating on Monday and four on Wednesday. Participants were expected to follow their assigned sequence unless an illness or personal circumstance kept them from participating. After each of the assigned sequences in a round was completed, participants were allowed to return for out-of-sequence makeup visits in order to complete each of their six total treatments.
Figure 1: Study design.
The crossover study design produced high internal validity. Each participant acted as their own control, which eliminated individual time-invariant factors (e.g. sex, race/ethnicity) as potential confounders. In addition, participants were divided into six groups with unique sequences of the treatments, which limited the potential effect that sequence of the treatments may have had on the outcomes. Some potential confounding factors may have varied across study days (e.g. ambient temperature and air pollution); however, these factors were unlikely to be associated with the individual treatments and were therefore unlikely to confound the associations in our analyses. Further details on statistical analyses and strengths of the study design are described below.
2.2. Participants and recruitment process
Participants (n=48) were recruited from the Fort Collins, Colorado area beginning in September of 2016. Specific eligibility criteria at the time of recruitment included age less than 36 years, body mass index (BMI) between 18 and 29 kg/m2, never-smoker, no regular exposure to air pollution above ambient levels (including secondhand tobacco smoke and recreational drugs), no self-reported history of chronic diseases (e.g. cardiopulmonary disease or diabetes), no recent surgery, no claustrophobia or fear of needles, not pregnant/breastfeeding or planning on becoming pregnant, ability and willingness to refrain from prescription and over-the-counter medication use during study sessions unless approved by the study physician, and willingness to comply with a strict study schedule. If participants passed eligibility screening they were asked to complete an individual health assessment conducted by a cardiologist to rule out any current or family history of cardiopulmonary disease. Further information on eligibility criteria and the individual health assessment is included in the Supplemental Materials.
All study procedures were approved by the Institutional Review Board at Colorado State University. Participants provided written consent for all study procedures.
2.3. Study sessions
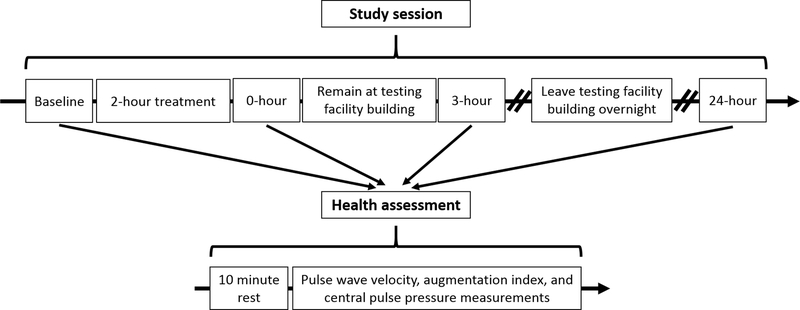
Study sessions consisted of four health assessments and the assigned treatment (Figure 2). Start times for the four participants in a session were staggered by 30 minutes beginning at 7:30am. Participants kept the same study day (i.e. Monday vs Wednesday) and start time throughout each of their sessions. In addition, participants kept the same daily schedule for each session they completed (i.e. the treatment and health assessments were completed at the same time during the day for each study session a participant completed). After arriving for a study session, participants were assessed by a cardiologist to ensure they were not currently or recently sick or suffering an inflammatory or allergic reaction. Once the cardiologist authorized participation, the study session began with a baseline health assessment and was followed immediately by the assigned 2-hour treatment. Another health assessment took place immediately after the treatment. Participants then had a lunch break during which they remained in the testing facility building. A third health assessment took place 3 hours after each participant finished their treatment. Participants then left the testing facility overnight before returning the next day for the fourth health assessment 24 hours after each participant finished their treatment.
Figure 2: Study session timeline and sequence of events.
Participants were asked to eat a consistent, low-fat diet and to refrain from alcohol and smoke exposure starting 24 hours prior to each study session and ending after the 24-hour follow-up health assessment. Unless approved by the study physician, participants were also asked to refrain from medication use starting 72 hours prior to each study session and ending after the 24-hour follow-up.
2.4. Health assessments and study outcomes
Each health assessment lasted approximately 1 hour and consisted of a 10-minute rest period (supine position) followed by a series of health measurements (full list of health measurements in Supplemental Materials). PWV, AIx, and CPP were measured using a non-invasive pressure waveform device (SphygmoCor XCEL, Atcor Medical, Australia) immediately following the 10-minute rest period. Study personnel were trained by a SphygmoCor representative and adhered to the manufacturer’s protocols (see Supplemental Materials). Quality control measures are integrated into the SphygmoCor software; only measurements that passed the instrument quality control guidelines were used for analysis. Heart rate was also measured during the AIx assessment and used as a covariate in some supplemental analyses. Height and weight were measured once at enrollment and used to calculate BMI (kg/m2).
2.5. Controlled treatments
Controlled treatments were administered in a specially designed facility on the Colorado State University campus called the Simulated Environmental Testing (SET) facility. The SET facility was large enough to house four participants simultaneously; participants remained seated throughout the 2-hour treatments. Participants were monitored for the entire duration of the controlled treatments by a registered nurse; the nurse also remotely (i.e. without entering the SET) measured blood pressure, heart rate, and oxygen saturation every 15 minutes while participants were in the SET facility. Participants were able to communicate with study staff if necessary via text message or intercom while inside the SET facility.
The six treatments included filtered air control (PM2.5 target level of 0 μg/m3), liquefied petroleum gas (LPG; 10 μg/m3), gasifier (35 μg/m3; fuel of pine wood chips), forced-draft fan rocket elbow (100 μg/m3; fuel of pine wood sticks), natural-draft rocket elbow (250 μg/m3; fuel of pine wood sticks), and three stone fire (500 μg/m3; fuel of pine wood sticks). Emissions for each of the treatments were extracted from a total-capture fume hood where the cookstove was operated by study personnel, and mixed with HEPA (high efficiency particulate air) filtered air to reach the target concentration for each respective treatment. A nephelometer (DustTrak DRX 8533, TSI Incorporated, USA) with a PM2.5 size-selective cyclone inlet and a gas analyzer (Siemens Ultramat 6E, Siemens AG, Germany) were used to monitor PM2.5, carbon monoxide (CO), and oxygen levels in the SET facility in real time. The DustTrak was calibrated to the wood and LPG stoves separately, based on gravimetric analysis of PM2.5 filter data collected within the SET facility prior to the study. Gravimetric filters were also collected on each sample day to ensure DustTrak accuracy and to detect any potential calibration drift. Humidity and temperature in the SET facility were also monitored (Omega HX94BC transmitter and Type K thermocouple, OMEGA Engineering, USA). A real-time control system (LabVIEW™, v15.0 32-bit, National Instruments, USA) was used to automate the flows of both dilution and pollution air based on real-time PM2.5 data received from the DustTrak to maintain target concentrations.
We conducted further testing to characterize additional pollutant levels inside the SET facility for each of the treatments, including the filtered air treatment. For each treatment, at least two 2-hour tests were conducted under the same conditions as a typical study session. No human participants were present in the SET facility during this additional testing. The pollutants characterized included PM2.5 mass, particle number size distributions (10 nm to 500 nm), PM2.5 elemental and organic carbon, nitrogen oxide, nitrogen dioxide, volatile organic compounds (VOCs), and carbonyls. Additional methods for the SET characterization have been previously published (Fedak et al. 2019).
2.6. Questionnaires and potential confounders
A questionnaire was administered during the initial study session to collect information on participant demographic characteristics. Additional questionnaires were administered prior to the baseline and 24-hour follow-up health assessments during each study session to collect information on potential confounding variables. Participants self-reported frequency of alcohol and caffeine consumption, exposures to smoke and ambient air pollution, medication use, physical activity, and sleep quality during the 24 hours leading up to the study session and for the period between the 3-hour post and 24-hour post health assessments when participants were away from the testing facility. Participants also self-reported mode of travel to the study facility. Hourly ambient temperature and PM2.5 concentrations were assessed as potential confounding factors in the analyses. PM2.5 data were downloaded from the U.S. EPA’s Air Quality Data API monitor in Fort Collins, Colorado (U.S. Environmental Protection Agency 2018), and ambient temperature data were downloaded from Colorado State University Atmospheric Science Department’s Christman Field Weather Station (Colorado State University 2018).
2.7. Statistical analysis
Data cleaning, descriptive statistics, and data visualization were performed in R version 3.5.0 (The R Project for Statistical Computing). We used the R package lme4 (Bates et al. 2015) to run linear mixed models.
Summary statistics (mean, standard deviation [sd], median, minimum, maximum) of participant baseline characteristics were calculated for the total population and by sex. For each treatment level, we estimated mean PM2.5 and CO exposures for each participant by averaging the PM2.5 and CO levels over the 2-hour periods they were inside the SET facility. We then averaged across all participants for each treatment level to produce the summary statistics. Paired t-tests were run to compare mean pre-treatment values of PWV, AIx, and CPP prior to control with mean pre-treatment values prior to the cookstove treatments.
We used linear mixed models to assess differences in outcomes for each cookstove treatment compared to control at the three post-treatment time points (0, 3, and 24 hours). Models included a fixed categorical term for treatment level, a fixed continuous term for baseline outcome measurement, a random term for participant, and a random term for date of the treatment. We included the baseline term to account for variations in the outcomes between treatments levels at the beginning of each study session (i.e. variations unrelated to the treatments), the term for participant to account for repeated measures within each participant, and the term for date to account for correlation that may have occurred between participants who were part of the same study session. Terms for sequence and visit were not used in the statistical models for the primary analyses because we included out-of-sequence makeup visits in the primary dataset. Sensitivity analyses were performed on a subset of the data which participants completed in sequence; models in these analyses included terms for sequence and visit number. In addition, we conducted sensitivity analyses to assess for potential confounding by including questionnaire variables and ambient temperature and PM2.5 concentrations during the 24 hours prior to each health assessment as covariates in the statistical models (see Supplemental Materials for further details).
Diagnostic plots (i.e. QQ plots and residuals vs fitted values plots) were evaluated and met assumptions for linear models.
3. Results
3.1. Participants
Baseline characteristics for the study population are presented in Table 1. The 48 participants (26 males and 22 females) had a mean age at baseline of 28 years (sd = 4) and a mean BMI at baseline of 23 kg/m2 (sd = 2). The study population largely identified as non-Hispanic white (42/48 participants).
Table 1:
Participant characteristics
| Variable | All participants (n = 48) | Females (n = 22) | Males (n = 26) |
|---|---|---|---|
| mean (sd), min, max | |||
| Age at study start, years | 27 (4), 21, 36 | 28 (3), 23, 34 | 27 (4), 21, 36 |
| Body mass index at study start, kg/m2 | 23 (2), 19, 29 | 23 (3), 20, 29 | 23 (2), 19, 26 |
| Baseline* pulse wave velocity, m/s | 6.0 (0.6), 4.8, 7.2 | 5.9 (0.6), 4.8, 7.1 | 6.1 (0.6), 4.8, 7.2 |
| Baseline* augmentation index, % | 8 (12), −31, 34 | 11 (14), −31, 34 | 5 (10), −12, 24 |
| Baseline* central pulse pressure, mmHg | 31 (5), 19, 46 | 30 (5), 19, 39 | 32 (6), 22, 46 |
| n (%) | |||
| Non-Hispanic white ethnicity/race | 42 (88) | 18 (82) | 24 (92) |
| Participants present for all 6 treatments+ | 39 (81) | 19 (86) | 20 (77) |
| Participants present for 5 or 6 treatments+ | 45 (94) | 22 (100) | 23 (88) |
Baseline means are the average values across all participants for the pre-treatment measurement of each participant’s first study visit.
Participant included if present for baseline health assessment, treatment, and at least one follow-up health assessment.
sd = standard deviation
Twenty-two of 48 participants completed all six treatments in their assigned sequence; missed sessions were typically due to illness or unforeseen scheduling conflicts. Including out-of-sequence makeup sessions, 45 of 48 participants completed at least five of the treatments and 39 of 48 participants completed all six treatments. Three participants dropped out of the study for personal reasons after two sessions (one participant) and three sessions (two participants); the sessions they completed were included in primary analyses. Including additional missing observations due to technical reasons or scheduling conflicts, total missing data was 6.3% for PWV and 6.9% for AIx and CPP.
3.2. Controlled treatments
PM2.5 exposure concentrations experienced by the participants were generally close to the targets (Table 2). The mean percent differences from the target PM2.5 level for the fan rocket, rocket elbow, and three stone fire cookstove treatments were all less than 9%. The mean percent differences from the target PM2.5 level for the gasifier and LPG cookstove treatments were 31% and 18%, respectively, which equate to concentrations that were 11 μg/m3 higher than the target value of 35 μg/m3 for the gasifier treatment and 2 μg/m3 lower than the target value of 10 μg/m3 for the LPG treatment (Table 2). The mean PM2.5 concentration for the control treatment was less than 1 μg/m3 (target concentration of 0 μg/m3). Mean CO mixing ratios within the SET facility were less than 10 ppm for all treatments and generally increased as target PM2.5 concentrations increased (Table 2).
Table 2:
SET facility pollution concentrations compared to target levels of fine particulate matter
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Control | LPG | Gasifier | Fan rocket | Rocket elbow | Three stone fire | |
| Fine particulate matter target concentration | ||||||
| 0 μg/m3 | 10 μg/m3 | 35 μg/m3 | 100 μg/m3 | 250 μg/m3 | 500 μg/m3 | |
| Participants with completed treatment, n | 47 | 45 | 44 | 44 | 45 | 47 |
| Mean (sd) PM2.5 concentration, μg/m3 | 1 (2) | 8 (3) | 46 (9) | 95 (9) | 254 (9) | 462 (41) |
| Mean difference from target level, μg/m3 | 1 | −2 | 11 | −5 | 4 | −38 |
| Maximum difference from target level, μg/m3 | 9 | 7 | 42 | 23 | 26 | 133 |
| Mean percent difference from target level, % | −18 | 31 | −5 | 2 | −8 | |
| Mean (sd) CO mixing ratio*, ppm | 2 (2) | 3 (1) | 5 (3) | 8 (2) | 6 (2) | 9 (4) |
SET = Simulated Environmental Testing; LPG = liquefied petroleum gas; sd = standard deviation; CO = carbon monoxide
CO did not have a target level. This row is showing the measured mean CO mixing ratio for each treatment.
Concentrations of additional pollutants measured in the SET characterization analysis generally increased as treatment PM2.5 target concentrations increased. Further results from the SET characterization have been previously published (Fedak et al. 2019).
3.3. Health outcomes
Mean baseline (i.e. pre-treatment) values for the health outcomes are presented in Table 1: mean PWV was 6.0 m/s (sd = 0.6), mean AIx was 7% (sd = 13), and mean CPP was 31 mmHg (sd = 5). There were small differences in baseline PWV and CPP between the treatments (Table S1). AIx also varied at baseline across the six treatments and had standard deviations as large as or larger in magnitude than the mean values. Based on paired t-tests between each cookstove treatment and control, only the baseline value of CPP for female participants prior to the rocket elbow cookstove treatment was significantly different (p-value < 0.05) from the baseline value prior to control (Table S1).
Linear mixed model estimates and 95% confidence intervals (CI) for the difference between each cookstove treatment compared to control at three post-treatment time points are presented in Table 3. At the immediate post-treatment and 3-hour post-treatment time points, differences for all cookstove treatments compared to control were generally consistent with a null association for all outcomes (Table 3; Figures 3–5). There were some exceptions to this trend, including higher PWV following the gasifier cookstove treatment at the 3-hour post-treatment time point (0.18 m/s; 95% CI: 0.01, 0.36). AIx was higher at the 3-hour post-treatment time point following the three stone fire cookstove treatment (2.9%; 95% CI: −0.4, 6.1), and lower at the immediate post-treatment time point following the gasifier cookstove treatment (−2.5%; 95% CI: −5.5, 0.6). CPP was between 0.1 and 1.1 mmHg lower at the immediate-post treatment time point for the four highest cookstove treatments compared to control (Table 3; Figure 5).
Table 3:
Differences in health outcomes following cookstove treatments compared to control at three post-treatment time points using linear mixed models*
| Health measurement time point | Treatment | |||||
|---|---|---|---|---|---|---|
| Control | LPG | Gasifier | Fan rocket | Rocket elbow | Three stone fire | |
| Pulse wave velocity (m/s) | ||||||
| Mean (sd) | Difference compared to control (95% confidence interval) | |||||
| 0-hour post-treatment | 6.16 (0.69) | 0.02 (−0.16, 0.21) | 0.08 (−0.11, 0.26) | −0.06 (−0.25, 0.12) | −0.07 (−0.25, 0.11) | −0.11 (−0.29, 0.07) |
| 3-hour post-treatment | 6.05 (0.81) | −0.04 (−0.22, 0.14) | 0.18 (0.01, 0.36) | −0.04 (−0.21, 0.14) | 0.06 (−0.12, 0.24) | 0.00 (−0.17, 0.18) |
| 24-hour post-treatment | 5.90 (0.64) | 0.15 (−0.02, 0.32) | 0.16 (−0.01, 0.33) | 0.09 (−0.08, 0.26) | 0.08 (−0.08, 0.25) | 0.15 (−0.02, 0.31) |
| Augmentation index (%) | ||||||
| Mean (sd) | Difference compared to control (95% confidence interval) | |||||
| 0-hour post-treatment | 10.5 (10.8) | −0.6 (−3.7, 2.4) | −2.5 (−5.5, 0.6) | 0.0 (−3.1, 3.1) | −0.4 (−3.4, 2.6) | 0.7 (−2.3, 3.7) |
| 3-hour post-treatment | 4.9 (8.5) | 0.0 (−3.3, 3.2) | 0.6 (−2.7, 3.8) | 1.6 (−1.7, 4.9) | 0.8 (−2.5, 4.0) | 2.9 (−0.4, 6.1) |
| 24-hour post-treatment | 10.8 (9.3) | −1.1 (−4.3, 2.2) | 0.6 (−2.7, 3.9) | 0.3 (−3.0, 3.6) | −0.6 (−3.8, 2.6) | −1.5 (−4.7, 1.8) |
| Central pulse pressure (mmHg) | ||||||
| Mean (sd) | Difference compared to control (95% Confidence Interval) | |||||
| 0-hour post-treatment | 32.6 (5.1) | 0.0 (−1.4, 1.5) | −1.1 (−2.6, 0.4) | −0.1 (−1.6, 1.4) | −0.6 (−2.0, 0.9) | −0.9 (−2.4, 0.6) |
| 3-hour post-treatment | 31.5 (4.8) | 0.5 (−1.0, 1.9) | 0.3 (−1.2, 1.8) | −0.5 (−2.0, 1.0) | −0.3 (−1.8, 1.2) | −0.6 (−2.0, 0.9) |
| 24-hour post-treatment | 31.1 (5.5) | 1.3 (−0.2, 2.7) | 1.5 (0.0, 3.0) | 1.6 (0.1, 3.0) | 1.0 (−0.5, 2.4) | 0.6 (−0.8, 2.1) |
LPG = liquefied petroleum gas; sd = standard deviation
Model terms include cookstove treatment level (fixed) + baseline health measurement (fixed) + date (random) + participant (random)
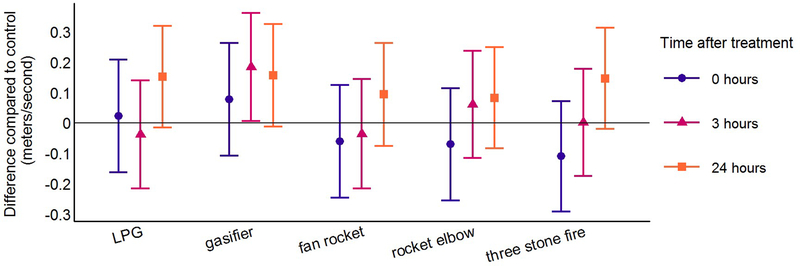
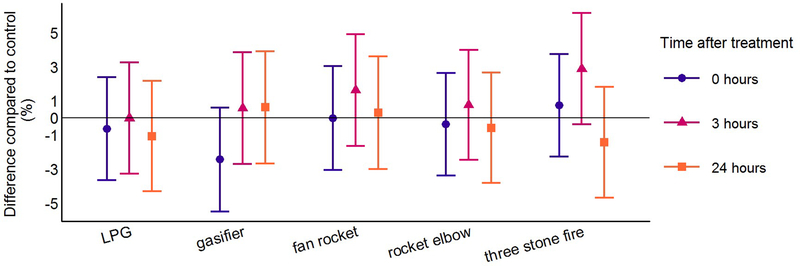
Figure 3: Differences in pulse wave velocity for each cookstove treatment compared to control at the three post-treatment time points using linear mixed models*.
LPG = liquefied petroleum gas
*Model terms include cookstove treatment level (fixed) + baseline health measurement (fixed) + date (random) + participant (random)
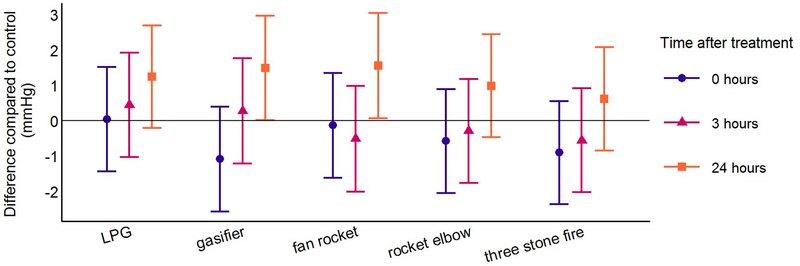
Figure 5: Differences in central pulse pressure for each cookstove treatment compared to control at the three post-treatment time points using linear mixed models*.
LPG = liquefied petroleum gas
*Model terms include cookstove treatment level (fixed) + baseline health measurement (fixed) + date (random) + participant (random)
PWV and CPP were higher 24 hours after all cookstove treatments compared to control (Table 3, Figures 3 and 5). The magnitude of the differences compared to control was similar across all cookstove treatments. Differences compared to control for PWV were between 0.08 and 0.16 m/s and differences compared to control for CPP were between 0.6 and 1.6 mmHg. Highlighting results following the treatments with the lowest and highest target PM2.5 concentrations (LPG and three stone fire), PWV was 0.15 m/s higher (95% CI: −0.02, 0.31) 24 hours after the three stone fire cookstove treatment and 0.15 m/s higher (95% CI: −0.02, 0.32) 24 hours after the LPG cookstove treatment compared to control. CPP was 0.6 mmHg higher (95% CI: −0.8, 2.1) 24 hours after the three stone fire cookstove treatment and 1.3 mmHg higher (95% CI: −0.2, 2.7) 24 hours after the LPG cookstove treatment compared to control. Differences compared to control for AIx at the 24-hour post-treatment time point were consistent with a null association for all cookstove treatment levels (Table 3; Figure 4).
Figure 4: Differences in augmentation index for each cookstove treatment compared to control at the three post-treatment time points using linear mixed models*.
LPG = liquefied petroleum gas
*Model terms include cookstove treatment level (fixed) + baseline health measurement (fixed) + date (random) + participant (random)
Results from sensitivity analyses are presented in the Supplemental Materials. None of the sensitivity analyses or inclusion of potential confounders resulted in meaningfully different model estimates compared to the primary model estimates presented in Table 3 and Figures 3–5.
4. Discussion
In the first study to assess the health impacts of air pollution emitted from multiple cookstove technologies using a crossover design, our results suggest that PWV and CPP were marginally higher 24 hours after the cookstove treatments compared to a filtered air control. PWV was between 0.08 and 0.16 m/s higher for each cookstove treatment compared to control at the 24-hour post-treatment time point; CPP was between 0.6 and 1.6 mmHg higher for each cookstove treatment compared to control at the same time point. There were no trends of higher or lower values across the cookstove treatment levels for AIx at any post-treatment time point. Our study design had strong internal validity that limited the impact of potential confounders, as confirmed by multiple sensitivity analyses.
Our results add to the small body of evidence that household air pollution can adversely impact central hemodynamics and arterial stiffness. Our study is the first to assess outcomes of central hemodynamics and arterial stiffness following controlled exposures to cookstove-generated air pollution; a study in China is the only study to date to evaluate this association in a field setting (Baumgartner et al. 2018). Among 205 women in the study in China, increased PM2.5 exposures (1-ln μg/m3) were associated with 1.1 percentage points higher AIx (95% CI: −0.2, 2.4) (Baumgartner et al. 2018). Among 102 women aged 50 years or more, increased PM2.5 exposures were associated with 2.9 mmHg higher CPP (95% CI: 0.8, 5.1) (Baumgartner et al. 2018). After 1.5 years of follow-up, however, the authors reported that a government sponsored semi-gasifier cookstove intervention did not improve hemodynamic outcomes compared to control participants, likely due to other improved cookstove adoption in the control group (Clark et al. 2019). Other studies have found associations between PWV and AIx and particulate and gaseous air pollution from ambient sources (Zanoli et al. 2017). Our results add further consistency that air pollution in general, and specifically household air pollution, can adversely impact markers of central hemodynamics and arterial stiffness.
Central hemodynamic indices and measures of arterial stiffness are strongly associated with future cardiovascular events and all-cause mortality (Vlachopoulos et al. 2010a; Vlachopoulos et al. 2010b). Measures of central hemodynamics are pathophysiologically more relevant than peripheral indices because central pressures are a better indicator of cardiac workload and overall cardiovascular health (Vlachopoulos et al. 2010a). In a meta-analysis of longitudinal studies, Vlachopoulos et al. found that AIx predicts clinical events independently of peripheral blood pressure, and that CPP predicts clinical events better than peripheral pulse pressure (Vlachopoulos et al. 2010a). Numerous studies have shown the importance of PWV as an indicator of arterial stiffness and a predictor of future cardiovascular events and all-cause mortality (Townsend et al. 2015; Vlachopoulos et al. 2010b).
Potential biological pathways initiated by PM2.5 exposure could help explain the higher values of PWV and CPP we observed 24 hours after each cookstove treatment. Inflammatory and oxidative stress pathways can be initiated within 24 hours after exposure to PM2.5 (Brook et al. 2010). Inflammatory cytokines and reactive oxygen species can cause an increase in vascular smooth muscle tone through endothelial dysfunction and reduced nitric oxide bioavailability (Huang and Vita 2006; Sprague and Khalil 2009). Higher vascular smooth muscle tone can lead to increased arterial stiffness and higher PWV and CPP (Avolio et al. 2011; Townsend et al. 2015). These same pathways could also influence AIx; however, changes in PWV and AIx can occur independently depending on which region of the arterial tree is most impacted by the exposure or stimulus of interest (Kelly et al. 2001). It is possible that the cookstove air pollution in our study impacted the vascular smooth muscle tone of the larger arteries more than the smaller, distal arterioles. It is also possible that AIx was impacted on a different timeframe than PWV and CPP so that changes in AIx were not captured at any of the three post-treatment time points in our study. In addition, AIx is a more complex measurement than PWV and can be influenced by a variety of factors within the arterial tree (Tomiyama and Yamashina 2010). This complexity may be expressed in our results in the large standard deviations that indicate high variability in the AIx measurements (Table 1 and Table S1). Since our study was powered based on other outcomes not reported here, it is possible that the high variability of AIx resulted in limited ability to detect changes in our statistical models.
Assessing short-term exposures and acute health outcomes in a group of young, healthy, and largely non-Hispanic white participants means that our results are not directly applicable to real-world cookstove users who are exposed repeatedly over the course of a lifetime. While generalizability is a weakness, the study setting allowed us to assess complex health outcomes resulting from exposures to pollution from multiple cookstove technologies in a controlled environment. This gives us a better understanding of the underlying acute differences in health resulting from household air pollution exposure. The strength of the crossover design also gave our study results high internal validity. For example, some potential confounding factors may have varied across study days (e.g. ambient air pollution); however, these factors were unlikely to be associated with the individual treatments and were, therefore, unlikely to confound the observed associations. Regardless, we performed numerous sensitivity analyses that included potential confounders as covariates, yet no meaningful differences from the primary analyses were observed (see Supplemental Materials). In addition, inclusion of the baseline health outcome values and the date of the study sessions in the analyses helped account for potential confounders that may have varied at random between study days. Further, assessing differences within person in the mixed models helped control for potential time-invariant confounding variables such as participant sex.
Logistically, all 48 study participants could not experience each treatment simultaneously and exposures could not be held perfectly at target levels; this means that groups of participants experienced different levels of exposure to air pollution for the same cookstove treatment. However, our data indicate that each participant was exposed to PM2.5 levels that were near target levels (Table 2), and that there was very little overlap in PM2.5 levels when assessing each individual’s personal mean exposure for each treatment level (see Figure S1 of Supplemental Materials). Our close control over pollution levels inside the SET facility should have minimized an impact on our reported results from overlapping treatment levels.
Our results indicate that each cookstove treatment (compared to control) had a similar effect on PWV and CPP even though the PM2.5 target concentrations for the cookstove treatments ranged from 10 to 500 μg/m3. In an attempt to explain these unexpected findings, we characterized additional pollutants for each treatment (i.e. PM2.5 mass, particle number size distributions (10 nm to 500 nm), PM2.5 elemental and organic carbon, nitrogen oxide, nitrogen dioxide, VOCs, and carbonyls); results from the additional pollutant characterization have been published previously (Fedak et al. 2019). We found that none of the pollutants we measured explained the similar effect each treatment had on PWV and CPP. For example, PM2.5 mass, particle numbers, concentrations of carbonyls, VOC levels, and concentrations of elemental and organic carbon generally increased as PM2.5 target levels for each treatment increased. An exception to these trends was the LPG treatment, which had higher concentrations of carbonyls than both the gasifier and fan rocket treatments and the highest number of particles in the 10 to 30 nm range out of any treatment. Additionally, while each cookstove treatment had higher levels of nitrogen oxide than control, levels for the fan rocket and rocket elbow treatments were several times higher than the other cookstove treatments. Finally, each treatment emitted similar levels of nitrogen dioxide. These results provide no clear evidence that any single pollutant was associated with the changes in PWV and CPP we observed in our results. It is possible that the complex nature of pollutants emitted from the cookstoves resulted in mixtures of pollution that impacted the health outcomes in a similar magnitude, or that the range of exposures experienced during the 2-hour treatments was not large enough to lead to detectable differences in the magnitude of the changes in PWV and CPP. Multipollutant characterization of a wider range of exposures in future studies may help provide clarity to these lingering uncertainties.
An alternative explanation for our results is that the filtered air control treatment was beneficial for the health outcomes we measured as opposed to the cookstove treatments being detrimental. Since the control treatment was the reference level in our analysis, it may have given the appearance that the cookstove treatments each had a similar adverse impact on PWV and CPP when, in fact, the control treatment had a beneficial impact. Air filter intervention studies have reported associations between air filtration and improved endothelial function and inflammatory markers (Allen et al. 2011), as well as improved blood pressure and stress hormones (Li et al. 2017). While these previous studies were designed to assess the impact of air filtration in real-world settings, as compared to our study in a laboratory setting, they do provide evidence of the potential health benefits of breathing filtered air.
In analyses published separately, we also observed higher brachial systolic blood pressure following the cookstove treatments compared to control (Fedak et al. 2019), giving a broader context and adding consistency to the results presented here. While these results may not be relevant for people exposed to air pollution emitted from indoor cookstoves over a lifetime, our findings are still informative for a number of reasons. Repeated exposures to air pollution experienced in real-world settings may result in a chronic, underlying environment in which cardiovascular disease can manifest (Brook et al. 2010). In addition, individuals with existing cardiovascular disease may be more susceptible to clinically meaningful adverse cardiovascular events as a result of acute exposures to air pollution (Brook et al. 2010). Our results may be indicative of such subclinical, underlying changes in health. Although the differences we observed were small, the similar magnitude of the differences following each cookstove treatment compared to control may indicate that acute exposures from even the cleanest cookstove technologies can adversely impact these subclinical markers of cardiovascular health. Further research is necessary to help us understand if cookstove technology is capable of reducing exposures enough to improve long term health.
5. Conclusion
Our results from a controlled exposure study with a crossover design are an important contribution to understanding the cardiovascular health effects resulting from exposure to household air pollution. Our findings suggest higher levels of PWV and CPP within 24 hours after 2-hour treatments to pollution from five different cookstove technologies. The similar differences in PWV and CPP we observed following each cookstove treatment compared to control may indicate that acute exposures from even the cleanest cookstove technologies can elicit adverse responses in markers of central hemodynamics and arterial stiffness. We recommend that future analyses also consider biomarkers that may be indicative of potential biological mechanisms.
Supplementary Material
Highlights.
Participants were exposed to pollution from five cookstoves and filtered air control
Pulse wave velocity was higher 24 hours after cookstove exposures vs control
Central pulse pressure was higher 24 hours after cookstove exposures vs control
Augmentation index did not change after cookstove exposures vs control
Results did not suggest an increased effect as particulate matter exposures increased
6. Acknowledgements
We are grateful to the volunteer participants of this study, the nurses and doctors from Heart Center of the Rockies who provided medical staffing during our exposure periods, and the students who aided in running our controlled exposure facility (Lizette VanZyl, Evan Guiderra, Kathryne Gutekunst, and Danny Stringer).
Funding:
This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under grant number R01ES023688 (Principal Investigators: Jennifer L. Peel and John Volckens).
Abbreviations
- PM2.5
fine particulate matter
- PWV
carotid-femoral pulse wave velocity
- AIx
central augmentation index
- CPP
central pulse pressure
- BMI
body mass index
- sd
standard deviation
- CI
confidence interval
- CO
carbon monoxide
- LPG
liquefied petroleum gas
- SET
simulated environmental testing
- VOC
volatile organic compound
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests:
The authors have no competing interests to declare.
Human Subjects Statement:
All study procedures were approved by the Institutional Review Board at Colorado State University. Participants provided written consent for all study procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, et al. 2011. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med 183:1222–1230. [DOI] [PubMed] [Google Scholar]
- Avolio A, Butlin M, Liu Y-Y, Viegas K, Avadhanam B, Lindesay G. 2011. Regulation of arterial stiffness: Cellular, molecular and neurogenic mechanisms. Artery Research 5:122–127. [Google Scholar]
- Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, et al. 2008. Experimental exposure to wood smoke: Effects on airway inflammation and oxidative stress. Occup Environ Med 65:319–324. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:48. [Google Scholar]
- Baumgartner J, Carter E, Schauer JJ, Ezzati M, Daskalopoulou SS, Valois MF, et al. 2018. Household air pollution and measures of blood pressure, arterial stiffness and central haemodynamics. Heart. [DOI] [PubMed] [Google Scholar]
- Bonlokke JH, Riddervold IS, Gronborg TK, Skogstrand K, Hougaard DM, Barregard L, et al. 2014. Systemic effects of wood smoke in a short-term experimental exposure study of atopic volunteers. J Occup Environ Med 56:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- Bruce N, Pope D, Rehfuess E, Balakrishnan K, Adair-Rohani H, Dora C. 2015. Who indoor air quality guidelines on household fuel combustion: Strategy implications of new evidence on interventions and exposure–risk functions. Atmospheric Environment 106:451–457. [Google Scholar]
- Clark SN, Schmidt AM, Carter EM, Schauer JJ, Yang X, Ezzati M, et al. 2019. Longitudinal evaluation of a household energy package on blood pressure, central hemodynamics, and arterial stiffness in china. Environ Res 177:108592. [DOI] [PubMed] [Google Scholar]
- Colorado State University. 2018. Department of atmospheric science christman field weather observations. Available: https://www.atmos.colostate.edu/fccwx/fccwx_latest.php [accessed 4 December 2018.
- Fatmi Z, Coggon D. 2016. Coronary heart disease and household air pollution from use of solid fuel: A systematic review. Br Med Bull 118:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedak KM, Good N, Walker ES, Balmes J, Brook RD, Clark ML, et al. 2019. Acute effects on blood pressure following controlled exposure to cookstove air pollution in the stoves study. Journal of the American Heart Association 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Vita JA. 2006. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med 16:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. 2001. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension 37. [DOI] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. 2017. Particulate matter exposure and stress hormone levels: A randomized, double-blind, crossover trial of air purification. Circulation 136:618–627. [DOI] [PubMed] [Google Scholar]
- McCracken JP, Wellenius GA, Bloomfield GS, Brook RD, Tolunay HE, Dockery DW, et al. 2012. Household air pollution from solid fuel use: Evidence for links to cvd. Glob Heart 7:223–234. [DOI] [PubMed] [Google Scholar]
- Quansah R, Semple S, Ochieng CA, Juvekar S, Armah FA, Luginaah I, et al. 2017. Effectiveness of interventions to reduce household air pollution and/or improve health in homes using solid fuel in low- and-middle income countries: A systematic review and meta-analysis. Environ Int 103:73–90. [DOI] [PubMed] [Google Scholar]
- Riddervold IS, Bonlokke JH, Olin AC, Gronborg TK, Schlunssen V, Skogstrand K, et al. 2012. Effects of wood smoke particles from wood-burning stoves on the respiratory health of atopic humans. Part Fibre Toxicol 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlstedt M, Dove R, Boman C, Pagels J, Swietlicki E, Londahl J, et al. 2010. Antioxidant airway responses following experimental exposure to wood smoke in man. Part Fibre Toxicol 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague AH, Khalil RA. 2009. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet (London, England) 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockfelt L, Sallsten G, Almerud P, Basu S, Barregard L. 2013. Short-term chamber exposure to low doses of two kinds of wood smoke does not induce systemic inflammation, coagulation or oxidative stress in healthy humans. Inhal Toxicol 25:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama H, Yamashina A. 2010. Non-invasive vascular function tests. Circulation Journal 74:24–33. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. 2015. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. 2018. Air quality data api. Available: https://aqs.epa.gov/api [accessed 4 December 2018.
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. 2010a. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur Heart J 31:1865–1871. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. 2010b. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- Zanoli L, Lentini P, Granata A, Gaudio A, Fatuzzo P, Serafino L, et al. 2017. A systematic review of arterial stiffness, wave reflection and air pollution. Mol Med Rep 15:3425–3429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.