Abstract
Epidemiological evidence suggests that exposure to air pollution is a leading risk factor for cardiovascular disease (CVD). However, there is little direct evidence linking exposure to vascular dysfunction. We conducted a cross-sectional study of 100 participants, recruited from the University of Louisville Clinics. Endothelial function was assessed by calculating the reactive hyperemia index (RHI). Oxidative stress was indexed by measuring urinary levels of isoprostanes (n=91). Inflammatory biomarkers were measured in the plasma (n=80). Daily average PM2.5 levels were obtained from regional monitoring stations. Adjusted associations between PM2.5 levels and measured outcomes were tested using generalized linear models. The average age of participants was 48 years (44% male, 62% white); 52% had a diagnosis of hypertension, and 44% had type-2 diabetes. A 12.4% decrease in RHI was associated with 10μg/m3 increase in PM2.5 (95% CI: −21.0, −2.7). The F-2 isoprostane metabolite showed a positive association of 28.4% (95% CI: 2.7, 60.3) increase per 10μg/m3 PM2.5. Positive associations were observed with angiopoietin 1 (17.4%; 95% CI: 2.8, 33.8), vascular endothelial growth factor (10.4%; 95% CI: 0.6, 21.0), placental growth factor (31.7%; 95% CI: 12.2, 54.5), intracellular adhesion molecule-1 (24.6%; 95% CI: 1.6, 52.8), and matrix metalloproteinase-9 (30.3%; 95% CI: 8.0, 57.5) per 10μg/m3 increase in PM2.5. Additionally, a 10μg/m3 increase in PM2.5 was associated with 15.9% decrease in vascular adhesion molecule-1 (95% CI:−28.3, −1.3). These findings suggest that exposure to PM2.5 is associated with impaired vascular function, which may result from oxidative stress and inflammation, thereby leading to a pro-atherogenic state.
Keywords: air pollution, endothelial dysfunction, PM2.5, isoprostanes, reactive hyperemia index, cytokines
INTRODUCTION
Extensive epidemiological evidence suggests that exposure to air pollution is associated with adverse health outcomes.1 A recent report by the World Health Organization (WHO), estimated that exposure to indoor and outdoor air pollution could be linked to 6.5 million deaths (approximately 12% of total deaths) per year worldwide.2 Furthermore, it has been estimated that 92% of the world’s population lives in areas where levels of the fine particulate matter fraction (PM2.5: 0.1 – 2.5 μm in diameter) exceeds the WHO-recommended annual mean concentration limit of 10μg/m3.2 Thus, exposure to polluted air is a current global health threat, especially in developing countries, and is likely to remain so in the future.
Polluted air is a complex mix of gases, including volatile organic compounds, metals and particles of various sizes. As air pollution particles initially contact the lung, pulmonary diseases (e.g. asthma, cancer, COPD) are common outcomes of these exposures. However, cardiovascular disease (CVD) has a higher prevalence in populations exposed to fine particulate (PM2.5) air pollution3, 4 Indeed, previous studies have shown that acute and chronic exposures promote CVD risk factors such as hypertension5, 6 and insulin resistance,7–9 thereby contributing to atherogenesis, thrombosis and the incidence of myocardial infarction, stroke and sudden cardiac death.4, 10, 11 These adverse cardiovascular outcomes likely result from a damaged or dysfunctional endothelium. Prior studies addressing the impact of PM2.5 exposure on vascular function have done so indirectly, or have done so using cohorts presenting with confounding factors. Even less is known about the impact of exposure on signaling molecules, which regulate endothelial function or angiogenesis. Nevertheless, in a young, healthy human cohort, we previously found that acute exposure to PM2.5 reduces the levels of proangiogenic growth factors and is associated with elevated levels of circulating endothelial microparticles, indicative of vascular apoptosis.12 We also identified a deficit of circulating endothelial progenitor cells (EPCs),13 a stem cell type that promotes vascular regeneration and tissue repair. Furthermore, mice exposed to concentrated ambient particles (CAPs) demonstrated impairments in both EPC mobilization and function.13–15 Nevertheless, these animal and human studies lack assessments of endothelial function and angiogenesis in response to PM2.5. Thus, the immediate physiological impact of exposure on the vasculature remains largely speculative and whether an at risk population is more susceptible to the effects of PM2.5 exposure remains uncertain.
To examine how PM2.5 exposure affects endothelial function, we calculated the reactive hyperemia index and measured angiogenic signaling molecules in a study group of individuals at risk of developing CVD. Our results support the notion that PM2.5 induces oxidative stress and may directly impair endothelial function. Attenuated endothelial function may form the basis of cardiovascular pathologies associated with PM2.5 exposure.
METHODS
Participant characteristics and specimen collection
Participants between the ages of 22 and 65 years were recruited from the Diabetes and Primary Care Clinics of the University of Louisville.16 The study period extended from June 2011 to May 2013. All participants signed informed consents and the Institutional Review Board of the University of Louisville approved the study. Participants were prescreened, and those with conditions known to effect peripheral blood cell counts and bone marrow function were excluded from the study. These conditions included malignancies, organ transplant, renal replacement therapy, type 1 diabetes, untreated thyroid disease, anemia, acute infections, HIV infection, hepatitis, and unhealed wounds. Participants on hormone replacement therapy or medications affecting bone marrow function or peripheral blood cell counts were also excluded from the study. We also excluded those unwilling or unable to provide informed consent, pregnant or lactating women, prisoners and other vulnerable populations. All menstruating females were in the luteal phase of their cycle. A total of 100 participants were enrolled in the study.
Prior to biospecimen collection, the participants fasted for a minimum of eight hours. At the clinic, each participant filled out a health and diabetes questionnaire and biometrics were recorded. Four ml of blood was obtained from the participants using citrate as an anti-coagulant and standard venipuncture procedures. Within 30 min of collection, the tubes were spun at 1000×g for 15 min, the plasma was collected and spun again at 10,000×g for 10 min. The plasma was collected and stored at −80°C until use. Standard clean catch urine specimens were obtained from participants, and frozen in multiple aliquots at −80°C until use.
Exposure assessment
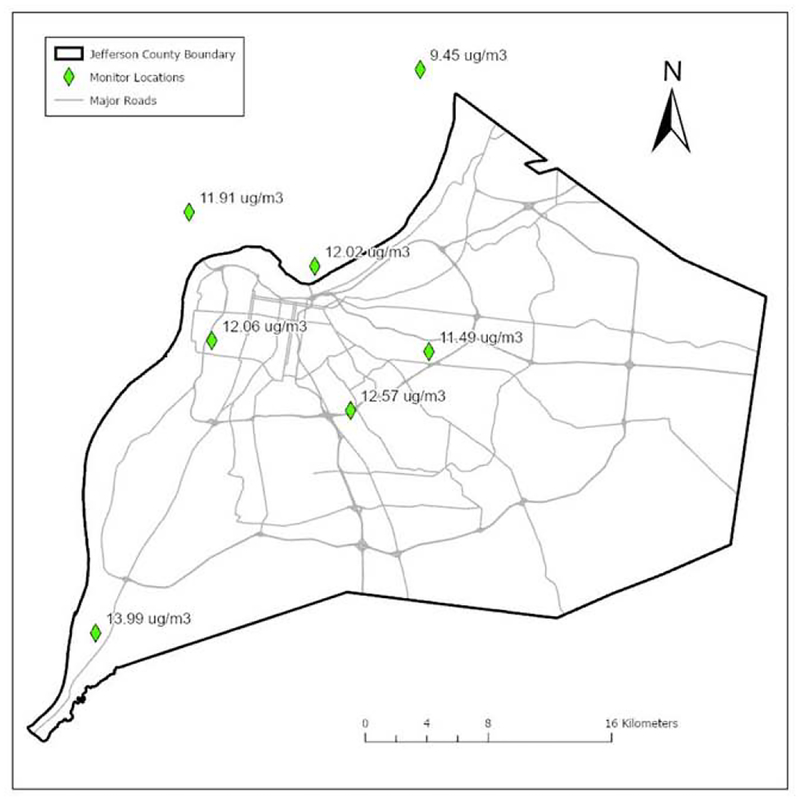
Ambient levels of PM2.5 in Louisville were obtained by calculating the daily average of five EPA-validated monitoring stations in Jefferson County, KY that report daily PM2.5 levels (Figure 1). Variations in PM2.5 between these monitors was limited, and over the study period, the mean PM2.5 concentration ranged from 11.49μg/m3 to 13.99μg/m3 (Figure 1). In general, PM2.5 measurements usually varied in accordance with each other and to approximately the same extent over time. However, we did observe higher values adjacent to a coal-fired power plant and lower values at a rural location. We observed that temporal variation was much higher than spatial variation between the monitors.
Figure 1. PM2.5 monitoring.
Illustrated are the approximate locations of EPA-validated monitoring sites in the Louisville metropolitan area and the measured, average PM2.5 concentrations over the study period.
To examine associations with measured parameters from our study participants, PM2.5 levels were obtained for the day the study participants visited the clinic, and for six days prior to their visit. From this we calculated 1-day, 3-day, and 7-day average PM2.5 levels for each participant.
Reactive hyperemia index
To assess endothelial function, we calculated a reactive hyperemia index (RHI) value on the study participants, where a lower value indicates diminished function This was accomplished using EndoPAT as stipulated by the manufacturer (Itamar Medical). After baseline recordings of the pulse wave amplitude from a fingertip on each arm, supra systolic pressure was applied to the non-dominant upper arm by a blood pressure cuff. The cuff was deflated after 5 min and reactive hyperemia recorded by the change in finger arterial pulse volume amplitude (PVA). The ratio between hyperemic and baseline PVA was normalized to the same ratio in the contralateral arm and reported as the reactive hyperemia index (RHI).17
Biochemical measurements
To examine the effects of PM2.5 on angiogenic signaling and inflammation, we examined the plasma levels of pro-angiogenic proteins (angiogenin, angiopoietin-1,2), growth factors, (vascular endothelial growth factors A,D,C, placental growth factor, stem cell factor, epidermal growth factor, platelet-derived growth factor, basic fibroblast growth factor, hepatocyte growth factor, erythropoietin), adhesion molecules (E-selectin, vascular cell adhesion molecule-1, intracellular adhesion molecule-1) and proteases (matrix metalloproteases 2, 9). The biomarkers were measured using laser microbead (Luminex) technology and specific kits from ThermoFisher. To examine systemic oxidative stress, we measured urinary levels of isoprostanes, which are formed from peroxidation of fatty acids and are indicative of oxidative stress in smokers.18 Urinary F2-isoprostane (Isop) and metabolite (Isom) were measured using GC-MS by the Vanderbilt University Eicosanoid Core Laboratory.19
Statistical analysis
Participant characteristics are presented as frequency (%) for categorical variables, and mean (±S.D.) for continuous variables. As outcome variables were positive, and heavily right-skewed, generalized linear models with the gamma distribution and log link were used to test for associations with PM2.5 levels. We performed both unadjusted and adjusted models for all outcomes. To control for potential meteorological impacts, all models were adjusted for average 24 h temperature on the day of the study visit. Additionally, because certain covariates may have unequal influence on the outcome variables, we tested for significant differences in covariates between high and low outcome groups using the median cutoff. Any variables significant at the p<0.1 level, as well as potential confounders and effect modifiers, were added to the final model, with final models chosen using the lowest Akaike Information Criterion value. Final models for RHI are adjusted for ambient temperature, sex, diabetes, hypertension, and waist size. Final models for isoprostanes are adjusted for ambient temperature, sex, diabetes, smoking status, systolic blood pressure, waist to hip ratio, hyperlipidemia, hsCRP, and aspirin use. Final models for cytokines are adjusted for ambient temperature, diabetes, smoking status, systolic blood pressure, waist to hip ratio, hsCRP, and ACE inhibitor use. We also included interaction terms to test for effect modification by sex and diabetes status, but interactions were not significant and were dropped from final models. As a sensitivity analysis, we performed augmented backward elimination for confounder identification (Supplemental Table 1).20 We also performed separate models using 3-day and 7-day PM2.5 levels (Supplemental Table 2), as well as adjustments for humidity and season (Supplemental Table 3). Seasonal variations were adjusted for by including sine and cosine terms of calendar dates for the day of blood draw. Predicted mean results were obtained for RHI, and plotted against PM2.5 levels, along with 95% confidence limits, and 95% prediction limits. All effect estimates were back transformed from the log scale to represent a percent change of the outcome, along with corresponding 95% confidence intervals per 10μg/m3 PM2.5. Statistical significance was accepted at p-value <0.05. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina) and GraphPad Prism, version 7 (GraphPad Software, La Jolla, California).
RESULTS
Participant characteristics
Participants in this study were of middle age (48.1 ± 10.6 yr), mostly female (56%), and overweight (avg. BMI=33.3) (Table 1). The racial makeup was representative of the metropolitan Louisville area as a whole (62% Caucasian, 28% Black). Our study group presented with a range of CVD risk levels where 21–52% of the participants presented with a CVD risk factor while 5–36% were on some medication to improve cardiovascular function. Total cholesterol levels were borderline high (190.3mg/dL), while HDL, LDL, and triglyceride levels were within normal ranges.
Table 1.
Cohort Demographics and Cardiovascular Disease History (n=100)
| Characteristics | Frequency |
|---|---|
| Sex | |
| Male | 44 |
| Race | |
| White | 62 |
| Black | 28 |
| Other | 10 |
| CVD Risk Factors | |
| RHI (<1.67) | 23 |
| Hypertension | 52 |
| Hyperlipidemia | 39 |
| Diabetes | 44 |
| Current smoker | 21 |
| Former smoker | 12 |
| Medications | |
| Metformin | 36 |
| Insulin | 28 |
| ACE | 31 |
| ARB | 9 |
| β-Blocker | 21 |
| Calcium-channel blocker | 17 |
| Diuretics | 35 |
| Statins | 36 |
| Vasodilators | 5 |
| Aspirin | 31 |
| Continuous Characteristics | Means (SD) |
| Age | 48.1 (10.6) |
| BMI | 33.26 (8.44) |
| WHR | 0.95 (0.16) |
| Waist size | 42.19 (8.72) |
| hsCRP | 3.51 (4.38) |
| Systolic BP (mmHg) | 128.0 (20.2) |
| Diastolic BP (mmHg) | 80.0 (10.6) |
| Lipid Levels (md/dL) | |
| Cholesterol | 190.3 (43.8) |
| HDL | 47.5 (16.4) |
| LDL | 102.1 (29.2) |
| Triglycerides | 147.3 (130.5) |
| Temperature (°C) | 11.63 (12.50) |
| Humidity (%) | 22.51 (13.33) |
| PM2.5 (μg/m3) | 11.45 (5.07) |
Abbreviations: BMI: body mass index (weight(kg)/height(m)2); WHR: waist to hip ratio; hsCRP: high sensititvity C-reactive protein; CVD: cardiovascular disease; ACE: angiotensin-converting enzyme inhibitor; ARB: angiotensin-receptor blockers.
Particulate matter air pollution and endothelial function
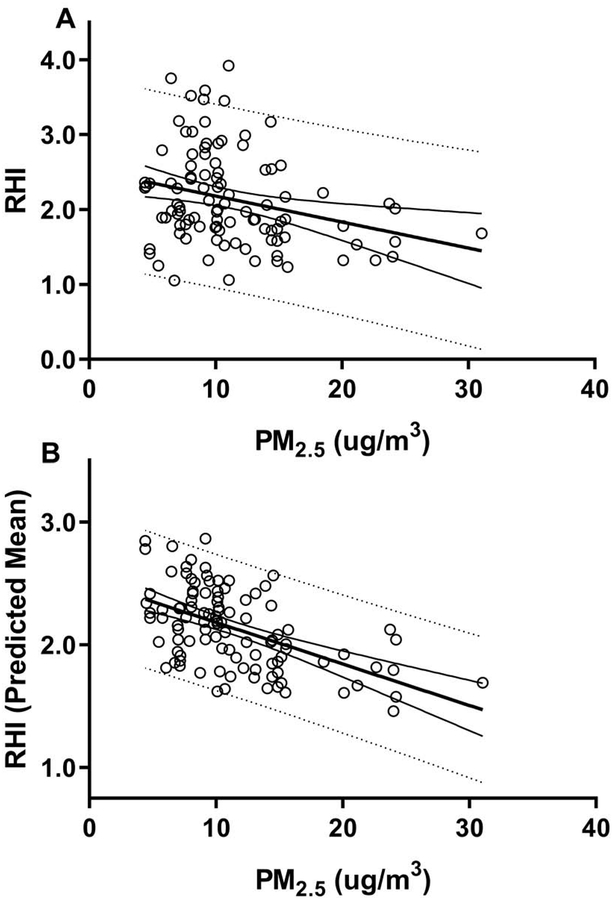
Using a generalized linear model with gamma distribution we determined associations between RHI and average PM2.5 levels on the day of blood draw. We found a statistically significant negative association between PM2.5 levels and RHI (Figure 2). Specifically, there was a 12.4% decrease in RHI for every 10μg/m3 increase in PM2.5 (Table 2). These negative associations were found for both the unadjusted RHI (Figure 2A) and for the adjusted model (Figure 2B). No association was observed when the analysis was done using 3- and 7-day PM2.5 exposure levels (Supplemental Table 2), but the associations were maintained when adjusting for humidity and season (Supplemental Table 3).
Figure 2. PM2.5 and RHI.
Illustrated are the regression analyses between PM2.5 and unadjusted RHI (A) and adjusted predicted mean RHI (B) with 95% confidence limits, and 95% prediction limits. The predicted mean results (B) were generated from a generalized linear model with gamma distribution, adjusting for temperature, sex, diabetes, hypertension, and waist size.
Table 2.
Associations between PM2.5, and reactive hyperemia index, isoprostanes, and cytokines.
| Model 1 (unadjusted) | Model 2 (adjusted) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | n | Mean (SD) | % Change* | 95% C.I. | P-Value | % Change* | 95% C.I. | P-Value |
| RHI | 100 | 2.14 (0.64) | −16.2 | −24.9, −6.6 | 0.002 | −12.4 | −21.0, −2.7 | 0.014 |
| Isop | 91 | 2.24 (2.26) | −7.3 | −30.0, 22.6 | 0.594 | 19.4 | −16.9, 71.6 | 0.339 |
| Isom | 91 | 0.93 (0.61) | 21.2 | −1.6, 49.2 | 0.071 | 28.4 | 2.7, 60.3 | 0.028 |
| Angiogenin | 80 | 121.10 (15.64) | −3.1 | −9.3, 3.5 | 0.347 | −4.7 | −10.4, 1.4 | 0.129 |
| Angiopoietin1 | 80 | 615.11 (190.44) | 24.5 | 7.5, 44.2 | 0.004 | 17.4 | 2.8, 33.8 | 0.017 |
| Ang2 | 80 | 3.33 (1.65) | 14.8 | −11.0, 48.1 | 0.286 | 3.1 | −19.2, 31.7 | 0.804 |
| VEGF | 80 | 14.70 (3.42) | 16.8 | 4.4, 30.5 | 0.007 | 10.4 | 0.6, 21.0 | 0.037 |
| VEGFd | 80 | 222.90 (15.71) | 1.9 | −1.6, 5.5 | 0.277 | 1.3 | −2.1, 4.7 | 0.468 |
| VEGFc | 80 | 103.34 (47.71) | 9.9 | −16.6, 44.6 | 0.505 | 5.4 | −19.5, 38.3 | 0.699 |
| PIGF | 80 | 3.35 (1.24) | 39.8 | 16.9, 67.0 | <0.001 | 31.7 | 12.2, 54.5 | <0.001 |
| SCF | 80 | 51.17 (14.83) | −3.8 | −17.1,11.5 | 0.602 | −6.7 | −19.6, 8.3 | 0.366 |
| EGF | 80 | 2.23 (1.06) | 10.4 | −12.3, 39.1 | 0.397 | 9.2 | −13.0, 37.0 | 0.449 |
| PDGFBB | 80 | 43.62 (20.55) | 21.9 | −3.1, 53.6 | 0.092 | 24.2 | −0.1, 54.5 | 0.051 |
| BFGF | 80 | 122.61 (11.60) | 1.2 | −3.4, 6.1 | 0.605 | −0.3 | −4.6, 4.2 | 0.893 |
| HGF | 80 | 64.65 (25.29) | 25.0 | 4.0, 50.4 | 0.017 | 10.1 | −3.3, 25.2 | 0.147 |
| Epo | 80 | 1.09 (0.11) | 5.1 | 0.1, 10.4 | 0.044 | 4.5 | −0.5, 9.7 | 0.079 |
| Eselectin | 80 | 25.71 (9.75) | 20.1 | −0.4, 44.6 | 0.055 | 11.0 | −4.3, 28.7 | 0.170 |
| VCAM1 | 80 | 581.11 (201.14) | −13.2 | −26.3, 2.1 | 0.088 | −15.9 | −28.3, −1.3 | 0.034 |
| ICAM1 | 80 | 193.49 (83.01) | 32.2 | 6.3, 64.5 | 0.012 | 24.6 | 1.6, 52.8 | 0.035 |
| MMP2 | 80 | 197.75 (58.47) | 4.5 | −17.6, 11.0 | 0.560 | −4.6 | −17.1, 9.9 | 0.516 |
| MMP9 | 80 | 18.22 (7.75) | 29.0 | 5.9, 57.5 | 0.012 | 30.3 | 8.0, 57.5 | 0.006 |
Represents % change per 10μg/m3 PM2.5. Generalized linear models with gamma distribution were used to test associations between outcomes and PM2.5. Isoprostanes and cytokines were measured in a subset of participants, n=91 and n=80, respectively. RHI was adjusted for temperature, sex, diabetes, hypertension, and waist size; isoprostanes were adjusted for temperature, sex, diabetes, current smokers, systolic blood pressure, waist to hip ratio, hyperlipidemia, hsCRRP, and aspirin use; cytokines were adjusted for temperature, diabetes, current smokers, systolic blood pressure, waist to hip ratio, hsCRP, and ACE inhibitor use. RHI indicated reactive hyperemia index; Isop, F2-isoprostane; Isom, F-2 isoprostane metabolite; Ang2, angiopoietin-2; VEGF, vascular endothelial growth factor; PlGF, placental growth factor; SCF, stem cell factor; EGF, epidermal growth factor; PDGFBB, platelet derived growth factor-BB; BFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; Epo, erythropoietin; VCAM1, vascular cellular adhesion molecule 1; ICAM1, intercellular adhesion molecule 1; MMP2, matrix metalloproteinase-2; MMP9, matrix metalloproteinase-9.
Particulate matter air pollution, pro-angiogenic molecules and oxidative stress
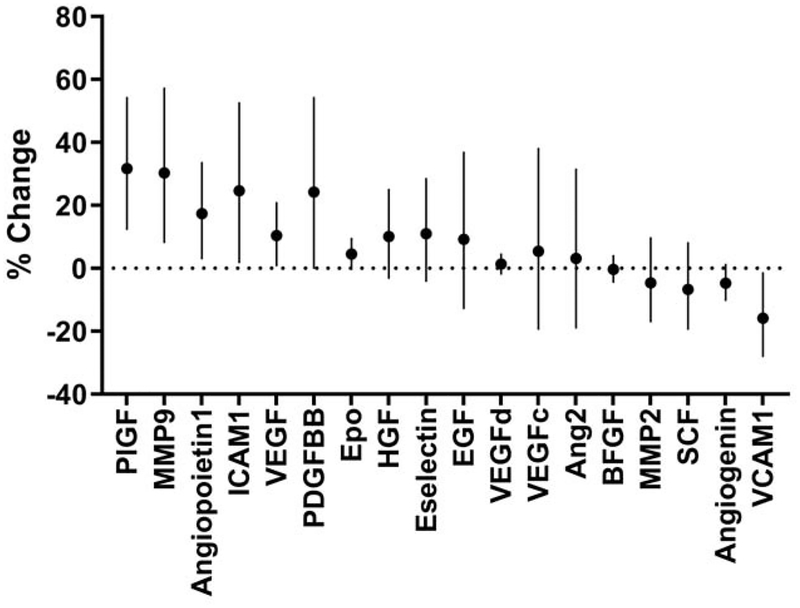
Circulating levels of PlGF, angiopoietin1, VEGF, and ICAM-1 showed a positive association with PM2.5 levels after adjustment for temperature, diabetes, smoking, systolic blood pressure, waist to hip ratio, hsCRP and ACE inhibitor use (Table 2; Figure 3). In addition, MMP-9, which supports new vessel growth, showed a positive association with PM2.5. In contrast, VCAM-1 demonstrated a negative association with air pollution levels (Table 2; Figure 3). Finally, when adjusted for temperature, sex, diabetes, smoking, systolic blood pressure, waist to hip ratio, hyperlipidemia, hsCRP and aspirin use, we found a positive association of PM2.5 with the F2 isoprostane metabolite (IsoM; Table 2) but not with F2-isoprostane (IsoP; Table 2). Fewer associations were observed when using 3- and 7-day PM2.5 exposure levels (Supplemental Table 2), but associations were maintained when adjusting for humidity and season (Supplemental Table 3).
Figure 3. Associations between plasma biomarkers and PM2.5.
The biomarkers include 18 analytes supportive of vascular function and angiogenesis or indicative of inflammation. Results are presented as percent change (and 95% confidence intervals) per 10 μg/m3 increase of PM2.5. Generalized linear models with gamma distribution were used to test associations between outcomes and PM2.5 (n=80). Models are adjusted for temperature, diabetes, current smokers, systolic blood pressure, waist to hip ratio, hsCRP, and ACE inhibitor use. Ang2 indicates angiopoietin-2; VEGF, vascular endothelial growth factor; PlGF, placental growth factor; SCF, stem cell factor; EGF, epidermal growth factor; PDGFBB, platelet derived growth factor-BB; BFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; Epo, erythropoietin; VCAM1, vascular cellular adhesion molecule 1; ICAM1, intercellular adhesion molecule 1; MMP2, matrix metalloproteinase-2; MMP9, matrix metalloproteinase-9.
DISCUSSION
While the associations between PM2.5 exposure and cardiovascular morbidity and mortality suggest that PM2.5 might directly affect endothelial integrity or function, there is little evidence to support this assertion. Although the direct application of PM2.5 particles to endothelial cells in culture induced oxidative stress and subsequent responses such as autophagy,21, 22 these outcomes were identified in a non-physiological model of exposure. Even in animal models, only the ex vivo constriction and relaxation of isolated endothelial vessels has been studied.23 In still other mouse or human studies, endothelial dysfunction has been assessed indirectly by measuring the expression levels of endothelin-1 and eNOS.24, 25 While some human-based studies do make direct assessments of endothelial function and identify associations with PM2.5 levels, these findings were limited to specific cohort populations. For instance, PM2.5 levels have been found to be negatively associated with endothelial dysfunction as identified by flow mediated dilation or a reactive hyperemia index, but these studies only used diabetics26, 27 or an elderly population,28 respectively. When using a more diverse cohort, another study identified negative associations with measured endothelial function but only in diabetics and not in non-diabetics.29 Still other, related studies found negative associations but only when analyzing one-year average PM2.5 exposure trends30 or found no association at all between measured exposure or intake and RHI.31 Thus, the present study has several advantages over previous human or animal studies in so far as it provides strong support to the idea that PM2.5 inhalation induces endothelial dysfunction. First, rather than quantifying biomarkers reflective of endothelial function, we assess function by calculating a reactive hyperemia index. Second, since our study group has a mix of CVD risk, our results indicate that the effects of PM2.5 on endothelial function is independent of CVD risk status. In fact, we continued to observe the association after adjustment for diabetes. Finally, we observed these associations when using measures of average daily PM2.5 levels. Thus, endothelial dysfunction resulting from acute PM2.5 inhalation may be pervasive in the general population.
We found that several growth factors (PlGF, angiopoietin-1, VEGF, PDGF-BB, erythropoietin) and signaling molecules (MMP9) that support angiogenesis showed a significant, or near significant, positive association with PM2.5 exposure. Our observed positive associations with VEGF and PDGF levels are consistent with the results from a recent meta-analysis of VEGF expression32 and a cell culture study of particle-induced PDGF expression,33 respectively. Similarly, positive associations with MMP-9 have also been observed in a cell culture model of exposure,34 in a human exposure study,35 and in endothelial36 and pulmonary37 tissues in murine models of exposure. Thus, persistent vascular injury resulting from exposure might stimulate the expression of several pro-angiogenic agonists to maintain vascular function. However, this assertion seems to be inconsistent with our measured assessment of vascular function, which identified impairments (decreased RHI) with increasing PM2.5. This apparent discrepancy may be due to resistance in downstream signaling that results in greater production of cytokines and growth factors. This is consistent with our previous findings in mice which showed that PM exposure induces resistance to VEGF signaling and limits EPC mobilization.15 Thus even in the presence of increased levels of VEGF, such as in the present study, reparative cells may have limited responsiveness to this agonist and angiogenesis or endothelial function would remain impaired. While a complete understanding of the mechanisms whereby PM2.5 impacts signaling downstream of the VEGF receptor is lacking, similar reductions in the efficacy of growth factor signaling might also account for the discrepancies between increased PlGF, angiopoietin1, PDGF-BB, and erythropoietin and impaired vascular function.
It is interesting to point out that the effect of PM2.5 on some growth factors differed between the current study and previous work from our group.12 Thus, while VEGF, PDGF, and EGF demonstrated an increase with PM2.5 in the current study (using participants residing in metropolitan Louisville, KY), we observed that these same growth factors decreased with PM2.5 in the previous study (using a Provo, Utah cohort). Although the basis for these differences is uncertain, they may be reflective of the different participants used in these studies as somewhat older participants of variable CVD risk were studied here, while healthy individuals participated in the previous study. Thus, while PM2.5 exposures may decrease VEGF (and other pro-angiogenic molecules) acutely in a healthy population, this outcome may be reversed in a more at-risk population presenting with other confounders and co-morbidities. Alternatively, the observed differences in growth factor expression may be reflective of differences in PM2.5 composition between the two locales. Little is known about the impact of individual PM2.5 components on growth factor expression or signaling competency. Finally, the observed differences in growth factor levels between the two studies might be reflective of experimental protocol and exposure duration. Blood in the Provo, UT study was collected at times of high PM2.5 during a wintertime inversion episode, when PM2.5 levels rapidly increase over a short time period (1–2 days). Thus while an acute increase in PM2.5 may deplete existing stores of certain growth factors in the Utah cohort, persistent exposure to lower levels of PM2.5 may serve as a stimulus for growth factor expression in the Louisville study participants.
Progressive atherosclerotic disease is a major adverse outcome associated with chronic exposure to PM2.5,38, 39 and extensive studies have shown that atherogenesis is facilitated by increase cell adhesion. In the present study we found a positive association of ICAM-1 with PM2.5, suggesting that exposure can increase atherogenesis by promoting cell adhesion. Indeed, increased adhesion molecule expression seems to be a common outcome of PM2.5 exposure.12, 40, 41 However, in contrast to the results obtained with ICAM-1, we observed a negative association with VCAM-1. While the responses of ICAM-1 and VCAM-1 are generally similar, the divergent outcomes in this study may result from some unique characteristic of our study population or local particle composition leading to the differential responses of these adhesion molecules. A clarification of these differing outcomes awaits further study.
The diverse pathological outcomes deriving from particulate inhalation may derive from the initiation of oxidative stress.42 It has been suggested that biomolecules (e.g. lipids) oxidized in the tissue of first exposure (lungs) are distributed throughout the vasculature to affect the function of distal cells and tissues.43 PM2.5-induced oxidative stress could also be a strong driver of the atherosclerotic response characteristic of chronic expsoures.44 Our earlier animal studies support the idea that pathologies associated with PM2.5 exposure result from oxidative stress. Specifically, we found that pulmonary over-expression of extracellular superoxide dismutase (ecSOD) in mice mitigated PM2.5-induced defects in EPC function and increased their availability as well.14 Similarly, ecSOD over-expression limited PM2.5-induced vascular insulin resistance.7 Nevertheless, the results derived from the use of these anti-oxidants are only correlative and direct evidence that PM2.5 exposure induces oxidative stress is minimal. To address this issue, we measured urinary levels of isoprostanes. These products of oxidized lipids have found widespread use as indicators of oxidative stress and their levels are increased upon acute exposure to particles generated from multiple sources.45–48 In this study, we specifically identified a positive association between PM2.5 levels and the F2-isoprostane metabolite, but not the parent compound, F2-isoprostane. Consistent with these findings, metabolite levels have been shown to be a more robust indicator of oxidative stress.49
Although our study provides strong support for an association between exposure to PM2.5 and endothelial dysfunction, it has some limitations. First, the size of the study was relatively small, consisting mostly of middle aged participants, which might limit the generalizability of our findings. Second, many participants had multiple risk factors for cardiovascular disease, necessitating adjustments for multiple confounders. Third, the study has a cross-sectional design and only single measures were obtained from each participant, precluding any causal inferences. Finally, we did not assess exposure at an individual level. Therefore, we cannot account for specific residential or occupational exposure to PM2.5 at levels which may deviate from that in the larger metropolitan Louisville area.
Nevertheless, we found a consistent and robust association between PM2.5 and impaired vascular function. Based on our measurements of multiple biomarkers, we speculate that these impairments result from oxidative stress and occur despite increases in pro-angiogenic signaling molecules. Taken together, the findings of this study provide important evidence suggesting that acute exposure to airborne particulate matter directly impacts endothelial function, thereby leading to an increase in the risk of cardiovascular morbidity and mortality.
Supplementary Material
Highlights.
Exposure to fine airborne particulate matter (PM2.5) is associated impaired vascular function.
Exposure to PM2.5 is associated with oxidative stress and changes in plasma levels of growth factors and cytokines.
These effects may underlie the association of PM2.5 exposure with increased cardiovascular morbidity and mortality.
Acknowledgements:
This work was supported in part by grants from the NIH (RO1 ES019217, P42 ES023716).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare that they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The study was approved by the Institutional Review Board (IRB number 10.0350) at the University of Louisville, and all participants gave written informed consent.
References
- 1.Chen H, Goldberg MS and Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23:243–97. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Ambient air pollution: a global assessment of exposure and burden of disease. 2016.
- 3.Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D and Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–7. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O’Neill MS, Herrington DM, Polak JF and Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). Journal of the American College of Cardiology. 2012;60:2158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Kopp A, Brook JR and Copes R. Spatial association between ambient fine particulate matter and incident hypertension. Circulation. 2014;129:562–9. [DOI] [PubMed] [Google Scholar]
- 7.Haberzettl P, O’Toole TE, Bhatnagar A and Conklin DJ. Exposure to Fine Particulate Air Pollution Causes Vascular Insulin Resistance by Inducing Pulmonary Oxidative Stress. Environ Health Perspect. 2016;124:1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC and Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S and Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope CA 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL and Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–8. [DOI] [PubMed] [Google Scholar]
- 11.Kunzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J and Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environmental health perspectives. 2005;113:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ and O’Toole T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ Res. 2016;119:1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, Bhatnagar A and Pope CA 3rd. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010;107:200–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haberzettl P, Conklin DJ, Abplanalp WT, Bhatnagar A and O’Toole TE. Inhalation of Fine Particulate Matter Impairs Endothelial Progenitor Cell Function Via Pulmonary Oxidative Stress. Arterioscler Thromb Vasc Biol. 2018;38:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberzettl P, Lee J, Duggineni D, McCracken J, Bolanowski D, O’Toole TE, Bhatnagar A and Conklin DJ. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ Health Perspect. 2012;120:848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zafar N, Krishnasamy SS, Shah J, Rai SN, Riggs DW, Bhatnagar A and O’Toole TE. Circulating angiogenic stem cells in type 2 diabetes are associated with glycemic control and endothelial dysfunction. PLoS One. 2018;13:e0205851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO and Lerman A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–86. [DOI] [PubMed] [Google Scholar]
- 19.Milne GL, Sanchez SC, Musiek ES and Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–6. [DOI] [PubMed] [Google Scholar]
- 20.Dunkler D, Plischke M, Leffondré K and Heinze G. Augmented backward elimination: a pragmatic and purposeful way to develop statistical models. PloS one. 2014;9:e113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deweirdt J, Quignard JF, Crobeddu B, Baeza-Squiban A, Sciare J, Courtois A, Lacomme S, Gontier E, Muller B, Savineau JP, Marthan R, Guibert C and Baudrimont I. Involvement of oxidative stress and calcium signaling in airborne particulate matter - induced damages in human pulmonary artery endothelial cells. Toxicol In Vitro. 2017;45:340–350. [DOI] [PubMed] [Google Scholar]
- 22.Wang JS, Tseng CY and Chao MW. Diesel Exhaust Particles Contribute to Endothelia Apoptosis via Autophagy Pathway. Toxicol Sci. 2017;156:72–83. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Qin X, Qiu L, Chen S, Zhou H, Xu Y, Hu Z, Zhang Y, Cao Q and Ying Z. Concentrated Ambient PM2.5-Induced Inflammation and Endothelial Dysfunction in a Murine Model of Neural IKK2 Deficiency. Environ Health Perspect. 2018;126:027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon-Garciduenas L, Franco-Lira M, D’Angiulli A, Rodriguez-Diaz J, Blaurock-Busch E, Busch Y, Chao CK, Thompson C, Mukherjee PS, Torres-Jardon R and Perry G. Mexico City normal weight children exposed to high concentrations of ambient PM2.5 show high blood leptin and endothelin-1, vitamin D deficiency, and food reward hormone dysregulation versus low pollution controls. Relevance for obesity and Alzheimer disease. Environ Res. 2015;140:579–92. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Ji X, Ku T and Sang N. Inflammatory response and endothelial dysfunction in the hearts of mice co-exposed to SO2, NO2, and PM2.5. Environ Toxicol. 2016;31:1996–2005. [DOI] [PubMed] [Google Scholar]
- 26.Schneider A, Neas L, Herbst MC, Case M, Williams RW, Cascio W, Hinderliter A, Holguin F, Buse JB, Dungan K, Styner M, Peters A and Devlin RB. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. 2008;116:1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanobetti A, Luttmann-Gibson H, Horton ES, Cohen A, Coull BA, Hoffmann B, Schwartz JD, Mittleman MA, Li Y, Stone PH, de Souza C, Lamparello B, Koutrakis P and Gold DR. Brachial artery responses to ambient pollution, temperature, and humidity in people with type 2 diabetes: a repeated-measures study. Environ Health Perspect. 2014;122:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM, Hasheminassab S, Pakbin P, Longhurst J, Sioutas C and Delfino RJ. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ Health. 2016;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES and Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–20. [DOI] [PubMed] [Google Scholar]
- 30.Erqou S, Clougherty JE, Olafiranye O, Magnani JW, Aiyer A, Tripathy S, Kinnee E, Kip KE and Reis SE. Particulate Matter Air Pollution and Racial Differences in Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol. 2018;38:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole CA, Carlsten C, Koehle M and Brauer M. Particulate matter exposure and health impacts of urban cyclists: a randomized crossover study. Environ Health-Glob. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Wang Y, Yuan S, Wen J, Li W, Yang L, Huang X, Mo Y, Zhao Y and Lu Y. Exposure to PM2.5 via vascular endothelial growth factor relationship: Meta-analysis. PLoS One. 2018;13:e0198813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou W, He F, Liu S, Pu J, Hu J, Sheng Q, Zhu T, Zhu T, Li B and Ran P. PM2.5 Induced the Expression of Fibrogenic Mediators via HMGB1-RAGE Signaling in Human Airway Epithelial Cells. Can Respir J. 2018;2018:1817398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun YJ, Piao MJ, Kang KA, Zhen AX, Madushan Fernando PDS, Kang HK, Ahn YS and Hyun JW. Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Mar Drugs. 2019;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Wang T, Liu S, Brook RD, Feng B, Zhao Q, Song X, Yi T, Chen J, Zhang Y, Wang Y, Zheng L, Rajagopalan S, Li J and Huang W. Extreme Levels of Air Pollution Associated with Changes in Biomarkers of Atherosclerotic Plaque Vulnerability and Thrombogenicity in Healthy Adults: The Beijing AIRCHD Study. Circ Res. 2019. [DOI] [PubMed] [Google Scholar]
- 36.Dai J, Chen W, Lin Y, Wang S, Guo X and Zhang QQ. Exposure to Concentrated Ambient Fine Particulate Matter Induces Vascular Endothelial Dysfunction via miR-21. Int J Biol Sci. 2017;13:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang B, Guo J and Xiao C. Effect of PM2.5 environmental pollution on rat lung. Environ Sci Pollut Res Int. 2018;25:36136–36146. [DOI] [PubMed] [Google Scholar]
- 38.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ and Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC and Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–10. [DOI] [PubMed] [Google Scholar]
- 40.Chiu YH, Garshick E, Hart JE, Spiegelman D, Dockery DW, Smith TJ and Laden F. Occupational vehicle-related particulate exposure and inflammatory markers in trucking industry workers. Environ Res. 2016;148:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilker EH, Alexeeff SE, Suh H, Vokonas PS, Baccarelli A and Schwartz J. Ambient pollutants, polymorphisms associated with microRNA processing and adhesion molecules: the Normative Aging Study. Environ Health. 2011;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC and Rajagopalan S. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci. 2009;111:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H and Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kattoor AJ, Pothineni NVK, Palagiri D and Mehta JL. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep. 2017;19:42. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr., Lin H, Vasan RS, Benjamin EJ and Mittleman MA. Short-Term Exposure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters B, Ballmann C, Quindry T, Zehner EG, McCroskey J, Ferguson M, Ward T, Dumke C and Quindry JC. Experimental Woodsmoke Exposure During Exercise and Blood Oxidative Stress. J Occup Environ Med. 2018;60:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossner P Jr., Svecova V, Milcova A, Lnenickova Z, Solansky I, Santella RM and Sram RJ. Oxidative and nitrosative stress markers in bus drivers. Mutat Res. 2007;617:23–32. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Lintelmann J, Klingbeil S, Li J, Wang H, Kuhn E, Ritter S and Zimmermann R. Determination of air pollution-related biomarkers of exposure in urine of travellers between Germany and China using liquid chromatographic and liquid chromatographic-mass spectrometric methods: a pilot study. Biomarkers. 2017;22:525–536. [DOI] [PubMed] [Google Scholar]
- 49.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng X, Franke A, Roberts LJ, Milne G, Zheng W and Dai Q. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. 2012;96:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.