Abstract
Type-1 diabetes (T1D) increases systemic inflammation, bone loss, and risk for bone fractures. Levels of the anti-inflammatory cytokine IL-10 are decreased in T1D, however their role in T1D-induced osteoporosis is unknown. To address this, diabetes was induced in male IL-10 knockout (KO) and wild-type (WT) mice. Analyses of femur and vertebral trabecular bone volume fraction (BVF) identified bone loss in T1D-WT mice at 4- and 12-weeks which in T1D-IL-10-KO mice was further reduced at 4 but not 12 weeks. IL-10 deficiency also increased the negative effects of T1D on cortical bone. Osteoblast marker, osterix was decreased while osteoclast markers were unchanged, suggesting that IL-10 promotes anabolic processes. MC3T3-E1 osteoblasts cultured under high glucose conditions displayed a decrease in osterix which was prevented by addition of IL-10. Together, our results suggest that IL-10 is important for promoting osteoblast maturation and reducing bone loss during early stages of T1D.
Introduction
Diabetes mellitus is a chronic metabolic disease that occurs when the pancreatic β-cells do not produce sufficient insulin or when the body is unable to utilize the insulin. It affects millions of people in the US and worldwide and its prevalence is increasing at an alarming rate [Centers for Disease Control and Prevention, 2014]. While type 1 diabetes (T1D) is characterized by destruction of pancreatic β-cells by autoreactive T-cells, type 2 diabetes (T2D) is characterized by insulin resistance [Atkinson and Maclaren, 1994][Lebovitz, 1999]. The systemic hyperglycemia, metabolic derrangements and inflammation that occur in diabetes lead to a number of complications including nephropathy, cardiomyopathy, neuropathy, retinopathy and osteoporosis. Bone loss is an increasing concern for diabetic patients, especially T1D, which has been reported to increase the risk of hip fracture by 2–8 fold [Hofbauer et al., 2007][Shah et al., 2016][Weber and Schwartz, 2016][Miao et al., 2005]. Male and female T1D animal models also display reduced bone formation and bone loss similar to humans [Eller-Vainicher et al., 2011][Coe et al., 2013][Bonds et al., 2006; Burghardt et al., 2010; Hanley et al., 2003; Petit et al., 2010; Strotmeyer et al., 2004][Martin and McCabe, 2007]. T1D-induced bone loss can be exacerbated by factors such as estrogen deficiency [Raehtz et al., 2017], drug therapies [Chen et al., 2015; Coe et al., 2015] and inflammation [Motyl et al., 2009].
Bone remodeling is a highly-regulated process that requires precise balance between osteoblasts (bone forming cells) and osteoclasts (bone resorbing cells). Imbalance in this process can lead to bone loss. We and others have shown that T1D bone loss is associated with an anabolic defect characterized by decreased osteoblast lineage selection and differentiation as well as increased osteoblast death [Motyl et al., 2009][Motyl and McCabe, 2009][Coe et al., 2013]. Both T1D patients and mouse models exhibit decreased levels of serum osteocalcin (osteoblast marker). In addition, as a result of altered lineage selection and lower Wnt10B signaling, T1D mice display increased bone marrow adiposity [Botolin and McCabe, 2007; Zhang et al., 2015][Pietschmann et al., 1988][Motyl and McCabe, 2009][Zhang et al., 2015][McCabe, 2007]. T1D effect on osteoclast activity however, is less clear and dependent on the specific animal model used and disease severity [McCabe et al., 2011][Verhaeghe et al., 2000][Motyl and McCabe, 2009]. Bone remodeling is also modulated by many factors including cytokines [Raehtz et al., 2017][Chiang et al., 1999; Irwin et al., 2013; Motyl et al., 2009; Zhang et al., 2001]. A role for inflammatory cytokines in T1D bone pathology has been suggested by systemic and local increases in cytokines during the pathogenesis of T1D [Erbagci et al., 2001][Snell-Bergeon et al., 2010][Motyl et al., 2009][Motyl et al., 2009][Schloot et al., 2002]. Circulating interferon gamma (IFN-γ) has been shown to be increased with age in the non-obese diabetic (NOD) animal model [Schloot et al., 2002]. However, IFN-γ deficient mice are not protected from T1D induced bone loss, suggesting potential compensation by, or role for other cytokines in T1D effects on bone density [Motyl et al., 2009]. Several studies including ours have shown that levels of tumor necrosis factor alpha (TNF-α) are increased and play an important role in diabetes induced bone loss [Qiao et al., 2017][Motyl et al., 2009][Raehtz et al., 2017][Ko et al., 2016]. Furthermore, we have also demonstrated an increase in expression of IFN-γ, interleukin-1 (IL-1) and interleukin-6 (IL-6) in the bone of T1D male mice [Motyl et al., 2009]. Also, during estrogen deficiency along with T1D, high levels of TNF-α correlated with bone loss and osteoblast death [Raehtz et al., 2017]. Studies have also shown that inhibition of TNF-α during diabetes can prevent a decrease in callus bone formation induced by T1D [Ko et al., 2016]. In contrast to increases in pro-inflammatory cytokines, the anti-inflammatory cytokine IL-10 has been shown to be markedly decreased prior to diabetic onset in the NOD mice [Schloot et al., 2002]. Interestingly, administration of IL-10 protected NOD mice from developing diabetes [Pennline et al., 1994]. Similar to NOD mice, diabetic patients exhibit significantly low serum IL-10 compared to non-diabetic subjects [Van Exel et al., 2002]. In spite of these studies on IL-10 levels in diabetes, role of IL-10 in the bone during diabetes is not known.
Previous studies have demonstrated an important role for IL-10 in bone homeostasis [Al-Rasheed et al., 2004][Alayan et al., 2007][Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, 2003][Claudino et al., 2010][Dresner-Pollak et al., 2004]. Studies have found that IL-10 KO mice, in addition to showing features of colitis, also demonstrate systemic skeletal disease with reduced bone mass, decreased bone formation and increased mechanical fragility [Al-Rasheed et al., 2004][Alayan et al., 2007][Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, 2003] [Claudino et al., 2010][Dresner-Pollak et al., 2004]. Bone loss in IL-10 KO mice was more severe in mice with colitis compared to mice without colitis [Dresner-Pollak et al., 2004]. Similarly, 9-months old IL-10 KO mice have accelerated alveolar bone loss compared to age matched WT controls [Al-Rasheed et al., 2004]. While the mechanisms by which IL-10 regulates bone health is not completely understood, IL-10 has been shown to have direct effects on both osteoblasts (increase in osteoblast differentiation) and osteoclasts (inhibit osteoclast differentiation) [Evans and Fox, 2007][Hong et al., 2010][Xu et al., 1995][Dresner-Pollak et al., 2004]. Even though past studies have shown that IL-10 levels are decreased during T1D and that IL-10 has direct effects on osteoblasts and osteoclasts, the role of IL-10 in T1D-mediated bone pathology is not known. We demonstrate here that IL-10 regulation of osteoblasts plays an important role in T1D-induced bone loss during early but not later stages of disease.
Materials and Methods
Animals and Experimental Design
B6.129P2-Il10tm1Cgn/J mutant mice (IL-10−/−) and C57BL/6J male mice (6–7 weeks old) were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were allowed to acclimate to animal facility for 1-week prior to start of the experiment. After this period mice were randomly divided into four groups (8–10 per group): wild-type control, wild-type type 1 diabetes, IL-10−/− control and IL-10−/− type 1 diabetes. To induce diabetes, mice were injected (intraperitoneally) with 65 mg/kg body weight streptozotocin (STZ) for 5 consecutive days. Control mice received 0.1 M sodium citrate buffer (vehicle) for the same time period. Insulin injections (0.1 unit) were given to mice that lost 15% of their body weight. Blood glucose was measured four weeks after the first injection with an AccuChek compact glucometer (Roche), by collecting a drop of blood from the pedal dorsal vein. Mice with a blood glucose of >250 mg/dL were considered diabetic. Mice (4 – 5 per cage) were maintained on a 12-hour light/dark cycle at 23°C and provided food and water ad libitum. Tissues were collected at 1- and 3-months after the first injection. All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee and conformed to NIH guidelines.
μCT Bone Imaging
At harvest, femur and vertebrate bones were fixed in 10% formalin for 24 hours. After 24 hours, bones were transferred to 70% ethanol and scanned using a GE Explore Locus microcomputed tomography (μCT) system at a voxel resolution of 20 μm obtained from 720 views. The beam angle of increment was 0.5, and beam strength was set at 80 peak kV and 450 μA. Each run included bones from WT, KO (control and T1D groups), as well as a calibration phantom to standardize gray scale values and maintain consistency. Bone measurements were performed blind. On the basis of auto-threshold and isosurface analyses of multiple bone samples, a fixed threshold (799) was used to separate bone from bone marrow. Femur trabecular bone analyses were performed from ~ 1% of the total length proximal to the growth plate, extending 10% of bone length toward the diaphysis and excluding the cortical bone. Trabecular bone mineral content (BMC), bone mineral density (BMD), percent bone volume fraction (% BVF), trabecular thickness (Tb. Th), spacing (Tb. Sp), and number (Tb. N) were computed using GE Healthcare MicroView software. Femoral trabecular isosurface images were taken from a region in the femur where analyses were performed measuring 1.0 mm in length and 1.0 mm in diameter. For vertebral analysis, the third lumbar vertebrae were used. Cortical measurements were performed in a 2×2×2 mm cube centered midway down the length of the bone.
Bone and Intestine RNA analysis
Immediately after euthanasia, bone samples were cleaned of all muscle and connective tissue, snap frozen in liquid nitrogen and stored at −80°C until RNA extraction [Raehtz et al., 2017]. Intestinal samples were flushed of their contents with 1X PBS and frozen in liquid nitrogen and stored at −80 C until further processing [Lee et al., 2013]. Frozen tissues were crushed under liquid nitrogen conditions using the Bessman Tissue Pulverizer (Spectrum Laboratories, Rancho Dominguez, CA). TriReagent (Molecular Research Center, Cincinnati, OH) was used to isolate RNA and RNA integrity was verified by agarose-gel electrophoresis. cDNA was synthesized by reverse transcription using Superscript II Reverse Transcriptase Kit and oligo dT primers (Invitrogen, Carlsbad, CA). Complementary DNA was amplified by PCR using iQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA). Real time PCR was carried out for 40 cycles (95° C for 15 seconds, 60° C for 30 seconds, and 72° C for 30 seconds) using an iCycler thermal cycler and data evaluated using the iCycler software. Negative controls included primers without cDNA. RNA levels of the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (HPRT) did not fluctuate with treatment and were used as an internal control. Primers used for real-time polymerase chain reaction are listed in Table 1.
Table 1:
Abbreviations: HPRT, Hypoxanthine guanine phosphoribosyl transferase; TRAP, Tartrate resistance acid phosphatase; TNFa, tumor necrosis alpha; IL-6, interleukin 6; aP2, adipocyte Protein 2; RUNX2, run-related transcription factor 2; IFNg, interferon gamma; TRPV6, transient receptor potential cation channel subfamily v member 6; TRPV5, transient receptor potential cation channel subfamily v member 5; CaBP9K, calbindin-d9k; CaBP28K, calbindin-d28k
| Gene | Forward (5’−3’) | Reverse (5’−3’) |
|---|---|---|
| HPRT | AAGCCTAAGATGAGCGCAAG | TTACTAGGCAGATGGCCACA |
| TNFa | AGGCTGCCCCGACTACGT | GACTTTCTCCTGGTATGAGATAGCAA |
| Osteocalcin | AAGCAGGAGGGCAATAAGGT | TAGGCGGTCTTCAAGCCAT |
| Osterix | CTGCGGAAAGGAGGCACA AAGAAG | GGGTTAAGGGGAGCAAAGTCAGAT |
| TRAP | AATGCCTCGACCTGGGA | CGTAGTCCTCCTTGGCTGCT |
| Wnt10b | AATGCGGATCCACAACAACA | TTCCATGGCATTTGCACTTC |
| aP2 | GCGTGGAATTCGATGAAATCA | CCCGCCATCTAGGGTTATGA |
| RUNX2 | GACAGAAGCTTGATGACTCTAAACC | TCTGTAATCTGACTCTGTCCTTGTG |
| IL-6 | ATCCAGTTGCCTTCTTGGGACTGA | TAAGCCTCCGACTTGTGAAGTGGT |
| IFNg | GGCTGTCCCTGAAAGAAAGC | GAGCGAGTTATTTGTCATTCGG |
| TRPV6 | GAGGATCCCGATGAGGTGGG | GGGCAGATCCACGTCATAGT |
| TRPV5 | CCAGGATAAGATCCGGCTGG | AAAGTAGCGAGAGGCACCAA |
| CaBP9K | TGGATAAGAATGGCGATGGAG | GCTAGAGCTTCAGGATTGGAG |
| CaBP28K | CCAGGTTACTACCAGTGCAGG | AGTGGTTGTGGTCACCAACTCT |
In vitro cell culture system
Preosteoblast MC3T3-E1 cells (CRL-2593; ATCC, Manassas, VA) (passages between 18 to 24) were plated at a density of 3.9 × 103 cm2 with α–minimal essential media (α-MEM) containing 10% fetal bovine serum (FBS) (Invitrogen and Atlanta Biologicals, Atlanta, GA) and 1% Penicillin-Streptomycin (Life Technologies). Cell media was changed every other day. After confluency, cells were treated with α-MEM with low (5 mM) and high (30mM) glucose for 3 days. Cells were then treated with 50 ng/ml recombinant murine IL-10 (Peprotech) for 24 hours. Cells were harvested with TriReagent (Molecular Research Center Inc.) and RNA extracted and analyzed as previously described.
In vitro cell protein extraction and Western blotting
Preosteoblast MC3T3-E1 cells (CRL-2593; ATCC, Manassas, VA) (passages between 18 to 24) were treated with low (5 mM) and high (30 mM) glucose for 3 days. Cells were then stimulated with 50 ng/ml recombinant murine IL-10 (Peprotech) for the indicated time points. Cells were lysed with lysis buffer containing 1% Triton X-100, protease and phosphatase inhibitors. Protein concentrations were determined using Bradford and equivalent protein (50 μg) was loaded into the wells of SDS-PAGE gels for western blot analysis. Blots were probed with antibodies diluted in LiCor blocking buffer (LiCor) or 5% BSA. Primary antibodies were against pERK1/2, p-JNK1/2, JNK1/2, and p-P38 (Cell Signaling Technology Inc. (Danvers, MA), ERK-2 and tubulin (Santa Cruz). For immunoblotting, the secondary1/2 antibodies were IR-dye or HRP conjugated and analyzed by Licor’s Odyssey or chemiluminescence respectively. The bands were quantified using Lico1/2r’s Odyssey program (for IR dye) or densitometry (Image J for chemiluminescence).
Statistical Analysis
Data are presented as mean ± standard error or as box-plots (5–95 percentile) as indicated. Statistical analysis was performed using Student’s t-test (two group comparisons) or ANOVA (more than 2 groups) with GraphPad Prism software version 7 (GraphPad, San Diego, CA, USA). Significant outliers (if present and indicated in figure legend) were removed using the ROUT test for outliers. A p-value < 0.05 was considered statistically significant.
Results
Wild type and IL-10 knockout mice exhibit similar blood glucose levels
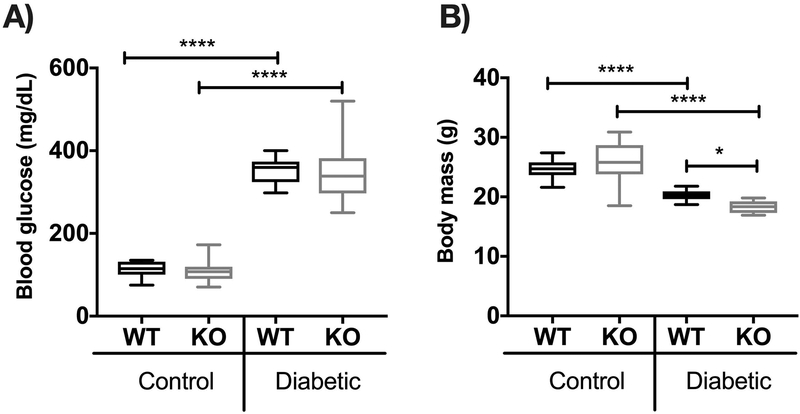
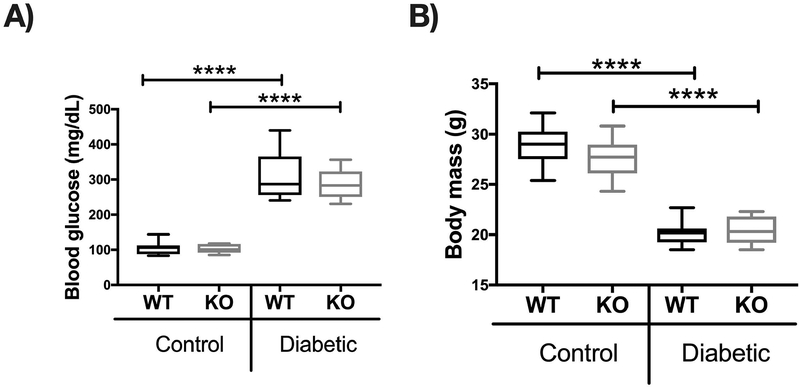
To induce T1D hyperglycemic conditions in mice, we used streptozotocin (STZ) a pharmacological compound (derived from Streptomyces achromogenes) that causes pancreatic β cell destruction and consequent marked hypoinsulinemia and hyperglycemia [Szkudelski, 2001]. Male mice, 7 to 8-week-old wild type (WT) and IL-10 knockout (KO) (both in C57BL/6J background), were injected with 65 mg of STZ per kilogram body weight for 5 consecutives days. Control mice received equal volume of vehicle (sodium citrate buffer). To confirm diabetes, blood glucose was measured 1 month after the first STZ injection. Blood glucose was significantly increased in both WT and KO groups (p<0.0001), whereas WT and KO control mice maintained normal blood glucose levels <200 mg/dL. No differences in blood glucose levels were observed between T1D WT and KO groups (Figure 1A). As expected, T1D WT mice had decreased body weight compared to corresponding control mice (p< 0.0001) [Motyl and McCabe, 2009]. Although control WT and KO groups did not differ in body weight, T1D KO mice lost significantly more weight compared to T1D WT mice at this time point (p< 0.05) (Figure 1B).
Figure 1. Induction of Type 1 diabetes with streptozotocin (STZ) in WT and IL-10 knockout mice.
C57BL/6 (WT) and IL-10 knockout (KO) mice were treated with citrate buffer (vehicle control) or STZ (diabetic) for 5 consecutives days. (A) Blood glucose levels (mg/dL) and (B) body weight in grams (g) were measured 4 weeks after the first STZ injection. Whiskers in the box plot represent 5–95 percentile values; n= 9–10 per group. *p<0.05, ****p<0.0001. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO= IL-10 knockout mice.
IL-10 deficiency enhances T1D-induced trabecular and cortical bone loss.
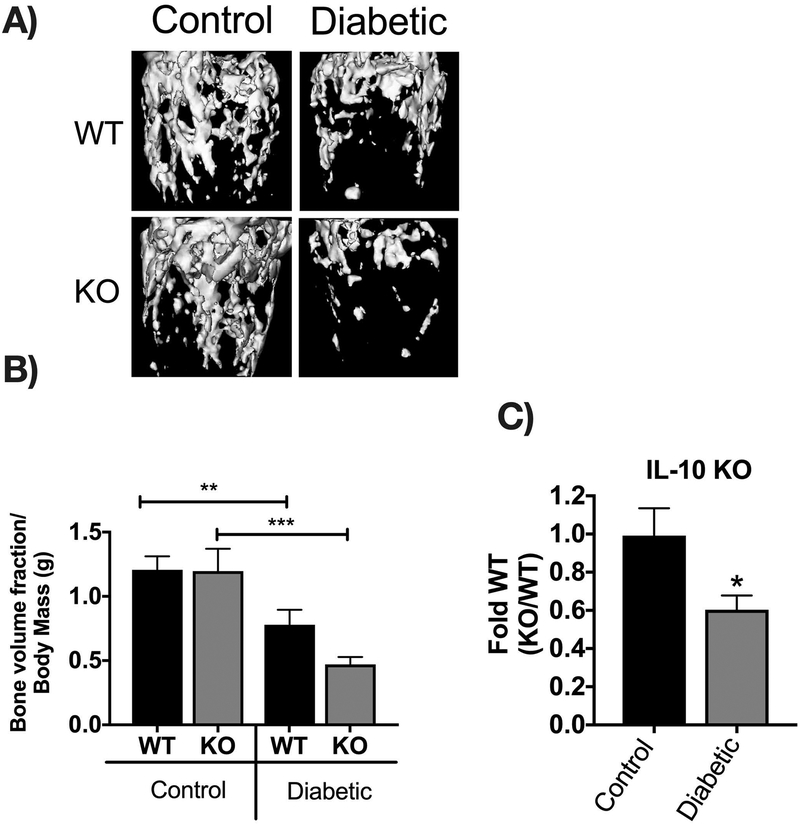
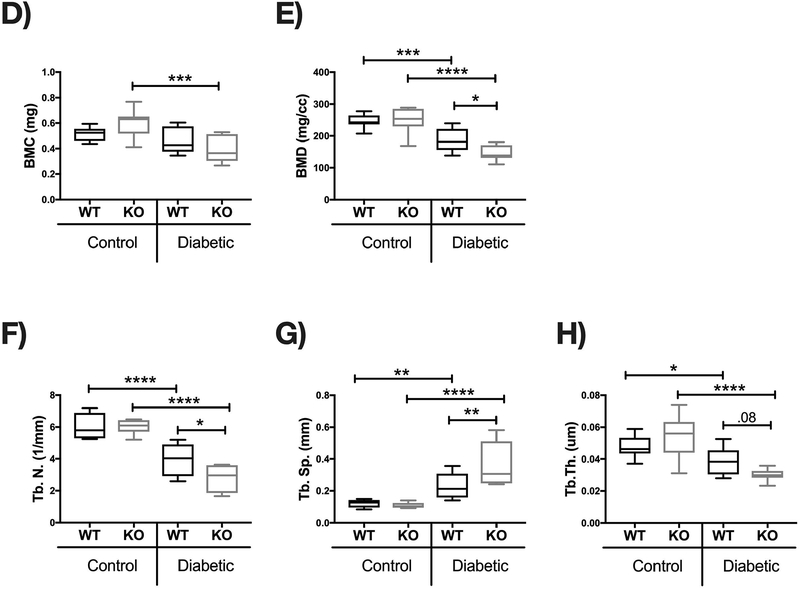
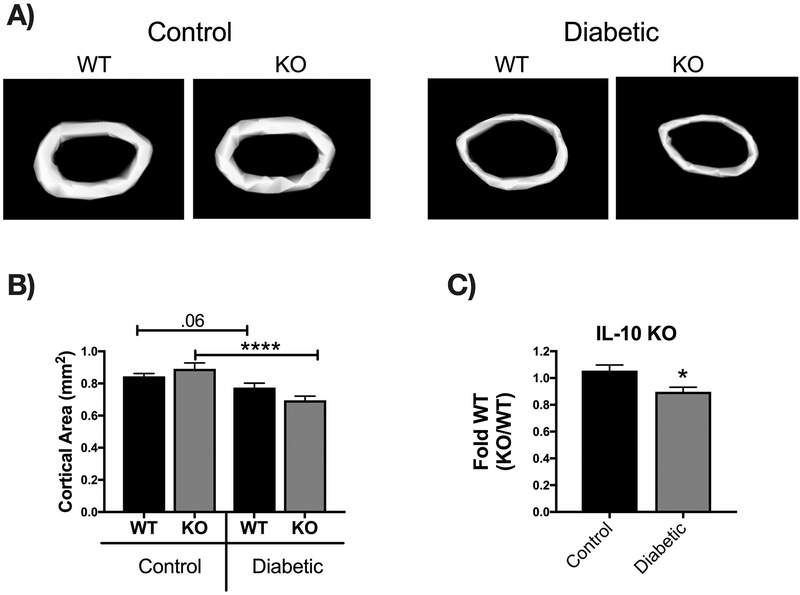
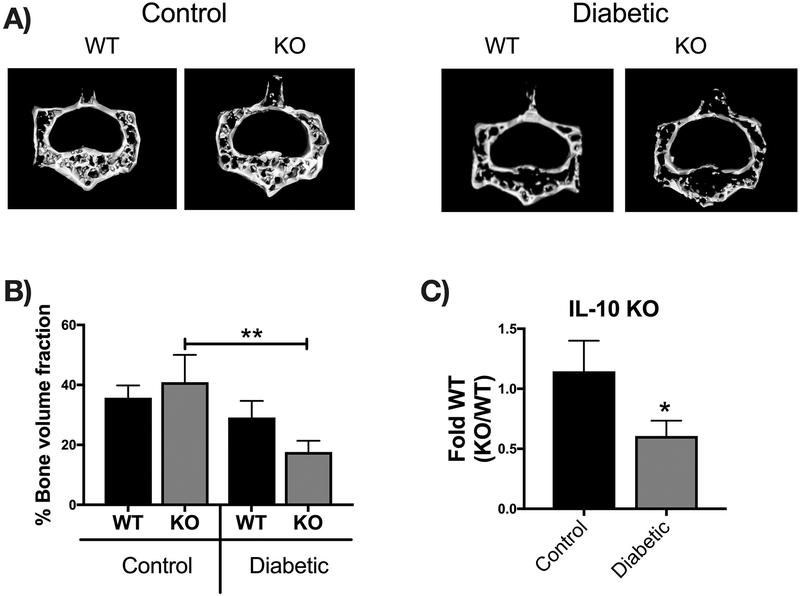
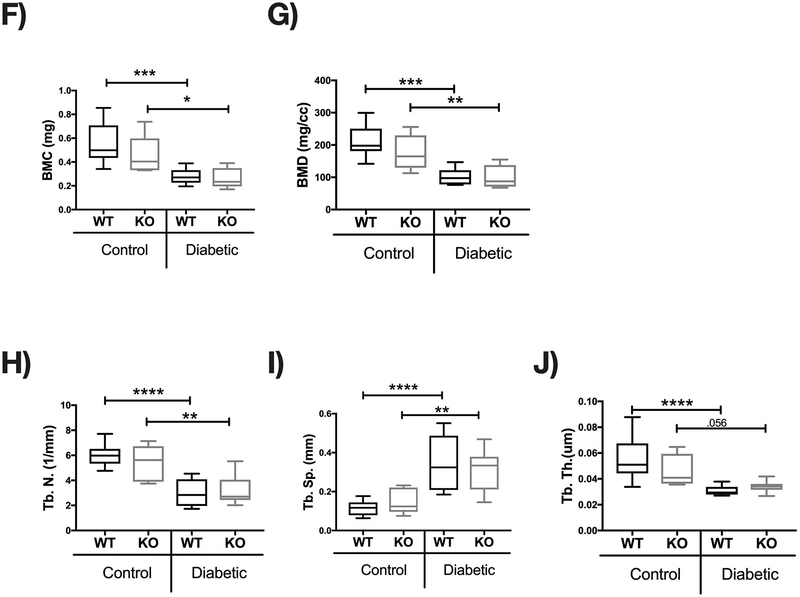
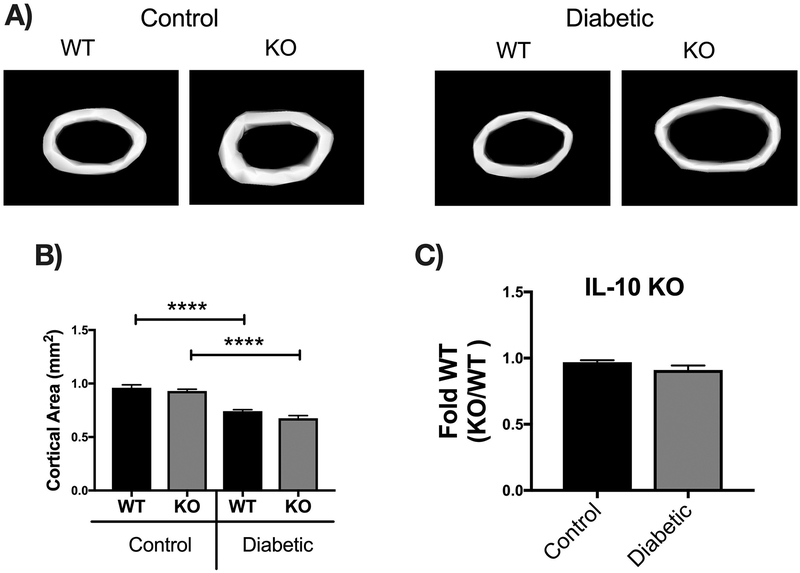
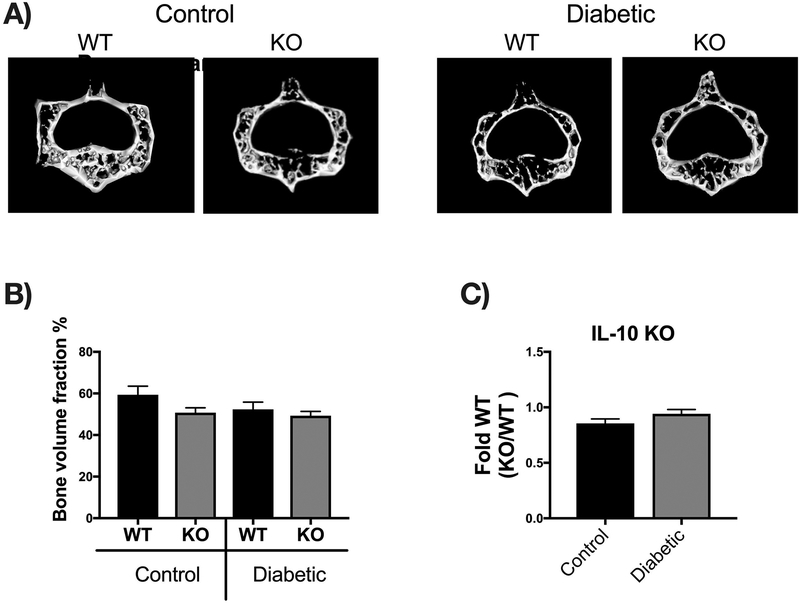
Bone density and structural parameters at the 1 month time point were assessed using microcomputed tomography (μCT). Distal femoral trabecular bone (Figure 2) and diaphyseal cortical bone (Figure 3) showed no significant differences between control WT and KO groups, suggesting that IL-10 is not essential for normal bone homeostasis in this age group. However, femur trabecular bone volume fraction (BVF corrected to body weight) was significantly decrease in T1D WT mice compared to control WT mice (p<0.01). Similarly, the KO diabetic group also displayed significant trabecular bone loss compared to KO control mice (p<0.01). When analyzed against the respective WT cohorts, trabecular bone loss in the T1D KO mice was significantly greater compared to the T1D WT mice (p<0.05; Figure 2C). These results suggest that IL-10 plays an important role in preventing femur trabecular bone loss during diabetes and that loss of IL-10 exacerbates T1D bone loss. Further analyses of bone trabecular micro-architecture indicated a decrease in bone mineral density, trabecular thickness, number and an increase in trabecular spacing in the WT diabetic group compared to WT control mice. These parameters were exacerbated in the KO diabetic compared to WT diabetic mice. (Figure 2 D–H). Deficiency of IL-10 also increased the negative effects of T1D on cortical bone. Specifically, KO mice displayed less cortical area (p<0.05; Figure 3C), bone mineral content (p<0.001), density (p<0.001, and thickness (p<0.05) when compared to the T1D WT group (Figure 3 A–I). To assess if these effects are bone site-specific we examined trabecular bone of the 3rd lumbar vertebrae using μCT analysis. Vertebrae from T1D WT mice showed a modest decrease in BVF compared to the WT controls (Figure 4 A–B). Importantly, the KO diabetic group exhibited a significant decrease in vertebral BVF compared to control KO mice vertebrae (Figure 4 A–B). When analyzed relative to the respective WT mouse groups, vertebral trabecular bone loss was significantly greater in the KO diabetic mice (Figure 4C). Together, these data demonstrate that lack of IL-10 can exacerbate T1D bone loss in femur and vertebrae.
Figure 2. IL-10 knockout exacerbates trabecular bone loss 1 month after diabetes induction.
Control and STZ treated WT and IL-10 knockout mice were euthanized 4 weeks after the first STZ injection. Femoral bones were collected and trabecular bone analyzed by uCT. (A) Representative micro-computed tomography isosurface images. (B) Bone volume fraction (corrected for weight loss). (C) Bone volume fraction (corrected for weight loss) from the KO group normalized to respective WT groups. * represent p<0.05 by T-test against WT diabetic group. (D) Bone mineral content (mg). (E) Bone mineral density (mg/cc). (F) Trabecular number (Tb. N, 1/mm). (G) Trabecular space (Tb. Sp, mm). (H) Trabecular thickness (Tb. Th, um). Bar graphs values are averages ± standard error; Whiskers in box plots represent 5–95 percentile values; n= 7–9 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO= IL-10 knockout mice.
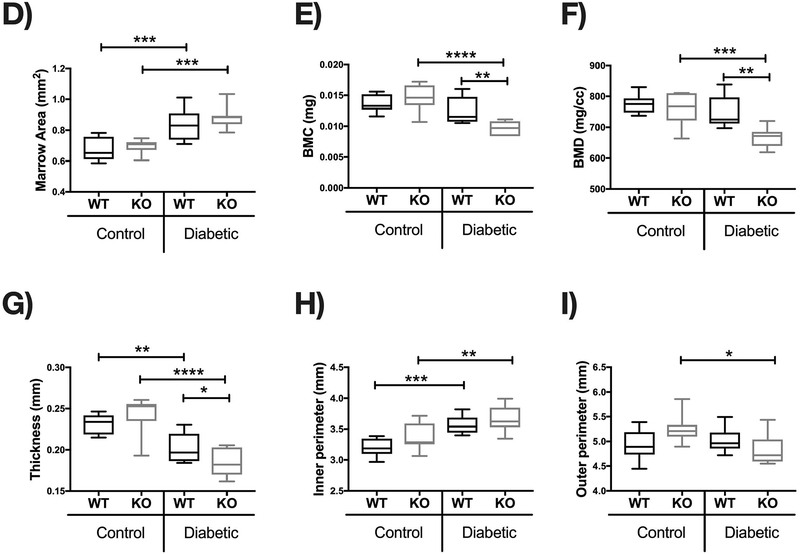
Figure 3. IL-10 deficiency exacerbates T1D-induced cortical bone loss 1 month after diabetes induction.
Cortical bone analysis were performed using μCT, on femoral bones described in Figure 2. (A) Representative micro-computed tomography isosurface images. (B) Cortical area (mm2). (C) Cortical area from the KO groups normalized to respective WT groups. * represent p<0.05 by T-test against WT diabetic group. (D) Marrow area (mm2). (E) Bone mineral content (mg). (F) Bone mineral density (mg/cc). (G) Cortical thickness (mm). (H) Inner perimeter (mm). (I) Outer perimeter. Bar graphs values are averages ± standard error; Whiskers in box plots represent 5–95 percentile values; n= 7–9 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO= IL-10 knockout mice.
Figure 4. IL-10 knockout exacerbates vertebral trabecular bone loss 1 month after diabetes induction.
Vertebral bones were collected from mice groups described in Fig 2 and μCT analysis performed from the third lumbar vertebral body to assess trabecular bone. (A) Representative micro-computed tomography isosurface images. (B) Percent bone volume fraction and (C) bone volume fraction from the KO groups normalized to respective WT groups. * represent p<0.05 by T-test against WT diabetic group. Bar graphs values are averages ± standard error; n= 5–7 per group. *p<0.05, **p<0.01. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO=IL-10 knockout mice.
Long term T1D effects on bone density are not affected by IL-10 deficiency
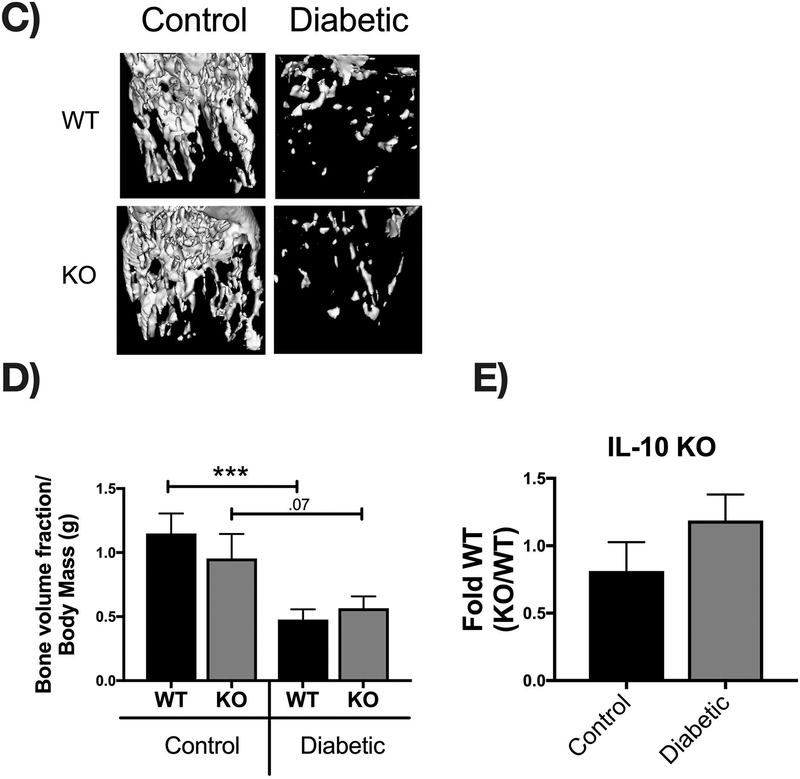
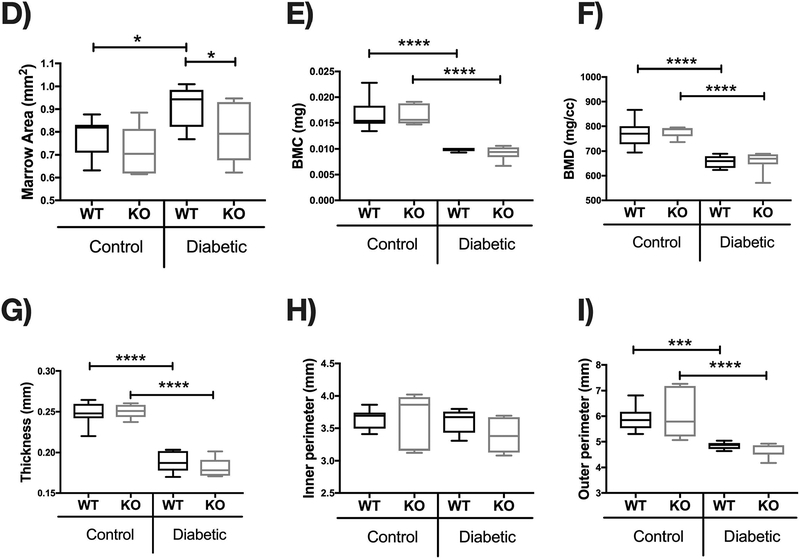
To determine if IL-10 KO diabetic mice continue to have excessive bone loss over-time (compared to WT diabetic mice), we examined mice 3 months after the first STZ injection. As seen in the 1 month experiments, blood glucose was similarly elevated in both T1D WT and KO mice (p<0.0001) (Figure 5 A). Compared to genotype controls, body weight was decreased in both WT and KO T1D mice (p<0.0001); however, unlike the 1 month time point, body weights were similar between the two genotypes (Figure 5 B). MicroCT analysis revealed that femur trabecular BVF was significantly decreased in the WT diabetic (p<0.0001) compared to WT control mice (Figure 5 C–D). Consistent with this, trabecular thickness (p<0.0001) and number (p<0.0001) were decreased and trabecular space (p<0.0001) increased markedly in the WT diabetic compared to WT control mice (Figure 5 D–J). Unlike the 1 month time point, when analyzed against the WT cohorts, KO diabetic showed similar trabecular bone loss compared to WT mice at the 3 months time point (Figure 5E). In the cortical bone, T1D significantly decreased cortical area, bone mineral density and content, thickness and outer perimeter measures in both WT and KO diabetic mice compared to the respective control groups (Figure 6 A–I). When analyzed against the WT cohorts, IL-10 deficiency did not exacerbate T1D effects on cortical thickness (Figure 6 A–C). Measurment of vertebral trabecular BVF demonstrated no significant difference between any of the groups at this time point (Figure 7). Our results suggest that IL-10 plays an important role in inhibiting trabecular and cortical bone loss only during early diabetic time point but not at the later time point.
Figure 5. IL-10 knockout does not affect trabecular bone loss 3 months after diabetes induction.
Control and STZ treated WT and IL-10 knockout mice were euthanized 12 weeks after the first STZ injection. Femoral bones were collected and trabecular bone analyzed by uCT. (A) Blood glucose levels (mg/dL) and (B) body weight in grams (g); n=9–10. (C) Representative micro-computed tomography isosurface images. (D) Bone volume fraction (corrected for weight loss). (E) Bone volume fraction (corrected for weight loss) from the KO group normalized to respective WT groups. (F) Bone mineral content (mg). (G) Bone mineral density (mg/cc). (H) Trabecular number (Tb.N, 1/mm). (I) Trabecular space (Tb.Sp, mm). (J) Trabecular thickness (Tb.Th, um). Bar graphs values are averages ± standard error; Whiskers in box plots represent 5–95 percentile values; n= 5–9 per group. **p<0.01, ****p<0.0001. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO=IL-10 knockout mice.
Figure 6. IL-10 knockout does not exacerbate T1D-induced cortical bone loss 3 months after diabetes induction.
Control and STZ treated WT and IL-10 knockout mice were euthanized 12 weeks after the first STZ injection. Femoral cortical bone analysis were performed using μCT. (A) Representative micro-computed tomography isosurface images. (B) Cortical area (mm2). (C) Cortical area from the KO groups normalized to respective WT groups. (D) Marrow area (mm2). (E) Bone mineral content (mg). (F) Bone mineral density (mg/cc). (G) Cortical thickness (mm). (H) Inner perimeter (mm). (I) Outer perimeter. Bar graphs values are averages ± standard error; Whiskers in box plots represent 5–95 percentile values; n= 5–9 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO= IL-10 knockout mice.
Figure 7. IL-10 knockout does not exacerbate vertebral trabecular bone loss 3 months after diabetes induction.
After 12 weeks of treatment vertebral bone was collected and the third lumbar vertebrae was analyzed via uCT. (A) Representative micro-computed tomography isosurface images. (B) Percentage bone volume fraction and (C) bone volume fraction from the KO groups normalized to respective WT groups. Bar graphs values are averages ± standard error; n= 5–7 per group. *p<0.05, **p<0.01. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. WT=wild type, KO=IL-10 knockout mice.
T1D effects on osteoblast gene expression are exacerbated in IL-10 knockout mice
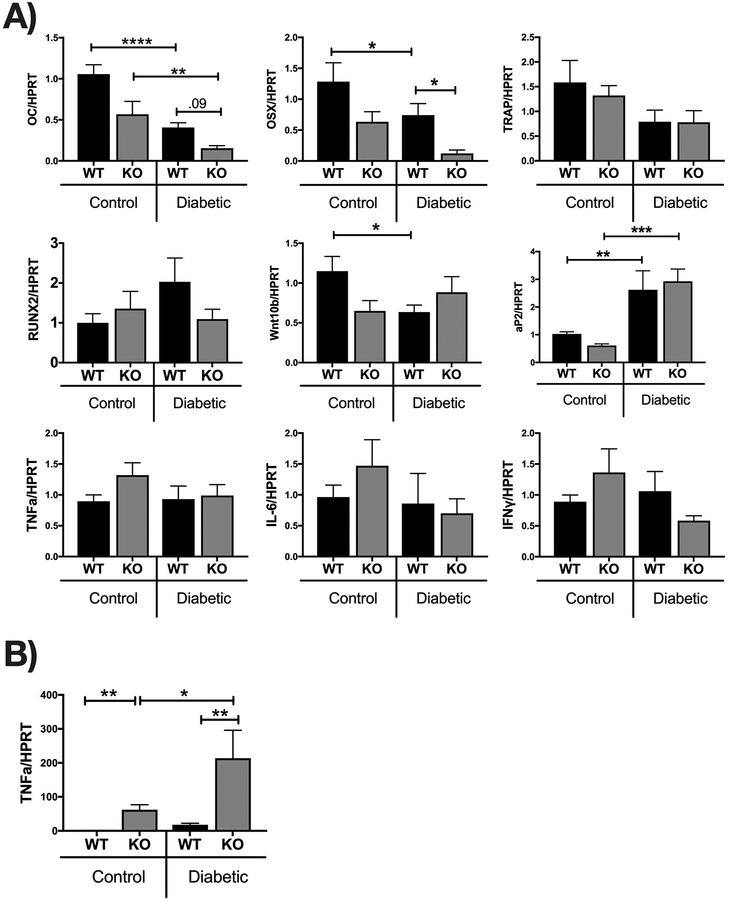
To understand the mechanisms by which IL-10 regulates T1D bone loss, especially during early stages of T1D (4 weeks), we examined mRNA markers of bone formation (osteoblasts) and bone resorption (osteoclasts). For this, we extracted RNA from tibia of the two genotypes under control and diabetic conditions and then examined the expression of bone formation markers (osteocalcin and osterix) and bone resorption markers (tartrate-resistant acid phosphatase, TRAP). As shown in Figure 8A, expression of both osteocalcin (osteoblast differentiation marker) and osterix (osteoblast transcription factor) were significantly decreased in WT T1D mice (p <0.05). Importantly, the KO diabetic mice displayed a significantly greater decrease in osterix than WT diabetic mice (Figure 8A, p<0.05). KO diabetic mice also had lower osteocalcin levels compared to WT diabetic mice, however the difference did not reach statistical significance. Together, these results suggest that the presence of IL-10 reduces the negative effects of T1D on anabolic bone responses. In contrast, expression of TRAP was variable and did not significantly differ between the various groups suggesting that IL-10 likely does not regulate bone resorption in T1D mice (Figure 8A).
Figure 8. Relative gene expression in the tibia and intestine from WT and IL-10 knockout mice.
Control and STZ treated WT and IL-10 knockout mice were euthanized 4 weeks after the first STZ injection. Tibia (A), colon (B) and jejunum (C) were collected, RNA extracted and mRNA analysis of indicated genes assessed by Q-RT-PCR. Genes were normalized to HPRT. Values are averages ± standard error; n= 8–10 per group. *p<0.05, **p<0.01, ****p<0.0001. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test. Outliers removed: OSX (1 outlier in the KO-C and WT-D),Wnt10b (1 outlier in the WT-D), RUNX2 (1 outlier in the WT-C and KO-D), TRAP (1 outlier in the WT-D), TNFA(1 outlier in the WT-C and WT-D), IL-6 (1 outlier in all of the groups), IFNY (1 outlier in the WT-C, WT-D, and KO-D), TNFA colon (2 outliers in the WT-control, 1 outlier in the KO-control).
Previous studies have shown that T1D can affect osteoblast lineage selection [Botolin and McCabe, 2007][Coe et al., 2013]. Therefore, we examined the expression of Wnt10b, a major enhancer of osteoblast differentiation [Bennett et al., 2007]. In addition, we assessed expression of aP2, a fatty acid adipocyte protein whose expression in bone is indicative of increase in marrow adiposity [Shan et al., 2013]. In previous studies we identified that bone Wnt10b is decreased while aP2 is increased in T1D mice [Raehtz et al., 2017][Zhang et al., 2015]. Similarly, in the current study, we found Wnt10b expression was significantly decreased in the WT diabetic (p<0.05) compared to WT control mice. In contrast to the WT, there was no significant difference between the KO control and KO diabetic mice. Note however, that Wnt10b expression was already significantly decreased in the KO control compared to the WT control bones (Figure 8A). Expression of aP2, on the other hand, was significantly enhanced in both T1D WT and KO mice compared to their respective control genotype mice (Figure 8A, p<0.05). These data suggest that under T1D conditions IL-10 effects on bone density are likely independent of Wnt10b and adipocyte lineage selection.
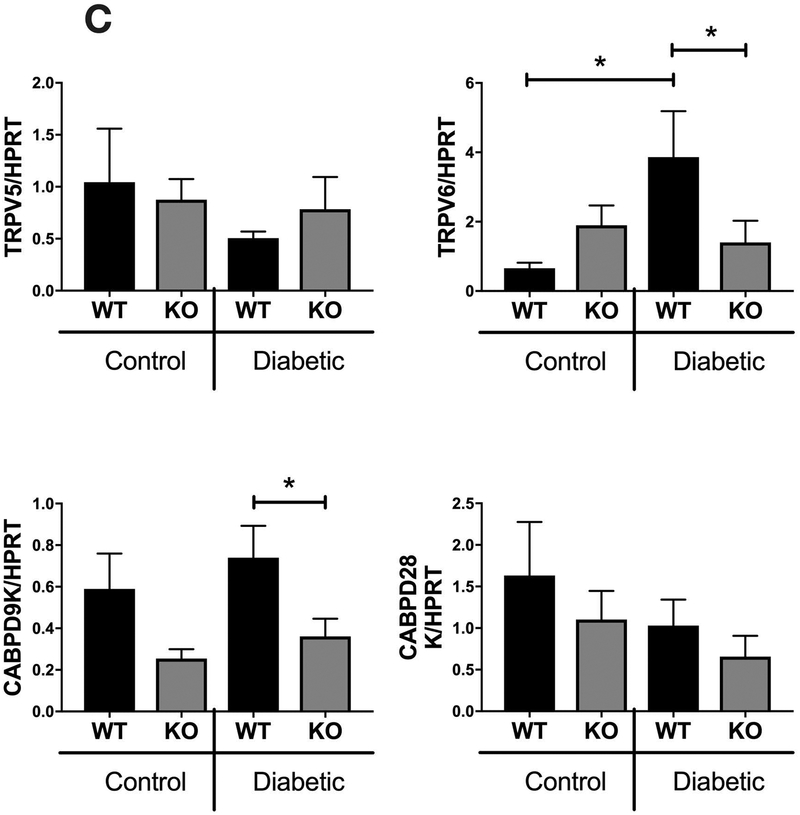
Since IL-10 is an important anti-inflammatory cytokine we next investigated the expression patterns of cytokines previously associated with T1D bone loss such as IFN-γ, TNF-α, and IL-6. Interestingly, our results showed no significant differences in expression of inflammatory cytokines in the bone between any of the groups (Figure 8A). Unlike the bone, intestinal expression of TNFα was significantly enhanced in the KO group (Fig 8B; p<0.01) (as expected [Rennick and Fort, 2000]), suggesting an enhanced pro-inflammatory environment in the gut but likely not the bone. To examine the effect of IL-10 on intestinal Ca uptake, we analyzed small intestinal expression of genes involved in calcium transport: TRPV5, TRPV6, CABPD9K and CABPD28K (Fig 8C). Levels of expression were not significantly different between the control WT and KO mice or between control KO and diabetic KO mice. Interestingly expression of TRPV6 and CABPD9K was different between diabetic WT and diabetic KO mice; this occurs as a result of T1D increasing expression of TRPV6 (significantly) and CABPD9K (trend) in WT mice but not KO mice. The impact of this on calcium absorption and bone health in T1D will be addressed in future studies.
IL-10 regulates osterix gene expression in osteoblasts via MAPK pathway
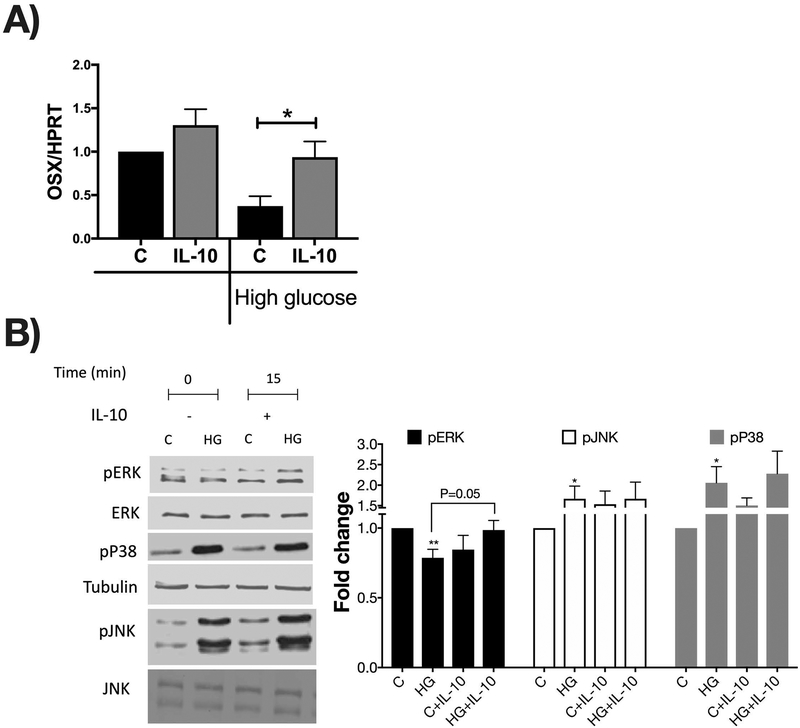
To further understand the mechanisms by which IL-10 regulates osteoblast markers under diabetic conditions, we used MC3T3-E1 osteoblasts treated with or without IL-10, under normal and high glucose conditions. Previous studies have shown in vitro that high glucose treatment of osteoblasts inhibits expression of anabolic genes [Zayzafoon et al., 2002][Zayzafoon et al., 2000][Balint et al., 2001][Terada et al., 1998]. Since our in vivo studies suggest a role for IL-10 in osterix gene expression in the tibia, we focused on regulation of expression of osterix in vitro. MC3T3-E1 cells were treated with normal (5 mM) or high glucose (30 mM) for 72 hours followed by stimulation without or with IL-10 for 24 hours. As expected, osterix gene expression was significantly reduced under high glucose in the absence of IL-10 (p<0.05). However, in the presence of IL-10 under high glucose conditions, osterix expression reversed to control levels. Interestingly, IL-10 did not significantly affect osterix expression in cells grown under normal glucose conditions (Figure 9A).
Figure 9. IL-10 prevents high glucose-mediated suppression of osterix expression via the ERK pathway.
(A) MC3T3-E1 cells were treated with high glucose (72 hours) +/− IL-10 for 24 hours. RNA extracted and mRNA analysis of osterix (OSX) gene assessed by Q-RT-PCR and normalized to HPRT. (B) MC3T3-E1 cells were treated with high glucose (72 hours) +/− IL-10 for 15 minutes. Representative blots show protein levels analyzed by Western Blot as well as loading controls (ERK and Tubulin). Quantification of western blots for p-ERK/ERK, p-p38, and p-JNK. Values are averages ± standard error; n= 4–5 per group, *p<0.05, **p<0.01. Statistical analysis performed by One-way ANOVA followed by Fisher’s LSD post-test.
To understand the signaling mechanisms by which IL-10 regulates osterix gene expression, we focused on the MAPK pathways (JNK, p38 and ERK). Previous studies have shown that osterix expression is regulated by JNK, p38 or ERK depending on the stimulus [Lu et al., 2006][Barbuto and Mitchell, 2013]. To understand the relationship between high glucose conditions and osterix in the context of these MAPK pathways, we first tested the effect of high glucose on the phosphorylation status of these three kinases. High glucose enhanced phosphorylation of JNK and p38 (as we have shown before [Zayzafoon et al., 2002]). Interestingly, high glucose decreased ERK phosphorylation (Figure 9B, p<0.05). The ERK pathway is known to be a key stimulator of osteoblast differentiation. Consequently, we examined if IL-10 treatment prevents the high glucose suppression of ERK signaling. As expected, treatment with IL-10 prevented high glucose suppression of ERK phosphorylation (Figure 9B p=0.05). These results indicate that IL-10 likely sustains osteoblast ERK signaling and osterix expression under T1D conditions.
Discussion
Studies in human and diabetic murine models have demonstrated changes in the levels of pro and anti-inflammatory cytokines with unmanaged diabetes [Erbagci et al., 2001][Snell-Bergeon et al., 2010][Lee et al., 2008][Motyl and McCabe, 2009][Motyl et al., 2009]. Several proinflammatory cytokines have been reported to be elevated in T1D patients and animal models. For example, serum IFN-y is increased with age in the non-obese diabetic mice (NOD) and it reaches the highest levels at the onset of diabetes [Schloot et al., 2002]. T1D patients also present with elevated TNF-α serum levels [Qiao et al., 2017]. Other cytokines such as IL-6 and interleukin 1 beta (IL-1β)are also elevated under diabetic conditions [Senn et al., 2002]. Interestingly, not only are these cytokines increased in the serum of diabetic mice, they are also expressed at high levels in the bone (e.g. IFN-y and TNF-α [Motyl and McCabe, 2009][Motyl et al., 2009]), providing a link between inflammatory cytokines and T1D-induced bone loss. Furthermore, proinflammatory cytokines such as TNFα, IL-1β and prostaglandin E2 have been shown to play an important role in diabetes-induced periodontal bone loss [Liu et al., 2006][Salvi et al., 1997a][Salvi et al., 1997b]. Inhibition of cytokine activity/levels, especially TNFα, has been shown to decrease osteoclast numbers and increase osteoblast and periodontal bone formation in a diabetes-induced periodontal bone loss model [Pacios et al., 2012].
Compared to pro-inflammatory cytokines, levels of the anti-inflammatory cytokine IL-10 has been shown to be decreased in the serum and pancreas during diabetes progression in NOD mice [Schloot et al., 2002]. Remarkably, administration of recombinant IL-10 via daily subcutaneous injections or non-viral gene delivery protects NOD mice against the development of insulinitis and diabetes [Pennline et al., 1994][Lee et al., 2006]. Similar to NOD mice, diabetic patients also exhibit lower IL-10 serum levels compared to non-diabetic subjects [Van Exel et al., 2002]. Given that other studies have shown a beneficial effect of IL-10 on bone mass [Zhang et al., 2014][Claudino et al., 2010] and that IL-10 levels are decreased during T1D [Van Exel et al., 2002] [Schloot et al., 2002], in the current report we investigated the importance of IL-10 in T1D-induced bone loss. Our results clearly demonstrate that during early stages of T1D-induced bone loss, IL-10 deficiency exacerbates bone loss in mice. Specifically, T1D IL-10-KO mice display a 60% decrease in bone volume fraction compared to only a 39% decrease in T1D wild type mice. Thus, reduced IL-10 levels during T1D in human patients may contribute to bone loss early on. However, our data also suggest that over time, IL-10 levels are not crucial to regulate bone density since by 6 months of age, diabetic IL-10 KO and WT mice exhibit similar levels of bone loss, suggesting that bone loss in the IL-10 KO diabetic group reaches a set point that is achieved by T1D WT mice at a later timepoint. Consistent with this, a progressive decline, over a 12-week period, in trabecular bone density, has been shown in T1D WT rats [Silva et al., 2009]. It should also be noted that our studies were performed in male mice. While both male and female T1D mice lose similar amounts of femoral and vertebral trabecular bone [Martin and McCabe, 2007], we do not know if IL-10 influences T1D bone loss in a sex dependent manner. Future studies are needed to assess the influence of sex hormones in the response.
Consistent with our findings that IL-10 can play a role in bone density, many studies have demonstrated the importance of IL-10 in regulating bone health in non-diabetic models. IL-10 has been shown to regulate alveolar bone loss in aged mice (~9 month) compared to younger mice (3 months old) [Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, 2003][Al-Rasheed et al., 2004]. In these studies, changes in bone density in the aged IL-10 KO mice were associated with down-regulation of osteoblast and osteocyte markers in the periodontal tissue [Claudino et al., 2010]. Another study looking at bone metabolism in IL-10 KO mice in the context of colitis development demonstrated a decrease in cancellous bone mass of the tibia as well as the trabecular bone surface and number, in the IL-10 KO mice compared to the WT group. Interestingly, these changes were most striking in IL-10 KO animals that showed colitis [Dresner-Pollak et al., 2004]. Although these authors demonstrated this phenotype in 8 to 12 week old mice, in our studies we did not observe any signs of colitis in IL-10 KO mice (as demonstrated by equivalent body weight in control WT and KO mice at both time points tested). In addition, IL-10 KO mice in our facility did not display any overt clinical signs of colitis (such as diarrhea, bleeding etc). Because IL-10 KO mice in our study did not display overt colitis, it is not surprising that non-diabetic IL-10 KO mice did not exhibit any bone pathology. It is important to note that a trend towards a lower femoral trabecular bone density in the IL-10 KO group was noted at the later time point (12 week treatment time @ which they are 6 months old). Even though we did not observe any clinical signs of colitis, colons from the IL-10 KO mice (non-diabetic and diabetic) showed markedly higher TNFα gene expression suggesting that the intestinal environment of these mice are skewed towards a pro-inflammatory state. This however, did not likely influence bone inflammatory environment since expression of inflammatory genes in the bone were similar between groups. These results suggest that the bone phenotype in the IL-10 KO diabetic group in this study is independent of changes in bone inflammation. Similar to our results, an increase in alveolar bone loss in IL-10 deficient mice was shown to be independent of changes in the inflammatory cytokines TNFα, IL1β, IL-6 and transforming growth factor beta (TGFβ) [Claudino et al., 2010].
We and others have shown that T1D-induced bone loss is associated with suppression of osteoblast and an increase in adipogenesis [Botolin and McCabe, 2007; Zhang et al., 2015]. Similarly, we show here that T1D decreased markers of osteoblast maturation and increased markers of adipogenesis in the WT mice. In the IL-10 KO diabetic mice, although osteoblast markers were suppressed even further compared to the WT diabetic mice, expression of adipogenic markers were similar to that of the WT mice. A previous study using an in vitro model has shown that IL-10 can suppress lipid accumulation and adipogenesis in 3T3-L1 fibroblasts [Kim and Pyo, 2019]. However, in our in vivo T1D model, it appears that IL-10 deficiency does not significantly influence T1D-induced adipogenesis markers. Thus, our results suggest that the effect of diabetes on bone density in the absence of IL-10 is likely independent of changes in adipocytes.
IL-10 can also negatively influence osteoclastogenesis [Claudino et al., 2010][Hong et al., 2010][Xu et al., 1995][Zhang et al., 2016][Zhang et al., 2014]. Specifically, IL-10 prevents the differentiation of osteoclast progenitors to preosteoclasts [Evans and Fox, 2007][Xu et al., 1995]. In a co-culture system of mouse bone marrow cells and primary osteoblastic cells, IL-10 treatment for 7 days prevented osteoclast differentiation [Hong et al., 2010]. In contrast, in IL-10 deficient mice (8 and 12 weeks old) osteoclast cell number were not affected [Dresner-Pollak et al., 2004]. Our results on TRAP (a marker of osteoclast) gene expression, suggests that neither T1D nor IL-10 significantly influence osteoclastogenesis. Although previous studies have demonstrated that proinflammatory cytokines can promote and enhance osteoclastogenesis [Kobayashi et al., 2000][Ries et al., 989][Azuma et al., 2000][Gao et al., 2007], deficiency of IL-10 did not upregulate pro-inflammatory cytokines in the bone. Accordingly, IL-10 deficiency did not affect TRAP gene expression in the control or diabetic mice. Together, these results are consistent with our previous studies showing that T1D does not influence osteoclast markers and further adds that IL-10 is not an important regulator of osteoclastogenesis in T1D model [Botolin and McCabe, 2007; Zhang et al., 2015].
To understand the direct mechanisms by which IL-10 regulates osteoblasts, we examined the effects of IL-10 in osteoblasts cultured under normal and high glucose conditions. While studies looking at the role of IL-10 on osteoblast cells is limited, our results demonstrate a direct role for IL-10 in regulating osterix gene expression in osteoblasts especially under high glucose conditions. Previous studies have shown that high glucose can negatively influence osteoblast differentiation [García-Hernández et al., 2012] and can decrease osteocalcin and osteoprotegerin gene expression is differentiated osteoblasts [Zayzafoon et al., 2000] [Shao et al., 2014]. Similarly, we found that high glucose exposure of pre-osteoblasts markedly decreases osterix gene expression. Osterix is an osteoblast-specific transcriptor factor that is required to induce preosteoblasts differentiation into mature osteoblasts [Sinha and Zhou, 2013]. The importance of osterix physiologically is underscored by osterix null mice which lack bone formation and have no trabeculae [Nakashima et al., 2002]. Consistent with our findings, gene expression analysis of human osteoblastic cells, isolated from diabetic patients with osteoporotic fractures express significantly low levels of osterix [Miranda et al., 2016]. Our studies further demonstrate that IL-10 can directly reverse high glucose-mediated suppression of osterix expression. In an effort to identify signaling mechanisms, our studies further show that IL-10 increases in osterix expression via regulation of the ERK pathway. Regulation of ERK pathway by IL-10 have been shown in different models [Hovsepian et al., 2013][Pereira et al., 2015]. In addition, previous studies using parathyroid hormone (PTH) and TNF-α have shown regulation of osterix gene expression via regulation of MAPK pathway [Barbuto and Mitchell, 2013][Lu et al., 2006]. Thus, consistent with these studies, our findings support a role for the ERK pathway in contributing to the regulation of osterix under high glucose conditions in the presence of IL-10.
In summary, the present study demonstrates that IL-10 knockout mice are more susceptible to T1D bone loss, especially during the early disease process and therefore, decreases in IL-10 observed in animal models and human patients with T1D may have a detrimental role to bone health. We identified that IL-10 is able to regulate osterix gene expression and influence bone effects during T1D primarily through the regulation of osteoblasts. Together, our studies suggest that IL-10 and its signaling pathways are potential therapeutic targets for T1D bone loss.
Acknowledgements:
The authors thank the staff of Campus Animal Resources for the excellent care of our animals. The work presented in this study was funded in part by the National Institute of Health, grants AT007695 (to LRM and NP), DK101050 (to LRM and NP) and EY024757 (to SM); NDR-A was supported by the William Townsend Porter Pre- doctoral Fellowship from the American Physiological Society.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Competing Interests: None
References:
- Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM TD. 2003. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res 82(8):632–635. [DOI] [PubMed] [Google Scholar]
- Al-Rasheed A, Scheerens H, Srivastava AK, Rennick DM, Tatakis DN. 2004. Accelerated alveolar bone loss in mice lacking interleukin-10: Late onset. J Periodontal Res 39(3):194–198. [DOI] [PubMed] [Google Scholar]
- Alayan J, Ivanovski S, Farah CS. 2007. Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J Periodontal Res 42(2):97–103. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Maclaren NK. 1994. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med 331(21):1428–36. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. 2000. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 275(7):4858–64. [DOI] [PubMed] [Google Scholar]
- Balint E, Szabo P, Marshall CF, Sprague SM. 2001. Glucose-induced inhibition of in vitro bone mineralization. Bone 28(1):21–28. [DOI] [PubMed] [Google Scholar]
- Barbuto R, Mitchell J. 2013. Regulation of the osterix (Osx, Sp7) promoter by osterix and its inhibition by parathyroid hormone. J Mol Endocrinol 51(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. 2007. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res 22(12):1924–1932. [DOI] [PubMed] [Google Scholar]
- Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. 2006. Risk of Fracture in Women with Type 2 Diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab 91(9):3404–3410. [DOI] [PubMed] [Google Scholar]
- Botolin S, McCabe LR. 2007. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 148(1):198–205. [DOI] [PubMed] [Google Scholar]
- Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. 2010. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95(11):5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2014. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Dep Heal Hum Serv; 1–20. [Google Scholar]
- Chen HH, Horng MH, Yeh SY, Lin IC, Yeh CJ, Muo CH, Sung FC, Kao CH. 2015. Glycemic control with thiazolidinedione is associated with fracture of T2DM patients. PLoS One 10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Kyritsis G, Graves DT, Amar S. 1999. Interleukin-1 and Tumor Necrosis Factor Activities Partially Account for Calvarial Bone Resorption Induced by Local Injection of Lipopolysaccharide. [DOI] [PMC free article] [PubMed]
- Claudino M, Garlet TP, Cardoso CRB, de Assis GF, Taga R, Cunha FQ, Silva JS, Garlet GP. 2010. Down-regulation of expression of osteoblast and osteocyte markers in periodontal tissues associated with the spontaneous alveolar bone loss of interleukin-10 knockout mice. Eur J Oral Sci 118(1):19–28. [DOI] [PubMed] [Google Scholar]
- Coe LM, Tekalur SA, Shu Y, Baumann MJ, Mccabe LR. 2015. Bisphosphonate treatment of type I diabetic mice prevents early bone loss but accentuates suppression of bone formation. J Cell Physiol 230(8):1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe LM, Zhang J, McCabe LR. 2013. Both spontaneous Ins2+/− and streptozotocin-induced type I diabetes cause bone loss in young mice. J Cell Physiol 228(4):689–695. [DOI] [PubMed] [Google Scholar]
- Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. 2004. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 127(3):792–801. [DOI] [PubMed] [Google Scholar]
- Eller-Vainicher C, Zhukouskaya VV, Tolkachev YV, Koritko SS, Cairoli E, Grossi E, Beck-Peccoz P, Chiodini I, Shepelkevich AP. 2011. Low bone mineral density and its predictors in type 1 diabetic patients evaluated by the classic statistics and artificial neural network analysis. Diabetes Care 34(10):2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbagci AB, Tarakcioglu M, Coskun Y, Sivasli E, Sibel Namiduru E. 2001. Mediators of inflammation in children with type I diabetes mellitus: cytokines in type I diabetic children. Clin Biochem 34(8):645–650. [DOI] [PubMed] [Google Scholar]
- Evans KE, Fox SW. 2007. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Exel E, Gussekloo J, De Craen AJM, Frölich M, Van Der Wiel AB, Westendorp RGJ. 2002. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85-plus study. Diabetes 51(4):1088–1092. [DOI] [PubMed] [Google Scholar]
- Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. 2007. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest 117(1):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Hernández A, Arzate H, Gil-Chavarría I, Rojo R, Moreno-Fierros L. 2012. High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone 50(1):276–288. [DOI] [PubMed] [Google Scholar]
- Hanley D a, Brown JP, Tenenhouse A, Olszynski WP, Ioannidis G, Berger C, Prior JC, Pickard L, Murray TM, Anastassiades T, Kirkland S, Joyce C, Joseph L, Papaioannou A, Jackson S a, Poliquin S, Adachi JD. 2003. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-sectional results from the Canadian Multicentre Osteoporosis Study. J Bone Miner Res 18(4):784–90. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Brueck CC, Singh SK, Dobnig H. 2007. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 22(9):1317–1328. [DOI] [PubMed] [Google Scholar]
- Hong MH, Williams H, Jin CH, Pike JW. 2010. The Inhibitory Effect of Interleukin-10 on Mouse Osteoclast Formation Involves Novel Tyrosine-Phosphorylated Proteins. J Bone Miner Res 15(5):911–918. [DOI] [PubMed] [Google Scholar]
- Hovsepian E, Penas F, Siffo S, Mirkin GA, Goren NB. 2013. IL-10 Inhibits the NF-κB and ERK/MAPK-Mediated Production of Pro-Inflammatory Mediators by Up-Regulation of SOCS-3 in Trypanosoma cruzi-Infected Cardiomyocytes. PLoS One 8(11):e79445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. 2013. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis 19(8):1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Pyo S. 2019. Interleukin-10 suppresses adipogenesis via Wnt5a signaling pathway in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 509(4):877–885. [DOI] [PubMed] [Google Scholar]
- Ko KI, Coimbra LS, Tian C, Alblowi J, Kayal RA, Einhorn T, Gerstenfeld LC, Pignolo RJ, Graves DT. 2016. Diabetes reduces mesenchymal stem cells in fracture healing through a TNFα-mediated mechanism. Diabetologia 25(3):289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. 2000. Tumor Necrosis Factor Stimulates Osteoclast Differentiation by a Mechanism Independent of the Odf/Rankl-Rank Interaction. J Exp Med 191(2):275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz HE. 1999. Type 2 Diabetes: An Overview. Clin Chem 45(8). [PubMed] [Google Scholar]
- Lee J-H, Lee W, Kwon OH, Kim J-H, Kwon OW, Kim KH, Lim J-B. 2008. Cytokine profile of peripheral blood in type 2 diabetes mellitus patients with diabetic retinopathy. Ann Clin Lab Sci 38(4):361–7. [PubMed] [Google Scholar]
- Lee M, Park H, Youn J, Eun TO, Ko K, Kim S, Park Y. 2006. Interleukin-10 plasmid construction and delivery for the prevention of type 1 diabetes. Ann N Y Acad Sci 1079:313–319. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee E, Irwin R, Lucas PC, McCabe LR, Parameswaran N. 2013. B-Arrestin-1 Deficiency Protects Mice From Experimental Colitis. Am J Pathol 182(4):1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Krothapalli N, Alyasii M, Luan Q, Graves DT. 2006. Diabetes Enhances Periodontal Bone Loss through Enhanced Resorption and Diminished Bone Formation. J Dent Res 85(6):510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Gilbert L, He X, Rubin J, Nanes MS. 2006. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NFKB pathways. J Biol Chem 281(10):6297–6306. [DOI] [PubMed] [Google Scholar]
- Martin LM, McCabe LR. 2007. Type I diabetic bone phenotype is location but not gender dependent. Histochem Cell Biol. 10.1007/s00418-007-0308-4. [DOI] [PubMed] [Google Scholar]
- McCabe L, Zhang J, Raehtz S. 2011. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr 21(2):187–206. [DOI] [PubMed] [Google Scholar]
- McCabe LR. 2007. Journal of Cellular Biochemistry. [Google Scholar]
- Miao J, Brismar K, Nyrén O, Ugarph-Morawski A, Ye W. 2005. Elevated Hip Fracture Risk in Type 1 Diabetic Patients. Diabetes Care 28(12):2850–2855. [DOI] [PubMed] [Google Scholar]
- Miranda C, Giner M, José Montoya M, Vázquez MA, Miranda MJ, Pérez-Cano R. 2016. Influence of high glucose and advanced glycation end-products (ages) levels in human osteoblast-like cells gene expression. BMC Musculoskelet Disord 17(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl K, McCabe LR. 2009. Streptozotocin, type 1 diabetes severity and bone. Biol Proced Online 11(1):296–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl KJ, Botolin S, Irwin R, Appledorn DM, Kadakia T, Amalfitano A, Schwartz RC, McCabe LR. 2009. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol 218(3):575–83. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. 2002. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29. [DOI] [PubMed] [Google Scholar]
- Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, Graves DT. 2012. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J 26(4):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennline K, Roque-Gaffney E, Monahan M. 1994. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol 71(2):169–175. [DOI] [PubMed] [Google Scholar]
- Pereira L, Font-Nieves M, Van den Haute C, Baekelandt V, Planas AM, Pozas E. 2015. IL-10 regulates adult neurogenesis by modulating ERK and STAT3 activity. Front Cell Neurosci 9(February):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit MA, Paudel ML, Taylor BC, Hughes JM, Strotmeyer ES, Schwartz AV, Cauley JA, Zmuda JM, Hoffman AR, Ensrud KE. 2010. Bone mass and strength in older men with type 2 diabetes: the Osteoporotic Fractures in Men Study. J Bone Miner Res 25(2):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann P, Schernthaner G, Woloszczuk W. 1988. Serum osteocalcin levels in diabetes mellitus: analysis of the type of diabetes and microvascular complications. Diabetologia 31(12):892–895. [DOI] [PubMed] [Google Scholar]
- Qiao YC, Chen YL, Pan YH, Tian F, Xu Y, Zhang X, Zhao HL. 2017. The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. PLoS One 12(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehtz S, Bierhalter H, Schoenherr D, Parameswaran N, McCabe LR. 2017. Estrogen Deficiency Exacerbates Type 1 Diabetes Induced Bone TNFα expression and Osteoporosis in Female Mice. Endocrinology. 10.1210/en.2016-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick DM, Fort MM. 2000. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol (278):829–833. [DOI] [PubMed] [Google Scholar]
- Ries WL, Seeds MC, Key L. 989 Interleukin-2 stimulates osteoclastic activity : Increased acid production and radioactive calcium release. J Periodont Res 24:242–246. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Collins JG, Yalda B, Arnold RR, Lang NP, Offenbacher S. 1997a. Monocytic TNFa secretion patterns in IDDM patients with periodontal diseases. J Clin Periodontol 4:8–16. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S. 1997b. Inflammatory Mediator Response as a Potential Risk Marker for Periodontal Diseases in Insulin-Dependent Diabetes Mellitus Patients. J Periodontol 68(2):127–135. [DOI] [PubMed] [Google Scholar]
- Schloot NC, Hanifi-Moghaddam P, Goebel C, Shatavi SV, Flohe S, Kolb H, Rothe H. 2002. Serum IFN-gamma and IL-10 levels are associated with disease progression in non-obese diabetic mice. Diabetes Metab Res Rev 18(1):64–70. [DOI] [PubMed] [Google Scholar]
- Senn JJ, Klover PJ, Nowak IA, Mooney RA. 2002. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51(12):3391–3399. [DOI] [PubMed] [Google Scholar]
- Shah VN, Shah CS, Pharm M, Snell-bergeon JK. 2016. Type 1 Diabetes and Risk for Fracture: Meta-analysis and Review of the Literature. Diabet Med 32(9):1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Liu W, Kuang S. 2013. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues 27:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Cao X, Song G, Zhao Y, Shi B. 2014. Metformin rescues the MG63 osteoblasts against the effect of high glucose on proliferation. J Diabetes Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD, Lynch MA, McKenzie JA, Tanouye KM, Nyman JS, Wang X. 2009. Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J bone Miner Res 24(9):1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha KM, Zhou X. 2013. Genetic and molecular control of osterix in skeletal formation. J Cell Biochem 114(5):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D’Agostino RB, Hamman RF, Dabelea D. 2010. Inflammatory markers are increased in youth with type 1 diabetes: The SEARCH case-control study. J Clin Endocrinol Metab 95(6):2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. 2004. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res 19(7):1084–1091. [DOI] [PubMed] [Google Scholar]
- Szkudelski T 2001. Physiological Research.
- Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, Otani S. 1998. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone 22(1):17–23. [DOI] [PubMed] [Google Scholar]
- Verhaeghe J, Thomsen JS, Van Bree R, Van Herck E, Bouillon R, Mosekilde L. 2000. Effects of exercise and disuse on bone remodeling, bone mass, and biomechanical competence in spontaneously diabetic female rats. Bone 27(2):249–256. [DOI] [PubMed] [Google Scholar]
- Weber DR, Schwartz G. 2016. Epidemiology of Skeletal Health in Type 1 Diabetes. Curr Osteoporos Rep 14(6):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LX, Kukita T, Kukita A, Otsuka T, Niho Y, Iijima T. 1995. Interleukin‐10 selectively inhibits osteoclastogenesis by inhibiting differentiation of osteoclast progenitors into preosteoclast‐like cells in rat bone marrow culture system. J Cell Physiol 165(3):624–629. [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Botolin S, McCabe LR. 2002. p38 and activating transcription factor-2 involvement in osteoblast osmotic response to elevated extracellular glucose. J Biol Chem 277(40):37212–37218. [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Stell C, Irwin R, McCabe LR. 2000. Extracellular glucose influences osteoblast differentiation and c-jun expression. J Cell Biochem 79(2):301–310. [DOI] [PubMed] [Google Scholar]
- Zhang J, Motyl KJ, Irwin R, MacDougald O a., Britton R a., McCabe LR. 2015. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic L. reuteri. Endocrinology (July):EN20151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ding Y, Rao GZ, Miao D. 2016. Effects of IL-10 and glucose on expression of OPG and RANKL in human periodontal ligament fibroblasts. Brazilian J Med Biol Res 49(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, Yang W. 2014. Interleukin-10 inhibits bone resorption: A potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed Res Int 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. 2001. Tumor necrosis factor-α (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem 276(1):563–568. [DOI] [PubMed] [Google Scholar]