Abstract
Research in the last decade has substantially advanced our understanding of the pathophysiology of heart failure with preserved ejection fraction (HFpEF). However, treatment options remain limited as clinical trials have largely failed to identify effective therapies. Part of this failure may be related to mechanistic heterogeneity. It is speculated that categorizing HFpEF patients based upon underlying pathophysiological phenotypes may represent the key next step in delivering the right therapies to the right patients. Echocardiography may provide valuable insight into both the pathophysiology and underlying phenotypes in HFpEF. Echocardiography also plays a key role in the evaluation of patients with unexplained dyspnea, where HFpEF is suspected but the diagnosis remains unknown. The combination of E/e’ and right ventricular systolic pressure has recently been shown to add independent value in the diagnostic evaluation of people suspected with HFpEF. Finally, echocardiography enables identification of different etiologies that mimic HFpEF but are treated differently, such as valvular heart disease, pericardial constriction, high output heart failure, or infiltrative myopathies such as cardiac amyloid. The purpose of this review is to summarize the current understanding of the pathophysiology and phenotyping of HFpEF with particular attention to the role of echocardiography in this context.
Keywords: diagnosis, diastolic function, echocardiography, filling pressure, heart failure, non-invasive
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is a common clinical syndrome that is increasing in prevalence. Rather than an isolated abnormality in left ventricular (LV) diastolic function, patients with HFpEF display multifaceted limitations in cardiac, vascular, and peripheral function.(1) Phenotyping based upon pathophysiology, comorbidities or some combination may provide targeted therapies to the specific HFpEF subpopulations that are positioned to derive the greatest benefit.(2).
Cardiovascular imaging plays a key role in the diagnosis and evaluation of HFpEF, particularly echocardiography, which allows for assessment of cardiac structure, function, and hemodynamics.(3) In this review, we will summarize the current understanding of the pathophysiology and phenotypes of HFpEF, with focus on the essential role of imaging for the evaluation and care of patients with HFpEF.
Pathophysiology of HFpEF: Beyond Diastolic Dysfunction
LV diastolic dysfunction plays a fundamental, overarching role in the pathophysiology of HFpEF.(1) LV diastolic dysfunction is defined by an impairment in relaxation, an increase in viscoelastic chamber stiffness, or some combination of the two,(4,5) and leads to symptomatic HF by causing elevated filling pressures at rest or with exertion.(6) Elevated filling pressures promote symptoms of dyspnea,(7) impair exercise capacity,(7,8) increase risk for HF hospitalization,(9) and decrease survival in HFpEF.(10) The importance and assessment of LV diastolic dysfunction in HFpEF are reviewed in detail in other articles in this issue.
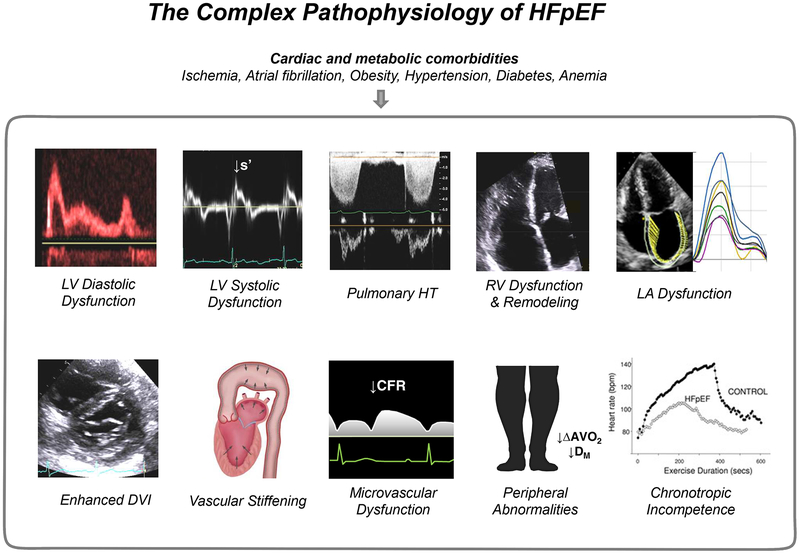
While diastolic dysfunction is central to HFpEF, it is also important to acknowledge that there are declines in LV relaxation and compliance with normal aging, or with cardiometabolic comorbidities such as obesity, insulin resistance, and hypertension.(11–13) Not all patients with diastolic dysfunction have or will develop clinical HFpEF.(14,15) Research in the past decade has demonstrated that, in addition to LV diastolic dysfunction, multiple non-diastolic abnormalities in cardiovascular system contribute to the syndrome of HFpEF. These include subtle LV systolic dysfunction, LA impairment, relative pericardial restraint, abnormal right ventricular-pulmonary artery coupling, pulmonary vascular disease, systemic vascular stiffening, coronary and peripheral microvascular dysfunction, and chronotropic incompetence (Figure 1).(1)
Figure 1. The Complex Pathophysiology of HFpEF:
Patients with HFpEF display impairments beyond diastolic dysfunction. The primary involvement in any one component may vary between patients. See text for details. Portions of this figure were adapted with permission from references 29 and 30. ΔAVO2, arterial-venous oxygen content difference; CFR, coronary flow reserve; DM, diffusive oxygen conductance; DVI, diastolic ventricular interdependence; LA, left atrial; LV, left ventricular; s’, systolic mitral annular tissue velocity; RV, right ventricular; and HT, hypertension.
Left Ventricular Systolic Dysfunction
Despite having a “preserved” EF, it is now well established that LV systolic performance is not normal in HFpEF, whether assessed by preload recruitable stroke work, stress-corrected endocardial and midwall shortening, twisting, or circumferential and longitudinal shortening using tissue Doppler or strain imaging.(6,16–20) There is evidence to support a causal link between metabolic and cardiac comorbidities and reduced LV longitudinal strain,(21) and a recent study has shown that HFpEF patients with microvascular dysfunction display more abnormal systolic mechanics by strain and tissue Doppler imaging.(22) This coronary microvascular dysfunction that develops secondary to metabolic comorbidities may contribute to subendocardial ischemia and impairments in LV longitudinal shortening during stress, especially in the setting of myocardial oxygen supply-demand imbalance.(23)
Multiple studies have demonstrated that subtle impairments in systolic function at rest become dramatic during exercise in patients with HFpEF, contributing to decreased exercise capacity, impaired early diastolic recoil/LV suction, impaired cardiac output and elevation in LV filling pressures.(6,17,24–26) While LV diastolic dysfunction is clearly present in HFpEF, the limitation in stroke volume reserve during exertion is not mediated by failure to increase LV end diastolic volume, but rather by inability to reduce end systolic volume.(24,27,28) This is related in part to abnormal peripheral vasorelaxation, which increases LV afterload,(6,24,29,30) but equally or more important are limitations in LV contractile reserve.(6,17,24) Impaired LV systolic mechanics in HFpEF also predict increased risk of adverse outcomes.(16,19)
Pulmonary Hypertension
Pulmonary hypertension (PH) is extremely common in HFpEF, seen in roughly 80% of patients, and mortality is increased in this cohort.(31–33) While PH is predominantly related to LA hypertension in the majority of HFpEF patients, a substantial number go on to develop pulmonary vascular disease, manifest by elevation in pulmonary vascular resistance and reduction in pulmonary arterial (PA) compliance.(34,35) Recent data have demonstrated that HFpEF patients with pulmonary vascular disease display adverse outcomes, worse exercise capacity, and unique hemodynamic features that develop during exercise, including impaired recruitment of LV preload due to excessive right heart congestion and blunted right ventricular (RV) systolic reserve.(34,36)
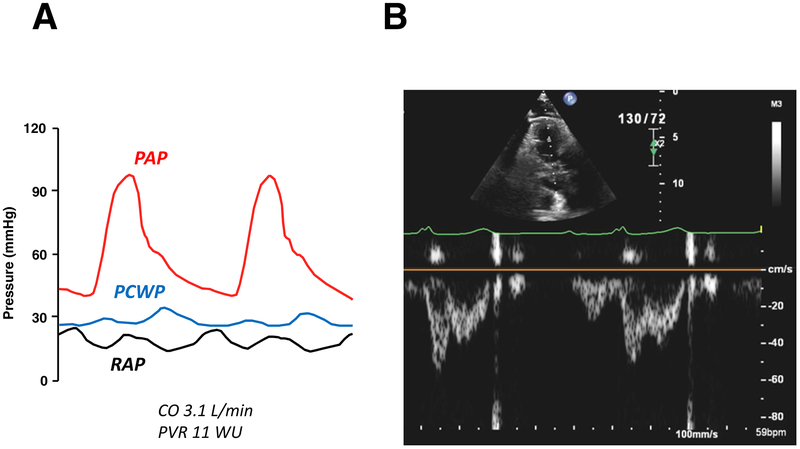
Some patients with HFpEF develop pulmonary vascular dysfunction that becomes manifest only during exercise.(6,35) This is evidenced by an increase in the slope of the pulmonary artery pressure-flow relationship,(37) or simply by failure to reduce pulmonary vascular resistance during exertion.(6) This pulmonary vascular reserve limitation is more common in the obese phenotype of HFpEF,(38) and has recently been linked to adverse clinical outcomes.(39) The presence of pulmonary vascular disease should be suspected from mid-systolic notching in the RV outflow Doppler profile, along with a short acceleration time caused by increased pulmonary arterial impedance with enhanced wave reflection (Figure 2).(40) Pulmonary vascular resistance can be estimated using a ratio of peak tricuspid regurgitation [TR] velocity to the RV outflow velocity-time integral,(41) but this has yet to be validated in HFpEF populations.
Figure 2. HFpEF with Pulmonary Vascular Disease:
Invasive pressure tracings and right ventricular outflow pulse-wave Doppler imaging in a HFpEF patient with severe pulmonary vascular disease. (A) There is severe elevation in mean pulmonary artery pressure [PAP] due to marked elevation in pulmonary capillary wedge pressure [PCWP] but also coexisting elevation in pulmonary vascular resistance (PVR 11.0 WU). Clinical right heart failure is also present, as evidenced by high right atrial pressure [RAP] (20 mmHg). (B) Right ventricular outflow pulse-wave Doppler in this patient demonstrates a distinct mid-systolic notch, presumably caused by backward traveling compression wave, with abbreviation of acceleration time. Abbreviations as in Figure 1.
Right Ventricular Dysfunction
The presence of PH eventually leads to RV systolic dysfunction, which is common and associated with adverse outcomes in HFpEF.(32,33) RV systolic function can be assessed by tricuspid annular plane systolic excursion (TAPSE), RV fractional area change, free wall strain, tricuspid annular s’ velocity or RV index of myocardial performance.(42) Recent data have shown that the response of the RV to PH (RV-PA coupling) is even more important, and RV-PA coupling can then be assessed by the ratio of RV function to right ventricular systolic pressure (RVSP).(42,43) Lower TAPSE/RVSP ratio (<0.36 mm/mmHg) is associated with adverse outcomes in HFpEF,(42,44,45) and may identify a patient cohort that will respond differently to interventions targeted to RV afterload.(44)
RV dysfunction in HFpEF is not exclusively mediated by afterload mismatch from PH.(32) Many patients with near-normal PA pressures at rest also have intrinsic RV dysfunction, particularly in the setting of AF and TR. Patients with HFpEF also display abnormal RV function during exercise, even when resting function appears normal.(6) This is remarkably similar to what is observed in the LV, where stress reserve is impaired even when resting function is normal,(17,24) emphasizing the fact that HFpEF is a biventricular disorder where stress reserve capacity is impaired.(1)
RV dysfunction is associated with RV (or right heart) remodeling. Recent longitudinal data have shown that RV diastolic area increases by 21% and RV fractional area change decreases by 10% over just 4 years of time in patients with HFpEF.(46) These changes greatly exceeded corresponding alterations in LV structure and function during the same time course. Development of incident RV dysfunction was associated with higher PA pressures, development of AF, coronary disease, and obesity, suggesting that it may be preventable.(46) Assessments of RV remodeling focus on dilation (RV basal, mid, and longitudinal dimensions and areas), but RV hypertrophy and right atrial (RA) dilation are also important.(47) Increased RV diameter, area, and RV wall thickness have been shown to predict adverse outcome in HFpEF.(32,48) RV and RA dilation lead to tricuspid annular dilation and resultant TR,(46) which causes greater ventricular interdependence (discussed below), more profound exercise limitations, and increased risk of death.(33,46,49) Echocardiography allows for identification of HFpEF patients with significant TR, and such patients may respond to structural interventions targeted to the valve insufficiency, though data are sparse at this time.
Left Atrial Dysfunction
LA remodeling and dysfunction secondary to increased LV filling pressure are common in HFpEF and associated with worse symptoms, more pulmonary vascular disease, greater RV dysfunction, depressed exercise capacity, and adverse outcomes, suggesting that patients with relatively greater “atrial myopathy” may also constitute a different phenotype within the HFpEF spectrum.(50–53) The LA may adapt to “protect” the pulmonary vasculature, and thus right heart, from the deleterious effects of high LV filling pressures caused by ventricular diastolic dysfunction.(50) This may partially explain why patients with HFpEF that develop AF, where LA booster function is lost, suffer from worse functional disability and increased risk of death,(54,55) as well as increase risk for developing RV dysfunction.(32,33,43,46) A number of patients develop LA myopathy (or elevation of LA pressure) out of proportion to LV diastolic dysfunction (or LV filling pressure), often referred to as the stiff LA syndrome.(56) This may occur after surgical or catheter ablation for AF. However, a number of patients with HFpEF also display relatively greater LA dysfunction in the absence of prior LA interventions, and echocardiography can play an important role in defining this aspect of the pathophysiology through volumetric and strain imaging.(57,58) Measurements of LA volume alone are important, but insufficient to identify LA dysfunction. LA deformation analysis, particularly LA reservoir strain, appears to be robust to detect LA dysfunction, and has been shown to carry prognostic value in patients with HFpEF.(52,53,59,60)
Vascular Stiffening
Increased vascular stiffness and arterial wave reflections increase LV afterload and unfavorably affects loading sequence, contributing to impaired LV early relaxation and contractile function, LV hypertrophy, and subsequent risk of incident HF.(29,61–66) Vascular stiffness and wave reflection are abnormally elevated in patients with HFpEF,(61,62) particularly during exercise.(29) Systemic hypertension and aging have been considered to be the primary causes of arterial stiffening, but recent data suggest that stiffening is related to comorbid conditions observed with HFpEF such as metabolic syndrome and obesity.(67,68) Vascular stiffening coupled with increased LV systolic elastance promotes blood pressure lability in HFpEF.(69) Arterial stiffening in HFpEF is partially reversible, as acute administration of NO donors can improve both arterial function and cardiac hemodynamics at rest and during exertion.(29)
Ventricular Interdependence
As the total epicardial heart volume enlarges there is greater stretch of the pericardium, which may exert a compressive external contract pressure on the LV, resulting in flattening of the interventricular septum as the two ventricles compete for space in the pericardial sac. The net result of this enhanced diastolic ventricular interaction is a reduction in LV volume (preload), even as intracavitary pressures transmitted back to the pulmonary capillaries increase.(38) The degree of ventricular interdependence is visually recognized as a D-shaped LV cavity in the short axis view, and this can be quantified by the ratio of the LV anteroposterior dimension to the septo-lateral dimension (the eccentricity index, higher values indicate greater interdependence). Typically, in RV pressure overload, the leftward septal shift occurs at both end-systole and enddiastole, with the most marked deformation at end-systole while there is the most marked shift of the septum at end-diastole with relatively sparing of its configuration at end-systole in RV volume overload.(70)
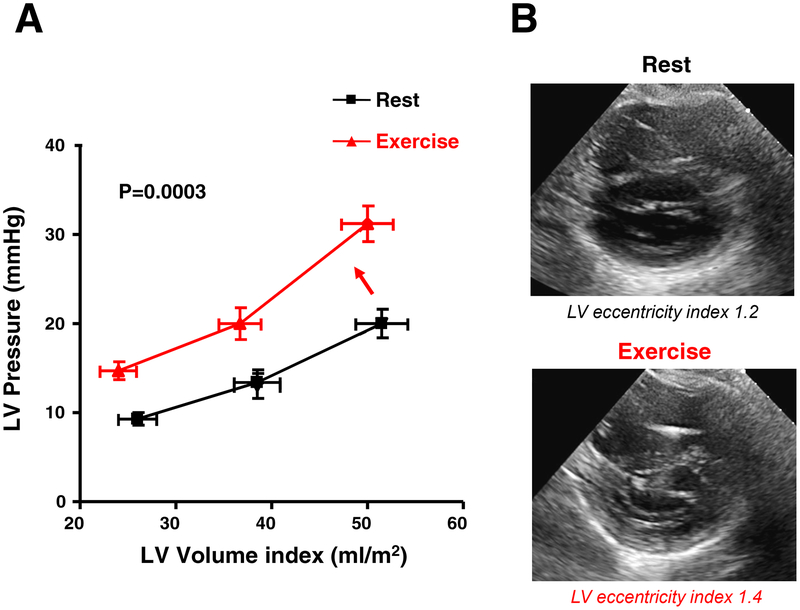
Constrictive physiology should be considered when evidence of enhanced interdependence is observed by echocardiography. A number of echocardiographic clues are useful to evaluate the presence of constrictive pericardial disease (Table 1). Pericardial restraint is an important contributor to the filling pressure elevation in patients with obese HFpEF, who display more RV enlargement and dysfunction, greater LV mass, and an increase in epicardial fat (Figure 3).(38) Conversely, RV unloading with lower body suction improves forward stroke volume because the LV filling is enhanced as pericardial restraint is released.(71) Pericardial restraint is often most dramatic during exercise, when venous return to the right heart is augmented. This is manifest by a parallel shift upward in the diastolic pressure volume relationship, suggesting increased external constraint on the heart (Figure 3).(72)
Table 1.
Differential Diagnoses of HFpEF and Their Clues and Subsequent Testings
| Differential Diagnosis | Echocardiographic Clues | Subsequent Testing Considered |
|---|---|---|
| Hypertrophic cardiomyopathy | Asymmetric hypertrophy, ↑↑LV wall thickness, LVOT obstruction, SAM | CMR, Genetic testing |
| Restrictive cardiomyopathy | Small LV cavity, ↑LV wall thickness, Sparkling myocardium, Apical sparing, Severely reduced tissue Doppler, PE, Hepatic vein diastolic flow reversal during inspiration | CMR, Biopsy, etc |
| Pulmonary arterial hypertension | ↑RVSP with no sign of elevated LV filling pressure (e.g., E/A ratio <1), Isolated right heart dilation, PA dilation, RVOT Doppler midsystolic notch | Right heart catheterization, High-resolution CT |
| Constrictive pericarditis | Pericardial thickening, Septal bounce, annulus paradoxus and annulus reversus, ↑Respiratory variation in mitral/tricuspid flow, Hepatic vein diastolic flow reversal during expiration, Absence of IVC collapse | CT, CMR, Right heart catheterization |
| Valvular heart disease | Morphological valvular abnormalities, Color Doppler | Detailed echocardiographic assessments for stenosis/regurgitation, TEE |
| Coronary artery disease | Regional wall motion abnormality and thinning | Coronary angiography |
| Chronic thromboembolic pulmonary hypertension | ↑RVSP with no sign of elevated LV filling pressure, Isolated right heart dilation, PA dilation, RVOT Doppler midsystolic notch | V/Q scan, High-resolution CT, Right heart catheterization ± Pulmonary angiography |
| High output heart failure | ↑Doppler-derived cardiac output, ↑4 cardiac chamber dilation | Right heart catheterization |
CMR, cardiac magnetic resonance; CT, computed tomography; HFpEF, heart failure with preserved ejection fraction; IVC, inferior vena cava; LVOT, left ventricular outflow obstruction; PA, pulmonary artery; RVSP, estimated right ventricular systolic pressure; PE, pericardial effusion; RVOT, right ventricular outflow; SAM, systolic anterior motion of the mitral valve; TEE, transesophageal echocardiography; and V/Q, ventilation/perfusion.
Figure 3. Obesity-related HFpEF with Enhanced Pericardial Restraint:
(A) Diastolic pressure-volume relationships of patients with HFpEF (mean BMI 30.2) at rest (black) and with exercise (red). With exercise, the diastolic pressure-volume relationship (DPVR) curve shifts upward. While chamber stiffness (linearized slope of the diastolic pressure-volume relationship) increases significantly with exercise in HFpEF, the majority of the increase in LV end diastolic pressure is related to parallel shift upward in the DPVR, suggesting increased external forces from the right heart and pericardium. Adapted with permission from reference 72. (B) Parasternal short axis-views at end-diastole in a patient with obese HFpEF (body mass index 42 kg/m2) demonstrating worsening pericardial restraint from rest to exercise. Note the D-shaped septum during 20 watts supine ergometer exercise with increase in the LV eccentricity index. Abbreviations as in Figure 1.
Heightened ventricular interdependence also plays an important role in HFpEF patients with pulmonary vascular disease and TR.(34,49) The presence of enhanced ventricular interdependence complicates interpretation of the mitral inflow profiles and E/e’ ratios in HFpEF, since there is a variable contribution of myocardial diastolic dysfunction versus relative pericardial restraint driving the raised filling pressure in these patients.(38) Recent studies have evaluated the potential efficacy of surgical approaches to target the pericardium in HFpEF.(73,74)
Coronary Microvascular Dysfunction and Myocardial Injury
Accumulating data suggest an important role for abnormalities in the coronary microcirculation in HFpEF.(22,23,75) Coronary microvascular inflammation secondary to systemic inflammation from metabolic comorbidities and loss of nitric oxide bioavailability is believed to cause the coronary microvascular dysfunction and rarefaction.(68) Even in the absence of epicardial coronary stenosis, this coronary microvascular dysfunction and rarefaction may promote cardiac injury by inducing myocardial supply-demand mismatch, especially during exercise, leading to systolic and diastolic reserve limitations, higher filling pressures during exercise, and more impaired exercise capacity.(23) The presence of coronary microvascular dysfunction is associated with markers of greater disease severity, including more profound right heart dysfunction and abnormal endothelium-dependent vasodilation in patients with HFpEF.(22)
Coronary microvascular dysfunction can be detected using echocardiography, although this method has not reached widespread use. (22) Other approaches including myocardial positron emission tomography and cardiac magnetic resonance, as well as the invasive measurement of coronary flow reserve are used more commonly in this evaluation at the current time.(76,77)
Abnormalities in the Periphery
Microvascular abnormalities have been observed in skeletal muscle, which plays a role in limiting functional capacity in HFpEF.(78,79) Indeed, the ability augment oxygen extraction is the periphery is an important determinant of exercise capacity in HFpEF.(27,80,81) This is supported by the observation that reduced capillary density in skeletal muscle is correlated with worse exercise capacity in patients with HFpEF.(78) Diffusive oxygen conductance in the periphery is impaired in patients with HFpEF, and may be favorably modified by exercise training in this cohort.(81,82)
Comorbidity-Based Phenotyping
The extent to which each of these pathophysiologic abnormalities is present in the individual patient can be quite variable, and there is accordingly substantial mechanistic heterogeneity in what is broadly defined as “HFpEF”. As described above, this heterogeneity has been speculated as a primary cause of the failure of prior clinical trials in HFpEF.(2) Accordingly, there is an unmet need to categorize different phenotypes within the broader spectrum of HFpEF into pathophysiologically homogenous groups. In addition to pathophysiology-based phenotypes described above, including pulmonary vascular disease, RV dysfunction, pericardial restraint, LA dysfunction, or chronotropic incompetence, there are key clinical phenotypes that demonstrate distinct pathophysiologic features compared to “garden variety of HFpEF”, which may have treatment strategies that might be better tailored to the underlying pathophysiology (Central illustration).(22,23,34,38,75,83)
Central illustration. The Phenotypes of HFpEF:
There are key clinical phenotypes that demonstrate distinct pathophysiologic features compared to “garden variety of HFpEF”. FA, fatty acid; fxn, function; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrial; LV, left ventricular; NO-cGMP, nitric oxide-cyclic guanosine monophosphate signaling; O2, oxygen; PA, pulmonary artery; PH, pulmonary hypertension; PV, pulmonary vascular; and RV, right ventricular.
Obese HFpEF
Obesity is a major risk factor for HFpEF,(11) and it is now recognized as one of the most important clinical phenotypes of HFpEF.(38) A number of key differences have been identified in obese HFpEF as compared to non-obese HFpEF patients, including greater relationships between body weight and cardiac filling pressures, greater plasma volume expansion, more ventricular remodeling, adverse hemodynamics, altered RV-PA coupling, worse exercise capacity, and enhanced pericardial restraint.(38) Visceral adiposity and ectopic fat deposit can also contribute to the obesity phenotype by altering hemodynamics, inducing systemic and local inflammation, myocardial substrate utilization, and causing direct mechanical effects. Weight gain and excess central adiposity contributes to ventricular stiffening and myocardial dysfunction that ultimately leads to HFpEF.(67,84)
Greater adiposity in certain regional fat depots may also be important,(85) including visceral,(86) intramuscular,(87) epicardial,(38,88,89) and intramyocardial fat.(89,90) There are several possible treatments targeting the HFpEF obesity phenotype. Weight loss achieved by caloric restriction has been shown to improve exercise capacity and quality of life in obese HFpEF,(91) and other studies evaluating weight loss strategies or therapies targeted to epicardial fat or increased pericardial restraint might also be effective.(73,74)
Ischemic HFpEF
The presence of epicardial coronary artery disease identifies a distinct HFpEF phenotype in view of its worse prognosis and potential for improvement through revascularization.(92) Ischemic stress imaging, including echocardiography, may be less accurate in patients with HFpEF.(92) This may in part be related to microvascular and hemodynamically mediated myocardial ischemia and injury from a supply-demand mismatch in HFpEF.(22,23,75) Patients with greater myocyte injury, whether due to ischemic insult or other processes, may be excellent candidates for phenotyping because they are readily identifiable by a blood test (high sensitivity troponin levels), and echocardiography,(22) and because the degrees of impairment in myocardial function, hemodynamic alterations, and exercise incapacity are all correlated with the magnitude of cardiac injury present.(23)
Cardiometabolic HFpEF
This cohort overlaps with the obese phenotype but may not require the presence of obesity, particularly in Asian populations who display metabolic abnormalities at lower body weights.(93) After adjusting for BMI, greater percentage of body fat is associated with ventricular-vascular stiffening, and this may be related to the metabolic sequelae of even “lean” adiposity.(94) Cardiometabolic duress leading to low grade inflammation and impaired nitric oxide metabolism has been proposed as a unifying mechanism of HFpEF.(68) Metabolic syndrome is associated with greater ventricular stiffening,(67) altered substrate utilization decreasing myocardial efficiency,(95) abnormal ventricular function,(84,96) and greater burden of pulmonary vascular disease.(97) The pathophysiologic evidence of combined, biventricular systolic and diastolic reserve impairment in HFpEF described above (6,17,24) suggests that a fundamental disorder in cardiac energy metabolism could play a role.
While mitochondrial function has not yet been assessed in human cardiomyocytes in HFpEF, there is evidence for impaired mitochondrial function in skeletal muscle.(79) Sarcopenia can lead to decreased muscle strength, reduced exercise capacity, and worse quality of life in HFpEF patients.(98,99) Sarcopenia may also coexist with obesity: “sarcopenic obesity”, which is characterized by excess fat mass and decreased muscle mass and it is known to be more related to cardiometabolic and functional abnormalities.(99,100)
Next Steps for Phenotyping
Numerous candidates for sub-phenotyping exist, based upon pathophysiology or comorbidities (Summary Figure), but none will gain traction unless there is an effective treatment identified that is specific to that phenotype. Further study is required to better standardize HFpEF phenotyping, including optimal discrimination between overlapping phenotypes, and determination of the best means to separate phenotypes. This may be based upon clinical presentation, characteristic changes in cardiac structure and function, hemodynamic signatures, mechanisms exercise intolerance (cardiac vs peripheral), the presence comorbidities, or some combination of each of these metrics (Table 2).
Table 2.
Key Questions and Gaps with Regard to the Understanding of HFpEF
| Key Questions | Gaps in Evidence | Future Studies Needed |
|---|---|---|
| Phenotyping & Pathophysiology | ||
| Phenotyping HFpEF is promising to better individualize therapy, but it remains unclear how HFpEF phenotypes should be defined? | There can be several approaches to HFpEF classification, including pathophysiologic, clinical presentation, comorbidities, cardiac structure, and machine-learning. What are the roles of non-invasive imaging, biomarkers, or other approaches? | Further study is required to standardize the HFpEF phenotyping, identify discrete phenotypes that behave similarly and respond to treatment similarly, and identify the optimal roles of non-invasive imaging and biomarkers to categorize them. |
| Diagnosis | ||
| Currently, echocardiography is a central role for diagnosis of HFpEF. What are the roles of different modalities in the evaluation for HFpEF? | Other imaging modalities such as cardiac magnetic resonance, cardiac computed tomography, and positron emission tomography may be promising, but the data to support their usefulness require further investigation. | Future studies are needed to investigate the roles for difference modalities in the evaluation for HFpEF. |
| What are the expected roles of novel imaging techniques for HFpEF? | Recent studies have reported the possibility of machine learning-based echocardiographic analysis for diagnosis and phenotyping in HFpEF. | Future studies are needed to establish the roles of machine learning-based imaging in HFpEF, with appropriate standards against which the machine-learning approaches can be compared. |
| Because hemodynamics are often normal at rest in HFpEF, diastolic stress echocardiography may be useful to enhance diagnosis, but is this ready for standard practice? | Evidence to support its utility across multiple studies in HFpEF is not sufficient. E/e’ may not change with changes in filling pressure during exercise. How should we deal with E-A fusion during exercise? What is the optimal cutoff of E/e’ during exercise? | Further research is needed to validate the utility of exercise stress echocardiography, ideally in multicenter studies using simultaneous assessment using echocardiography and invasive hemodynamics, with blinded interpretation from disinterested observers. |
Diagnosis of HFpEF
The diagnosis of HFpEF is obvious in the patient with overt congestion, but evaluation of the euvolemic patient with exertional dyspnea presents a greater challenge.(101–103) Part of this difficulty is related to the fact that filling pressures are often normal at rest, but become elevated only during the stress of exercise.(102,103) Invasive cardiopulmonary exercise testing has emerged as the gold standard to definitively identify or exclude HFpEF as the cause of dyspnea.(101–103) Expert consensus guidelines from the ESC have recommended a combination of different indices of diastolic dysfunction and natriuretic peptide testing. When tested prospectively, this approach has yielded good specificity, but poor sensitivity (Table 3).(101,102)
Table 3:
Recent Studies Providing Sensitivity and Specificity of the Current Guidelines for the Diagnosis of HFpEF among Patients with Unexplained Dyspnea
| Study | n | Guideline | Sensitivity | Specificity | AUC | Indeterminate |
|---|---|---|---|---|---|---|
| Reddy et al. 2018101 | 414 (HFpEF 267) | ESC | 57% | 78% | 0.67 | 0% |
| Obokata et al. 2017102 | 74 (HFpEF 50) | ASE/EACVI5 | 34% | 83% | 0.65 | 24% |
| ESC | 60% | 75% | 0.68 | 0% |
ASE/EACVI, Recommendations for the evaluation of left ventricular diastolic function by echocardiography from the American Society of Echocardiography and the European Association of Cardiovascular Imaging; AUC, area under the curve; ESC, 2016 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure (Ponikowski P. Eur Heart J. 2016;37:2129–2200.); and other abbreviations as in Table 1.
A recent study was performed to identify echocardiographic measures and clinical features that could independently predict the presence of HFpEF in >500 subjects presenting with exertional dyspnea, to help guide the diagnostic evaluation.(101) Case status (HFpEF or non-cardiac cause of dyspnea) was ascertained by invasive diastolic stress testing. The discriminatory ability of multiple echocardiographic parameters was evaluated to differentiate HFpEF from non-cardiac dyspnea in individual patients (Table 4). While many of these variables were predictive of HFpEF diagnosis, none were robust by themselves to distinguish the groups (all area under the curves [AUCs]<0.70).(101)
Table 4:
Operating Characteristics of Echocardiographic Parameters for the Diagnosis of HFpEF
| AUC | P | Sensitivity | Specificity | |
|---|---|---|---|---|
| Ejection fraction <55% | 0.52 | 0.09 | 8% | 96% |
| LV hypertrophy | 0.57 | 0.0006 | 26% | 88% |
| LA volume Index >34 ml/m2 | 0.66 | <0.0001 | 49% | 83% |
| E/e’ ratio (septal) >9 | 0.69 | <0.0001 | 78% | 59% |
| E/e’ ratio (septal) >13 | 0.66 | <0.0001 | 46% | 86% |
| Septal e’ velocity <7 cm/s | 0.62 | <0.0001 | 48% | 76% |
| Right atrial pressure >10 mmHg | 0.56 | <0.0001 | 16% | 97% |
| RV systolic pressure >35mmHg | 0.66 | <0.0001 | 46% | 86% |
| RV fractional area change <48% | 0.64 | <0.0001 | 39% | 88% |
| Tricuspid annular plane systolic excursion <16 mm | 0.54 | 0.0008 | 9% | 99% |
| Visual RV dysfunction | 0.58 | <0.0001 | 22% | 94% |
| Visual RV dilation | 0.60 | <0.0001 | 32% | 88% |
Table is compiled from data presented in reference #101.
E/e’, the ratio of early diastolic mitral inflow to mitral annular tissue velocities; LA, left atrial; LV, left ventricular; RV, right ventricular; and other abbreviations as in Table 1.
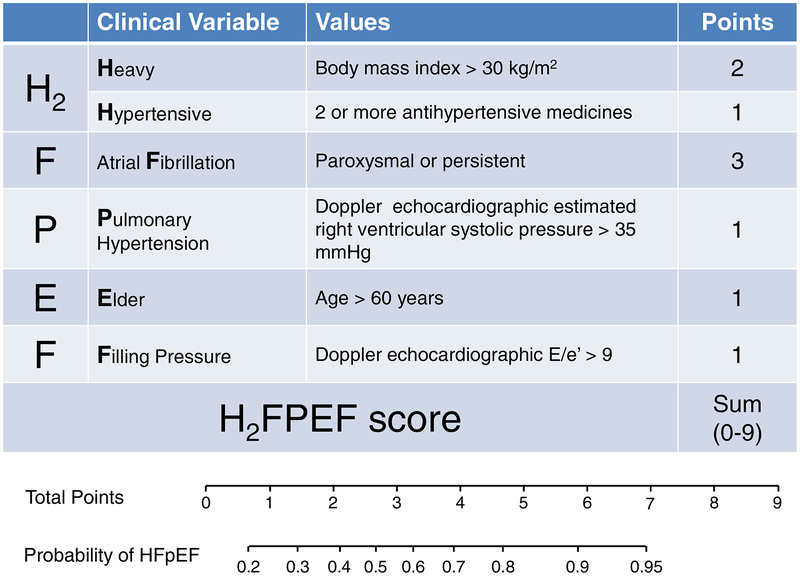
In multivariable logistic regression, only 2 echocardiographic variables remained significant predictors of HFpEF: septal E/e’>9 and RVSP>35 mmHg.(101) When combined with clinical characteristics independently associated with HFpEF, including AF, obesity, older age, and treatment with 2 or more antihypertensive medicines, this algorithm (H2FPEF score) was found to be a robust method to estimate the probability that HFpEF is the cause of dyspnea (Figure 4).(101) This scheme was then validated in an independent test cohort where it retained excellent discriminatory capacity (AUC 0.886; p<0.0001). Patients found to have an intermediate probability of HFpEF according to this scheme require additional evaluation with exercise testing.(101)
Figure 4. The H2FPEF score to Facilitate Diagnostic Evaluation in HFpEF:
In this score, the echocardiographic parameters that were independently predictive for HFpEF (E/e’ >9 and right ventricular systolic pressure >35mmHg) are incorporated with clinical characteristics to determine the probability that HFpEF is present in patients presenting with unexplained dyspnea. Adapted with permission from reference 101. Abbreviations as in Figure 1.
The H2FPEF score requires external validation in other centers, and patients with other causes of the clinical syndrome of HF were excluded from the original study (pericardial diseases, high output failure, valvular heart disease, etc.), so these alternative causes of dyspnea need to also be considered. Pulmonary arterial hypertension must be considered in patients meeting criteria by the score, since RVSP is elevated in these patients due to pulmonary vascular disease, and septal e’ may be reduced due to RV diastolic dysfunction, increasing septal E/e’. Like all diagnostic tests, the positive and negative predictive values will depend upon the pre-test probability of disease, and the utility will be optimized when applied to patients with intermediate probabilities.
Echocardiography to Identify Disorders that Mimic HFpEF
In addition to identifying discrete phenotypes within the broader HFpEF spectrum, echocardiography plays an essential role to rule out disorders that mimic HFpEF (Table 1). These include hypertrophic cardiomyopathy, primary valvular heart disease, non-Group 2 PH, cardiac amyloidosis, pericardial disease, and high output failure. After excluding the presence of depressed EF, assessing for these “masqueraders” is a critical step in evaluation, as each of the mimics have their own unique treatments that differ from “garden variety” HFpEF.
Conclusions and Future Directions
We now understand that HFpEF is a complex syndrome that has multiple pathophysiologic abnormalities. The extent to which each of these abnormalities is present in the individual patient is variable. In addition to multiple cardiac and metabolic comorbidities, this mechanistic heterogeneity makes it difficult to apply “one size fits all” approaches to people with HFpEF, and categorizing the patients based upon underlying clinical and pathophysiological phenotypes represents a key next step in delivering the right therapies to the right patient. Echocardiography plays an essential role in the evaluation for HFpEF and provides valuable information to assess pathophysiological mechanisms, phenotyping, and prognosis. Diagnosis of HFpEF non-invasively remains challenging in the absence of fluid retention and overt congestion, and among the large subset of HFpEF with exertional dyspnea, the diagnosis requires objective documentation of elevated LV filling pressure either noninvasively or invasively. Together with clinical characteristics, echocardiography can help determine the likelihood that HFpEF is present, and allow for informed decision making regarding the need for more advanced testing.
There are many unanswered questions and gaps in evidence with regard to the diagnosis and phenotyping in HFpEF (Table 2). Further study is required to identify how HFpEF phenotypes should be defined, establish the roles of non-invasive imaging and biomarkers for phenotyping, determine roles for different modalities and machine learning-based imaging in the evaluation for HFpEF,(104) and standardize diagnostic criteria, thereby allowing and targeted treatments and accurate diagnosis.
Highlights.
HFpEF is a heterogeneous syndrome and categorizing patients based upon pathophysiology may provide phenotype-specific therapies.
Echocardiography provides valuable information to assess pathophysiological mechanisms, phenotyping, as well as diagnosis in HFpEF.
Further study is needed to establish the HFpEF phenotyping and roles of non-invasive imaging in it.
Acknowledgments:
Dr. Borlaug is supported by the National Institutes of Health (R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262). Dr. Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
ABBREVIATIONS:
- AF
atrial fibrillation
- CAD
coronary artery disease
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- LA
left atrial
- LV
left ventricular
- PA
pulmonary artery
- RVSP
right ventricular systolic pressure
- PH
pulmonary hypertension
- RV
right ventricular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–15. [DOI] [PubMed] [Google Scholar]
- 2.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 4.Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol. 1993;22:318–25. [DOI] [PubMed] [Google Scholar]
- 5.Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med. 2004;55:373–94. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, Dyspnea, and Pulmonary Reserve in Heart Failure with Preserved Ejection Fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–44. [DOI] [PubMed] [Google Scholar]
- 10.Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 11.Savji N, Meijers WC, Bartz TM, et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Bibra H, Paulus WJ, St John Sutton M, Leclerque C, Schuster T, Schumm-Draeger PM. Quantification of diastolic dysfunction via the age dependence of diastolic function -impact of insulin resistance with and without type 2 diabetes. Int J Cardiol. 2015;182:368–74. [DOI] [PubMed] [Google Scholar]
- 13.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–3. [DOI] [PubMed] [Google Scholar]
- 14.Nayor M, Cooper LL, Enserro DM, et al. Left Ventricular Diastolic Dysfunction in the Community: Impact of Diagnostic Criteria on the Burden, Correlates, and Prognosis. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah AM, Claggett B, Kitzman D, et al. Contemporary Assessment of Left Ventricular Diastolic Function in Older Adults: The Atherosclerosis Risk in Communities Study. Circulation. 2017;135:426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–9. [DOI] [PubMed] [Google Scholar]
- 19.Shah AM, Claggett B, Sweitzer NK, et al. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasselberg NE, Haugaa KH, Sarvari SI, et al. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jellis C, Martin J, Narula J, Marwick TH. Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol. 2010;56:89–97. [DOI] [PubMed] [Google Scholar]
- 22.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMISHFpEF. Eur Heart J. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obokata M, Reddy YNV, Melenovsky V, et al. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biering-Sorensen T, Santos M, Rivero J, et al. Left ventricular deformation at rest predicts exercise-induced elevation in pulmonary artery wedge pressure in patients with unexplained dyspnoea. Eur J Heart Fail. 2017;19:101–110. [DOI] [PubMed] [Google Scholar]
- 26.Opdahl A, Remme EW, Helle-Valle T, et al. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation. 2009;119:2578–86. [DOI] [PubMed] [Google Scholar]
- 27.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abudiab MM, Redfield MM, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 31.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammed SF, Hussain I, Abou Ezzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorter TM, Obokata M, Reddy YN, Melenovsky V, Borlaug BA. Exercise Unmasks Distinct Pathophysiologic Features in Heart Failure with Preserved Ejection Fraction and Pulmonary Vascular Disease. Eur Heart J. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J. 2017;38:2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–9. [DOI] [PubMed] [Google Scholar]
- 38.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W, Oliveira RKF, Lei H, Systrom DM, Waxman AB. Pulmonary Vascular Resistance During Exercise Predicts Long-Term Outcomes in Heart Failure With Preserved Ejection Fraction. J Card Fail. 2017;24:169–176. [DOI] [PubMed] [Google Scholar]
- 40.Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:268–76. [DOI] [PubMed] [Google Scholar]
- 41.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–7. [DOI] [PubMed] [Google Scholar]
- 42.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–81. [DOI] [PubMed] [Google Scholar]
- 43.Hussain I, Mohammed SF, Forfia PR, et al. Impaired Right Ventricular-Pulmonary Arterial Coupling and Effect of Sildenafil in Heart Failure With Preserved Ejection Fraction: An Ancillary Analysis From the Phosphodiesterase-5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) Trial. Circ Heart Fail. 2016;9:e002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorter TM, van Veldhuisen DJ, Voors AA, et al. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425–432. [DOI] [PubMed] [Google Scholar]
- 45.Guazzi M, Dixon D, Labate V, et al. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. [DOI] [PubMed] [Google Scholar]
- 46.Obokata M, Reddy YN, Melenovsky V, Pislaru SV, Borlaug BA. Deterioration in right ventricular structure and function over time in heart failure with preserved ejection fraction. Eur Heart J. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 48.Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. 2014;7:911–7. [DOI] [PubMed] [Google Scholar]
- 50.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 51.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. [DOI] [PubMed] [Google Scholar]
- 52.Santos AB, Kraigher-Krainer E, Gupta DK, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freed BH, Daruwalla V, Cheng JY, et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imag. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients with Preserved Ejection Fraction: A Community-Based Study. Circulation. 2013;128:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reddy YNV, El Sabbagh A, Packer D, Nishimura RA. Evaluation of shortness of breath after atrial fibrillation ablation-Is there a stiff left atrium? Heart Rhythm. 2018;15:930–935. [DOI] [PubMed] [Google Scholar]
- 57.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5. [DOI] [PubMed] [Google Scholar]
- 58.Freed BH, Shah SJ. Stepping Out of the Left Ventricle’s Shadow: Time to Focus on the Left Atrium in Heart Failure With Preserved Ejection Fraction. Circ Cardiovasc Imag. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obokata M, Negishi K, Kurosawa K, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–58. [DOI] [PubMed] [Google Scholar]
- 60.Santos AB, Roca GQ, Claggett B, et al. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber T, Wassertheurer S, O’Rourke MF, et al. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–83. [DOI] [PubMed] [Google Scholar]
- 62.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zamani P, Bluemke DA, Jacobs DR Jr., et al. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: the multi-ethnic study of atherosclerosis. Hypertension. 2015;65:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chirinos JA, Kips JG, Jacobs DR Jr., et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah SJ, Wasserstrom JA. Increased arterial wave reflection magnitude: a novel form of stage B heart failure? J Am Coll Cardiol. 2012;60:2178–81. [DOI] [PubMed] [Google Scholar]
- 66.Chirinos JA, Segers P, Rietzschel ER, et al. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension. 2013;61:296–303. [DOI] [PubMed] [Google Scholar]
- 67.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Failure. 2014;2:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 69.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–51. [DOI] [PubMed] [Google Scholar]
- 70.Louie EK, Rich S, Levitsky S, Brundage BH. Doppler echocardiographic demonstration of the differential effects of right ventricular pressure and volume overload on left ventricular geometry and filling. J Am Coll Cardiol. 1992;19:84–90. [DOI] [PubMed] [Google Scholar]
- 71.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–4. [DOI] [PubMed] [Google Scholar]
- 72.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borlaug BA, Carter RE, Melenovsky V, et al. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borlaug BA, Schaff HV, Pochettino A, et al. Pericardiotomy Enhances Left Ventricular Diastolic Reserve With Volume Loading in Humans: Implications for the Surgical Management of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Indorkar R, Kwong RY, Romano S, et al. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovasc Imaging. 2018. [Google Scholar]
- 78.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molina AJ, Bharadwaj MS, Van Horn C, et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of Exercise Intolerance in Heart Failure with Preserved Ejection Fraction: The Role of Abnormal Peripheral Oxygen Extraction. Circ Heart Fail. 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selvaraj S, Martinez EE, Aguilar FG, et al. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haykowsky MJ, Nicklas BJ, Brubaker PH, et al. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsujimoto T, Kajio H. Abdominal Obesity Is Associated With an Increased Risk of All-Cause Mortality in Patients With HFpEF. J Am Coll Cardiol. 2017;70:2739–2749. [DOI] [PubMed] [Google Scholar]
- 87.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng ACT, Strudwick M, van der Geest RJ, et al. Impact of Epicardial Adipose Tissue, Left Ventricular Myocardial Fat Content, and Interstitial Fibrosis on Myocardial Contractile Function. Circ Cardiovasc Imaging. 2018;epub ahead of press. [DOI] [PubMed] [Google Scholar]
- 90.Sarma S, Carrick-Ranson G, Fujimoto N, et al. Effects of age and aerobic fitness on myocardial lipid content. Circ Cardiovasc Imaging. 2013;6:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–27. [DOI] [PubMed] [Google Scholar]
- 93.Tromp J, Teng TH, Tay WT, et al. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail. 2018. [DOI] [PubMed] [Google Scholar]
- 94.Fernandes-Silva MM, Shah AM, Claggett B, et al. Adiposity, body composition and ventricular-arterial stiffness in the elderly: the Atherosclerosis Risk in Communities Study. Eur J Heart Fail. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. [DOI] [PubMed] [Google Scholar]
- 96.Bello NA, Cheng S, Claggett B, et al. Association of Weight and Body Composition on Cardiac Structure and Function in the ARIC Study (Atherosclerosis Risk in Communities). Circ Heart Fail. 2016;9:pii: e002978. doi: 10.1161/CIRCHEARTFAILURE.115.002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lai YC, Tabima DM, Dube JJ, et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation. 2016;133:717–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bekfani T, Pellicori P, Morris DA, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–46. [DOI] [PubMed] [Google Scholar]
- 99.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2015;12:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kohara K, Ochi M, Tabara Y, Nagai T, Igase M, Miki T. Arterial stiffness in sarcopenic visceral obesity in the elderly: J-SHIPP study. Int J Cardiol. 2012;158:146–8. [DOI] [PubMed] [Google Scholar]
- 101.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Gajjala S, Agrawal P, et al. Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation. 2018;138:1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]