Abstract
Muscular dystrophies are a group of genetic muscle disorders that cause progressive muscle weakness and degeneration. Within this group, Duchenne muscular dystrophy (DMD) is the most common and one of the most severe. DMD is an X chromosome linked disease that occurs to 1 in 3,500 to 1 in 5,000 boys. The cause of DMD is a mutation in the dystrophin gene, whose encoded protein provides both structural support and cell signaling capabilities. So far, there are very limited therapeutic options available and there is no cure for this disease. In this review, we discuss the existing cell therapy research, especially stem cell-based, which utilize myoblasts, satellite cells, bone marrow cells, mesoangioblasts and CD133+ cells. Finally, we focus on human pluripotent stem cells (hPSCs) which hold great potential in treating DMD. hPSCs can be used for autologous transplantation after being specified to a myogenic lineage. Over the last few years, there has been a rapid development of isolation, as well as differentiation, techniques in order to achieve effective transplantation results of myogenic cells specified from hPSCs. In this review, we summarize the current methods of hPSCs myogenic commitment/differentiation, and describe the current status of hPSC-derived myogenic cell transplantation.
Keywords: Duchenne muscular dystrophy (DMD), stem cell therapy, hiPSC, myogenic lineage specification
Muscular dystrophies (MDs) are a group of more than 50 heterogeneous genetic diseases, marked by degeneration of skeletal muscle and progressive weakness. The different MDs vary in terms of groups of muscles involved, age at disease onset, progression, and ultimate level of disability. Furthermore, several MDs show compromised physiology of other organs, such as the heart and brain in Duchenne muscular dystrophy (DMD) (Connuck et al., 2008; Rahimov and Kunkel, 2013; Yoshioka et al., 1980).
The most common form of MD is DMD, a fatal disease affecting around 1 in 3,500 to 1 in 5,000 live male births (Emery, 2002; Mendell et al., 2012). Boys with DMD usually lose the ability to walk in early teenage years, lose the ability to feed themselves in late teenage years, and die from respiratory insufficiency or cardiomyopathy in early adulthood (Emery, 2002; Mavrogeni et al., 2015).
Current standard of care includes the use of corticosteroids, cardioprotective treatment, ventilatory support, and physical therapy (Mah, 2016). However, these treatments have limitations and side effects, and are only able to delay the progression of the disease. No curative therapies are available for DMD.
In recent years, considerable research effort has been directed to developing new therapeutic options to treat DMD. Exon skipping, gene therapy and cell therapy have received considerable research attention. Antisense oligonucleotide (AON) mediated exon skipping that restores partial but functional dystrophin protein has advanced significantly during recent years with several AONs in clinical trials. Among them, Eteplirsen (for exon 51 skipping, affecting 13% of DMD patients) has been conditionally approved by the FDA in 2016 (Lim et al., 2017; Nakamura et al., 2017). Gene therapy that aims to produce a mini-dystrophin in muscle fibers is also in clinical trials with promising initial results, while gene editing using the CRISPR/Cas9 systems, to correct the dystrophin gene, may not be far behind. In this review, we present current stem cell-based therapies whose goal is to replenish the muscle stem cell pool with dystrophin-competent cells, with the focus on human pluripotent stem cells (hPSCs), which allows autologous cell therapies.
1. Pathological features of DMD
DMD is due to mutations in the dystrophin gene. The dystrophin gene is translated into a 427kDa protein, which is part of the Dystrophin Glycoprotein Complex (DGC) that provides a structural and signaling link between the cytoskeleton of the muscle fiber and the extracellular matrix (Ervasti, 2007). In healthy individuals, the dystrophin protein stabilizes the plasma membrane of the striated muscle fibers. However, in patients with DMD or the allelic Becker muscular dystrophy (BMD), mutations in the dystrophin gene cause the complete loss of the dystrophin protein (in DMD), or the production of a truncated, partially-functional, dystrophin protein (in BMD) (Rahimov and Kunkel, 2013).
In DMD patients, loss of a functional DGC leads to damage of the sarcolemma upon muscle contraction, which results in loss of sarcoplasmic proteins from the muscle fiber, and extensive damage of the muscle (Ervasti, 2007). As a consequence, DMD muscles are subject to chronic cycles of necrosis and regeneration, in the attempt to replenish the damaged fibers with new, functional fibers (Ervasti, 2007).
Muscle regeneration starts with the activation of the muscle stem cells, the satellite cells. These cells are embedded between the sarcolemma and the basal lamina, in a quiescent state (Charge and Rudnicki, 2004; Mauro, 1961). Upon activation, satellite cells enter the cell cycle, start to migrate toward the regenerating areas of the muscle, and give rise to more functionally committed cells, the myoblasts, which differentiate to generate new myofibers (Charge and Rudnicki, 2004). Satellite cells usually generate their progeny by asymmetric cell division, when specific, mostly unknown fate determinants are segregated in a polarized manner between the two daughter cells, to generate a new satellite cell and a myoblast (Gurevich et al., 2016; Kuang et al., 2007; Rocheteau et al., 2012; Wang et al., 2019).
In DMD, muscle regeneration is compromised because the continuous rounds of muscle degeneration and regeneration deplete the pool of satellite cells (Lu et al., 2014). Moreover, because dystrophin is also expressed in satellite cells, its loss results in distorted polarity of the satellite cells, deficits in their asymmetric division, and precocious differentiation (Dumont et al., 2015; Wang et al., 2019). Consequently, the DMD muscle becomes progressively unable to build new muscle fibers, which further contributes to its wasting. Furthermore, DMD muscle fibers are gradually replaced by fat and fibrotic tissue, which further hampers the mechanical and physiological activity of the skeletal muscle (Alvarez et al., 2002; Rahimov and Kunkel, 2013; Serrano and Munoz-Canoves, 2010; Villalta et al., 2011).
In healthy muscle, degeneration and regeneration is orchestrated by a strong, localized, inflammatory response, in which T cells, macrophages, and neutrophils infiltrate the muscle after injury, and release elevated levels of pro-inflammatory cytokines and regulatory cues (Shin et al., 2013). However, in the DMD muscle, chronic inflammation leads to excessive levels of intramuscular reactive oxygen species, which further contribute to the muscle wasting, and hampers the regenerative power of the satellite cells (Shin et al., 2013).
Muscle regeneration also requires the action of the fibro-adipogenic progenitors (FAPs), a population of muscle interstitial cells (Joe et al., 2010; Uezumi et al., 2010). Inflammatory cells regulate activation and proliferation of the FAPs, which, in turn, coordinate the regenerative action of the satellite cells (Heredia et al., 2013; Joe et al., 2010; Lemos et al., 2015; Uezumi et al., 2010; Uezumi et al., 2011). However, in DMD muscles, the continuous rounds of muscle degeneration and regeneration increase the FAPs’ differentiation into adipocytes or fibroblasts, the accumulation of which further compromises the mechanical features of the muscle (Lemos et al., 2015; Malecova et al., 2018; Mozzetta et al., 2013; Saccone et al., 2014).
2. Skeletal muscle determination in the vertebrate embryo
Muscle commitment and differentiation are mainly controlled by the regulated spatio-temporal expression of a set of four proteins (Myf5, MyoD1, Myogenin, and Mrf4) termed the muscle regulatory factors (MRFs) (Buckingham and Rigby, 2014). The MRFs are transcription factors that drive the expression of a multitude of genes regulating establishment and maintenance of the myogenic fate.
In embryonic development, myogenic cells originate from mesodermal precursors that colonize the paraxial mesoderm (PM), and that initially become part of the anterior area of the primitive streak (PS) (Buckingham and Rigby, 2014; Chang and Kioussi, 2018; Pourquie, 2004). The initial mesodermal differentiation is controlled by specific signaling proteins and molecules emanating from the anatomical regions surrounding the developing PM (Cunningham et al., 2015; Hamade et al., 2006). As a result, these morphogenetic gradients mark the first distinction between the anterior and posterior muscle groups of the future body (Chang and Kioussi, 2018).
A population of cells termed neuromesodermal progenitors (NMPs) is considered the precursor of the PM. NMPs initially reside in the anterior PS, and give rise to the presomitic mesoderm (PSM) on both sides of the neural tube (Gouti et al., 2017; Tzouanacou et al., 2009). For their role as PSM precursors, NMPs are considered the source of the first muscle precursors during development (Pourquie et al., 2018).
Several signaling pathways govern the patterning of the PM towards a mesodermal or neural fate. In particular, signaling by the transforming growth factor β (TGF-β)/Nodal, and the bone morphogenetic proteins (BMPs), play an inductive role on the early specification of the PM in the PS (Robertson, 2014). Initially, NMPs express both the mesodermal marker T/Brachyury and the neural marker Sox2 (Gouti et al., 2017; Henrique et al., 2015; Olivera-Martinez et al., 2012; Takemoto et al., 2011; Tzouanacou et al., 2009; Wymeersch et al., 2016). Subsequently, proliferating NMPs downregulate Sox2, sequentially express the transcription factors Tbx6, Snai1, and Mesogenin 1 (Msgn1), and progressively downregulate T/Brachyury expression (Chalamalasetty et al., 2011; Chalamalasetty et al., 2014; Chapman et al., 1996; Gouti et al., 2017). These transcriptional changes, in both NMPs and PSM cells, seem to be regulated by an oscillatory activity of the Notch, FGF, and Wnt signaling (Hubaud and Pourquie, 2014). As a result, NMPs express the transcription factors Mesp1 and Mesp2, which in turn, activate a transcriptional program leading to the segmentation of the PSM into somites (Hubaud and Pourquie, 2014; Morimoto et al., 2005). At this stage, PSM cells downregulate the expression of Msgn1 and Tbx6, and begin to express the paired-box domain transcription factor Pax3, which marks the first transition toward a true muscle cell commitment (Aulehla et al., 2008).
Somitogenesis is the most important step towards the complete determination of the muscle progenitors. Similar to formation of the PM, the segmentation of the PSM, as the first step of somitogenesis, is controlled by extracellular gradients of specific signaling molecules and proteins. The morphological processes underlying the separation of each somite are regulated, among them, by FGF8 and Wnt3 emanating from the caudal portion of the embryo, retinoic acid (RA) released by the rostral region of the forming somites, and the notochord-released sonic hedgehog (Shh) (Chang and Kioussi, 2018).
Somites initially appear as spherical clusters of epithelial-like cells that differentiate into two main regions: (i) a ventro-medial, mesenchymal-like, sclerotome/syndetome, from which derive vertebral bones, ribs cartilage, and the tendons of the trunk, and (ii) a dorso-lateral, epithelial-like, dermomyotome (DM). From the DM derive the precursor cells of the skeletal muscle of trunk and limbs, of brown fat, of dermis, and part of the endothelial and smooth muscle cells of the blood vessels (Buckingham and Rigby, 2014). The DM further differentiates into three distinct domains: (i) a dorsomedial (epaxial) DM, close to the neural tube, (ii) a central DM (dermatome), and (iii) a ventromedial (hypaxial) DM (Chang and Kioussi, 2018).
During the above process, the descendants of the mesodermal precursors continue their progressive myogenic determination. The Pax3+ cells of the DM, which derive from the PSM, begin to express Pax7 (Buckingham and Relaix, 2015). The resulting double Pax3+/Pax7+ cells will generate the muscle precursors that will give rise to the fetal myogenesis, and to the perinatal satellite cells (Buckingham and Relaix, 2015; Gunther et al., 2013; Seale et al., 2000; von Maltzahn et al., 2013). The pro-myogenic action of Pax3 and Pax7 is finely tuned by the intracellular and extracellular stimuli of FGFs, Wnts and Shh (Buckingham and Rigby, 2014).
The initial expression of the MRFs can be traced in the epaxial DM, where Myf5 is detected at day E8.0 in the mouse embryo (Ott et al., 1991). Shortly after, Myf5 is also expressed in the hypaxial DM (Buckingham and Rigby, 2014). These two groups of Myf5+ cells then express MyoD1, to further support myogenic specification (Sassoon et al., 1989). Very soon, cells residing in the epaxial and lateral lips of the DM begin to migrate toward its ventral face to form the myotome, the first skeletal muscle of the body, and the source of the muscle precursor cells that will be incorporated in the trunk (Buckingham and Rigby, 2014). Around the same time, Pax3+ cells delaminate from the hypaxial DM to migrate into distinct anatomical fields where they will give rise to vertebral and abdominal muscles, the diaphragm, and limb muscles (Chang and Kioussi, 2018).
In addition to pro-myogenic inputs, the anatomical structures surrounding the somites release negative cues to restrain the potentially massive differentiation of the early muscle precursors, and to preserve their survival and proliferation for the future waves of muscle formation. For example, Bmp4 coming from the lateral mesoderm, and Bmp7 secreted by the neural tube, restrain the muscle differentiation in the somite (Amthor et al., 2002; Pourquie et al., 1996; Reshef et al., 1998; Wang et al., 2010). In a similar way, the local activation of Notch in some of the muscle precursors of the DM/myotome inhibits their differentiation (Hirsinger et al., 2001; Schuster-Gossler et al., 2007; Vasyutina et al., 2007).
In summary, the balanced combination of signaling molecules secreted from the anatomical structures surrounding the PM, the somites, and the migrating muscle precursors, orchestrates the setting of the muscle commitment, and its maintenance during the cell divisions.
3. Cell therapy for DMD
Cell therapy is based on the heterologous, or autologous, transplantation of cells, with the goal of regenerating the damaged tissue or organ of the patient, and replenishing specific stem cell populations. In the case of DMD, the main goal is to reconstitute the satellite cell pool with dystrophin competent cells, and thereby restore muscle function due to the presence of dystrophin expressing muscle fibers. The source of the therapeutic cells can be healthy, histocompatible donors, or genetically corrected autologous cells. Thus far, a number of different cell types have been applied in transplantation experiments in DMD animal models, and in DMD clinical trials.
3a. Myoblasts and satellite cells
Experimental myoblast transplantation dates back to 1989, when research from groups led by Kunkel and Partridge pioneered the intramuscular (IM) transplantation of normal neonatal mouse myoblasts into mdx mice, a dystrophin deficient DMD mouse model (Morgan et al., 1990; Partridge et al., 1989). Subsequent experiments in humans and mice showed that IM-injected normal human or mouse myoblasts formed new dystrophin+ fibers, with the partial reconstitution of a normal muscle morphology (Gussoni et al., 1992; Huard et al., 1992; Kinoshita et al., 1994).
However, these early successful results in mice did not extend into the clinic. Following transplantation of muscle stem cells harvested from healthy immunocompatible donors, only small percentages of normal dystrophin (0–5% (Karpati et al., 1993); 0–10.3% (Mendell et al., 1995); 0–3.6% (Tremblay et al., 1993); 0% (Morandi et al., 1995); 0–80% (Huard et al., 1992)) were detected in patient’s biopsies post transplantation. With the exception of one study (Huard et al., 1992), DMD patients receiving heterologous, partially immune-compatible, human myoblasts, did not show any functional improvements of the transplanted limb. These results can be explained by immune-rejection, the limited number and scarce migration of injected cells, and by massive cell death after transplantation (Skuk and Tremblay, 2003).
Satellite cells have a strong therapeutic advantage over myoblasts, because of their self-renewal capability, which maintains their stemness. Indeed, mouse muscles transplanted with a single mouse muscle fiber, containing around seven satellite cells, or even with a single mouse satellite cell, showed a much better muscle engraftment than was the case with transplanted myoblasts (Collins et al., 2005; Sacco et al., 2008). Similarly, human satellite cells isolated by fluorescence-activated cell sorting (FACS) from muscle biopsies, and transplanted into immune-compromised mice, also on a mdx background, led to stable engraftments, formation of mouse fibers expressing human markers, and colonization of the mouse satellite cell niche, the latter being a key feature for the regeneration of a chronically damaged muscle (Garcia et al., 2018; Xu et al., 2015).
However, many practical limitations and safety concerns still affect the use of human satellite cells in the clinic. Four issues are of particular note: (i) only a very small amount of satellite cells can be isolated from a biopsy, especially from the dystrophic muscle, thus only a very limited number of recipient muscles can be transplanted; (ii) cultured satellite cells show a reduced transplantation efficiency; (iii) most satellite cells die after transplantation, and their dissemination rate is negligible; and (iv) delivery of the satellite cells to muscles such as the diaphragm requires a systemic delivery. However, when injected into the bloodstream, satellite cells aggregate as micro-thrombi inside small vessels, and do not colonize the muscle. Thus, to be therapeutically viable, satellite cells must be given the ability to survive inside the blood, to extravasate from circulation to enter the muscles, and to migrate inside the muscle.
New protocols have been designed to overcome these hurdles, for example by using hydrogels or hypoxia conditioning (Gilbert et al., 2010; Liu et al., 2012), by expressing proteins regulating cell migration (Morgan et al., 2010), and by elongating the telomeres to increase cell proliferation (Zhu et al., 2007). However, despite the promising potential of muscle cell transplantation and numerous efforts to optimize cell culture conditions in a lab setting, the use of myoblasts or satellite cells to treat DMD in the clinic has not been realized yet.
3b. Bone marrow cells and mesoangioblasts
To overcome the therapeutic limitations of satellite cells, researchers sought, and found, other cell populations with myogenic capability, such as those inside the bone marrow (BM) (Ferrari et al., 1998; Gussoni et al., 1999). BM-derived myogenic cells can migrate into the regenerating muscle via the circulation, suggesting their potential use for DMD treatment. However, BM transplantation (BMT) in mdx mice, in canine models of DMD, and in a DMD patient, did not show improved dystrophin production, nor amelioration of muscle function (Dell’Agnola et al., 2004; Ferrari et al., 2001; Gussoni et al., 2002). These results indicate that the BM-derived myogenic cells either do not support muscle regeneration, or make only a negligible contribution.
The finding of circulating cells with myogenic potential increased the scientific interest in identifying new types of non-muscle cells with the potential to contribute to muscle regeneration. That led to the discovery of mesoangioblasts (De Angelis et al., 1999). Originally isolated from the embryonic dorsal aorta of the mouse embryo (De Angelis et al., 1999; Minasi et al., 2002), mesoangioblasts contribute to postnatal muscle development (De Angelis et al., 1999), and are considered the developmental precursors of pericytes, perivascular cells resident in the adult muscle (Dellavalle et al., 2007; Minasi et al., 2002). While mesoangioblasts derived from mouse embryo aorta express myogenic markers such as MyoD1, and endothelial markers such as VE-cadherin and CD31 (De Angelis et al., 1999), pericytes express neither, rather they express markers such as NG2, PDGFRβ and CD146 (Birbrair et al., 2013; Dellavalle et al., 2007). Nevertheless, pericytes can differentiate into muscle when exposed to low serum conditions, or when co-cultured with myoblasts (Dellavalle et al., 2007).
The potential plasticity of pericytes was also confirmed by their ability to leave the perivascular niche, and adopt the fate of the recipient local tissue (Minasi et al., 2002). This evidence further suggested their therapeutic use for DMD. Indeed, the intra-vascular injection of mouse mesoangioblasts, or of human pericytes, in both mouse and canine models of DMD and of limb-girdle muscular dystrophy, demonstrated their ability to colonize the muscle (Dellavalle et al., 2011; Dellavalle et al., 2007; Sampaolesi et al., 2006; Sampaolesi et al., 2003).
In a phase I/IIa clinical trial, five DMD individuals were injected intra-arterially with donor HLA-matched normal mesoangioblasts (Cossu et al., 2015). The trial showed in one individual a band corresponding to the full-length dystrophin by immunoblotting that could not be explained by revertant fibers, but no functional improvement in any of the patients (Cossu et al., 2015). All patients had undetectable or extremely low immunological responses against dystrophin protein domains.
3c. CD133+ cells
CD133+ cells, first isolated from human peripheral blood, are multipotent stem cells with the capacity to repopulate the BM, and differentiate into endothelial cells (Torrente et al., 2004). CD133+ cells have also myogenic potential, as they express myogenic markers, and can give rise to satellite cells and to dystrophin positive myofibers after IM or intra-arterial transplantation into immunocompromised dystrophic mice (Meng et al., 2014; Negroni et al., 2009; Torrente et al., 2004). In 2007, a clinical trial of IM transplantation of DMD autologous CD133+ cells showed increased muscle vascularization, but no integration of the donor cells in the muscle fibers (Torrente et al., 2007).
4. Pluripotent stem cells
Vertebrate pluripotent stem cells (PSCs) retain their ability to differentiate into the three germ layers of the embryo: ectoderm, mesoderm, and endoderm. Typical PSCs are the embryonic stem cells (ESCs) (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998), and the induced PSC (iPSCs) (Takahashi et al., 2007; Takahashi and Yamanaka, 2006).
The generation of iPSCs opened up new avenues in stem cell therapy, and solved many problems associated with use of ESCs. For example, while human ESCs (hESCs) can only be isolated from the inner cell mass of an early embryo, which incurs numerous technical and ethical problems, human iPSCs (hiPSCs) can be generated from somatic cells, thereby allowing for the possibility of designing autologous, patient-specific, cell therapeutic strategies. This feature, along with the ability to be expanded indefinitely in vitro, and the plasticity to differentiate into any cell type, make hiPSCs a unique source for therapy, and for the study of the mechanisms of development and diseases.
4a. iPSCs
Initially, iPSCs were generated by transducing mouse or human somatic fibroblasts with lentiviruses expressing the four “Yamanaka” factors (Oct3/4, Sox2, c-Myc, and Klf4) (Takahashi et al., 2007; Takahashi and Yamanaka, 2006; Wernig et al., 2007). Subsequently, to reduce the risk of DNA mutagenesis and to improve the rate of reprogramming, protocols were created to model the iPSCs for clinical purposes (Fusaki et al., 2009; Kim et al., 2009; Soldner et al., 2009; Warren et al., 2010).
For therapy of muscular dystrophies, hiPSCs hold great promise. Transplantation of therapeutic cells differentiated from hiPSCs generated from the patient’s own cells will not induce immune rejection as in heterologous transplantation. Furthermore, patient’s-derived iPSCs make it possible to model in vitro the etiology, and the pathophysiological progression, of different muscular dystrophies, to perform automated pre-clinical drugs screenings, and to set up in vitro protocols of gene editing before in vivo testing (Abujarour et al., 2014; Choi et al., 2016; Dick et al., 2013; Li et al., 2015; Long et al., 2018; Maffioletti et al., 2018; Mondragon-Gonzalez and Perlingeiro, 2018; Shoji et al., 2015; Uchimura et al., 2017; Young et al., 2016). With patient-specific hiPSCs, we should be able to identify new correlations between the established etiologic cause of each type of muscular dystrophy and the presence of genetic and epigenetic modifiers in the human genome, information which is crucial for design more efficacious pharmacological therapies.
5. Muscle linage specification systems
One of the strategies to achieve a direct myogenic specification of PSCs is to replicate in the culture dish the inductive stimuli which underlie the muscle determination in the developing embryos. To accomplish this goal, one approach is for monolayer PSCs to be treated in vitro with the specific cytokines and growth/morphogenetic factors that orchestrate the specification of the mesoderm in vivo, the somitogenesis, and the commitment of the early muscle progenitors (Chal et al., 2015). A second, simpler approach to convert the PSCs into myogenic cells is through the over-expression of key myogenic transcription factors, to induce a pro-myogenic transcriptome, which in turn, can make the PSCs more sensitive to myogenic inductive stimuli.
5a. Early attempts to achieve myogenic differentiation of the PSCs in vitro
The first attempt to direct ESCs into a myogenic fate was carried out by inducing the formation of the embryoid body (EB) in vitro. The EBs are clusters of PSCs that can spontaneously differentiate into precursor cells of the three germ layers of the embryo (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998). For example, mouse ESCs (mESCs) were allowed to aggregate in EBs, that in turn, were cultivated in hanging drops for two days, then in suspension for three days. After five days, the EBs were plated, with the myotubes detected four days later, showing the capability of the ESCs to acquire the myogenic fate almost spontaneously (Rohwedel et al., 1998; Rohwedel et al., 1994). However, a large proportion of cells in these mouse EBs (mEBs) differentiated into neuronal cells (Bain et al., 1995; Rohwedel et al., 1998). Similar results were obtained using human EBs (hEBs) cultured in growth medium supplemented with dexamethasone, insulin-transferrin-selenium (ITS), glutamine, and epidermal growth factor (EGF) (Zheng et al., 2006). However, the myogenic precursors induced in this study differentiated properly only when transplanted in the regenerating muscle of a recipient mouse (Zheng et al., 2006).
These early experiments demonstrated that to obtain homogenous and consistent muscle commitment and differentiation in vitro, it was necessary to identify the proper combination of pro-myogenic cues. A seminal advancement in this direction was achieved by the Studer group, which, for the first time, reported the successful differentiation of hESCs into CD73+ mesenchymal progenitor cells, and then into myoblasts (Barberi et al., 2007; Barberi et al., 2005). In these studies, hESCs were cultured at low-density in serum-free medium supplemented with ITS (Barberi et al., 2007). Importantly, the progressive increase of serum concentration over the course of the cell culture increased the percentage of CD73+ cells, and allowed the mesenchymal precursors to progress toward different mesodermal fates, such as bone and muscle, through a transient endodermal/mesodermal stage (Barberi et al., 2007). Eventually, this process resulted in the generation of muscle-committed cells, positive for the neural cell adhesion molecule (NCAM+), an established marker of human myogenic cells. Sorted NCAM+ cells generated myotubes in vitro, and colonized the regenerating muscle of recipient, immunocompromised, mice (Barberi et al., 2007; Barberi et al., 2005). Subsequently, Sakurai et al. were able to generate myogenic cells from mESCs, via the induction of an early mesodermal lineage, using serum-free, chemically-defined, culture media supplemented with the synchronized addition of Bmp4 and lithium chloride (LiCl) (Sakurai et al., 2009). These pioneer studies set the foundation for step-wise systems for the myogenic commitment in vitro.
5b. Step-wise differentiation systems
According to the process of muscle commitment in the vertebrate embryo, the first logical step to differentiate the PSCs into the myogenic linage is to induce them to a PM-, NMPs-like fate. In this regard, several groups successfully generated PM cells by treating mouse and human PSCs with the inhibitor of the glycogen synthase kinase3-β (Gsk3-β) CHIR99021 (Chal et al., 2015; Mendjan et al., 2014). Since Gsk3-β inhibits the nuclear internalization of β-catenin, treating the cells with CHIR99021 mimics the addition of Wnt in the culture medium. Additional protocols showed that the induction of a PM fate can be further supported by supplementing the culture media of CHIR99021-treated PSCs with FGF (Gouti et al., 2014; Turner et al., 2014), or with activin, a TGF-β pathway activator (Loh et al., 2016). However, other reports showed FGF and activin to be dispensable for the acquisition of the NMP fate (Henrique et al., 2015; Pourquie et al., 2018), probably because the activation of the Wnt signaling in the PSCs results in the production of FGFs by the cells (Denham et al., 2015). These findings suggest that intracellular activation of Wnt signaling is sufficient for PSCs to acquire the PM/NMP fate (Pourquie et al., 2018).
The next step is to induce the differentiation of the NMP-like cells into PSM-like cells. As presented earlier (section 2), PSM cells express serially Tbx6 and Msgn1 (Chalamalasetty et al., 2014; Gouti et al., 2017), and several groups showed that activation of the Wnt signaling with CHIR99021 is sufficient to induce the expression of these two PSM markers in the PSCs. For example, Chal et al. (Chal et al., 2015) showed that by inhibiting BMP and activating Wnt, both mouse and human ESCs can differentiate into PSM progenitors. In contrast, Shelton et al. (Shelton et al., 2014) reported that the treatment of the same types of cells with CHIR99021 alone robustly induced the PSM in serum-free media. These two investigations, and similar work from other labs including our own, indicate that the initial activation of the Wnt pathway by CHIR99021 is sufficient to differentiate the PSCs to the PSM stage (Choi et al., 2016; Henrique et al., 2015; Loh et al., 2016; Sudheer et al., 2016; Xi et al., 2017).
From this step onward, the cell culture conditions used to induce the full myogenic commitment in vitro vary among the different protocols, including further treatments to increase the muscle programming efficiency, via the addition of hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1) and FGF2 to the culture medium (Chal et al., 2015; Shelton et al., 2014). When treated in such a way, mESCs generate Pax7+ myogenic cells, which give rise to Myogenin+ myoblasts and fuse into myosin heavy chain (MyHC)+ myotubes that show contractile activity in vitro (Chal et al., 2015).
A simplified protocol of muscle commitment has recently been devised in our lab by treating normal, and DMD-derived, hiPSCs (DMD-hiPSCs) with a Notch inhibitor (DAPT), after an initial treatment with CHIR99021 (Choi et al., 2016). In this study, we identified a defect in myotube formation in the DMD-hiPSCs caused by the up-regulation of the BMP and TGF-β signaling in the DMD myoblasts. The addition of a TGF-β inhibitor into the medium significantly improved the fusion of the muscle programmed DMD-hiPSCs (Choi et al., 2016). Increased myogenic linage differentiation of the healthy hPSCs was also observed by using different TGF-β inhibitors on CHIR99021 pre-treated hiPSCs in vitro, or by treating mice transplanted with muscle programmed, genetically corrected DMD-hiPSCs (Hicks et al., 2018).
The protocols that involve human iPSC step-wise differentiation without EB formation are compared in Table 1 (Chal et al., 2016; Chal et al., 2015; Choi et al., 2016; Sakai-Takemura et al., 2018; Wu et al., 2018).
Table 1.
human iPSC step-wise differentiation
| Study | Induction factor | Medium | Duration of muscle commitment and differentiation (days) | Cell composition | Purification | In vitro | In vivo |
|---|---|---|---|---|---|---|---|
| Chal et al., 2015 | days 0–6 CHIR99021 and LDN193189, insulin-transferrin-selenium; days 3–6 addition of FGF-2; days 6–8 IGF-1, HGF, FGF2 and LDN193189; days 8–12 IGF-1; days 12–50 IGF-1 and HGF |
DMEM based medium; days 0–6 serum free, thereafter + knock-out serum replacement (KSR) |
20–30 | ~22% of nuclei were MYOG+, and 23% of nuclei were PAX7+ | No purification | MyHC+, MYOG+ fibers, PAX7+ cells, organized sarcomeres, with the periodic distribution of the sarcomeric proteins Titin and fast MyHC |
No |
| Chal et al., 2016 | days 0–6 CHIR99021 and LDN193189, insulin-transferrin-selenium; days 3–6 addition of FGF-2; days 6–8 IGF-1, HGF, FGF2 and LDN193189; days 8–12 IGF-1; days 12–50 IGF-1 and HGF |
DMEM based medium; days 0–6 serum free, thereafter + KSR |
30 | Most MYOG+ cells, MyHC+ myofibers. PAX7+ satellite-like cells. | No purification | Sub-culturing of human myo-progenitors by day 28. MyHC+, MYOG+ fibers, PAX7+ cells, organized sarcomeres. Fast MyHC+ and h-Dystrophin+ fibers in muscle construct. |
No |
| Choi et al., 2016 | days 0–4 CHIR99021; days 4–12 DAPT |
Serum-free N2 medium | 30 | ~15% NCAM+ HNK1-myoblasts, 61.5% MYOG+ and 63.6% MyHC+ cells |
NCAM+ HNK1- |
MyHC, TITIN, DES, DYSTROPHIN, α-ACTININ | PAX7+ cells, h-Dystrophin+ fibers |
| Hicks et al., 2018 | days 0–2 CHIR99021; days 12–20 FGF2, TGF-β inhibitor when in differentiation condition |
days 0–12, days 20–35 E6 medium, days 12–20 StemPro-34 medium, days 35–50 DMEM/F12 + 1% ITS medium |
30–50 | PAX7, MYF5, MYOD, MYOGENIN and spontaneously contracting myotubes | ERBB3+NGFR+ | Enriched for PAX7 and MYF5 | h-LaminA/C+, h-Dystrophin+ fibers. In vivo engraftment of ERBB3+ hiPSC-SMPCs restored dystrophin to levels approaching uncultured fetal muscle |
| Sakai-Takemura et al., 2018 | days 0–12 Chal et al. 2016 method; days 12–42 floating culture; days 42–70 adhesion culture |
days 0–12 DMEM, days 12–70 10% FBS/DMEM |
70 | MYOGENIN+ myotubes | CD57(−) CD108(−) CD271(+) ERBB3(+) cells |
MYOGENIN, MyHC |
h-LaminA/C+ (nuclear membrane) and h-Spectrin+ (sarcolemma) myofibers |
| Wu et al., 2018 | days 1–4 CHIR99021, BMP inhibitor, TGF-β inhibitor; days 5–15 BMP inhibitor, TGF-β inhibitor |
MDM-I medium days1–4, MDM-II medium day 5–15, MDM-III medium for terminal differentiation |
15 | at day 4 99.4% PAX7+, at day 15, 50–55% MYF5+ |
CD10+CD24- | MyHC+ myotubes | myofibers expressing human markers (maximum of 50–60 fibers positive for h-Dystrophin and h-Lamin A/C) |
5c. Direct-programming of mPSCs and hPSCs into the myogenic lineage
Muscle specification in PSCs can also be induced through the forced expression of specific myogenic transcription factors such as MyoD1, Pax3 and Pax7. As previously mentioned, these transcription factors play critical roles in myogenic specification during development, and in the adult. Initial attempts to induce myogenesis in somatic cells, as well as in ESCs, used the ectopic expression of MyoD1 by means of cell transfection or viral transduction (Davis et al., 1987; Ozasa et al., 2007). Subsequent studies were carried out by over-expressing MyoD1, Pax3, or Pax7 into mouse and human iPSCs (Abujarour et al., 2014; Darabi et al., 2012; Filareto et al., 2013; Goudenege et al., 2012). In particular, by using a doxycycline inducible system, Perlingeiro and colleagues over-expressed Pax3, or Pax7, in mouse and human ESCs and hiPSCs (iPax3, iPax7 cells), and successfully isolated myogenic progenitors by sorting the EB-derived cells for the platelet-derived growth factor receptor α (Pdgfrα), an early PM marker that discriminates between the skeletal and the cardiac muscle fate in vitro (Darabi et al., 2008; Darabi et al., 2011; Magli et al., 2014). Importantly, iPax3 and iPax7 cells can generate muscle fibers, and colonize the satellite cell niche upon transplantation in the mouse dystrophic muscle (Darabi et al., 2012; Magli et al., 2017).
5d. hPSCs-derived myogenic cell transplantations
As an ideal autologous cell source for therapy of muscular dystrophies, hiPSCs can be generated from patient’s somatic cells, processed for genetic correction, differentiated in vitro, and then transplanted back into the patient. The step-wise differentiation system has some advantages. For example, it is inherently transgene-free, thus avoiding mutagenic risks for the patient, and can be standardized according to good manufacturing practices. However, there are some limitations. One of the major issues of the step-wise differentiation systems is the generation in vitro of mixed cell populations, including terminally differentiated myotubes and other non-muscle cell types, such as neurons. Consequently, the presence of a potentially large percentage of contaminating, non-myogenic, cells in vitro strongly reduces the engraftment efficiency of the therapeutic cells in vivo, and results in low rates of satellite cell niche colonization and muscle regeneration (Kim et al., 2017). In comparison, the directly-programmed iPAX7/iPAX3 hiPSC-derived myogenic progenitors, which comprise mostly PAX7+/PAX3+ cells, resulted in far better muscle engraftment (Darabi et al., 2012; Darabi et al., 2011; Kim et al., 2017). The differences in engraftment efficiency between the direct-programming and step-wise differentiation protocols can be explained by the fact that the iPAX7/iPAX3 hiPSCs may represent a purer and more homogeneous myogenic population than the myogenic cells generated through the step-wise differentiation systems. In addition, iPAX7/iPAX3 hiPSCs can have a cellular status more similar to muscle progenitors than myoblasts.
Evidence indicates that the Pax7+ myogenic progenitors, such as freshly isolated satellite cells, hold a better regeneration capacity than do the myoblasts, which allows the former cells to enter the muscle stem cell niche, a key prerequisite for long-term therapeutic effects. Indeed, both satellite cells and iPax7/iPax3 PSCs-derived myogenic cells show comparable engraftment rates in mouse recipient muscles, and contribution to serial cycles of muscle regeneration (Incitti et al., 2019; Magli et al., 2017; Sacco et al., 2008). In addition, it is now clear that the human myogenic progenitor cells derived from step-wise differentiation cultures are more similar to the muscle progenitors of the early fetal stages than they are to the adult satellite cells, as shown by assays of in vitro differentiation and transcriptomic analysis (Chal et al., 2015; Choi et al., 2016; Hicks et al., 2018; Shelton et al., 2014). Moreover, recent results show that the iPax7/iPax3 PSCs-derived myogenic progenitors increase their myogenic potential after the transplantation in the muscle of immunocompromised mice, and, once in the satellite cell niche, they show a molecular signature comparable to that of adult satellite cells (Incitti et al., 2019). The above evidence indicates that the muscle environment in vivo instructs the PSCs-derived myogenic cells to progress from a fetal/perinatal-like status into an adult-like myogenic status (Incitti et al., 2019). Nevertheless, the molecular basis of this maturing process is still unknown.
Recently, several groups have started to identify new surface markers characterizing the human muscle precursors, to improve the engraftment rates of the hiPSCs-derived myogenic precursors, with the goal of standardizing the in vitro procedures for clinical applications. For example, Hicks et al. purified PAX7+ myogenic progenitors from hPSCs-derived myogenic cells in vitro, based on the expression of the Erb-B2 receptor tyrosine kinase 3, and the nerve growth factor receptor (Hicks et al., 2018). Similarly, Magli et al. and Wu et al. identified CD10, CD24, CD54, Integrin α9β1, and Syndecan 2, as markers useful for purifying hiPSC-derived myogenic progenitors in vitro, by using MYF5 and PAX7 double reporter hESCs, or iPAX7-hPSCs (Magli et al., 2017; Wu et al., 2018). These studies reported better engraftment results using cells isolated with these markers than non-isolated cells.
6. Genetic engineering of hiPSCs to restore functional dystrophin expression
In order to generate dystrophin expressing muscle fibers, hiPSCs derived from DMD patients can be genetically corrected to express functional dystrophin for autologous cell replacement therapy. CRISPR-Cas9 mediated gene editing is currently being investigated as a tool to perform such correction. It involves two components: a single guide RNA (sgRNA) and the Cas9 endonuclease. Cas9 endonuclease associates with the sgRNA at the genomic target sequence to create DNA double strand breaks leading to homology-directed repair (HDR), or nonhomologous end-joining (NHEJ) repair (Garcia-Doval and Jinek, 2017; Hochheiser et al., 2018). Thereafter, this technique could, theoretically, correct most of the DMD mutations including point mutations, deletions and insertion, re-establishing the correct sequence of the dystrophin gene, or at least its translational frame after RNA transcription (Amoasii et al., 2018; Bengtsson et al., 2017; Wong and Cohn, 2017). The clear advantage of CRISPR-Cas9 over other strategies, is that it affords permanent repair of the dystrophin gene (Amoasii et al., 2018; Bengtsson et al., 2017; Long et al., 2015; Nelson et al., 2016; Tabebordbar et al., 2015). To date, there have not been any clinical trials of genetically corrected DMD-hiPSCs for cell transplantation therapy. However, this concept has been proven feasible by successful restoration of dystrophin protein expression, and DGC formation, from engraftment of myotubes derived from genetically corrected DMD-hiPSCs in immunocompromised mdx mice (Young et al., 2016).
7. Potential limitations of using hiPSCs for DMD treatment
The use of hiPSCs for treatment of muscular dystrophies is very promising. However, before proceeding to clinical trials, four key limitations must be overcome, and potential safety issues addressed. (i) We have to identify the patients’ best somatic cell type from which to generate the hiPSCs, and we have to improve the hiPSCs muscle commitment protocols, for example by generating the myogenic cells more quickly in vitro, and by using culture media free of animal factors. (ii) We have to optimize the delivery route of the therapeutic cells, to get the highest rate of muscle engraftment in vivo. Delivery could use innovative methods of systemic dissemination, thereby avoiding the dangerous accumulation of injected cells in highly vascularized organs such as liver and lungs. Furthermore, transplanted cells could be instructed to selectively cross the muscle-endothelial barrier, to fuse with the regenerating muscle fibers. (iii) For long-term benefits, we have to ensure that the therapeutic cells will stably colonize the satellite cell niche. (iv) The gene editing tools, which will be used to correct the causative genetic defect, must be without off-target effects.
Progress on overcoming these limitations is being made, including the development of DNA-free-based protocols for myogenic induction in vitro using a combination of chemical compounds and specific growth factors/morphogens, the identification of specific cell surface markers to separate the myogenic cells for transplantation from the other types of cells in the differentiation culture, and the use of new generation CRISPR-based systems (Fu et al., 2014; Hicks et al., 2018; Magli et al., 2017; Wu et al., 2018).
8. Future directions of the hiPSCs therapy development for the MDs
To develop clinically applicable hiPSCs-based therapies, researchers have focused on deriving cells that have high potency in terms of regenerating and self-renew, i.e. cells that have similar features to those of adult satellite cells (Incitti et al., 2019) (Figure 1). Based on this, progenitor cells can acquire a higher clinical potential. As discussed earlier, hiPSCs-derived myogenic progenitors have a molecular profile that is similar to fetal-stage myoblasts. Therefore, one of the most critical experiments to do is the induction, in vitro, of the progression of these cells toward more mature myogenic stages. We can achieve this goal by seeking compounds that can mature myogenic progenitors, or we can co-culture these cells with primary myotubes to mimic the in vivo muscle environment. Moreover, it is important to consider whether the hiPSCs-derived myogenic cells could be delivered systemically. In this respect, Gerli et al. (Gerli et al., 2019) recently demonstrated that modulating NOTCH and PDGF pathways can endow satellite cells with the ability to migrate trans-endothelially. Based on this finding, we can predict that a proper combination of modulatory growth factors and cytokines in vitro can instruct the hiPSCs-derived myogenic cells to reach all the muscles of the body via the bloodstream.
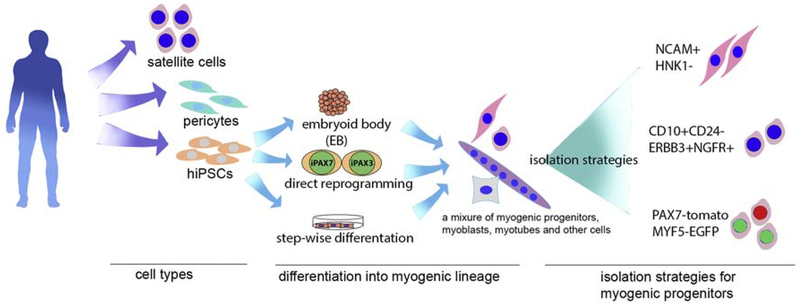
Figure 1.
Overview of potential stem cell therapy strategies for DMD that have been studied. Three type of cells, satellite cells, pericytes and hiPSCs have the highest potential for clinical application. Satellite cells and pericytes can be directly transplanted after isolation, hiPSCs require myogenic lineage differentiation, for which three methods are shown. The final cell product after myogenic commitment/differentiation can be isolated by FACS using the combination of surface markers or fluorescent reporter proteins.
9. Conclusion
Stem cells, due to their advantageous regeneration capability, bring the promise for cell transplantation therapy (Fig.1 summary of cells that can be used for stem cell-based therapies for muscular dystrophies). hiPSCs that can be derived from patients open the avenue for autologous cell therapy. With the rapid development of serum-free lineage specification protocols, expandable myogenic progenitor cells can be differentiated from hiPSCs. This population of cells has similar characteristics to stem cells and has superior muscle regeneration capability compared with myoblasts. In combination with gene editing techniques, hiPSC-derived myogenic progenitor cells hold potential as an efficacious therapeutic avenue for MDs.
Highlights:
Myogenic progenitor cells could provide a source for cell therapy for Duchenne muscular dystrophy (DMD) to regenerate and replace the diseased tissue.
An orchestration of signaling molecules, including Notch, FGF, Wnt and TGF-β, directs the myogenic lineage determination during an embryo’s development, and sets the basis for the hPSC (human pluripotent stem cell) myogenic linage specification in vitro.
There were several attempts of available cell-based therapies for DMD with limited success, therefore, there is a need to develop more cell therapy options (e.g. iPSC based).
This review describes the most recent studies of human induced pluripotent stem cells muscle lineage specification, and the potential of their clinical application to treat muscular dystrophies.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not for-profit sectors.
Abbreviations:
- hPSCs
human pluripotent stem cells
- hiPSCs
human induced pluripotent stem cells
- PSC
pluripotent stem cells
- AON
antisense oligonucleotide
- FAPs
fibro-adipogenic progenitors
- DGC
Dystrophin Glycoprotein Complex
- PM
paraxial mesoderm
- PS
primitive streak
- NMPs
neuromesodermal progenitors
- PSM
presomitic mesoderm
- TGF-β
transforming growth factor β
- BMPs
bone morphogenetic proteins
- RA
retinoic acid
- Shh
sonic hedgehog
- DM
dermomyotome
- EB
embryoid body
- ITS
insulin-transferrin-selenium
- NCAM+
neural cell adhesion molecule
- Gsk3-β
glycogen synthase kinase3-β
References
- Abujarour R, Bennett M, Valamehr B, Lee TT, Robinson M, Robbins D, Le T, Lai K, and Flynn P (2014). Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem Cells Transl Med 3, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez K, Fadic R, and Brandan E (2002). Augmented synthesis and differential localization of heparan sulfate proteoglycans in Duchenne muscular dystrophy. J Cell Biochem 85, 703–713. [DOI] [PubMed] [Google Scholar]
- Amoasii L, Hildyard JCW, Li H, Sanchez-Ortiz E, Mireault A, Caballero D, Harron R, Stathopoulou TR, Massey C, Shelton JM, et al. (2018). Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Christ B, Rashid-Doubell F, Kemp CF, Lang E, and Patel K (2002). Follistatin regulates bone morphogenetic protein-7 (BMP-7) activity to stimulate embryonic muscle growth. Dev Biol 243, 115–127. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, Lewandoski M, and Pourquie O (2008). A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat Cell Biol 10, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, and Gottlieb DI (1995). Embryonic stem cells express neuronal properties in vitro. Dev Biol 168, 342–357. [DOI] [PubMed] [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, and Studer L (2007). Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med 13, 642–648. [DOI] [PubMed] [Google Scholar]
- Barberi T, Willis LM, Socci ND, and Studer L (2005). Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2, e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson NE, Hall JK, Odom GL, Phelps MP, Andrus CR, Hawkins RD, Hauschka SD, Chamberlain JR, and Chamberlain JS (2017). Corrigendum: Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun 8, 16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, and Delbono O (2013). Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, and Relaix F (2015). PAX3 and PAX7 as upstream regulators of myogenesis. Semin Cell Dev Biol 44, 115–125. [DOI] [PubMed] [Google Scholar]
- Buckingham M, and Rigby PW (2014). Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell 28, 225–238. [DOI] [PubMed] [Google Scholar]
- Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, Cherrier T, Nesmith AP, Parker KK, and Pourquie O (2016). Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc 11, 1833–1850. [DOI] [PubMed] [Google Scholar]
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, et al. (2015). Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat Biotechnol 33, 962–969. [DOI] [PubMed] [Google Scholar]
- Chalamalasetty RB, Dunty WC Jr., Biris KK, Ajima R, Iacovino M, Beisaw A, Feigenbaum L, Chapman DL, Yoon JK, Kyba M, et al. (2011). The Wnt3a/beta-catenin target gene Mesogenin1 controls the segmentation clock by activating a Notch signalling program. Nat Commun 2, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamalasetty RB, Garriock RJ, Dunty WC Jr., Kennedy MW, Jailwala P, Si H, and Yamaguchi TP (2014). Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development 141, 4285–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CN, and Kioussi C (2018). Location, Location, Location: Signals in Muscle Specification. J Dev Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, and Papaioannou VE (1996). Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol 180, 534–542. [DOI] [PubMed] [Google Scholar]
- Charge SB, and Rudnicki MA (2004). Cellular and molecular regulation of muscle regeneration. Physiol Rev 84, 209–238. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lim H, Estrellas K, Mula J, Cohen TV, Zhang Y, Donnelly CJ, Richard JP, Kim YJ, Kim H, et al. (2016). Concordant but Varied Phenotypes among Duchenne Muscular Dystrophy Patient-Specific Myoblasts Derived using a Human iPSC-Based Model. Cell Rep 15, 2301–2312. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, and Morgan JE (2005). Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301. [DOI] [PubMed] [Google Scholar]
- Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM, Wilkinson JD, Orav EJ, Cuniberti L, Salbert BA, et al. (2008). Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J 155, 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, et al. (2015). Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med 7, 1513–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Brade T, Sandell LL, Lewandoski M, Trainor PA, Colas A, Mercola M, and Duester G (2015). Retinoic Acid Activity in Undifferentiated Neural Progenitors Is Sufficient to Fulfil l Its Role in Restricting Fgf8 Expression for Somitogenesis. PLoS One 10, e0137894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, and Perlingeiro RC (2012). Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, and Perlingeiro RC (2008). Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med 14, 134–143. [DOI] [PubMed] [Google Scholar]
- Darabi R, Santos FN, Filareto A, Pan W, Koene R, Rudnicki MA, Kyba M, and Perlingeiro RC (2011). Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors. Stem Cells 29, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, and Lassar AB (1987). Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, and Cossu G (1999). Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol 147, 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Agnola C, Wang Z, Storb R, Tapscott SJ, Kuhr CS, Hauschka SD, Lee RS, Sale GE, Zellmer E, Gisburne S, et al. (2004). Hematopoietic stem cell transplantation does not restore dystrophin expression in Duchenne muscular dystrophy dogs. Blood 104, 4311–4318. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, et al. (2011). Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2, 499. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. (2007). Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9, 255–267. [DOI] [PubMed] [Google Scholar]
- Denham M, Hasegawa K, Menheniott T, Rollo B, Zhang D, Hough S, Alshawaf A, Febbraro F, Ighaniyan S, Leung J, et al. (2015). Multipotent caudal neural progenitors derived from human pluripotent stem cells that give rise to lineages of the central and peripheral nervous system. Stem Cells 33, 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick E, Kalra S, Anderson D, George V, Ritso M, Laval SH, Barresi R, Aartsma-Rus A, Lochmuller H, and Denning C (2013). Exon skipping and gene transfer restore dystrophin expression in human induced pluripotent stem cells-cardiomyocytes harboring DMD mutations. Stem Cells Dev 22, 2714–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, and Rudnicki MA (2015). Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 21, 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery AE (2002). The muscular dystrophies. Lancet 359, 687–695. [DOI] [PubMed] [Google Scholar]
- Ervasti JM (2007). Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta 1772, 108–117. [DOI] [PubMed] [Google Scholar]
- Evans MJ, and Kaufman MH (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, and Mavilio F (1998). Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528–1530. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Stornaiuolo A, and Mavilio F (2001). Failure to correct murine muscular dystrophy. Nature 411, 1014–1015. [DOI] [PubMed] [Google Scholar]
- Filareto A, Parker S, Darabi R, Borges L, Iacovino M, Schaaf T, Mayerhofer T, Chamberlain JS, Ervasti JM, McIvor RS, et al. (2013). An ex vivo gene therapy approach to treat muscular dystrophy using inducible pluripotent stem cells. Nat Commun 4, 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sander JD, Reyon D, Cascio VM, and Joung JK (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, and Hasegawa M (2009). Efficient induction of transgene - free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 85, 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SM, Tamaki S, Lee S, Wong A, Jose A, Dreux J, Kouklis G, Sbitany H, Seth R, Knott PD, et al. (2018). High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Reports 10, 1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Doval C, and Jinek M (2017). Molecular architectures and mechanisms of Class 2 CRISPR-associated nucleases. Curr Opin Struct Biol 47, 157–166. [DOI] [PubMed] [Google Scholar]
- Gerli MFM, Moyle LA, Benedetti S, Ferrari G, Ucuncu E, Ragazzi M, Constantinou C, Louca I, Sakai H, Ala P, et al. (2019). Combined Notch and PDGF Signaling Enhances Migration and Expression of Stem Cell Markers while Inducing Perivascular Cell Features in Muscle Satellite Cells. Stem Cell Reports 12, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, and Blau HM (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, Gekas J, Rousseau J, and Tremblay JP (2012). Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol Ther 20, 2153–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M, Delile J, Stamataki D, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, and Briscoe J (2017). A Gene Regulatory Network Balances Neural and Mesoderm Specification during Vertebrate Trunk Development. Dev Cell 41, 243–261 e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, and Briscoe J (2014). In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol 12, e1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S, Kim J, Kostin S, Lepper C, Fan CM, and Braun T (2013). Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell 13, 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich DB, Nguyen PD, Siegel AL, Ehrlich OV, Sonntag C, Phan JM, Berger S, Ratnayake D, Hersey L, Berger J, et al. (2016). Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science 353, aad9969. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Bennett RR, Muskiewicz KR, Meyerrose T, Nolta JA, Gilgoff I, Stein J, Chan YM, Lidov HG, Bonnemann CG, et al. (2002). Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest 110, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, and Blau HM (1992). Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature 356, 435–438. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, and Mulligan RC (1999). Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390–394. [DOI] [PubMed] [Google Scholar]
- Hamade A, Deries M, Begemann G, Bally-Cuif L, Genet C, Sabatier F, Bonnieu A, and Cousin X (2006). Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev Biol 289, 127–140. [DOI] [PubMed] [Google Scholar]
- Henrique D, Abranches E, Verrier L, and Storey KG (2015). Neuromesodermal progenitors and the making of the spinal cord. Development 142, 2864–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, and Chawla A (2013). Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MR, Hiserodt J, Paras K, Fujiwara W, Eskin A, Jan M, Xi H, Young CS, Evseenko D, Nelson SF, et al. (2018). ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat Cell Biol 20, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsinger E, Malapert P, Dubrulle J, Delfini MC, Duprez D, Henrique D, Ish-Horowicz D, and Pourquie O (2001). Notch signalling acts in postmitotic avian myogenic cells to control MyoD activation. Development 128, 107–116. [DOI] [PubMed] [Google Scholar]
- Hochheiser K, Kueh AJ, Gebhardt T, and Herold MJ (2018). CRISPR/Cas9: A tool for immunological research. Eur J Immunol 48, 576–583. [DOI] [PubMed] [Google Scholar]
- Huard J, Bouchard JP, Roy R, Malouin F, Dansereau G, Labrecque C, Albert N, Richards CL, Lemieux B, and Tremblay JP (1992). Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve 15, 550–560. [DOI] [PubMed] [Google Scholar]
- Hubaud A, and Pourquie O (2014). Signalling dynamics in vertebrate segmentation. Nat Rev Mol Cell Biol 15, 709–721. [DOI] [PubMed] [Google Scholar]
- Incitti T, Magli A, Darabi R, Yuan C, Lin K, Arpke RW, Azzag K, Yamamoto A, Stewart R, Thomson JA, et al. (2019). Pluripotent stem cell-derived myogenic progenitors remodel their molecular signature upon in vivo engraftment. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, and Rossi FM (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M, et al. (1993). Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol 34, 8–17. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. (2009). Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Magli A, Chan SSK, Oliveira VKP, Wu J, Darabi R, Kyba M, and Perlingeiro RCR (2017). Expansion and Purification Are Critical for the Therapeutic Application of Pluripotent Stem Cell-Derived Myogenic Progenitors. Stem Cell Reports 9, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Guerette B, Asselin I, Roy R, and Tremblay JP (1994). Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle Nerve 17, 1407–1415. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, and Rudnicki MA (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, and Rossi FM (2015). Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21, 786–794. [DOI] [PubMed] [Google Scholar]
- Li HL, Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T, Tanaka M, Amano N, Watanabe A, Sakurai H, et al. (2015). Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports 4, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KR, Maruyama R, and Yokota T (2017). Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther 11, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, and Kuang S (2012). Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 139, 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, et al. (2016). Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 166, 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, and Olson EN (2015). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Li H, Tiburcy M, Rodriguez-Caycedo C, Kyrychenko V, Zhou H, Zhang Y, Min YL, Shelton JM, Mammen PPA, et al. (2018). Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci Adv 4, eaap9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Poddar M, Tang Y, Proto JD, Sohn J, Mu X, Oyster N, Wang B, and Huard J (2014). Rapid depletion of muscle progenitor cells in dystrophic mdx/utrophin−/− mice. Hum Mol Genet 23, 4786–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, Moyle LA, Steele-Stallard H, Cappellari O, Wells KE, Ferrari G, et al. (2018). Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering. Cell Rep 23, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli A, Incitti T, Kiley J, Swanson SA, Darabi R, Rinaldi F, Selvaraj S, Yamamoto A, Tolar J, Yuan C, et al. (2017). PAX7 Targets, CD54, Integrin alpha9beta1, and SDC2, Allow Isolation of Human ESC/iPSC-Derived Myogenic Progenitors. Cell Rep 19, 2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli A, Schnettler E, Swanson SA, Borges L, Hoffman K, Stewart R, Thomson JA, Keirstead SA, and Perlingeiro RC (2014). Pax3 and Tbx5 specify whether PDGFRalpha+ cells assume skeletal or cardiac muscle fate in differentiating embryonic stem cells. Stem Cells 32, 2072–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah JK (2016). Current and emerging treatment strategies for Duchenne muscular dystrophy. Neuropsychiatr Dis Treat 12, 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecova B, Gatto S, Etxaniz U, Passafaro M, Cortez A, Nicoletti C, Giordani L, Torcinaro A, De Bardi M, Bicciato S, et al. (2018). Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat Commun 9, 3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America 78, 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A (1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9, 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogeni S, Markousis-Mavrogenis G, Papavasiliou A, and Kolovou G (2015). Cardiac involvement in Duchenne and Becker muscular dystrophy. World J Cardiol 7, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et al. (1995). Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med 333, 832–838. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, Kneile K, Dunn DM, Duval B, Aoyagi A, et al. (2012). Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 71, 304–313. [DOI] [PubMed] [Google Scholar]
- Mendjan S, Mascetti VL, Ortmann D, Ortiz M, Karjosukarso DW, Ng Y, Moreau T, and Pedersen RA (2014). NANOG and CDX2 pattern distinct subtypes of human mesoderm during exit from pluripotency. Cell Stem Cell 15, 310–325. [DOI] [PubMed] [Google Scholar]
- Meng J, Chun S, Asfahani R, Lochmuller H, Muntoni F, and Morgan J (2014). Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther 22, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, et al. (2002). The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 129, 2773–2783. [DOI] [PubMed] [Google Scholar]
- Mondragon-Gonzalez R, and Perlingeiro RCR (2018). Recapitulating muscle disease phenotypes with myotonic dystrophy 1 induced pluripotent stem cells: a tool for disease modeling and drug discovery. Dis Model Mech 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi L, Bernasconi P, Gebbia M, Mora M, Crosti F, Mantegazza R, and Cornelio F (1995). Lack of mRNA and dystrophin expression in DMD patients three months after myoblast transfer. Neuromuscul Disord 5, 291–295. [DOI] [PubMed] [Google Scholar]
- Morgan J, Rouche A, Bausero P, Houssaini A, Gross J, Fiszman MY, and Alameddine HS (2010). MMP-9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve 42, 584–595. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Hoffman EP, and Partridge TA (1990). Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol 111, 2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Takahashi Y, Endo M, and Saga Y (2005). The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature 435, 354–359. [DOI] [PubMed] [Google Scholar]
- Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, et al. (2013). Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med 5, 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Shiba N, Miyazaki D, Nishizawa H, Inaba Y, Fueki N, Maruyama R, Echigoya Y, and Yokota T (2017). Comparison of the phenotypes of patients harboring in-frame deletions starting at exon 45 in the Duchenne muscular dystrophy gene indicates potential for the development of exon skipping therapy. J Hum Genet 62, 459–463. [DOI] [PubMed] [Google Scholar]
- Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, Torrente Y, Butler-Browne GS, and Mouly V (2009). In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther 17, 1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera-Martinez I, Harada H, Halley PA, and Storey KG (2012). Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol 10, e1001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MO, Bober E, Lyons G, Arnold H, and Buckingham M (1991). Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development 111, 1097–1107. [DOI] [PubMed] [Google Scholar]
- Ozasa S, Kimura S, Ito K, Ueno H, Ikezawa M, Matsukura M, Yoshioka K, Araki K, Yamamura KI, Abe K, et al. (2007). Efficient conversion of ES cells into myogenic lineage using the gene-inducible system. Biochem Biophys Res Commun 357, 957–963. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, and Kunkel LM (1989). Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 337, 176–179. [DOI] [PubMed] [Google Scholar]
- Pourquie O (2004). The chick embryo: a leading model in somitogenesis studies. Mech Dev 121, 1069–1079. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Al Tanoury Z, and Chal J (2018). The Long Road to Making Muscle In Vitro. Curr Top Dev Biol 129, 123–142. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Breant C, Francis-West P, Brickell P, Tessier-Lavigne M, and Le Douarin NM (1996). Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell 84, 461–471. [DOI] [PubMed] [Google Scholar]
- Rahimov F, and Kunkel LM (2013). The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol 201, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef R, Maroto M, and Lassar AB (1998). Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev 12, 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EJ (2014). Dose-dependent Nodal/Smad signals pattern the early mouse embryo. Semin Cell Dev Biol 32, 73–79. [DOI] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, and Tajbakhsh S (2012). A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148, 112–125. [DOI] [PubMed] [Google Scholar]
- Rohwedel J, Guan K, Zuschratter W, Jin S, Ahnert-Hilger G, Furst D, Fassler R, and Wobus AM (1998). Loss of beta1 integrin function results in a retardation of myogenic, but an acceleration of neuronal, differentiation of embryonic stem cells in vitro. Dev Biol 201, 167–184. [DOI] [PubMed] [Google Scholar]
- Rohwedel J, Maltsev V, Bober E, Arnold HH, Hescheler J, and Wobus AM (1994). Muscle cell differentiation of embryonic stem cells reflects myogenesis in vivo: developmentally regulated expression of myogenic determination genes and functional expression of ionic currents. Dev Biol 164, 87–101. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, and Blau HM (2008). Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone V, Consalvi S, Giordani L, Mozzetta C, Barozzi I, Sandona M, Ryan T, Rojas-Munoz A, Madaro L, Fasanaro P, et al. (2014). HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes Dev 28, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai-Takemura F, Narita A, Masuda S, Wakamatsu T, Watanabe N, Nishiyama T, Nogami K, Blanc M, Takeda S, and Miyagoe-Suzuki Y (2018). Premyogenic progenitors derived from human pluripotent stem cells expand in floating culture and differentiate into transplantable myogenic progenitors. Sci Rep 8, 6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Inami Y, Tamamura Y, Yoshikai T, Sehara-Fujisawa A, and Isobe K (2009). Bidirectional induction toward paraxial mesodermal derivatives from mouse ES cells in chemically defined medium. Stem Cell Res 3, 157–169. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. (2006). Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444, 574–579. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. (2003). Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301, 487–492. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, and Buckingham M (1989). Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature 341, 303–307. [DOI] [PubMed] [Google Scholar]
- Schuster-Gossler K, Cordes R, and Gossler A (2007). Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci U S A 104, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, and Rudnicki MA (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786. [DOI] [PubMed] [Google Scholar]
- Serrano AL, and Munoz-Canoves P (2010). Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316, 3050–3058. [DOI] [PubMed] [Google Scholar]
- Shelton M, Metz J, Liu J, Carpenedo RL, Demers SP, Stanford WL, and Skerjanc IS (2014). Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Reports 3, 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Tajrishi MM, Ogura Y, and Kumar A (2013). Wasting mechanisms in muscular dystrophy. Int J Biochem Cell Biol 45, 2266–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji E, Sakurai H, Nishino T, Nakahata T, Heike T, Awaya T, Fujii N, Manabe Y, Matsuo M, and Sehara-Fujisawa A (2015). Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci Rep 5, 12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D, and Tremblay JP (2003). Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil 24, 285–300. [PubMed] [Google Scholar]
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al. (2009). Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudheer S, Liu J, Marks M, Koch F, Anurin A, Scholze M, Senft AD, Wittler L, Macura K, Grote P, et al. (2016). Different Concentrations of FGF Ligands, FGF2 or FGF8 Determine Distinct States of WNT-Induced Presomitic Mesoderm. Stem Cells 34, 1790–1800. [DOI] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al. (2015). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, and Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, and Kondoh H (2011). Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470, 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, and Jones JM (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Marchesi C, D’Antona G, Cogiamanian F, Pisati F, Gavina M, Giordano R, Tonlorenzi R, Fagiolari G, et al. (2007). Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant 16, 563–577. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, et al. (2004). Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest 114, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, and Richards CL (1993). Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant 2, 99–112. [DOI] [PubMed] [Google Scholar]
- Turner DA, Hayward PC, Baillie-Johnson P, Rue P, Broome R, Faunes F, and Martinez Arias A (2014). Wnt/beta-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development 141, 4243–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, and Nicolas JF (2009). Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell 17, 365–376. [DOI] [PubMed] [Google Scholar]
- Uchimura T, Otomo J, Sato M, and Sakurai H (2017). A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem Cell Res 25, 98–106. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, and Tsuchida K (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, et al. (2011). Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124, 3654–3664. [DOI] [PubMed] [Google Scholar]
- Vasyutina E, Lenhard DC, Wende H, Erdmann B, Epstein JA, and Birchmeier C (2007). RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc Natl Acad Sci U S A 104, 4443–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, and Tidball JG (2011). Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet 20, 790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J, Jones AE, Parks RJ, and Rudnicki MA (2013). Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A 110, 16474–16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Noulet F, Edom-Vovard F, Tozer S, Le Grand F, and Duprez D (2010). Bmp signaling at the tips of skeletal muscles regulates the number of fetal muscle progenitors and satellite cells during development. Dev Cell 18, 643–654. [DOI] [PubMed] [Google Scholar]
- Wang YX, Feige P, Brun CE, Hekmatnejad B, Dumont NA, Renaud JM, Faulkes S, Guindon DE, and Rudnicki MA (2019). EGFR-Aurka Signaling Rescues Polarity and Regeneration Defects in Dystrophin-Deficient Muscle Stem Cells by Increasing Asymmetric Divisions. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, and Jaenisch R (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324. [DOI] [PubMed] [Google Scholar]
- Wong TWY, and Cohn RD (2017). Therapeutic Applications of CRISPR/Cas for Duchenne Muscular Dystrophy. Curr Gene Ther 17, 301–308. [DOI] [PubMed] [Google Scholar]
- Wu J, Matthias N, Lo J, Ortiz-Vitali JL, Shieh AW, Wang SH, and Darabi R (2018). A Myogenic Double-Reporter Human Pluripotent Stem Cell Line Allows Prospective Isolation of Skeletal Muscle Progenitors. Cell Rep 25, 1966–1981 e1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymeersch FJ, Huang Y, Blin G, Cambray N, Wilkie R, Wong FC, and Wilson V (2016). Position-dependent plasticity of distinct progenitor types in the primitive streak. Elife 5, e10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H, Fujiwara W, Gonzalez K, Jan M, Liebscher S, Van Handel B, Schenke-Layland K, and Pyle AD (2017). In Vivo Human Somitogenesis Guides Somite Development from hPSCs. Cell Rep 18, 1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wilschut KJ, Kouklis G, Tian H, Hesse R, Garland C, Sbitany H, Hansen S, Seth R, Knott PD, et al. (2015). Human Satellite Cell Transplantation and Regeneration from Diverse Skeletal Muscles. Stem Cell Reports 5, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Okuno T, Honda Y, and Nakano Y (1980). Central nervous system involvement in progressive muscular dystrophy. Arch Dis Child 55, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CS, Hicks MR, Ermolova NV, Nakano H, Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack JA, et al. (2016). A Single CRISPR-Cas9 Deletion Strategy that Targets the Majority of DMD Patients Restores Dystrophin Function in hiPSC-Derived Muscle Cells. Cell Stem Cell 18, 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JK, Wang Y, Karandikar A, Wang Q, Gai H, Liu AL, Peng C, and Sheng HZ (2006). Skeletal myogenesis by human embryonic stem cells. Cell Res 16, 713–722. [DOI] [PubMed] [Google Scholar]
- Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, Di Santo JP, Butler-Browne GS, and Wright WE (2007). Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 6, 515–523. [DOI] [PubMed] [Google Scholar]