Abstract
Objective:
Sleep apnea has been associated with anxiety, but the mechanisms of the sleep apnea-anxiety relationship are unresolved. Sleep apnea causes oxidative stress, which might enhance anxiety-like behavior in rodents. To clarify the apnea-anxiety connection, we tested the effect of intermittent hypoxia, a model of sleep apnea, on the anxiety behavior of mice.
Methods:
The rodents were exposed daily to 480 one-minute cycles of intermittent hypoxia to a nadir of 7±1% inspiratory oxygen fraction or to a sham procedure with room air. After 7 days, the mice from both groups were placed in an elevated plus maze and were video recorded for 10 min to allow analysis of latency, frequency, and duration in open and closed arms. Glyoxalase-1 (Glo1) and glutathione reductase-1 (GR1) were measured in the cerebral cortex, hippocampus, and striatum by Western blotting.
Results:
Compared to controls, the intermittent hypoxia group displayed less anxiety-like behavior, perceived by a statistically significant increase in the number of entries and total time spent in open arms. A higher expression of GR1 in the cortex was also observed.
Conclusion:
The lack of a clear anxiety response as an outcome of intermittent hypoxia exposure suggests the existence of additional layers in the anxiety mechanism in sleep apnea, possibly represented by sleepiness and irreversible neuronal damage.
Keywords: Sleep, biological markers, molecular biology, stress, anxiety
Introduction
Obstructive sleep apnea (OSA) is often associated with psychiatric and neurobehavioral symptoms, including depression and anxiety.1-4 Indeed, the presence of mood disorders or cognitive dysfunction is a diagnostic criterion for OSA according to the International Classification of Sleep Disorders.5 The prevalence of anxiety disorders among OSA patients varies from 11 to 70%, and a remarkable overlap of anxiety and OSA symptoms is frequently observed.6 This wide range in the prevalence of anxiety among OSA patients could be a result of different definitions and methods of assessment.
The main possible mechanism for exacerbating anxiety in sleep apnea is the recognized association of apnea with sympathetic hyperactivity.7,8 This could enhance “fight or flight” mechanisms, leading to anxiety-like behavior.9 However, mechanisms for the emergence of apnea-induced anxiety behavior are still unclear. Besides sympathetic hyperactivity, a possible pathway to explain the concomitance of anxiety and sleep apnea could be the response to oxidative stress triggered by intermittent hypoxia in OSA patients. Intermittent hypoxia is a well-established cause of oxidative stress,10,11 which in turn is associated with neuronal cell loss in rodents11-13 and with alterations similar to those observed in patients with residual sleepiness after effective OSA treatment.14,15 This interplay between intermittent hypoxia and oxidative stress might explain part of the neurobehavioral phenotype observed in OSA patients.
Oxidative stress has been proposed as a common factor for anxiety and other long-term consequences and co-morbidities of OSA, such as hypertension and insulin resistance.16 Among all oxidative stress mediators, an association between anxiety-related phenotype and overexpression of two genes, glyoxalase-1 (Glo1) and glutathione reductase-1 (GR1), was originally described by Hovatta et al. in rats.17 GR is the chief redox buffer in most cells, being critical for the balance of oxidative processes and resistance to oxidative stress,18 while Glo1 is an important enzyme for the neutralization of cytotoxic byproducts of glycolysis.19 Despite their relevance, Hovatta et al.’s17 findings were, at least in part, contradicted by subsequent results.20-22 Animals treated with L-buthionine-(S,R)-sulfoximine (BSO) for 7 days to produce oxidative stress showed lower expression of Glo1 and glutathione than controls.23 After 7 days of BSO, the time in open arms was lower, indicating higher anxiety; lower levels of glutathione were also observed in the hippocampus and amygdala. Rats receiving the antioxidant tempol, used to counteract the effect of BSO, behaved similarly to controls in anxiety tests.24 This finding suggests that these proteins, involved in oxidative stress regulation, are potential anxiety-like behavior mediators.17,25
We hypothesized that intermittent hypoxia may be associated with reduced Glo1 and GR1 expression and with higher anxiety-like behavior. The present study aimed to investigate anxiety mechanisms that may involve oxidative stress and alterations in Glo1 and GR1 levels in mice submitted to 7 days of intermittent hypoxia.
Methods
Animals
Two groups of two-month-old male Balb/c mice were maintained in an isolated room under controlled environmental conditions (22±1 °C; 12:12-h light-dark cycle) and received food and water ad libitum. These groups, 19 mice in total, were subjected to intermittent hypoxia or sham hypoxia for 7 days. All experimental procedures were approved by the institutional ethics committee (protocol 09-482). The experimental protocols were designed in accordance with the Guide for the Care and Use of Laboratory Animals.26
Intermittent hypoxia system
The intermittent hypoxia system utilized in the present study has been previously described elsewhere.27,28 In brief, 13 mice were exposed to a protocol of intermittent hypoxia for 7 days, 8 h/day (09:00 a.m. to 05:00 p.m.) in the light phase, totaling 480 cycles of hypoxia/reoxygenation per day. The mice were submitted to cycles of 30 s of hypoxia induced by insufflation in a sealed cage with a gas mixture (92% nitrogen and 8% CO2) that reduced the inspired oxygen fraction from 21 to 7±1% and increased the CO2 concentration to 5±1%, which was followed by 30 s of reoxygenation when room air was again insufflated into the cages. An automated system controlled the gas cycling. Intermittent hypoxia was conducted for 7 consecutive days. Six control animals were submitted to sham intermittent hypoxia, being housed in similar cages and conditions as the intermittent hypoxia group, except for the insufflated gas mixture being room air.
Anxiety assessment
Anxiety behavior was assessed after 7 days of intermittent hypoxia using the elevated plus-maze test.29,30 All behavioral tests were performed between 09:00 a.m. and noon. To habituate the mice to the testing room conditions, they were moved there 30 min prior to the test. The apparatus utilized for the test was elevated 50 cm from the floor and consisted of two open arms (25 x 5 x 0.5 cm) and two closed arms (25 x 5 x 16 cm). The mice were placed at the center of the platform (5 x 5 x 0.5 cm) with their nose directed towards a closed arm. The animals were allowed to explore the apparatus for 10 min while their behavior was recorded by a video camera. Posterior video analyses were performed semi-manually, with the assistance of data-counting software. Entrances into any space in the apparatus (open arms, closed arms, or center) were only counted when the animal placed all the four paws in it. Latency, frequency, and duration in the open and closed arms were analyzed. The total frequency (TF) of entries was calculated by adding the frequencies in the open and closed arms.
Molecular analyses
After 7 days of intermittent hypoxia exposure and anxiety assessment, the animals were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The mice were euthanized by cervical dislocation, and the brains were immediately removed. The hippocampus, cerebral cortex, and striatum were separated and rapidly frozen in liquid nitrogen. These three brain regions were selected because they are involved in the regulation of anxiety and fear.17 The samples were stored at -80 °C until the moment of analysis. Glo1 and GR1 (Santa Cruz Biotechnology, Santa Cruz, USA) levels were determined by Western blotting in the cerebral cortex, hippocampus, and striatum. The protein bands were visualized by ethidium bromide (0.5 µg.mL-1) (Vilber Lourmat apparatus, Marne-la-Vallée, France).The images were captured using an L-Pix Chemi photo digitizer (Loccus Biotecnologia, Cotia, Brazil) for subsequent editing and analysis with a digitizer (Loccus Biotecnologia).
Statistical analysis
All statistical analyses were conducted using SPSS version 18. The results from elevated plus-maze test and the protein levels in the hippocampus, cerebral cortex, and striatum were expressed as median and interquartile range. The comparisons between the two groups were performed using Mann-Whitney U tests. Differences with p < 0.05 for alpha error were considered significant.
Results
Body weight
The average baseline body weights in the hypoxia and control groups were 23.2 g (22.0-24.9) and 23.5 g (22.9-25.2), respectively, with no significant difference observed. The body weights were stable throughout the experimental period in both groups.
Elevated plus-maze test
Mice exposed to intermittent hypoxia scored lower on the anxiety behavior indices than the control group (Table 1). Differences in behavior were indicated by higher frequencies of open-arm entries and by longer duration and higher frequency of head-dipping, which indicate anxiolysis and increased risk-taking behavior. There were no significant differences in total entries, frequency of closed arm entry, time spent in open and closed arms, or stretching duration and frequency.
Table 1. Effect of intermittent hypoxia on elevated plus-maze parameters.
| Control (n=6) | Intermittent hypoxia (n=13) | p-value | |
|---|---|---|---|
| Total entries (frequency) | 4.00 (1.00-13.00) | 11.50 (6.25-18.00) | 0.11 |
| Open entries (frequency) | 0.00 (0.00-2.00) | 3.00 (1.25-6.50) | 0.04 |
| Closed entries (frequency) | 4.00 (1.00-10.00) | 9.00 (4.00-11.00) | 0.20 |
| Time in open arms (s) | 0.00 (0.00-10.00) | 23.00 (2.00-70.75) | 0.09 |
| Time in closed arms (min) | 4.00 (2.40-5.00) | 2.90 (2.10-3.70) | 0.20 |
| Stretching (frequency) | 11.00 (6.00-13.00) | 7.50 (3.50-11.75) | 0.19 |
| Stretching (duration, s) | 32.00 (15.00-72.00) | 27.00 (6.00-51.00) | 0.37 |
| Head-dipping (frequency) | 0.00 (0.00-5.00) | 13.00 (2.75-19.25) | 0.03 |
| Head-dipping (duration, s) | 0.00 (0.00-6.00) | 14.50 (5.00-25.00) | 0.02 |
Data presented as median and 25-75% quartiles.
p-values from the Mann-Whitney U test.
Bold font indicates statistical significance.
Western blot analysis
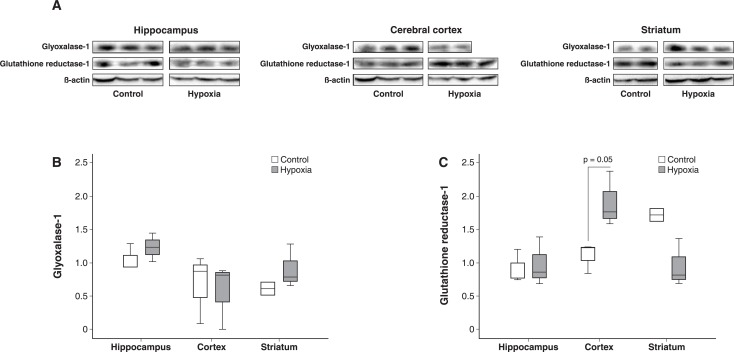
The GR1 level was 43% higher in the cerebral cortex (p = 0.05), 8% higher in the hippocampus (p = 0.51), and 52% lower in the striatum (p = 0.13) of the intermittent hypoxia group than the control group. The Glo1 level was 6% lower in the cerebral cortex (p = 0.56), 32% higher in the hippocampus (p = 0.51), and 28% higher in the striatum (p = 0.08) of the intermittent hypoxia than the control group (Figure 1).
Figure 1. The molecular findings for glutathione reductase-1 and glyoxalase-1 levels. A) Western blots from three nervous system areas showing the expression of glyoxalase-1, glutathione reductase-1, and the structural protein beta-actin used as a control between groups. B) Glyoxalase-1 levels in the hippocampus, cerebral cortex, and striatum were similar between the hypoxia (n=13) and control groups (n=6); C) Glutathione reductase-1 levels were 42% higher in the cerebral cortex of animals exposed to intermittent hypoxia.
Discussion
Our results demonstrated decreased anxiety-like behavior, which is contrary to previous hypotheses. The plus-maze parameters that reached significance in the intermittent hypoxia group also indicate compatibility with increased impulsivity, risk-taking, or mania-like behavior. Regarding the enzymes analyzed, unlike previous assumptions, no significant results were observed for GR1 or Glo1 expression in the analyzed areas. The only borderline difference was in GR1 expression level in the cerebral cortex, which was also unexpected. These results suggest the existence of additional factors in the relationship between enzyme expression and anxiety, and the results should be interpreted in light of the peculiarities of different intermittent hypoxia models.
The elevated plus maze is based on an approach-avoidance paradigm built on the opposition of two natural rodent behaviors: the propensity to explore the environment (mainly the open arms, denoting anxiolysis) and the propensity to remain in a safe environment (the closed arms, denoting anxiogenesis). Sleep restriction leads to increased anxiety as part of a complex phenotype, including inattention, impulsivity, and mania-like behavior. In this method, anxiety and impulsivity/mania are opposite and mutually exclusive, since the former is associated with increased time in the closed arms, while the latter corresponds to increased time in the open arms. Thus, in our experiment, reduced anxiety could represent a mania-like state induced by intermittent hypoxia, as a primary or secondary consequence of brain damage.
Similar experiments evaluating the effects of intermittent hypoxia on anxiety-like behavior in an elevated plus maze have already been performed. Zhu et al.31 submitted adult rats to an intermittent hypoxia model, rather than sleep apnea, using hypobaric exposure for 4 h per day, with an oxygen pressure equivalent to an inspiratory fraction of 12% oxygen. After 14 days, an antidepressant-like effect and reduced anxiety were observed after a chronic mild stress paradigm. Furthermore, Leconte et al.32 showed that repeated hypoxic episodes to an 8% FIO2 nadir, applied for 1 h and repeated three times per week for 6 weeks, decreased anxiety-like behavior in the plus-maze and light/dark transition tests. A similar nadir of inspired O2 was employed in our study in an intermittent hypoxia system, and the frequency of open arm entries in the elevated plus-maze test was similar in our results. Finally, Perry et al.33 demonstrated that intermittent hypoxia to a 10% FIO2 nadir for 3 days does not change anxiety behavior in the elevated plus-maze test in comparison with controls or with animals exposed to paradoxical sleep deprivation. It should be pointed out that all these experiments have slightly different hypoxia protocols. Thus, ours is the only study design utilizing a 7-day intermittent hypoxia protocol that mimics an apnea-hypopnea index of 60/h with a nadir of inspired O2 of 7%. Despite the dissimilar intermittent hypoxia protocols used in these studies, the observed anxiolysis agrees with our results. These data attest that, contrary to our hypothesis, intermittent hypoxia might have no effect on or lead to decreased anxiety in rodents.
The absence of an increase in anxiety levels might be explained by confounders such as sleep restriction or the effect of sleepiness on anxiety. It should be pointed out that the intermittent hypoxia protocol in rodents leads to sleep restriction, which is less distressing than total sleep deprivation. A recent meta-analysis of clinical studies demonstrated that sleep deprivation has significant anxiogenic potential in humans, although no such effect was observed with sleep restriction.34 In addition, the increase in anxiety level is proportional to the duration of sleep loss. Considering that we employed a period of intermittent hypoxia shorter than that of similar studies, this might have affected the results. Furthermore, rodent studies evaluating the relationship between sleep and anxiety have failed to reproduce the sleep deprivation-induced anxiety observed in humans.35-37 While sleep deprivation increases anxiety in humans,34 a meta-analysis of 49 rodent studies37 found reduced anxiety-like behavior in both mice and rats, with no species-specific effects. The discrepancy between our results and those of the meta-analysis of preclinical studies might be due to use of the elevated plus-maze, which despite being the gold-standard technique for anxiety-like behavior in rodents, has led to most of the conflicting data in this field of study. These comparisons indicate that there might be additional factors modulating the relationship between sleep deprivation and hypoxia.
Furthermore, the possible reduction in anxiety might be directly related to intermittent hypoxia, which acts over and above simple sleep restriction. It is well-documented that intermittent hypoxia causes oxidative damage to neurons.13 Higher locomotion to stimulate anxiolysis might occur as a direct result of brain damage, rather than as an effect of sleep restriction. Intermittent hypoxia might lead to impulsivity or mania-like events, just as classically observed in REM sleep deprivation.38,39 In this case, the increased locomotion and apparent anxiolysis might be a direct effect of sleep deprivation or a brain damage-induced transient maniac state.
Sleepiness may be acting as a confounder in this context, partially explaining the observed anxiolysis. In sleepy women scoring over 10 on the Epworth sleepiness scale, the frequency of depression is higher than in non-sleepy women, although the frequency of anxiety is similar.40 Rodents exposed to intermittent hypoxia increase sleep time by more than 3 h per day, indicating sleepiness. Even 2 weeks after the discontinuation of intermittent hypoxia exposure, the total sleep time remains higher than normal.14 This finding suggests that sleepiness attenuates anxiety-like behavior.
Regarding oxidative stress effects, in a review, Bouayed et al.25 observed a link between oxidative stress and high-anxiety behaviors, but no causal relationship. Oxidative stress produced by the intermittent hypoxia model may increase the expression of antioxidant enzymes.17,21,41 A study by Ntalapascha et al.42 found increased oxidative burden, possibly via the protein oxidation pathway, in OSA patients without comorbidities. The antioxidant enzymes Glo1 and GR1 are significantly lower in the hippocampus of rats submitted to social stress.43 These enzymes have been described as overexpressed in the cerebral cortex of animals with high anxiety-related behavior.17 The expression of these enzymes is increased after 24-h sleep deprivation.44 This effect is corroborated by our findings and may be explained by an initial antioxidant response elicited to face the immediate rise in oxidative stress. Differently from injected substances that remain circulating for prolonged periods, intermittent hypoxia is promptly reversible as soon as the procedure is interrupted. Instead of consuming antioxidant enzymes, intermittent hypoxia may lead to their overexpression and higher tissue levels immediately after ceasing the experiment. In the present study, we observed a 43% higher level of GR1 in the cerebral cortex, indicating that this compensatory mechanism may be causing the apparent inverse relationship between the enzyme and anxiety.
The limited number of anxiety-related oxidative stress markers assessed and the problems entailed in anxiety-like behavior assessment in rodents are the present study’s main limitations. The small sample size and time of outcome assessment might also have impacted the results, although previous data have suggested that there is no circadian effect on the elevated plus maze.45 The use of intermittent hypoxia mimics only part of the features of OSA. The pattern of hypercapnia induced by CO2 insufflation in the cage is different from that observed in humans, and the hypoxic events remain present even during waking periods. Moreover, the duration of the hypoxic period might have been short, especially considering that OSA is chronic disease, usually diagnosed years after the onset of symptoms. However, articles about the short-term effects of Continuous Positive Airway Pressure withdrawal provide interesting insights about the effects of being under intermittent hypoxic conditions for short periods, impacting both behavioral performance and oxidative stress-related enzymatic expression.46,47
Further investigation with longer intermittent hypoxia exposure and a greater range of molecular and behavioral evaluations is necessary to ensure the validity of the present results. In conclusion, mice submitted to intermittent hypoxia show no increase in anxiety behavior. The mechanism for this change may involve the anxiety-modulating effects of antioxidants like GR1.
Disclosure
DM is co-owner of a sleep medicine clinic. GNP is currently an employee of Springer Nature, although this position has no relation with the present article or any of this author’s academic activities. The other authors report no conflicts of interest.
Acknowledgements
This study was supported by Fundo de Incentivo à Pesquisa e Eventos (FIPE)/HCPA. The sponsor had no role in the design or conduct of this study. AC received a grant from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), LJK received a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2015/19136-5), and CZF and DM received grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- 1.Rajaratnam SM, Barger LK, Lockley SW, Shea SA, Wang W, Landrigan CP, et al. Sleep disorders, health, and safety in police officers. JAMA. 2011;306:2567–78. doi: 10.1001/jama.2011.1851. [DOI] [PubMed] [Google Scholar]
- 2.Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 3.Bardwell WA, Berry CC, Ancoli-Israel S, Dimsdale JE. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47:583–96. doi: 10.1016/s0022-3999(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 4.Bardwell WA, Norman D, Ancoli-Israel S, Loredo JS, Lowery A, Lim W, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine . International classification of sleep disorders, 3rd ed. Darien: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 6.Saunamäki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2007;116:277–88. doi: 10.1111/j.1600-0404.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–5. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- 9.Maes M, Meltzer HY, Suy E, Minner B, Calabrese J, Cosyns P. Sleep disorders and anxiety as symptom profiles of sympathoadrenal system hyperactivity in major depression. J Affect Disord. 1993;27:197–207. doi: 10.1016/0165-0327(93)90007-7. [DOI] [PubMed] [Google Scholar]
- 10.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–53. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–16. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 13.Baronio D, Martinez D, Fiori CZ, Bambini-Junior V, Forgiarini LF, Pase da Rosa D, et al. Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respir Physiol Neurobiol. 2013;185:217–21. doi: 10.1016/j.resp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 15.Lim DC, Veasey SC. Neural injury in sleep apnea. Curr Neurol Neurosci Rep. 2010;10:47–52. doi: 10.1007/s11910-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 16.Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010;1359:178–85. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–6. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 18.Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:27–42. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Distler MG, Palmer AA. Role of glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front Genet. 2012;3:250. doi: 10.3389/fgene.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ditzen C, Jastorff AM, Kessler MS, Bunck M, Teplytska L, Erhardt A, et al. Protein biomarkers in a mouse model of extremes in trait anxiety. Mol Cell Proteomics. 2006;5:1914–20. doi: 10.1074/mcp.M600088-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Thornalley PJ. Unease on the role of glyoxalase 1 in high-anxiety-related behaviour. Trends Mol Med. 2006;12:195–9. doi: 10.1016/j.molmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Krömer SA, Kessler MS, Milfay D, Birg IN, Bunck M, Czibere L, et al. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J. Neurosci. 2005;25:4375–84. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, et al. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res. 2011;1404:63–71. doi: 10.1016/j.brainres.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–52. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 25.Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2:63–7. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Research Council . Guide for the care and use of laboratory animals. Washington: National Academy; 2011. [Google Scholar]
- 27.Martinez D, Fiori CZ, Baronio D, Carissimi A, Kaminski RS, Kim LJ, et al. Brown adipose tissue: is it affected by intermittent hypoxia? Lipids Health Dis. 2010;9:121. doi: 10.1186/1476-511X-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiori CZ, Martinez D, Baronio D, da Rosa DP, Kretzmann NA, Forgiarini LF, et al. Downregulation of uncoupling protein-1 mRNA expression and hypoadiponectinemia in a mouse model of sleep apnea. Sleep Breath. 2014;18:541–8. doi: 10.1007/s11325-013-0916-2. [DOI] [PubMed] [Google Scholar]
- 29.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;(22):1088. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos AC, Fogaça MV, Aguiar DC, Guimarães FS. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr. 2013;35:S101–11. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 31.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–63. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leconte C, Léger M, Boulouard M, Tixier E, Fréret T, Bernaudin M, et al. Repeated mild hypoxic exposures decrease anxiety-like behavior in the adult mouse together with an increased brain adrenomedullin gene expression. Behav Brain Res. 2012;230:78–84. doi: 10.1016/j.bbr.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 33.Perry JC, D'Almeida V, Antunes IB, Tufik S. Distinct behavioral and neurochemical alterations induced by intermittent hypoxia or paradoxical sleep deprivation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:87–94. doi: 10.1016/j.pnpbp.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Pires GN, Bezerra AG, Tufik S, Andersen ML. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med. 2016;24:109–18. doi: 10.1016/j.sleep.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184:1305–12. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise training on anxiety, spatial memory, and associated neurobiological measures in mice. Behav Brain Res. 2013;250:74–80. doi: 10.1016/j.bbr.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pires GN, Bezerra AG, Tufik S, Andersen ML. Effects of experimental sleep deprivation on anxiety-like behavior in animal research: systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;68:575–89. doi: 10.1016/j.neubiorev.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ, et al. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase α3 sodium pump. Proc Natl Acad Sci U S A. 2011;108:18144–9. doi: 10.1073/pnas.1108416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. B. J Pharmacol. 2011;164:1263–84. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayley AC, Williams LJ, Berk M, Kennedy GA, Jacka FN, Pasco JA. The relationship between excessive daytime sleepiness and depressive and anxiety disorders in women. Aust N Z J Psychiatry. 2013;47:772–8. doi: 10.1177/0004867413490036. [DOI] [PubMed] [Google Scholar]
- 41.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68:261–75. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Ntalapascha M, Makris D, Kyparos A, Tsilioni I, Kostikas K, Gourgoulianis K, et al. Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath. 2013;17:549–55. doi: 10.1007/s11325-012-0718-y. [DOI] [PubMed] [Google Scholar]
- 43.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–40. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Beeler JA, Prendergast B, Zhuang X. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol Behav. 2006;87:870–80. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, Phillips CL, Melehan KL, Rogers NL, Seale JP, Grunstein RR. Effects of short-term CPAP withdrawal on neurobehavioral performance in patients with obstructive sleep apnea. Sleep. 2006;29:545–52. doi: 10.1093/sleep/29.4.545. [DOI] [PubMed] [Google Scholar]
- 47.Antoniades C, Lee R, Kohler M, Stradling J. Paradoxical decrease in isoprostane and increase in superoxide dismutase following CPAP withdrawal in OSA. Eur Respir J. 2016;47:1014–5. doi: 10.1183/13993003.01725-2015. [DOI] [PubMed] [Google Scholar]