Abstract
Background
The prevalence of Achromobacter xylosoxidans lung isolation in cystic fibrosis (CF) patients has increased, but the impact on lung function is controversial. The aim of this study was to evaluate the long-term effects of A. xylosoxidans isolation on respiratory function of adult patients with CF in the first 3 years after identification of A. xylosoxidans isolation.
Methods
This was a case–control retrospective study performed at a single CF centre in Lille, France. Data for 36 patients with CF who had at least one sputum culture positive for A. xylosoxidans (Ax+) were evaluated and compared with control CF patients uninfected by A. xylosoxidans (Ax−). Respiratory function and exacerbation frequency were evaluated between 1 year prior to and 3 years after A. xylosoxidans isolation.
Results
Compared with the Ax− group, the Ax+ group had a lower forced expiratory volume in 1 s (FEV1) at baseline (median (interquartile range): 55.2% (50.6–59.8%) versus 73.8% (67.2–80.4%); p=0.005), a greater decline in FEV1 (±se) in the first year after A. xylosoxidans identification (−153.6±16.1 mL·year−1 versus −63.8±18.5 mL·year−1; p=0.0003), and more exacerbations in the first 3 years after A. xylosoxidans identification (9 (7–12) versus 7 (5–10); p=0.03). Ax+ patients co-colonised with Pseudomonas aeruginosa (n=27, 75%) had a greater FEV1 decline (p=0.003) and more exacerbations in the year after A. xylosoxidans identification (p=0.037) compared with patients colonised with A. xylosoxidans alone. Patients with chronic A. xylosoxidans isolation (n=23, 64%) had more exacerbations than intermittently colonised patients in the 3 years after A. xylosoxidans identification (p=0.012).
Conclusion
A. xylosoxidans isolation is associated with a decline in respiratory function in patients with CF. Chronic A. xylosoxidans isolation and P. aeruginosa co-isolation may be markers of more severe respiratory disease in Ax+ patients.
Short abstract
Respiratory isolation of Achromobacter xylosoxidans exacerbates the decline in respiratory function in CF. Chronic A. xylosoxidans isolation and Pseudomonas cocolonisation may be markers of more severe disease in A. xylosoxidans-positive patients. http://bit.ly/2yJbSOS
Introduction
Cystic fibrosis (CF) is a monogenic disease caused by mutations in the CF transmembrane conductance regulator gene and is the most common autosomal recessive genetic disease in France [1,2]. The predominant respiratory features of CF are bronchiectasis, bacterial isolation, and recurrent infections. Staphylococcus aureus and Haemophilus influenzae are the most prevalent bacteria in the sputum of young patients with CF, whereas Pseudomonas aeruginosa predominates in later decades. Other opportunistic pathogens, including Achromobacter xylosoxidans, Burkholderia cepacia and Stenotrophomonas maltophilia, are increasingly detected in patients lungs with CF [3]. Respiratory impairment is the major cause of death in patients with CF [4], underscoring the need for a greater understanding of how bacterial isolation and co-isolation patterns, particularly the interplay between “classical” and “emerging” pathogens, influence pulmonary function in these patients.
In France, the prevalence of A. xylosoxidans in the sputum of patients with CF has increased from 5.6% in 2014 to 6.3% in 2016 [1]. A. xylosoxidans is a strict aerobic Gram-negative bacillus [5] with broad natural resistance and frequent acquired resistance to antibiotics. These features are shared with P. aeruginosa, which in some cases has resulted in the inadvertent misidentification of A. xylosoxidans as P. aeruginosa [6]. However, while P. aeruginosa is a well-characterised agent of lung disease in patients with CF, the clinical significance of A. xylosoxidans isolation on the decline in lung function of patients with CF remains controversial. Lambiase et al. [7] and De Baets et al. [8] did not detect differences in the rate of decline in lung function of A. xylosoxidans-colonised (referred to as Ax+) and uncolonised (Ax−) patients with CF. In contrast, a recent Spanish study of CF patients found that airway isolation with Achromobacter spp. accelerated lung function decline and increased the number of pulmonary exacerbations [9]. Thus, further studies are necessary to clarify the clinical impact of Achromobacter spp. isolation.
To address this knowledge gap, we performed a case–control study by comparing the respiratory function of 36 Ax+ and 36 Ax− patients with CF (controls matched for age, sex, and P. aeruginosa isolation status) during the year before and the first 3 years after isolation of A. xylosoxidans. The main study objective was to determine the longitudinal effect of A. xylosoxidans isolation on respiratory function and exacerbation frequency in patients with CF. Secondary objectives were to evaluate these features in Ax+ patient subgroups with or without P. aeruginosa co-isolation and with intermittent versus chronic A. xylosoxidans infection.
Materials and methods
Patients
Between 2011 and 2017, a total of 275 adult subjects with CF were followed at the Lille Cystic Fibrosis Centre (Centre de Ressources et de Compétences pour la Mucoviscidose) in France. The Observatory Research Protocol Evaluation Committee reviewed the study and granted permission to analyse patient data without the need for informed consent. The Institutional Review Board of the French Language Pulmonary Society (CEPRO 2012-009) approved the study. CF diagnosis criteria were sweat chloride concentration >60 mmol·L−1, and/or genetic confirmation of two CF-associated mutations, and two clinical features consistent with CF [10]. Of the 275 patients followed over the 7-year period, data from 36 patients with and 36 patients without A. xylosoxidans isolation were analysed for this report. Ax+ patients were defined as having at least one Ax+ sputum sample confirmed by cytobacteriological examination between 2011 and 2017. Control subjects were individually case matched by age (±4 years), sex and P. aeruginosa isolation status. Data were collected from 1 year prior to until 3 years after baseline, which was defined as the date on which A. xylosoxidans was first detected or, for the case-matched controls, the same age (±4 years) as the Ax+ patients at baseline.
Measures and data collection
All patients were evaluated every 3 months, at which time they provided a sputum sample and underwent spirometry using the MICRO spirometer 5000 (Medisoft; Sorinnes, Belgium). Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were expressed as the percentage of predicted normal values [11]. The numbers of exacerbations and antibiotic treatment courses were recorded for each year. An exacerbation was defined as a respiratory condition requiring antibiotics regardless of the type of antibiotic, the duration of treatment or the route of administration. Chronic isolation was defined as at least two Ax+ sputum samples within 6 months. Long-term antibiotic therapy (usually oral azithromycin or aerosol colimycin) was defined as treatment for ≥6 months. Sputum samples were analysed for A. xylosoxidans, P. aeruginosa, B. cepacia, non-tuberculous mycobacteria, methicillin-sensitive S. aureus, and methicillin-resistant S. aureus.
Statistical analysis
Categorical variables are expressed as number and percentage. Continuous variables are reported as median and interquartile range (IQR) or mean±sd. Normality of continuous variables was checked graphically and by Shapiro–Wilk's test. Baseline characteristics of the Ax+ and Ax− groups were compared using a McNemar test for categorical variables and a Wilcoxon's signed rank test for continuous variables. Subgroups stratified by P. aeruginosa co-isolation and intermittent versus chronic A. xylosoxidans isolation were compared using the Chi-squared test (or Fisher's exact test when expected cell frequency was <5) for categorical variables and the Mann–Whitney U-test for continuous variables. Changes in FEV1 were compared between groups and/or subgroups using linear mixed models. Group, time, and group×time interaction were included as fixed effects and patients as a random effect in all comparisons to account for the correlation between repeated measures. For the comparisons between Ax+ and Ax− groups, we added a second random effect to account for the matched sets, and comparisons were adjusted for age. The total number of exacerbations during the first, the first 2, and 3 years after A. xylosoxidans isolation were compared between groups using a generalised linear mixed model (Poisson distribution, log link function) unadjusted and adjusted for age, including matched sets as a random effect.
Statistical testing was conducted at the two-tailed α-level of 0.05. Data were analysed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
The study cohort consisted of 72 out of the 275 adult patients with CF who were followed at our centre from 2011 to 2017. On average, patients received a consultation every 3 months, at which time they provided a sputum sample and underwent spirometry. Of the 72 patients, 36 had at least one sample positive for A. xylosoxidans. Each A. xylosoxidans+ patient was matched by age (±4 years), sex and P. aeruginosa isolation status to a control patient who remained A. xylosoxidans− throughout the 4-year study period. Baseline characteristics are reported in table 1. The median age at baseline of the A. xylosoxidans− group was significantly younger than that of the A. xylosoxidans+ group (19.5 versus 23.5 years; p<0.001); this difference can likely be attributed to the criteria used for age matching (±4 years). In both study groups, the sex ratio was balanced and 75% of patients were co-colonised with P. aeruginosa. A. xylosoxidans+ patients had a significantly lower baseline FEV1 than the A. xylosoxidans− patients (median 55.2% versus 73.8%; p=0.005), whereas baseline FVC did not differ significantly (p=0.22).
TABLE 1.
Baseline characteristics of Achromobacter xylosoxidans-colonised and matched uncolonised patients with cystic fibrosis
| Characteristics | Control subjects (n=36) | Patients (n=36) | p-value |
| Female | 19 (52.8) | 19 (52.8) | 1.00 |
| Age years | 19.5 (17.0–27.0) | 23.5 (20.0–31.0) | <0.001 |
| BMI kg·m−2 | 20.6 (19.2–21.8) | 19.5 (17.7–21.0) | 0.12 |
| Homozygous F508del | 14 (41.2) | 14 (41.2) | 1.00 |
| Heterozygous F508del | 13 (36.1) | 17 (47.2) | 0.28 |
| Pancreatic insufficiency | 30 (83.3) | 30 (83.3) | 1.00 |
| Diabetes | 8 (22.2) | 11 (30.6) | 0.44 |
| Long-term azithromycin | 10 (27.8) | 14 (38.9) | 0.28 |
| Transplantation during follow-up | 0 (0.0) | 7 (19.4) | 0.011 |
| Lumacaftor+ivacaftor treatment | 7 (19.4) | 6 (16.6) | NA |
| Pseudomonas aeruginosa isolation | 27 (75.0) | 27 (75.0) | 0.56 |
| MSSA isolation | 21 (58.3) | 22 (61.1) | 0.82 |
| MRSA isolation | 5 (13.9) | 8 (22.2) | 0.37 |
| Burkholderia cepacia isolation | 4 (11.1) | 3 (8.3) | NA |
| Non-tuberculous mycobacteria | 2 (5.5) | 4 (11.1) | NA |
| Allergic bronchopulmonary aspergillosis | 11 (30.6) | 18 (50.0) | 0.071 |
| Intravenous antibiotherapy courses in the previous year n | 1.5 (0.0–5.0) | 2.0 (0.0–4.0) | 0.71 |
| Long-term (>6 months) maintenance antibiotherapy in the previous year n | 13 (40.6) | 19 (52.8) | 0.27 |
| Exacerbations in the previous year n | 2.0 (1.0–4.0) | 2.0 (1.0–3.5) | 0.77 |
| FEV1 at baseline % | 73.8 (67.2–80.4) | 55.2 (50.6–59.8) | 0.005 |
| FVC at baseline % | 88.1 (81.3–94.9) | 76.2 (71.5–81.0) | 0.22 |
Data are presented as n (%) or median (interquartile range), unless otherwise stated. BMI: body mass index; MSSA: methicillin-sensitive Staphylococcus aureus; MRSA: methicillin-resistant S. aureus; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; NA: not analysed.
Respiratory impact of A. xylosoxidans isolation
To assess how A. xylosoxidans isolation affected respiratory function, we measured the annual rate of change in FEV1 and FVC from baseline to the end of year 3. Over this period, a total of 812 evaluations were performed (439 in the Ax+ group, 373 in the Ax− group), which corresponds to an average of 2.8 evaluations per patient per year. As shown in table 2, the Ax+ group had a larger annual decline in FEV1 (−153.6 mL·year−1 versus −63.8 mL·year−1; p<0.001; −5.27%·year−1 versus −3.05%·year−1; p=0.002). Although a similar trend was observed for FVC, the difference between the Ax+ and Ax− groups did not reach statistical significance (−101.0 mL·year−1 versus −160.0 mL·year−1; p=0.060) (table 2). Moreover, as shown in figure 1, the ventilatory function of Ax+ patients was poorer than that of Ax− patients at baseline and declined further over the next 3 years (p=0.02). In contrast, FEV1 remained stable in the Ax− patients over the same time period.
TABLE 2.
Differences in respiratory function and exacerbations between patients with cystic fibrosis who had at least one sputum culture positive for Achromobacter xylosoxidans (Ax+) and cystic fibrosis patients uninfected by A. xylosoxidans (Ax−)
| Characteristics | Control subjects (n=36) | Patients (n=36) | p-value |
| Lung function | |||
| ΔFEV1 %·year−1 | −3.05±0.54 | −5.27±0.47 | 0.002# |
| ΔFEV1 mL·year−1 | −63.8±18.5 | −153.6±16.1 | <0.001# |
| ΔFVC %·year−1 | −2.42±0.86 | −3.58±0.56 | 0.18 |
| ΔFVC mL·year−1 | −101.0±32.1 | −160.0±21.0 | 0.06 |
| Exacerbations n | |||
| Year before baseline | 1.5 (0.0–5.0) | 2.0 (1.0–3.5) | 0.77¶ |
| Year after baseline | 0.5 (0.0–4.0) | 2.0 (1.0–4.0) | 0.16¶ |
| Years 1 and 2 after baseline+ | 5.0 (4.0–6.0) | 6.0 (4.0–8.0) | 0.034¶ |
| Years 1–3 after baseline + | 7.0 (5.0–10.0) | 9.0 (7.0–12.0) | 0.033¶ |
Data are presented as β±se (slope of change calculated from the baseline (time of bacterial detection)) or median (interquartile range), unless otherwise stated. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: Calculated from the group×time interaction term of the linear mixed model;¶: calculated using a generalised linear mixed model (Poisson regression model) including matched sets as a random effect; +: cumulative number of exacerbations.
FIGURE 1.
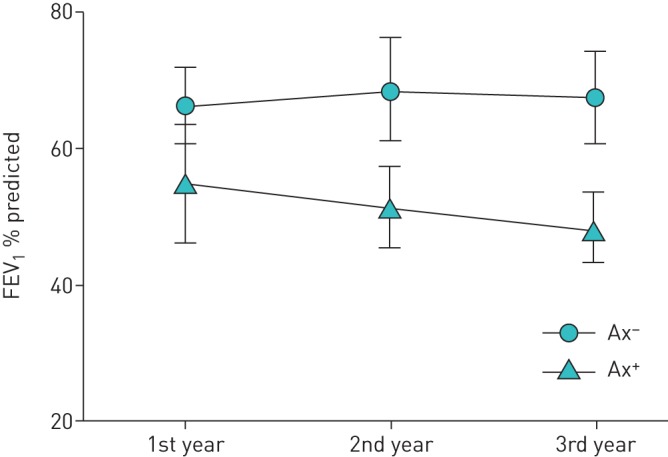
Change in forced expiratory volume in 1 s (FEV1) with time for patients with cystic fibrosis who had at least one sputum culture positive for Achromobacter xylosoxidans (Ax+) and cystic fibrosis patients uninfected by A. xylosoxidans (Ax−). Data are presented as the mean±sd of FEV1 recorded in the preceding year.
The exacerbation rate during the year before and the year after baseline were not significantly different between the Ax+ and Ax− patients (table 2). However, the cumulative number of exacerbations in the Ax+ group was significantly higher than that in the Ax− group by the end of the second year (median 6.0 versus 5.0; p=0.034) and third year (9.0 versus 7.0; p=0.033) after baseline (table 2). The median total number of exacerbations in the 3 years after baseline were 9 and 7 for the Ax+ and Ax− group, respectively. The same results were found when the statistical model was adjusted for age.
Respiratory impact of P. aeruginosa co-isolation with A. xylosoxidans
Next, we evaluated the baseline characteristics and decline in respiratory function of the Ax+ patients according to P. aeruginosa isolation status (table 3). As noted, 27 (75%) of the Ax+ group were colonised by P. aeruginosa, with a median time between the first detection of P. aeruginosa and A. xylosoxidans of 66 months (range: 4–288 months). The annual rate of FEV1 decline was greater for the A. xylosoxidans and P. aeruginosa co-colonised patients than for those colonised with A. xylosoxidans alone (−169.2 mL·year−1 versus −105.4 mL·year−1; p=0.092; −6.05%·year−1 versus −2.66%·year−1; p=0.003). In contrast, the numbers of exacerbations in the year before baseline and in years 2 and 3 after baseline were comparable between the two groups, and a significant difference was detected only in the first year after A. xylosoxidans detection (Ax+ 3.0 versus Ax− 2.0; p=0.037) (table 3).
TABLE 3.
Baseline characteristics and outcomes of Achromobacter xylosoxidans-colonised patients with cystic fibrosis stratified by Pseudomonas aeruginosa co-isolation status
| Characteristics or outcomes | Co-isolation status | p-value | |
| Ax+ Pa− (n=9) | Ax+ Pa+ (n=27) | ||
| Baseline characteristics | |||
| Female | 5 (55.6) | 14 (51.9%) | 1.00 |
| Homozygous F508del | 3 (33.3) | 11 (44.0) | 0.70 |
| Pancreatic insufficiency | 6 (66.7) | 24 (88.9) | 0.15 |
| Long-term azithromycin | 3 (33.3) | 11 (40.7) | 1.00 |
| MRSA isolation | 1 (11.1) | 7 (25.9) | 0.65 |
| Allergic bronchopulmonary aspergillosis | 5 (55.6) | 13 (48.1) | 1.00 |
| Age years | 26.0 (21.0–36.0) | 22.0 (20.0–28.0) | 0.30 |
| BMI kg·m−2 | 18.0 (17.7–20.8) | 19.6 (17.7–21.6) | 0.83 |
| Suppressive antibiotherapy in the previous year | 1 (11.1) | 18 (66.7) | 0.006 |
| Intravenous antibiotherapy courses in the previous year | 0.0 (0.0–2.0) | 2.0 (1.0–4.0) | 0.099 |
| Outcomes | |||
| Lung function | |||
| ΔFEV1 %·year−1 | −2.66±0.98 | −6.05±0.54 | 0.003 |
| ΔFEV1 mL·year−1 | −105.4±32.77 | −169.2±18.68 | 0.092 |
| Number of exacerbations: | |||
| Year before baseline, median (IQR) | 2.0 (1.0–2.0) | 3.0 (1.0–4.0) | 0.11 |
| Year after baseline, median (IQR) | 2.0 (1.0–3.0) | 3.0 (2.0–5.0) | 0.037 |
| Years 1 and 2 after baseline, median (IQR)# | 5.0 (3.0–8.0) | 6.5 (4.0–9.0) | 0.26 |
| Years 1–3 after baseline, median (IQR)# | 8.0 (7.0–12.0) | 9.0 (6.0–14.0) | 0.40 |
Data are presented as n (%), median (interquartile range) or β±se (slope of change calculated from the baseline (time of bacterial detection)), unless otherwise stated. MRSA: methicillin-resistant Staphylococcus aureus; BMI: body mass index; FEV1: forced expiratory volume in 1 s.#: cumulative number of exacerbations.
Comparison of respiratory function in patients with intermittent or chronic A. xylosoxidans isolation
Of the 36 Ax+ patients examined, 23 (63.9%) had at least two positive sputum samples within the 6 months prior to baseline and were considered to be chronically infected. The baseline characteristics and decline in respiratory function of patients with intermittent versus chronic A. xylosoxidans infection are shown in table 4. At baseline, chronically A. xylosoxidans-infected patients were significantly younger, had lower body weights and had been treated with significantly more azithromycin cycles than the intermittently infected patients. There were no significant differences between the two groups in either FEV1 at baseline (median 58.0 versus 49.0%; p=1.00) or the annual rate of decline in FEV1 in the 3 years after baseline (−135.9 mL·year−1 versus −157.2 mL·year−1; p=0.062). In contrast, the chronically infected group had a significantly higher cumulative number of exacerbations compared with the intermittently infected group by the end of both the second year (7 versus 4; p=0.049) and the third year (9.5 versus 6; p=0.012) after baseline.
TABLE 4.
Baseline characteristics and outcomes of patients with cystic fibrosis stratified by intermittent or chronic Achromobacter xylosoxidans isolation
| Characteristics or outcomes | Intermittent (n=13) | Chronic (n=23) | p-value |
| Baseline characteristics | |||
| Female | 6 (46.2) | 13 (56.5) | 0.55 |
| Age at Achromobacter xylosoxidans detection years | 28.0 (26.0–36.0) | 21.0 (19.0–28.0) | 0.019 |
| Weight kg | 63.0 (47.0–67.0) | 49.0 (44.0–56.0) | 0.032 |
| BMI kg·m−2 | 21.1 (17.8–22.4) | 18.7 (17.6–20.1) | 0.052 |
| Homozygous F508del | 6 (46.2) | 8 (38.1) | 0.64 |
| Pancreatic insufficiency | 11 (84.6) | 19 (82.6) | 1.00 |
| Diabetes | 2 (15.4) | 9 (39.1) | 0.26 |
| Azithromycin | 2 (15.4) | 12 (52.2) | 0.030 |
| Pseudomonas aeruginosa isolation | 10 (76.9) | 17 (73.9) | 1.00 |
| Allergic bronchopulmonary aspergillosis | 6 (46.2) | 12 (52.2) | 0.73 |
| FEV1 the year before Achromobacter xylosoxidans detection % | 58.0 (30.0–75.0) | 49.0 (31.0–77.0) | 1.00 |
| Pseudomonas aeruginosa antibiotic courses in year before baseline | 2.0 (1.0–3.0) | 3.0 (2.0–5.0) | NA |
| Outcomes | |||
| Lung function | |||
| ΔFEV1 %·year−1 | −3.82±1.16 | −5.55±0.52 | 0.17 |
| ΔFEV1 mL·year−1 | −135.9±38.56 | −157.2±17.96 | 0.62 |
| Number of exacerbations: | |||
| Year before baseline | 1.0 (1.0– 3.0) | 3.0 (2.0–4.0) | 0.066 |
| Year after baseline | 2.0 (1.0–4.0) | 3.0 (2.0–4.0) | 0.22 |
| Years 1 and 2 after baseline# | 4.0 (3.0–6.0) | 7.0 (5.0–9.0) | 0.049 |
| Years 1–3 after baseline# | 6.0 (4.0–6.0) | 9.5 (7.5–13.0) | 0.012 |
Data are presented as n (%), median (interquartile range) or β±se (slope of change calculated from the baseline (time of bacterial detection)), unless otherwise stated. BMI: body mass index; FEV1: forced expiratory volume in 1 s. #: Cumulative number of exacerbations.
Discussion
Emerging evidence suggests that the lungs of patients with CF are colonised by a broader range of pathogens than previously recognised. The presence of Achromobacter spp. has been investigated in several cohorts of patients with CF, but the results have been controversial. Our study of French patients with CF makes several important points. First, A. xylosoxidans-colonised patients had a lower ventilatory function at baseline, a greater annual rate of decline in FEV1, and an increased exacerbation frequency within the 3 years after A. xylosoxidans detection than did patients who remained Ax−. Second, patients with both A. xylosoxidans and P. aeruginosa had a greater rate of decline in FEV1 and more exacerbations than patients without P. aeruginosa isolation. Finally, patients with chronic A. xylosoxidans isolation had more exacerbations from the second year after A. xylosoxidans detection compared with the intermittently colonised patients.
The prevalence of A. xylosoxidans in our cohort was 13.1%, which is higher than the 6.3% reported by the French Cystic Fibrosis Registry [1] but comparable to cohorts in studies performed in other European countries such as Spain [9] and Italy [7]. Although the risk factors for A. xylosoxidans isolation are unclear, advanced age and chronic P. aeruginosa isolation seem to be common among CF patients with chronic A. xylosoxidans infection [12, 13]. The baseline characteristics of our study cohort were typical according to the French Cystic Fibrosis Registry [1]; for example, 41.6% of our patients were homozygous for F508del, and 83.3% had exocrine pancreatic insufficiency. Similarly, with respect to potential genetic confounding factors, the proportion of patients in our Ax− cohort (13 out of 36, 36.1%) and Ax+ cohort (17 out of 36, 47.2%) were heterozygous for F508del, which are comparable to the national registry data (42%). The small inter-group difference in our study (four patients) is thus unlikely to have impacted the results. A higher proportion of the Ax+ cohort than the Ax− cohort in our study presented with allergic bronchopulmonary aspergillosis (50% versus 31%). While this difference was not statistically significant, a higher prevalence of allergic bronchopulmonary aspergillosis has also been noted in Ax+ compared with Ax− patients with CF in another case–control study [12].
In our study, Ax+ patients had worse lung function at baseline than matched Ax− patients, which is in agreement with previous studies [8, 14] and suggests that severe lung disease may be associated with increased susceptibility to A. xylosoxidans isolation. We also found that A. xylosoxidans infection was associated with a significantly greater decline in FEV1 in the first 3 years after A. xylosoxidans detection. Some studies have reported that FEV1 is the respiratory function parameter that best correlates with mortality in patients with CF [15, 16]. In particular, the rate of FEV1 decline seems to be a good predictor of prognosis for patients with CF [17]. Thus, our results could suggest that A. xylosoxidans isolation is a predictor of worse prognosis.
An association between decline in lung function and A. xylosoxidans isolation has also been observed in other studies of patients with CF. A recent Spanish study of 21 patients with CF found that the presence of A. xylosoxidans was associated with an increased decline in lung function parameters (FVC and FEV1), and with more exacerbations [9]. Similarly, in a study of six French Ax+ patients and 11 matched controls, Godbert et al. [12] showed that FEV1 declined significantly faster and to a greater extent among the Ax+ patients compared with the controls. A study of 16 Ax+ and Ax− patients with CF in Denmark found the same trend in worsening respiratory function for Ax+ patients with high levels of anti-A. xylosoxidans antibodies [18]. In stark contrast, no significant differences in the rate of FEV1 decline were reported by Lambiase et al. [7], who compared six A. xylosoxidans-colonised and six P. aeruginosa-colonised patients with CF, and by De Baets et al. [8], who compared 8 Ax+ with 16 Ax− patients with CF, and by TAN et al. [19] who compared 13 chronic A. xylosoxidans-colonised children with controls.
Our finding that A. xylosoxidans isolation significantly increased the cumulative number of exacerbations within 3 years of A. xylosoxidans detection underscores the potential importance of this pathogen for patient prognosis. Indeed, in a 5-year modelling study, the detrimental effect of each exacerbation was predicted to be equivalent to a 12% loss of FEV1 [20]. These results are consistent with previous studies showing that A. xylosoxidans-colonised patients with CF had more hospitalisations and intravenous antibiotic treatment courses than matched Ax− patients (n=8 per group) [8]. The aforementioned case–control study by Godbert et al. [12] also found an association between A. xylosoxidans isolation and more frequent hospitalisations and antibiotic courses. Nevertheless, one study has shown no link between A. xylosoxidans infection and pulmonary exacerbations. Among a cohort of 1103 Canadian patients with CF followed for 18 years, exacerbations were more frequent among the 48 patients with chronic A. xylosoxidans infection compared with those with intermittent or no history of infection, but this difference was not statistically significant after adjustment for potential confounders [21].
The majority (75%) of our 72-patient cohort was colonised with P. aeruginosa, and subgroup analysis revealed that the presence of P. aeruginosa was associated with a significantly greater annual decline in FEV1. Diamantea et al. [22] discovered a similar trend in their cohort of 11 Ax+ Pa+ and four Ax+ Pa− patients with CF. These findings indicate that isolation by P. aeruginosa is detrimental to the lung function of Ax+ patients with CF, although it should be noted that the extent of chronic lung inflammation, as measured by cytokine production, was found to be comparable in Ax+ patients and Pa+ patients with CF [23].
Our comparison of patients with intermittent versus chronic A. xylosoxidans isolation identified no effect on FEV1, but the chronically infected patients did have significantly more exacerbations than the intermittently infected group in the 3 years after A. xylosoxidans identification (9.5 versus 6.0; p=0.012). The proportion of chronically A. xylosoxidans-colonised patients in our study (23 out of 36, 64%) was comparable to that in the aforementioned Spanish study, which found the same association between chronic A. xylosoxidans infection and frequency of exacerbations [9]. In a North American case–control study of 32 patients with CF, chronic A. xylosoxidans isolation was associated with significantly more intravenous antibiotic courses within an 18-month period, but not with a difference in FEV1, compared with intermittently infected patients [24]. Firmida et al. [25] also reported a trend towards lower FEV1 in chronically A. xylosoxidans-infected compared with either intermittently A. xylosoxidans-infected patients or non-carriers, but the differences were not statistically significant. Similarly, a Canadian study of 34 A. xylosoxidans-colonised patients with CF found no significant differences between persistently infected (n=10) and intermittently infected (n=24) or matched control subjects (n=18) in either FEV1 or number of exacerbations [24]. Finally, in a Belgian study, fewer than a third of the Ax+ cohort of CF patients was chronically colonised, and although this group exhibited an increased hospitalisation rate, their lung function decline was not significantly different from that of patients infected with A. xylosoxidans only once [26].
This study has several limitations. First, the retrospective observational nature of the analysis has well-known limitations. Second, the number of statistical tests performed was limited, and we cannot exclude that some differences between cohorts may have been missed. Missing data are another potentially confounding factor that cannot be ruled out, even in a case-matched study. Third, the sample size was small, with only 36 patients identified over the 7-year study period. Nevertheless, this study is relevant because isolation by A. xylosoxidans is a rare event among patients with CF. Moreover, previous studies have generally included even smaller patient numbers. Fourth, the monocentric nature of the recruitment hinders generalisation of the results, and additional multicentre studies on this subject are warranted. Finally, we chose a time period of ±4 years for age matching between Ax+ and Ax− patients, which is a sufficiently large window that age-related differences in lung function may be a potential confounding factor [27]. Indeed, the A. xylosoxidans-colonised patients were significantly older than the Ax− patients in this study (23.5 versus 19.5 years; p<0.001), which could be explained by the tendency for A. xylosoxidans isolation to occur at older ages. An age difference of similar magnitude was also seen in the aforementioned American study, in which the median age of transiently A. xylosoxidans-infected, chronically A. xylosoxidans-infected and control CF patients was 25, 24 and 22 years of age, respectively [24].
To conclude, this study provides further evidence that lung isolation by A. xylosoxidans in patients with CF has a detrimental effect on respiratory function, as reflected by a larger decline in FEV1, a higher number of exacerbations, and an increased need for intravenous antibiotic courses compared with Ax− patients. Co-isolation with P. aeruginosa and chronic A. xylosoxidans isolation are also associated with increased severity of respiratory disease. These results highlight the need for clinicians to be vigilant in monitoring the presence of lung pathogens in CF patients, and suggest that the time may have come for standardised management strategies for the control, or even systematic eradication, of primary A. xylosoxidans infections, as currently recommended for P. aeruginosa.
Acknowledgements
The authors wish to thank Anne M. O'Rourke (O'Rourke Scientific and Medical Writing, San Diego, CA, USA) for editing a version of the manuscript.
Footnotes
Conflict of interest: M. Tetart has nothing to disclose.
Conflict of interest: F. Wallet has nothing to disclose.
Conflict of interest: M. Kyheng has nothing to disclose.
Conflict of interest: S. Leroy has nothing to disclose.
Conflict of interest: T. Perez reports being the principal investigator of a clinical study evaluating a device for chest clearance in cystic fibrosis (Simeox, PhysioAssist).
Conflict of interest: O. Le Rouzic reports personal fees and nonfinancial support from AstraZeneca, Boehringer Ingelheim, Chiesi, Lilly and Novartis, and nonfinancial support from GlaxoSmithKline, MundiPharma, Pfizer, Teva, the Santelys Association, Vertex and Vitalaire, outside the submitted work.
Conflict of interest: B. Wallaert reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: A. Prevotat reports personal fees from Vertex and GSK, and congress invitations from Teva and Novartis, outside the submitted work.
References
- 1.Bellis G, Dehillotte C, Lemonier L, et al. French Cystic Fibrosis Registry - 2016 Data Review. [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 3.Abbott IJ, Peleg AY. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: antimicrobial resistance and therapeutic strategies. Semin Respir Crit Care Med 2015; 36: 99–110. [DOI] [PubMed] [Google Scholar]
- 4.Ratjen F, Döring G. Cystic fibrosis. Lancet Lond Engl 2003; 361: 681–689. [DOI] [PubMed] [Google Scholar]
- 5.Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol 1971; 15: 477–481. [DOI] [PubMed] [Google Scholar]
- 6.Kidd TJ, Ramsay KA, Hu H, et al. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J Clin Microbiol 2009; 47: 1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambiase A, Catania MR, del Pezzo M, et al. Achromobacter xylosoxidans respiratory tract infection in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis 2011; 30: 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Baets F, Schelstraete P, Van Daele S, et al. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 2007; 6: 75–78. [DOI] [PubMed] [Google Scholar]
- 9.Recio R, Branas P, Martinez MT, et al. Effect of respiratory Achromobacter spp infection on pulmonary function in patients with cystic fibrosis. J Med Microbiol 2018; 67: 952–956. [DOI] [PubMed] [Google Scholar]
- 10.Smyth AR, Bell SC, Bojcin S, et al. European Cystic Fibrosis Society Standards of Care: Best Practice guidelines. J Cyst Fibros Off J Eur Cyst Fibros Soc 2014; 13: Suppl. 1, S23–S42. [DOI] [PubMed] [Google Scholar]
- 11.Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J 1993; 6: Suppl. 16, 1–100. [PubMed] [Google Scholar]
- 12.Godbert B, Briault A. Cystic fibrosis: Achromobacter xylosoxidans colonized patients have more severe respiratory disease. Eur Respir J 2013; 42: Suppl. 57, P1175. [Google Scholar]
- 13.Vongthilath R, Dehillotte C, Lemonnier L, et al. PYOnever study: adults patients with cystic fibrosis caracteristics without lung Pseudomonas aeruginosa colonization. Rev Mal Respir 2017; 34: A33. [Google Scholar]
- 14.Hansen C R, Pressler T, Høiby N, et al. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J Cyst Fibros 2006; 5: 245–251. [DOI] [PubMed] [Google Scholar]
- 15.Szczesniak R, Heltshe SL, Stanojevic S, et al. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: a statistical perspective for the clinical researcher. J Cyst Fibros 2017; 16: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992; 326: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 17.Que C, Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax 2006; 61: 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen CR, Pressler T, Høiby N, et al. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J Cyst Fibros 2006; 5: 245–251. [DOI] [PubMed] [Google Scholar]
- 19.Tan K, Conway SP, Brownlee KG, et al. Alcaligenes infection in cystic fibrosis. Pediatr Pulmonol 2002; 34: 101–104. [DOI] [PubMed] [Google Scholar]
- 20.Sanders DB, Bittner RCL, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010; 182: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somayaji R, Stanojevic S, Tullis DE, et al. Clinical outcomes associated with Achromobacter species infection in patients with cystic fibrosis. Ann Am Thorac Soc 2017; 14: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 22.Diamantea F, Petta V, Kotsifas K, et al. 57 Achromobacter xylosoxidans chronic infection and its clinical significance in a cohort of cystic fibrosis adult patients. J Cyst Fibros 2016; 15: S66. [Google Scholar]
- 23.Hansen CR, Pressler T, Nielsen KG, et al. Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J Cyst Fibros 2010; 9: 51–58. [DOI] [PubMed] [Google Scholar]
- 24.Edwards BD, Greysson-Wong J, Somayaji R, et al. Prevalence and Outcomes of Achromobacter species infections in adults with cystic fibrosis: a North American cohort study. J Clin Microbiol 2017; 55: 2074–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firmida MC, Pereira RHV, Silva EASR, et al. Clinical impact of Achromobacter xylosoxidans colonization/infection in patients with cystic fibrosis. Braz J Med Biol Res 2016; 49: e5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christiaens L, Goeminne P, Dupont L. Achromobacter xylosoxidans: friend or foe? Eur Respir J 2011; 38: Suppl. 55, 4292. [Google Scholar]
- 27.Kerem E, Viviani L, Zolin A, et al. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS Patient Registry. Eur Respir J 2014; 43: 125–133. [DOI] [PubMed] [Google Scholar]


