Abstract
Objective:
Amantadine blocks N-methyl-D-aspartate (NMDA) receptors and has dopaminergic and noradrenergic action, a neurochemical profile that suggests its potential as an antidepressant drug. We conducted a systematic review of preclinical and clinical studies addressing the effects of amantadine in animal models of depression and in patients with depression.
Methods:
PubMed, Science Direct, and Web of Science were searched up to September 1, 2017 to identify clinical and preclinical studies. The following search terms were used: “amantadine AND depress*”; “amantadine AND mood”; “amantadine AND animal models AND antidepres*”; and “amantadine AND (forced swim, learned helplessness, reserpine, chronic mild stress, anhedonia, sucrose preference).”
Results:
Amantadine had antidepressant-like effects in animal models and appeared to potentiate the antidepressant effects of other antidepressants. These preclinical findings have received some support from the results of small open-label clinical trials, suggesting that amantadine can reduce depressive symptomatology and potentiate the antidepressant effects of monoaminergic drugs. In addition to its glutamatergic and dopaminergic effects, the potential antidepressant-like effects of amantadine have been linked to molecular and cellular actions, such as increased expression of neurotrophic factors (e.g., brain-derived neurotrophic factor), activation of σ1 receptors, decreased corticosterone levels, and decreased inflammatory response to stress.
Conclusion:
Amantadine is an interesting candidate as new antidepressant drug for the treatment of depression.
Keywords: Amantadine, animal models, antidepressant, clinical trial, glutamate
Introduction
According to the World Health Organization,1 at least 350 million people live with depression, a mental disorder that is characterized by sadness, loss of interest or pleasure (i.e., anhedonia), inappropriate feelings of guilt, low self-esteem, sleep and appetite disorders, psychomotor retardation, and decreased concentration.1,2 Major depression is difficult to diagnose and treat, negatively affecting quality of life and causing social and economic losses. A major challenge is to discover treatments for this disease that are both quick and effective.1,3 In addition to the compromised efficacy of treatment if onset is delayed, some patients respond only partially or not at all to antidepressant treatment.4
Glutamate is the main excitatory neurotransmitter in the central nervous system. Alterations in glutamatergic transmission have been associated with the pathophysiology of mood disorders and the mechanism of action of antidepressant treatments.5,6 Drugs that reduce N-methyl-D-aspartate (NMDA) receptor activity have been proposed for the treatment of depression. Both ketamine (a noncompetitive NMDA receptor antagonist) and lamotrigine (which reduces glutamate release) have been shown to produce clinical antidepressant effects.5 Ketamine has a very interesting clinical profile. Its antidepressant effect can be detected 24-48 h after administration and is effective in depressed patients who are refractory to monoaminergic antidepressants.4,5 However, the clinical use of ketamine has some drawbacks, such as its mode of administration (i.e., intravenous), psychotic-like side effects, and potential for drug abuse.4,7
Amantadine hydrochloride, a synthetic tricyclic amine, was used for the first time in the 1960s as a prophylactic antiviral agent and for the treatment of influenza A virus infection. Amantadine’s main clinical indication is currently for the treatment of Parkinson's disease, both as a monotherapy and combined with levodopa or dopaminergic agonists.8 Other indications for neuropsychiatric disorders include drug-induced extrapyramidal side effects, motor fluctuations during L-DOPA treatment, attention-deficit/hyperactivity disorder, traumatic brain injury, and autistic spectrum disorders.9
Amantadine is well absorbed orally. After oral administration, the maximal plasma level of amantadine is observed at approximately 3.3 h after administration, with a half-life of approximately 16 h. Approximately 90% of amantadine is excreted unchanged in urine. The usual clinical dose of amantadine is 100 mg twice daily, which can be increased to up to 400 mg/day.9 As an antiviral agent, amantadine acts through blockade of M2 ion channels, inhibition of virus entry into the cell, and inhibition of virus replication.9-11 Other effects of this drug include increases in norepinephrine and serotonin (5-hydroxytryptamine [5-HT]) neurotransmission pre- and postsynaptically10 (Table 1). However, some of these effects have been observed at doses that are greater than the doses that are used clinically, leading some authors to question their clinical relevance.10,12 In addition, amantadine acts as a weak noncompetitive NMDA receptor antagonist and indirectly increases dopamine release.10,12,13 Thus, amantadine-induced NMDA receptor blockade has been proposed as a relevant mechanism of action at therapeutically relevant doses.10,12
Table 1. General pharmacodynamics of amantadine.
| Dopaminergic system | Amantadine acts on the presynaptic membrane, enhancing dopamine release14,15 and inhibiting its reuptake in brain homogenates at higher doses.16,17 Several of these dopaminergic effects are seen at concentrations higher than those that are used clinically in humans.18-20 The effects of amantadine on dopaminergic transmission are still debatable. Some results show that amantadine has a direct action on D2 receptors,20-22 but other studies have reported no significant actions on catecholaminergic receptors.23-25 Moresco et al.26 evaluated patients who received amantadine (200 mg/day, 10-14 days) and were being treated with L-DOPA, which was suspended the night before positron emission tomography scans were performed. The patients were free from dopaminergic agonists, anticholinergics, and antidepressants. The patients presented an increase in [11C]raclopride binding in the caudate and putamen. This suggests that amantadine increases the neosynthesis of D2 receptors, which may represent one mechanism of action. The effects of amantadine are also related to the inhibition of dopamine reuptake. Its antiparkinsonian activity may be attributable to the inhibition of dopamine reuptake into presynaptic neurons or an increase in dopamine release from presynaptic fibers.27 |
| Noradrenergic system | Amantadine appears to have pharmacological actions that are similar to those of tricyclic antidepressants.10 A significant increase in norepinephrine levels was observed after an oral dose of amantadine in healthy subjects.28 Noradrenergic mechanisms may also be involved in the actions of amantadine.29,30 |
| Glutamatergic system | Amantadine is an N-methyl-D-aspartate (NMDA) receptor antagonist that is commonly used for the treatment of Parkinson’s disease. It is thought to inhibit NMDA receptor activation through the stabilization of ion channels and more rapid channel closure.30,31 |
| Immunomodulation | Amantadine may also have immunomodulatory properties. It restored the production of interleukin (IL)-2, which is dysfunctional in Parkinson’s disease patients.32,33 IL-2 levels did not correspond to clinical improvement, so the significance of these findings is unclear. Other studies33,34 showed that amantadine treatment was an independent predictor of improved survival in Parkinson’s disease. |
Considering the pharmacological effects of amantadine and its current clinical use, we reviewed its potential for the treatment of depression. For that, we performed a systematic review of clinical and preclinical studies focusing on the antidepressant and antidepressant-like effects of amantadine.
Methods
This review of preclinical and clinical studies was performed by searching the PubMed, Science Direct, Web of Science, and MEDLINE databases. We selected articles that were published in English up to September 1, 2017, articles on the use of amantadine for the treatment of mood disorders, or animal studies of depression using amantadine. The inclusion criteria for preclinical studies were as follows: using validated animal models of depression or focus on antidepressant-like drug screening tests. The inclusion criteria for clinical studies were: having a controlled design or being an open-label study or a case series evaluating depression (or depressive symptoms). Exclusion criteria were non-English language, being a review article or a preclinical article that did not use models of depression or antidepressant-like drug screening tests, and being a clinical article that did not evaluate depression/depressive symptoms.
The following search terms were used: “amantadine AND depress*”; “amantadine AND mood”; “amantadine AND animal models AND antidepres*”; and “amantadine AND (forced swim, learned helplessness, reserpine, chronic mild stress, anhedonia, sucrose preference).” The titles and abstracts of all of the results were reviewed. Articles that did not meet the inclusion criteria or that met the exclusion criteria were withdrawn from further analysis. We manually reviewed the reference lists of the selected articles (IFR-B and RA reviewed preclinical and JCG and RA reviewed clinical studies).
Results and discussion
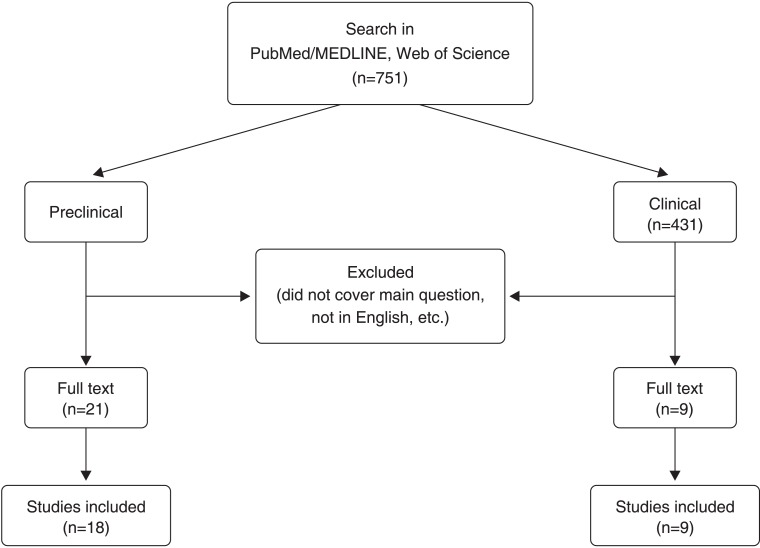
Using the search strategy described, 751 articles (Figure 1) were electronically retrieved (“amantadine AND depress*”: 366 articles; “amantadine AND mood”: 293 articles; “amantadine AND animal models AND antidepres*”: 10 articles; “amantadine AND [forced swim, learned helplessness, reserpine, chronic mild stress, anhedonia, sucrose preference]”: 112 articles; some of these articles were found in more than one search group). Restricting the search to studies performed in humans yielded 431 articles. After reviewing the titles and abstracts of all of the articles, the number of articles was reduced to 21 preclinical studies and nine clinical studies. Finally, after reading the original articles, three were withdrawn from the preclinical analysis and none were removed from the clinical analysis. Thus, 18 preclinical and nine clinical articles were included in the final analysis.
Figure 1. Flow chart of search strategy and results.
Antidepressant-like activity in preclinical studies
Preclinical studies evaluating amantadine in animal models of depression were grouped into three categories: i) studies that evaluated the antidepressant-like effect of amantadine alone in animal models of depression; ii) studies that evaluated the potential of amantadine to potentiate the antidepressant effects of monoaminergic antidepressants; and iii) studies that evaluated the antidepressant-like effects of amantadine on depressive-like behaviors associated with other comorbidities.
Studies evaluating the antidepressant-like effect of amantadine alone in animal models of depression
These studies evaluated the effects of amantadine in animal models of depression, including the forced swim test (FST), models of chronic mild stress that induced anhedonia, and the reserpine syndrome model (Table 2). The FST is well validated and the most frequently used animal model for screening antidepressant drugs.35,36 In this model, a rodent (rat or mouse) is placed in a cylinder that is filled with water and from which the animal cannot escape. After initial escape-directed behavior, the animal acquires an immobile posture. Antidepressant drugs reduce immobility at doses that do not increase spontaneous locomotor activity35,36 (in the FST, false-positive results are related to an increase in locomotor activity). In the mouse FST, Moryl et al.29 found that amantadine (20-80 mg/kg) exerted an anti-immobility effect. In that study, amantadine decreased locomotor activity in the open field test. Rogóz et al. repeatedly showed that amantadine (20 mg/kg) decreased immobility time in rats in the FST.37,38 In another series of studies, amantadine was co-administered with antidepressant drugs. Rogóz et al. found that amantadine administration alone at a lower dose (10 mg/kg) was ineffective in the FST, although it potentiated the effects of antidepressant drugs such as fluoxetine and imipramine.39-42 The authors also found that amantadine did not affect locomotor activity in the open field,37,38 which suggests that locomotor activity did not influence behavior in the FST.
Table 2. Pharmacological effects of amantadine in animal models of depression.
| Reference | Animal | Amantadine effective dose(mg/kg)* | Treatment(route) | Depression induction | Animal model | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| FST | |||||||
| Moryl et al.29 | Rat (male) | 20, 40, 80 | Acute (i.p.) | FST | FST: ↓ immobility time Locomotor activity (OFT): ↓ ambulation, peeping, rearing, and walking time | Antidepressant-like effects | |
| Rogoz et al.37 | Rat (male) | (10), 20 | Acute (i.p.) | FST | FST: ↓ immobility time Locomotor activity (OFT): no effect | Antidepressant-like effects | |
| Rogoz, et al.38 | Rat (male) | (10), 20 | Acute (i.p.) | FST | FST: ↓ immobility time Locomotor activity (OFT): no effect | Antidepressant-like effects | |
| CUMS | |||||||
| Yu et al.43 | Rat (male) | 25 | Chronic (oral) | CUMS | SPTMWMBody weight loss | SPT: ↑ sucrose consumption MWM: ↓ escape latency Body weight loss: prevented Locomotor activity (swim velocity in MWM): no effect | Antidepressant-like effects |
| Reserpine syndrome | |||||||
| Maj et al.21 | Rat (male and female) | (20), 40, 80 | Acute (i.p.) | Reserpine 5 mg/kg | Locomotor activity Catalepsy | ↓ reserpine-induced hypolocomotion ↓ catalepsy Locomotor activity (AA): no effect | Antidepressant-like effects |
| Jurna et al.44 | Rat (unspecified sex) | 50 | Acute (i.v.) | Reserpine 10 mg/kg | Electromyography | ↓ muscle rigidity Locomotor activity: NE | Antidepressant-like effects |
| Lassen45 | Rats (female) | 25, 50 | Acute (s.c.) | Reserpine 7.5 mg/kg | Locomotor activity | Did not affect reserpine-induced hypolocomotion AA: ↑ locomotor activity | Ineffective |
| Colpaert et al.46 | Rats (female) | 24-110 (ED50) | Acute (oral) | Reserpine 40 mg/kg | Hypokinesia Catalepsy | Prevented reserpine-induced hypokinesia ↓ catalepsy Locomotor activity: NE | Antidepressant-like effects |
| Goldstein et al.47 | Rats (male) | 21.8 (ED50) | Acute (s.c.) | Reserpine 5 mg/kg | Reserpine-induced hindlimb rigidity | Prevented reserpine-induced rigidity Locomotor activity: NE | Antidepressant-like effects |
| Cox & Tha48 | Mouse (male and female) | (25) | Acute (i.p.) | Reserpine 5 mg/kg | Reserpine-induced hypothermia | No effect Locomotor activity: NE | Ineffective |
| Messiha49 | Mouse (male) | 100 | Acute (i.p.) | Reserpine 0.2 mg/kg | Locomotor activity | ↑ reserpine-induced hypolocomotion Locomotor activity (AA): ↓ | Ineffective |
| Moryl et al.29 | Mouse (male) | (20), 40, 80 | Acute (i.p.) | Reserpine 2.5 mg/kg | Hypothermia | ↓ reserpine-induced hypothermia Locomotor activity: NE (for mice) | Antidepressant-like effects |
| Comorbidity | |||||||
| Tan et al.50 | Rat (male) | 45-135 | Chronic (28 days) (i.p.) | Depressive-like behavior induced by traumatic brain injury | SPTFST | SPT: ↑ sucrose preference FST: ↓ immobility time Locomotor activity: NE | Antidepressant-like effects |
| Co-administration | |||||||
| Skuza & Rogóz39 | Rat (male) | (10) | Acute (i.p.) | + σ1 receptor agonist SA4503 or σ2 receptor agonist siramesine | FST | FST: ↓ immobility time Locomotor activity (AA): no effect | Potentiated antidepressant-like effects of σ receptor agonists |
| Rogoz et al.37 | Rat (male) | (10) | Acute (i.p.) | + venlafaxine, imipramine, or fluoxetine | FST | FST: ↓ immobility time Locomotor activity (OFT): no effect or ↓ locomotor activity | Potentiated antidepressant-like effects |
| Rogoz et al.38 | Rat (male) | 20 | Acute (i.p.) | + imipramine | FST | FST: ↓ immobility time Locomotor activity (OFT): ↓ locomotor activity | Potentiated antidepressant-like effects |
| Kubera et al.51 | Rat (male) | (10) | Acute (i.p.) | + imipramine | FST | FST: ↓ immobility time Locomotor activity: NE | Potentiated antidepressant-like effects |
| Skuza & Rogóz40 | Rat (male) | (10) | Acute (i.p.) | + σ1 receptor agonist SA4503 | FST | FST: ↓ immobility time Locomotor activity (AA): no effect | Potentiated antidepressant-like effects of σ1 receptor agonist |
| Rogóz et al.41 | Rat (male) | (10) | Acute (i.p.) | + fluoxetine | FST | FST: ↓ immobility time Locomotor activity: NE | Potentiated antidepressant-like effects of fluoxetine |
| Skuza et al.52 | Mouse (male) | (10) | Acute (i.p.) | + σ1 receptor agonist PB190 | FSTTST | FST: ↓ immobility time TST: no effect Locomotor activity: NE | σ1 receptor agonist had no effect on antidepressant-like effects of amantadine |
AA = automated locomotor activity meter (i.e., chamber with photobeams); CUMS = chronic unpredictable mild stress; ED = effective dose; FST = forced swim test; i.p. = intraperitonial; i.v. = intravenous; MWM = Morris water maze; NE = not evaluated; OFT = open field test; s.c. = subcutaneous; SPT = sucrose preference test; TST = tail suspension test.
Doses in parentheses were ineffective.
Chronic unpredictable mild stress (CUMS) is a model of depression that induces several types of depressive-like behavior (e.g., anhedonia, sleep disturbances, and cognitive impairment) that can be reversed by repeated but not acute administration of standard monoaminergic antidepressants, although some NMDA antagonists (e.g., ketamine) can reverse CUMS-induced anhedonia faster than monoamine-based antidepressants.53,54 Thus, the CUMS model is particularly useful for evaluating the time required for drugs to exert antidepressant-like effects. Yu et al.43 studied the effects of amantadine on anhedonia and cognitive deficits that were induced by CUMS. They found that rats that were subjected to CUMS exhibited a decrease in sucrose preference (i.e., an index of anhedonia) and an increase in the latency to find a hidden platform in the Morris water maze, indicating memory impairment. Amantadine (25 mg/kg, orally, for 20 days) reversed anhedonia and impairments in the Morris water maze in stressed rats. Moreover, rats that were subjected to CUMS exhibited less weight gain than non-stressed and stressed rats that were treated with amantadine. Amantadine also reversed the stress-induced reduction of hippocampal postsynaptic density-95 protein expression, which is related to synaptic plasticity.43 Unfortunately, in this study, amantadine administration started at the beginning of the stress procedure (day 3), thus precluding an evaluation of the time necessary to reach an antidepressant-like effect and indicating that amantadine prevented the depressant-like effect of CUMS rather than reversed it.
Reserpine-induced behavioral alterations (e.g., lower mobility) and physiological changes (e.g., lower body temperature) have been used as a model to screen antidepressant drugs.55 Reserpine blocks the reuptake and storage of monoamines (e.g., serotonin, norepinephrine, and dopamine) in synaptic vesicles, resulting in monoamine depletion. Antidepressant drugs that affect monoamines are able to reverse these reserpine-induced effects.55 Maj et al.21 found that amantadine (40-80 mg/kg) reversed catalepsy and hypoactivity that were induced by reserpine in rats. Similarly, Jurna et al.44 employed electromyography and found that amantadine (50 mg/kg) blocked muscle rigidity that was induced by reserpine. Lassen et al.45 found that amantadine reversed reserpine-induced hypomotility but did not alter ptosis. Colpaert46 also found that amantadine prevented reserpine-induced hypokinesia in female rats. Goldstein et al.47 found that amantadine (30-60 mg/kg) reversed hindlimb rigidity that was induced by reserpine (5 mg/kg). Furthermore, amantadine at 40 and 80 mg/kg, but not 20 mg/kg, attenuated reserpine-induced hypothermia.29 Cox & Tha48 found that amantadine (25 mg/kg) failed to affect hypothermia that was induced by reserpine (5 mg/kg), although amphetamine (a multitarget drug that increases monoaminergic transmission) and the dopamine D2 receptor agonist apomorphine reversed it. Moreover, Messiha49 found that amantadine (100 mg/kg) enhanced the reserpine-induced suppression of locomotor activity.
These animal studies suggest that amantadine has a behavioral profile that is indicative of an antidepressant-like drug. Several of these antidepressant-like effects were seen at doses (e.g., 20-50 mg/kg) that correspond to clinically relevant doses.12
Studies evaluating the potential of amantadine to potentiate the antidepressant effects of monoaminergic antidepressants
These studies tested the hypothesis that the co-administration of amantadine with established antidepressant drugs increases their effectiveness. Rogóz et al.37,38,41,51 evaluated the effects of acute administration of amantadine (10 or 20 mg/kg) alone or combined with imipramine (5 and 10 mg/kg; serotonin and norepinephrine reuptake inhibitor), venlafaxine (10 and 20 mg/kg; serotonin and norepinephrine reuptake inhibitor), and fluoxetine (5 and 10 mg/kg; selective serotonin reuptake inhibitor) in the FST. When administered alone, all of these drugs, with the exception of fluoxetine, decreased immobility time in rats at the highest dose tested. When administered together with amantadine, all of these antidepressants decreased immobility time at both lower and higher doses, suggesting that amantadine had a synergistic effect with these monoamine-based antidepressants. To assess whether the mechanism of synergism between amantadine and imipramine involves pharmacokinetic interactions, Rogóz et al.38 measured plasma and brain concentrations of imipramine (5 and 10 mg/kg) and its active metabolite desipramine (selective norepinephrine reuptake inhibitor) after the administration of imipramine alone or combined with amantadine (20 mg/kg) in rats that were tested in the FST. No significant changes in plasma or brain concentration of imipramine or desipramine were found, suggesting that no pharmacokinetic interaction occurred.
Using the same approach, Skuza et al.39,40 found that the co-administration of ineffective doses of amantadine and σ receptor agonists (i.e., σ1 receptor agonist SA4503 and σ2 receptor agonist siramesine) reduced immobility time in the FST. Co-administration of amantadine with PB190, another σ1 receptor agonist, had no effect.52
Studies evaluating the antidepressant-like effects of amantadine on depressive-like behaviors associated with other comorbidities
Depression is a common psychiatric disorder after traumatic brain injury. Tan et al.50 evaluated the effects of amantadine on depression that was induced by traumatic brain injury in rats. Lesioned rats presented depressive-like behaviors (i.e., lower sucrose preference and increased immobility time in the FST) that were reversed by amantadine treatment (45 and 135 mg/kg for 28 days).
Preclinical studies evaluating the molecular and cellular effects of amantadine related to its antidepressant-like effect
Studies have evaluated the effects of amantadine alone and combined with antidepressant drugs on molecular and cellular mechanisms that are related to depression (e.g., neurotrophins and immune responses to stress). Brain-derived neurotrophic factor (BDNF) is a neurotrophin that is associated with the pathophysiology of depression and its treatment.56 Lower BDNF expression has been related to depression, and antidepressant treatments increase the activity of the BDNF signaling pathway (e.g., BDNF activates TrkB receptors). Interestingly, ketamine increases BDNF translation.57 Amantadine treatment alone increased BDNF mRNA expression in the cerebral cortex.58,59 When amantadine was co-administered with fluoxetine, BDNF mRNA expression increased in the cerebral cortex. When amantadine was co-administered with imipramine, BDNF mRNA expression increased in the hippocampus.41,58
Another approach that has been used to study the role of specific receptors in the antidepressant-like effects of amantadine involves the induction of a behavioral syndrome by drugs that target specific receptors. For example, 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OHDPAT), a 5-HT1A receptor agonist, induces characteristic behaviors (e.g., forepaw treading and flat posture) that can be influenced by drugs that either directly or indirectly act on these receptors (e.g., alter 5-HT release). A low dose of amantadine (10 mg/kg) did not alter the behavioral syndrome that was induced by 8-OHDPAT in rats. It also did not influence head twitches that were induced by the 5-HT2A receptor agonist (±)DOI.59 The co-administration of amantadine with fluoxetine attenuated the behavioral syndromes that were induced by 8-OHDPAT and (±)DOI.41 The co-administration of amantadine with imipramine reduced head twitches that were induced by (±)DOI, but this combined treatment also slightly increased the behavioral effects of 8-OHDPAT.59 A low dose of amantadine (10 mg/kg) that was administered repeatedly for 7 days did not alter hyperlocomotion that was induced by amphetamine and the dopamine D2/3 receptor agonist quinpirole.60 Repeated administration of amantadine combined with fluoxetine enhanced hyperlocomotion that was induced by quinpirole and amphetamine.60 This latter behavioral effect is consistent with the finding that repeated amantadine administration increases D2/3 (quinpirole) receptor binding and D2 mRNA expression in the nucleus accumbens.61 Tan et al.50 employed a model of depression that was induced by traumatic brain injury. Amantadine reversed dopamine cell loss in the substantia nigra and restored the reduction of dopamine levels in the striatum.
Another behavioral approach is the use of specific receptor antagonists to study the involvement of these receptors in a given effect. The dopamine D2 receptor antagonist sulpiride blocked the antidepressant-like effect of amantadine alone or combined with imipramine in the FST. The α1 adrenergic receptor antagonist prazosin blocked the effects of both imipramine treatment alone and combined with amantadine.38 Sulpiride, prazosin, and the σ receptor antagonists progesterone and BD1047 blocked the anti-immobility effect produced by the co-administration of amantadine with the σ receptor agonists SA4503 and siramesine in the FST.40,52
Pretreatment with α-methyl-p-tyrosine, which blocks dopamine and norepinephrine synthesis, attenuated the effect of amantadine on hypoactivity, but did not alter its anticataleptic effect.21 These results suggest that some of the effects of amantadine do not completely depend on monoamine synthesis or release.
An increase in plasma glucocorticoid levels is frequently found in patients with depression. Amantadine attenuated the increase in plasma corticosterone levels that was induced by the FST in rats.51 This effect was also seen with co-administration of amantadine and fluoxetine.41
A series of studies have evaluated the effects of co-administration of amantadine (10 mg/kg) plus imipramine and fluoxetine on immunological parameters.37,41,51,62 The co-administration of amantadine with antidepressants was also shown to increase the production of interleukin (IL)-10 (a cytokine with antiinflammatory action) and decrease the proinflammatory activity of macrophages. Amantadine administration alone also increased IL-10.41,51 These effects on inflammatory response may be involved in the improvement of depressive symptoms that is induced by amantadine.
Discussion of preclinical studies
The studies reviewed herein suggest that amantadine has antidepressant-like effects. However, there are some considerations when attempting to translate these findings to a clinical setting. First, locomotor activity is an important confounding factor in behavioral tests, such as the FST. The open-field test and automated activity meter (e.g., a chamber with photobeam sensors) were frequently used to evaluate locomotor activity in the studies that were reviewed herein (Table 2). Generally, an antidepressant-like effect was presumed when the drug did not affect locomotor activity. An increase in locomotor activity can lead to false-positive results in the FST, which is seen with psychostimulant drugs.35,63 Locomotor activity impairment may be seen with drugs that induce muscle relaxation or muscle rigidity, increase emotionality (e.g., fear), or induce sedation.64-67 At the doses tested, amantadine either decreased or did not change locomotor activity in most of the studies that were reviewed herein (Table 2), although one study found an increase in locomotor activity. Sedative or motor-impairing drugs can lead to false-negative results (e.g., sedation or motor impairment may reduce activity in the FST). Interestingly, however, some antidepressant drugs (e.g., tricyclics and ketamine) can reduce immobility in the FST but decrease open-field activity.64
Other considerations that are related to the translational value of potential antidepressant drugs in preclinical studies include i) parenteral drug administration, ii) the response to acute administration, and iii) the dose that is necessary for the observed effect. Several preclinical studies employ intraperitoneal drug administration, which is different from their intended clinical use, which is generally oral. This issue is particularly important for new drugs because little is known about their pharmacokinetics (e.g., drug stability in gastric fluids, first-pass metabolism, bioavailability, etc.). However, the pharmacokinetics of amantadine after oral administration and its central nervous system activity (e.g., antiparkinsonian effect) are already known. Moreover, one study that evaluated the antidepressant-like effect of amantadine used the oral route of administration.43,55
Another consideration is studies of the neurobiology of depression or the mechanism of action of antidepressant drugs that use animal models or tests (e.g., the FST) that are sensitive to acute drug administration. Antidepressant drugs often take several days to achieve clinical improvement in patients with major depression. The predictive validity of the FST had been well established, even with acute drug administration (e.g.,35,36). Thus, the results of acute amantadine administration in the FST are also a good indication of its potential clinical effect. However, a possible issue is the occurrence, or lack thereof, of tolerance to antidepressant-like effects that are observed in acute experiments. Some studies that evaluated repeated amantadine administration did not observe signs of tolerance to its antidepressant-like effects.43,55 However, these studies were not designed to evaluate tolerance. Another limitation of the present models that are used to screen antidepressant drugs is that some of these models were initially validated using monoaminergic drugs, which may reduce their ability to detect antidepressant-like effects of non-monoaminergic drugs. However, these models were sensitive to the antidepressant-like effects of ketamine and tianeptine (e.g.,68,69), suggesting their utility in detecting the effects of drugs beyond classic monoaminergic antidepressants.
The doses that are used in preclinical studies are also an important consideration for the translation of preclinical results. Amantadine generally showed dose-dependent effects. For example, in rats, 10 mg/kg was ineffective, and doses above 40 mg/kg were effective, with 20 mg/kg showing mixed results (Table 2). Using the allometric calculation,70 a rat dose of 40 mg/kg would correspond to a human dose of 3.24 mg/kg, which is near the clinical dose of amantadine (e.g., ∼190 mg for a 60 kg person).
Thus, the results of preclinical studies of amantadine that utilized animal models of depression or performed antidepressant-like drug screening suggest the good antidepressant potential of amantadine. However, the present review also identified some important gaps in these preclinical studies, such as potential sex-specific effects, the role of NMDA receptor antagonism in the antidepressant-like effect of amantadine, the effect of chronic amantadine administration, and the use of different animal models of depression to detect the onset of antidepressant-like actions.
Clinical trials
Studies of amantadine for the treatment of depressive states began in the 1970s but, in the present review, we identified only eight studies to date. Each of these studies had a small sample size, and most of them used an open-label design, which prevented reliable conclusions (Table 3).
Table 3. Clinical studies of potential antidepressant effect of amantadine.
| Reference | Disorder | Design | n | Evaluation scale | Amantadine dose (mg/day) | Treatment duration (weeks) | Result | Comments |
|---|---|---|---|---|---|---|---|---|
| Antidepressant studies | ||||||||
| Vale et al.71 | Chronic depressive syndrome | DB, R, C (placebo) | 16-18/group | Zung SRS | 100-200 | 4 | 67% of patients improved (vs. 25% in placebo group) | Diagnostic criteria not used Response: score below median |
| Stryjer et al.72 | MD (refractory) | Open Add-on | 8 | HDRS CGI | 100-300 | 4 | 50% of patients responded (≥ 50% reduction) | Pre vs. post comparison |
| Antidepressant potentiation studies | ||||||||
| Rogóz et al.58 | MD (refractory) | Open + imipramine | 25/group | HDRS | 100 | 6 | Potentiated effect of imipramine | Comparison between imipramine and imipramine + amantadine |
| Comorbidity/secondary depression | ||||||||
| Ferszt et al.73 | MD, bipolar depression, dysthymia + BDV infection | Open Add-on | 30 | MADRS | 200-350 | 8-12 | 63% of patients improved (≥ 40% reduction) | Better response associated with Ag2 BDV antigen |
| Dietrich et al.74 | MD, bipolar depression + BDV infection | Open Add-on | 25 | HDRS OCPCRIT | 100-300 | 11 (mean) | 68% of patients improved (≥ 50% reduction or two steps on OCPCRIT) | |
| Ziedonis & Kosten75 | Depression secondary to cocaine addiction | DB, R, C (placebo/ desipramine) | 5-9/group | BDI | 300 | 12 | Prevented increase in depression score | Reduced cocaine craving and consumption |
| Quarentini et al.76 | Depression induced by interferon-α | Single-blind add-on | 6-8/group | HADS | 200 | 24 | Prevented depression | Exclusion criteria: history of depression |
| Kronenberger et al.77 | Depression induced by interferon-α | DB, R, C (placebo) | 131-136/group | POMS | 200 | 48 | No effect on POMS depression factor | Prevented depressive symptoms in a subset of patients |
BDI = Beck Depression Inventory; BDV = Borna disease virus; C = comparative (drug comparator); CGI = Clinical Global Impressions; DB = double-blind; HADS = Hospital Anxiety and Depression Scale; HDRS = Hamilton Depression Rating Scale; MADRS = Montgomery-Asberg Rating Scale; MD = major depression; OCPCRIT = Operationalized Diagnostic Criteria System; POMS = Profile of Mood States; R = randomized; SRS = Self-Rating Scale.
Vale et al.71 evaluated 40 patients. The patients continued their usual antidepressant medications and were randomized into three groups. In one group, 10 patients initially received 100 mg amantadine in the first week, followed by 200 mg in the second week until the study ended at 4 weeks. In the second group, 10 patients initially received 200 mg amantadine in the first week, followed by 100 mg in the second week until the study ended at 4 weeks. The remaining 20 patients did not receive amantadine. The authors observed an improvement of depressive symptoms compared with placebo, but this beneficial effect did not remain after amantadine was discontinued.
Two studies evaluated the efficacy of amantadine in treatment-resistant depression. Stryjer et al.72 evaluated eight patients with treatment-resistant major depression. The patients continued to take their current antidepressant medication (e.g., fluoxetine, venlafaxine, or paroxetine) for 4 weeks. The patients received adjunct treatment with amantadine that reached a dose of 300 mg/day over the study. No significant improvement in symptoms of depression or anxiety was observed.
Rogóz et al.58 evaluated 50 patients with treatment-resistant unipolar depression. After a 2-week washout period, the patients were randomized into two groups: 25 patients received only imipramine (100 mg/day) for 12 weeks and 25 patients received imipramine (100 mg/day) and amantadine (150 mg/day, twice daily). The patients were evaluated using the Hamilton Depression Rating Scale (HDRS). Imipramine treatment alone did not alter HDRS scores. In the group that was treated with imipramine plus amantadine, HDRS scores decreased at 6 weeks. Additionally, amantadine did not alter the pharmacokinetics of imipramine or its active metabolite desipramine in plasma. A previous report from this group76 described 12 patients who were also included in this latter study.
These were the only clinical studies we found on the effects of amantadine for the treatment of depression. Additional studies were identified that evaluated the treatment of secondary depression in patients with hepatitis C who were receiving interferon treatment.75,77 Three studies investigated the treatment of secondary depression in cocaine addiction73 and depression in patients with Borna disease virus infection.74,78 In all of these studies, amantadine combined with the patients’ usual medication significantly improved depressive symptomatology compared with the control groups (Table 3). The dose of amantadine in these studies ranged from 100 to 300 mg/day.
None of these studies reported significant adverse reactions. However, Rizzo et al.79 planned to evaluate amantadine in an open-label study with depressive patients, but amantadine caused aggressive behavior in the first patients with depression who were included in this study; this adverse effect quickly resolved with the discontinuation of treatment. These reactions led the researchers to abandon the study. Other adverse effects that have been associated with amantadine are anticholinergic-like effects (e.g., blurred vision, nausea, dry mouth, urinary retention, and constipation), livedo reticularis, confusion, nervousness, drowsiness, difficulty concentrating, and insomnia.8-10 Interestingly, some case reports suggest that amantadine can be useful for the treatment of sexual dysfunction that is induced by serotonergic antidepressants.80,81 A case series with three patients suggested that high-dose amantadine induced a switch to mania in bipolar patients,82 a side effect that has also been reported for several antidepressant drugs.
Discussion of clinical studies
Although these preliminary studies suggest a potential antidepressant effect of amantadine, well-controlled clinical trials with large samples are needed to evaluate the possible beneficial effects of amantadine alone or combined with traditional antidepressants for the treatment of depressive disorders.
The antagonism of NMDA receptors is a promising target for new antidepressants, and the effect ketamine in refractory depressed patients was faster. Amantadine appears to have potential advantages, such as an oral route of administration, compared with intravenous ketamine administration. Amantadine also has a well-characterized adverse-effect profile and pharmacokinetic interactions compared with new NMDA receptor antagonists.
Surprisingly, although the clinical efficacy of amantadine in Parkinson’s disease has been demonstrated, and Parkinson’s disease is frequently associated with depression, we were unable to locate any studies evaluating the effect of amantadine in patients with comorbid depression and Parkinson’s disease. Moreover, no preclinical studies have evaluated sex differences regarding the effect of amantadine on depression. Other gaps in the literature include the influence of specific clinical characteristics, such as depression severity, depression subtype diagnosis, and the presence of psychotic symptoms, among others.
Conclusion
Considering the pharmacological profile of amantadine, some studies have evaluated its effects in animal models, which consistently found antidepressant-like effects in different models (e.g., FST, chronic mild stress paradigm, and reserpine syndrome test) and species (e.g., rats and mice). These beneficial effects have received some support from small open-label studies. Moreover, preliminary data suggest that amantadine can potentiate the clinical effects of other antidepressants, suggesting its possible use in refractory depression.
Pharmacologically, amantadine appears to exert antidepressant effects through multiple systems, such as the dopaminergic system (e.g., D2 receptors), σ receptor transmission (e.g., σ1 receptors), the noradrenergic system, possibly glutamatergic neurotransmission (although this has not been evaluated), and BDNF mediation or modulation. Amantadine is already in clinical use and may be a promising choice for the treatment of major depression. However, further controlled studies are still necessary to confirm this hypothesis.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
RA, JCG, and MAV are recipients of research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). IFR-B is the recipient of a graduate fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- 1.World Health Organization (WHO). Depression: let’s talk [Internet]. [cited 2015 Sep 04]. www.who.int/topics/depression/en/ [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Vásquez CE, Riener R, Reynolds E, Britton GB. NMDA receptor dysregulation on chronic state: a possible mechanism underlying depression with BDNF downregulation. Neurochem Int. 2014;79:88–97. doi: 10.1016/j.neuint.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–66. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 5.Mathew SJ, Keegan K, Smith L. Glutamate modulators as novel interventions for mood disorders. Rev Bras Psiquiatr. 2005;27:243–8. doi: 10.1590/s1516-44462005000300016. [DOI] [PubMed] [Google Scholar]
- 6.Sanacora G, Schatzberg AF. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology. 2015;40:1307. doi: 10.1038/npp.2014.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correia-Melo FS, Silva SS, Araújo-de-Freitas L, Quarantini LC. S-(+)-ketamine-induced dissociative symptoms as a traumatic experience in patients with treatment-resistant depression. Rev Bras Psiquiatr. 2017;39:188–9. doi: 10.1590/1516-4446-2016-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311:1670–83. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 9.Cordioli AV, Gallois CB, Isolan L. Psicofármacos, consulta rápida. 5a ed. Porto Alegre: Artmed; 2015. [Google Scholar]
- 10.Huber TJ, Dietrich DE, Emrich HM. Possible use of amantadine in depression. Pharmacopsychiatry. 1999;32:47–55. doi: 10.1055/s-2007-979191. [DOI] [PubMed] [Google Scholar]
- 11.Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology. 2012;78:1096–9. doi: 10.1212/WNL.0b013e31824e8f0d. [DOI] [PubMed] [Google Scholar]
- 12.Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents: preclinical studies. Neurosci Biobehav Rev. 1997;21:455–68. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 13.Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55–60. [PMC free article] [PubMed] [Google Scholar]
- 14.Scatton B, Cheramy A, Besson MJ, Glowinski J. Increased synthesis and release of dopamine in the striatum of the rat after amantadine treatment. Eur J Pharmacol. 1970;13:131–3. doi: 10.1016/0014-2999(70)90194-9. [DOI] [PubMed] [Google Scholar]
- 15.Von Voigtlander PF, Moore KE. Dopamine: release from the brain in vivo by amantadine. Science. 1971;174:408–10. doi: 10.1126/science.174.4007.408. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EA, Redfern PH. The effect of amantadine on the uptake of dopamine and noradrenaline by rat brain homogenates. J Pharm Pharmacol. 1970;22:957–9. doi: 10.1111/j.2042-7158.1970.tb08486.x. [DOI] [PubMed] [Google Scholar]
- 17.Heimans RL, Rand MJ, Fennessy MR. Effects of amantadine on uptake and release of dopamine by a particulate fraction of rat basal ganglia. J Pharm Pharmacol. 1972;24:875–9. doi: 10.1111/j.2042-7158.1972.tb08906.x. [DOI] [PubMed] [Google Scholar]
- 18.Baldessarini RJ, Lipinski JF, Chace KV. Effects of amantadine hydrochloride on catecholamine metabolism in the brain of the rat. Biochem Pharmacol. 1972;21:77–87. doi: 10.1016/0006-2952(72)90252-3. [DOI] [PubMed] [Google Scholar]
- 19.Heikkila RE, Cohen G. Evaluation of amantadine as a releasing agent or uptake blocker for H3-dopamine in rat brain slices. Eur J Pharmacol. 1972;20:156–60. doi: 10.1016/0014-2999(72)90144-6. [DOI] [PubMed] [Google Scholar]
- 20.Stone TW. Responses of neurones in the cerebral cortex and caudate nucleus to amantadine, amphetamine and dopamine. Br J Pharmacol. 1976;56:101–10. doi: 10.1111/j.1476-5381.1976.tb06964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maj J, Sowińska H, Baran L. The effect of amantadine on motor activity and catalepsy in rats. Psychopharmacologia. 1972;24:296–307. doi: 10.1007/BF00403648. [DOI] [PubMed] [Google Scholar]
- 22.Chopra YM, Dandiya PC. Potentiation of anticatatonic effect of antidepressants by amantadine. Indian J Med Res. 1977;66:142–9. [PubMed] [Google Scholar]
- 23.Stromberg U, Svensson TH, Waldeck B. On the mode of action of amantadine. J Pharm Pharmacol. 1970;22:959–62. doi: 10.1111/j.2042-7158.1970.tb08487.x. [DOI] [PubMed] [Google Scholar]
- 24.Farnebo LO, Fuxe K, Goldstein M, Hamberger B, Ungerstedt U. Dopamine and noradrenaline releasing action of amantadine in the central and peripheral nervous system: a possible mode of action in Parkinson's disease. Eur J Pharmacol. 1971;16:27–38. doi: 10.1016/0014-2999(71)90053-7. [DOI] [PubMed] [Google Scholar]
- 25.Henkel JG, Hane JT, Gianutsos G. Structure-anti-Parkinson activity relationships in the aminoadamantanes. Influence of bridgehead substitution. J Med Chem. 1982;25:51–6. doi: 10.1021/jm00343a010. [DOI] [PubMed] [Google Scholar]
- 26.Moresco RM, Volonte MA, Messa C, Gobbo C, Galli L, Carpinelli A, et al. New perspectives on neurochemical effects of amantadine in the brain of parkinsonian patients: a PET - [11C]raclopride study. J Neural Transm (Vienna). 2002;109:1265–74. doi: 10.1007/s00702-002-0694-7. [DOI] [PubMed] [Google Scholar]
- 27.Cacabelos R. Parkinson's disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechin F, van der Dijs B, Pardey-Maldonado B, Rivera JE, Baez S, Lechin ME. Effects of amantadine on circulating neurotransmitters in healthy subjects. J Neural Transm (Vienna). 2010;117:293–9. doi: 10.1007/s00702-010-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moryl E, Danysz W, Quack G. Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol. 1993;72:394–7. doi: 10.1111/j.1600-0773.1993.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 30.Dutta A, McKie S, Deakin JF. Ketamine and other potential glutamate antidepressants. Psychiatry Res. 2015;225:1–13. doi: 10.1016/j.psychres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25:3312–22. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wandinger KP, Hagenah JM, Klüter H, Rothermundt M, Peters M, Vieregge P. Effects of amantadine treatment on in vitro production of interleukin-2 in de-novo patients with idiopathic Parkinson's disease. J Neuroimmunol. 1999;98:214–20. doi: 10.1016/s0165-5728(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 33.Warren N, Burn DJ. The use of amantadine in Parkinson’s disease and other akinetic-rigid disorders. Adv Clin Neurosci Rehabil. 2004;4:38–41. [Google Scholar]
- 34.Uitti RJ, Rajput AH, Ahlskog JE, Offord KP, Schroeder DR, Ho MM, et al. Amantadine treatment is an independent predictor of improved survival in Parkinson's disease. Neurology. 1996;46:1551–6. doi: 10.1212/wnl.46.6.1551. [DOI] [PubMed] [Google Scholar]
- 35.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl). 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 36.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 37.Rogóz Z, Skuza G, Maj J, Danysz W. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology. 2002;42:1024–30. doi: 10.1016/s0028-3908(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 38.Rogóz Z, Skuza G, Kusmider M, Wójcikowski J, Kot M, Daniel WA. Synergistic effect of imipramine and amantadine in the forced swimming test in rats. Behavioral and pharmacokinetic studies. Pol J Pharmacol. 2004;56:179–85. [PubMed] [Google Scholar]
- 39.Skuza G, Rogóz Z. Effect of combined treatment with selective σ ligands and amantadine in the forced swimming test in rats. Pol J Pharmacol. 2002;54:699–702. [PubMed] [Google Scholar]
- 40.Skuza G, Rogóz Z. The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swimming test in rats. J Physiol Pharmacol. 2006;57:217–29. [PubMed] [Google Scholar]
- 41.Rogóz Z, Kubera M, Rogóz K, Basta-Kaim A, Budziszewska B. Effect of co-administration of fluoxetine and amantadine on immunoendocrine parameters in rats subjected to a forced swimming test. Pharmacol Rep. 2009;61:1050–60. doi: 10.1016/s1734-1140(09)70167-7. [DOI] [PubMed] [Google Scholar]
- 42.Roman A, Rogóz Z, Kubera M, Nawrat D, Nalepa I. Concomitant administration of fluoxetine and amantadine modulates the activity of peritoneal macrophages of rats subjected to a forced swimming test. Pharmacol Rep. 2009;61:1069–77. doi: 10.1016/s1734-1140(09)70169-0. [DOI] [PubMed] [Google Scholar]
- 43.Yu M, Zhang Y, Chen X, Zhang T. Antidepressant-like effects and possible mechanisms of amantadine on cognitive and synaptic deficits in a rat model of chronic stress. Stress. 2016;19:104–13. doi: 10.3109/10253890.2015.1108302. [DOI] [PubMed] [Google Scholar]
- 44.Jurna I, Grossmann W, Nell T. Depression by amantadine of drug-induced rigidity in the rat. Neuropharmacology. 1972;11:559–64. doi: 10.1016/0028-3908(72)90011-1. [DOI] [PubMed] [Google Scholar]
- 45.Lassen JB. The effect of amantadine and (+)-amphetamine on motility in rats after inhibition of monoamine synthesis and storage. Psychopharmacologia. 1973;29:55–64. doi: 10.1007/BF00421211. [DOI] [PubMed] [Google Scholar]
- 46.Colpaert FC. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–40. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein JM, Barnett A, Malick JB. The evaluation of anti-Parkinson drugs on reserpine-induced rigidity in rats. Eur J Pharmacol. 1975;33:183–8. doi: 10.1016/0014-2999(75)90154-5. [DOI] [PubMed] [Google Scholar]
- 48.Cox B, Tha SJ. The role of dopamine and noradrenaline in temperature control of normal and reserpine-pretreated mice. J Pharm Pharmacol. 1975;27:242–7. doi: 10.1111/j.2042-7158.1975.tb10693.x. [DOI] [PubMed] [Google Scholar]
- 49.Messiha FS. Effect of amantadine on chlorpromazine and reserpine-induced behavioral depression in the mouse. Neurosci Biobehav Rev. 1988;12:219–22. doi: 10.1016/s0149-7634(88)80046-0. [DOI] [PubMed] [Google Scholar]
- 50.Tan L, Ge H, Tang J, Fu C, Duanmu W, Chen Y, et al. Amantadine preserves dopamine level and attenuates depression-like behavior induced by traumatic brain injury in rats. Behav Brain Res. 2015;279:274–82. doi: 10.1016/j.bbr.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 51.Kubera M, Basta-Kaim A, Budziszewska B, Rogóz Z, Skuza G, Leskiewicz M, et al. Effect of amantadine and imipramine on immunological parameters of rats subjected to a forced swimming test. Int J Neuropsychopharmacol. 2006;9:297–305. doi: 10.1017/S1461145705005663. [DOI] [PubMed] [Google Scholar]
- 52.Skuza G, Sadaj W, Kabziński M, Cassano G, Gasparre G, Abate C, et al. The effects of new sigma (σ) receptor ligands, PB190 and PB212, in the models predictive of antidepressant activity. Pharmacol Rep. 2014;66:320–4. doi: 10.1016/j.pharep.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Slattery DA, Cryan JF. Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology (Berl). 2017;234:1451–65. doi: 10.1007/s00213-017-4552-6. [DOI] [PubMed] [Google Scholar]
- 54.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourin M. Is it possible to predict the activity of a new antidepressant in animals with simple psychopharmacological tests? Fundam Clin Pharmacol. 1990;4:49–64. doi: 10.1111/j.1472-8206.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 56.Castrén E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 2017;97:119–26. doi: 10.1016/j.nbd.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogóz Z, Skuza G, Daniel WA, Wojcikowski J, Dudek D, Wrobel A. Amantadine as an additive treatment in patients suffering from drug-resistant unipolar depression. Pharmacol Rep. 2007;59:778–84. [PubMed] [Google Scholar]
- 59.Rogóz Z, Skuza G, Legutko B. Repeated co-treatment with fluoxetine and amantadine induces brain-derived neurotrophic factor gene expression in rats. Pharmacol Rep. 2008;60:817–26. [PubMed] [Google Scholar]
- 60.Rogóz Z, Skuza G. Effect of repeated co-treatment with fluoxetine and amantadine on the behavioral reactivity of the central dopamine and serotonin system in rats. Pharmacol Rep. 2009;61:924–9. doi: 10.1016/s1734-1140(09)70150-1. [DOI] [PubMed] [Google Scholar]
- 61.Rogóz Z, Dlaboga D, Dziedzicka-Wasylewska M. Effect of combined treatment with imipramine and amantadine on the central dopamine D2 and D3 receptors in rats. J Physiol Pharmacol. 2003;54:257–70. [PubMed] [Google Scholar]
- 62.Kubera M, Maes M, Budziszewska B, Basta-Kaim A, Leśkiewicz M, Grygier B, et al. Inhibitory effects of amantadine on the production of pro-inflammatory cytokines by stimulated in vitro human blood. Pharmacol Rep. 2009;61:1105–12. doi: 10.1016/s1734-1140(09)70173-2. [DOI] [PubMed] [Google Scholar]
- 63.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 64.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–14. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 65.Hall CS. Emotional behavior in the rat. III. The relationship between emotionality and ambulatory activity. J Comp Psychol. 1936;22:345–52. [Google Scholar]
- 66.Stanford SC. The open field test: reinventing the wheel. J Psychopharmacol. 2007;21:134–5. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- 67.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 68.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–4. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 69.Kelly JP, Leonard BE. The effect of tianeptine and sertraline in three animal models of depression. Neuropharmacology. 1994;33:1011–6. doi: 10.1016/0028-3908(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 70.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 71.Vale S, Espejel MA, Dominguez JC. Amantadine in depression. Lancet. 1971;2:437–437. doi: 10.1016/s0140-6736(71)90153-x. [DOI] [PubMed] [Google Scholar]
- 72.Stryjer R, Strous RD, Shaked G, Bar F, Feldman B, Kotler M, et al. Amantadine as augmentation therapy in the management of treatment-resistant depression. Int Clin Psychopharmacol. 2003;18:93–6. doi: 10.1097/00004850-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Ziedonis DM, Kosten TR. Pharmacotherapy improves treatment outcome in depressed cocaine addicts. J Psychoactive Drugs. 1991;23:417–25. doi: 10.1080/02791072.1991.10471612. [DOI] [PubMed] [Google Scholar]
- 74.Ferszt R, Kühl KP, Bode L, Severus EW, Winzer B, Berghöfer A, et al. Amantadine revisited: an open trial of amantadinesulfate treatment in chronically depressed patients with Borna disease virus infection. Pharmacopsychiatry. 1999;32:142–7. doi: 10.1055/s-2007-979220. [DOI] [PubMed] [Google Scholar]
- 75.Kronenberger B, Berg T, Herrmann E, Hinrichsen H, Gerlach T, Buggisch P, et al. Efficacy of amantadine on quality of life in patients with chronic hepatitis C treated with interferon-α and ribavirin: results from a randomized, placebo-controlled, double-blind trial. Eur J Gastroenterol Hepatol. 2007;19:639–46. doi: 10.1097/MEG.0b013e3281ac20ca. [DOI] [PubMed] [Google Scholar]
- 76.Rogóz Z, Dziedzicka-Wasylewska M, Daniel WA, Wójcikowski J, Dudek D, Wróbel A, et al. Effects of joint administration of imipramine and amantadine in patients with drug-resistant unipolar depression. Pol J Pharmacol. 2004;56:735–42. [PubMed] [Google Scholar]
- 77.Quarantini LC, Miranda-Scippa A, Schinoni MI, Sampaio AS, Santos-Jesus R, Bressan RA, et al. Effect of amantadine on depressive symptoms in chronic hepatitis C patients treated with pegylated interferon: a randomized, controlled pilot study. Clin Neuropharmacol. 2006;29:138–43. doi: 10.1097/01.WNF.0000220824.57769.E5. [DOI] [PubMed] [Google Scholar]
- 78.Dietrich DE, Bode L, Spannhuth CW, Lau T, Huber TJ, Brodhun B, et al. Amantadine in depressive patients with Borna disease virus (BDV) infection: an open trial. Bipolar Disord. 2000;2:65–70. doi: 10.1034/j.1399-5618.2000.020110.x. [DOI] [PubMed] [Google Scholar]
- 79.Rizzo PM, Biandrate P, Tognoni G, Morselli PL. Amantadine in depression: relationship between behavioural effects and plasma levels. Eur J Clin Pharmacol. 1973;5:226–8. [Google Scholar]
- 80.Shrivastava RK, Shrivastava S, Overweg N, Schmitt M. Amantadine in the treatment of sexual dysfunction associated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1995;15:83–4. doi: 10.1097/00004714-199502000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Balon R. Intermittent amantadine for fluoxetine-induced anorgasmia. J Sex Marital Ther. 1996;22:290–2. doi: 10.1080/00926239608404410. [DOI] [PubMed] [Google Scholar]
- 82.Sodré LA, Bücker J, Zortéa K, Sulzbach-Vianna MF, Gama CS. Mania switch induced by amantadine in bipolar disorder: report of three cases. Rev Bras Psiquiatr. 2010;32:467–9. doi: 10.1590/s1516-44462010000400029. [DOI] [PubMed] [Google Scholar]