Abstract
Objective:
The findings of telomere length (TL) studies in bipolar disorder (BD) are controversial. The aim of the present study was to detect TL, human telomerase reverse transcriptase (hTERT), and brain derived neurotrophic factor (BDNF) in severe mania and subsequent remission.
Methods:
Twenty-one medication-free male patients and 20 age and gender matched controls were recruited. The patients were followed in the inpatient clinic, and comparisons were made between the same patients in their remission state and controls. Patients received lithium plus antipsychotics during the follow-up period. Quantitative real-time polymerase chain reaction was performed to verify leukocyte TL and whole blood hTERT gene expression levels. Serum BDNF levels were verified by enzyme-linked immunosorbent assay (ELISA).
Results:
Compared to controls, manic patients presented shorter telomeres (p < 0.001) whose length increased with treatment (p = 0.001). Patients in the late stages showed shorter TL than those in the early stages and controls (p < 0.001). hTERT gene expression levels were up-regulated in mania and remission compared to controls (p = 0.03 and p = 0.01, respectively). BDNF changes did not reach statistically significant levels.
Conclusions:
TL and hTERT gene expression might reflect a novel aspect of BD pathophysiology and TL might represent a novel biomarker for BD staging.
Keywords: Bipolar disorder, telomere, TERT protein
Introduction
Bipolar disorder (BD) is characterized by alternating manic and depressive episodes.1 Neuroprogression in BD results in worsening cognitive performance and increased risk of suicide.2 Cumulative damage to the brain and body caused by stress and/or inefficient stress management in an effort to maintain homeostasis is called the “allostatic load,”3 and is hypothesized to influence neuroprogression in BD.4 In patients with BD, recurrent mood episodes may be responsible for allostatic load, which, in turn, may result in accelerated aging.5 BD is associated with reduced life expectancy, premature mortality, and high prevalence of comorbid age-related disorders, such as cardiovascular conditions, metabolic imbalance, and immunosenescence.6 In light of these findings, clarifying the dynamics of stress response is important for improving both clinical and psychiatric prognosis.
Telomere length (TL) is an important biological marker of cellular aging.7 Telomeres are nucleoprotein structures present at the ends of eukaryotic chromosomes formed of long nucleotide repeats (TTAGGG) that protect chromosome ends from depredation and fusion. They shorten with each cell division and prevent replication of damaged or genomically unstable cells. Accelerated telomere shortening can be caused by exposure to stress and has been observed in several chronic and age-related disorders, including psychiatric disorders.8,9 Telomerase, an enzyme that extends telomeric nucleotide repeats, consists of two core components: telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC). Telomerase, with its catalytic subunit TERT, counteracts telomere shortening.7 Human TERT (hTERT), a catalytic subunit bearing the enzymatic activity of telomerase, is the rate-limiting determinant of human telomerase activity, whereas the other subunits are constitutively expressed.10
hTERT and brain derived neurotrophic factor (BDNF) are both neurotrophic factors that have roles in neuronal survival, inhibition of apoptosis, and reduction of excitotoxicity.11 hTERT has been demonstrated to successfully immortalize various types of cells.12 TERT mediates the neuroprotective effects of BDNF via inhibiting apoptotic pathways.13 Lithium promotes the expression of BDNF, which, in turn enhances TERT expression.14 Even TERT- and BDNF-modified umbilical cord blood mesenchymal stem cells may promote the recovery of neurological function following hypoxic-ischemic brain damage.11
Shorter leukocyte TL(LTL) has been associated with major depressive disorder, bipolar disorder, schizophrenia, and anxiety disorders (especially post-traumatic stress disorder).9 In a meta-analysis of the association between psychiatric disorders and TL, a robust effect size was observed, with a smaller effect size for BD than depressive and anxiety disorders.15 A recent meta-analysis about TL in BD found no differences between patients and controls, and concluded that studies should control for potential confounders such as clinical characteristics, the assays used to measure TL, and age-gender matching of BD patients with healthy control samples.16
The purpose of this study was to compare LTL in unmedicated patients with mania and matched healthy controls, and to determine whether the stage of the disorder has any effect on LTL. To assess whether TL change is due to increased shortening or decreased length replenishment by telomerase, we determined hTERT gene expression levels. Serum BDNF was also assessed due to known interaction with TERT. LTL, hTERT, and BDNF were also evaluated during the same patients’ subsequent remission state.
Methods
Sample
The sample comprised 21 medication-free, manic male patients and 20 age and gender matched healthy control subjects who resided in the same region as the patients. Males were selected for the study group to eliminate menstrual cycle effects on the studied parameters. Clinical diagnosis of BD type I was ascertained using the Structured Clinical Interview for DSM-IV (SCID-I). Inclusion criteria for the manic patients were a Young Mania Rating Scale (YMRS) total score ≥ 25 (signifying that a patient is markedly ill)17 and lithium response documentation. Remission was defined as a YMRS total score of < 4.18 Early stage BD was defined as having less than 5 previous episodes, and late stage BD was defined as having more than 10 episodes.19 The control subjects were volunteers with no history of psychiatric disorder or medical illness and who had no first-degree relatives with BD, schizophrenia, or other psychotic disorders.
Patients who had used oral psychotrophic drugs within the last 2 weeks or parenteral psychotrophic drugs within the last month were excluded. Patients who used mood stabilizers were not accepted for the study unless their blood levels were 0. Patients with comorbid axis I disorders (except from nicotine dependence), mental retardation, neurological and/or medical illnesses including metabolic syndrome were excluded. An attempt was made to minimize the factors affecting TL by excluding females, people below age 20 or above 40, and patients with body mass index (BMI) scores below 18.5 kg/m2 or above 24.9 kg/m2.
All of the patients and controls stated that they were not on a diet, had not recently engaged in heavy exercise, and were not on any kind of medication, including vitamins, dietary supplements, and/or energy drinks. Selected patients also stated that they had not been exposed to viral or bacterial pathogens or radiation or had used alcohol or illegal substances in the past week. Patients or controls with abnormal blood analyses (complete blood count, alkaline phosphatase, aspartate aminotransferase, gama glutamil transpeptidase, blood urea nitrogen, creatinine, uric acid, total protein, albumin, sodium, potassium, chloride, c-reactive protein, sedimentation rate, or thyroid function tests) were also excluded from the study.
The study was approved by the local ethics committee (TÜTF-BAEK 2014/198) and was supported by the Trakya University Scientific Research Project Committee (TÜBAP 2015/08). The study conforms to the provisions of the Declaration of Helsinki; only subjects who gave informed consent participated in the study.
Measures
All 41 participants completed the study. Blood samples were taken from the patients and controls by trained nurses between 08:00 and 10:00 a.m. The patients’ first blood samples were taken the day after they were admitted to the inpatient clinic. Only lorazepam was allowed before blood collection. The second blood samples were taken when the patients had fulfilled the remission criteria. The patients were followed in the inpatient clinic and comparison between the same patients in their remission state and controls were made. The patients received lithium and antipsychotics during the follow-up period.
The measurements were done at the laboratories of the Technology Research and Development Center of Trakya University (TÜTAGEM). For TL measurement and hTERT gene expression analysis, peripheral blood samples were collected in tubes with ethylenediaminetetraacetic acid.
For TL measurements, DNA was extracted from frozen whole blood using standard methods. TL was measured using quantitative real-time polymerase chain reaction (qRT-PCR) via a previously used and described method.20 The beta-hemoglobin gene (36B4) served as a reference single-copy gene. TL was expressed as a ratio of telomere copy number over single gene copy number (T/S). SYBR Select Master Mix (Life Technologies, Carlsbad, CA, USA) on an ABI Step One Plus Real-Time PCR system was used for TL and hTERT gene expression measurements. Cycle threshold 21 calculations were made. The relative amounts of TL and hTERT were calculated according to the 2−ΔΔCt method.21 Each reaction was performed in triplicate for each sample.
For hTERT gene expression measurements, total RNA was isolated using a PureLink RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer instructions. The extracted RNA concentrations were measured with a Qubit Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The first strand of cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) in a Veriti™ 96-well thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA). TERT gene expressions were determined as fold changes compared to controls.
For determination of BDNF serum level, peripheral blood samples were collected in tubes with heparin. BDNF serum levels were assessed using enzyme-linked immunosorbent assay kits (Boster Biological Technologies, Fremont, CA, USA) according to manufacturer instructions.
Statistical analysis
All statistical analyses were conducted with SPSS version 20.0 for Windows with α = 0.05. The statistics were done on the level of ΔCt (cycle treshold) values for the analysis of TL and hTERT gene expression measurements. The Kolmogorov-Smirnov test was used to check for normal sample distribution. The demographic variable with normal distribution (age) was tested using an independent samples t-test. Demographic variables without normal distribution (BMI, smoking, years of education, paid employment) were tested using the Mann-Whitney test. Comparisons of studied parameters between groups were made using an independent samples t-test. The manic and remission samples were compared to controls using a paired samples t-test. One-way analysis of variance (ANOVA) was used to compare early-BD, late-BD, and controls. Correlations between variables were assessed using Pearson’s or Spearman’s correlation coefficients.
Results
Demographics
Both patient (n=21) and control (n=20) groups were composed of male subjects. No subject from either group had comorbid diseases. The mean age between groups was similar (t = -0.66, p = 0.50). Both the patients and the controls had BMI within the normal range (z = -0.69, p = 0.48). The smoking rate between groups was similar (z = -0.76, p = 0.44). Years of education between groups were similar (z = -1.72, p = 0.08). Occupational status between the groups was similar (z = -1.38, p = 0.16) (Table 1). All patients were treated with lithium plus one antipsychotic drug and none of the patients received electroconvulsive therapy. All patients fullfilled the remission criteria. Demographic characteristics, including BD duration and number of manic and depressive episodes, are shown in Table 1.
Table 1. Demographic characteristics of the patients and controls.
| Patient group (n=21) | Control group (n=20) | p-value | |
|---|---|---|---|
| Age in years | 30.64 (8.07) | 32.30 (7.54) | 0.50* |
| Body mass index | 24.34 (3.98) | 24.85 (2.11) | 0.48 † |
| Cigarette smoking, % | 71 | 60 | 0.44 † |
| Years of education | 8.47 (3.77) | 10.50 (3.59) | 0.08 † |
| Current paid employment, % | 38 | 60 | 0.16 † |
| BD duration (years) | 8.10 (1.86) | - | |
| Number of manic episodes | 4.57 (3.31) | - | |
| Number of depressive episodes | 3.57 (3.26) | - | |
| Subjects with previous suicide attempts, n (%) | 8.00 (38.09) | - | |
| Time until remission (weeks) | 8.04 (1.93) | - | |
| Baseline YMRS | 27.57 (8.62) | - | |
| Endpoint YMRS | 2.10 (1.22) | - | |
| Antipsychotics used, n (%) | |||
| Olanzapine | 2.00 (9.52) | - | |
| Quetiapine | 9.00 (42.85) | - | |
| Risperidone | 7.00 (33.33) | - | |
| Haloperidol | 3.00 (14.28) | - |
Data presented as mean (standard deviation), unless otherwise specified.
BD = bipolar disorder; YMRS = Young Mania Rating Scale.
Independent samples t test;
Mann-Whitney test.
Baseline (manic sample) comparison with controls
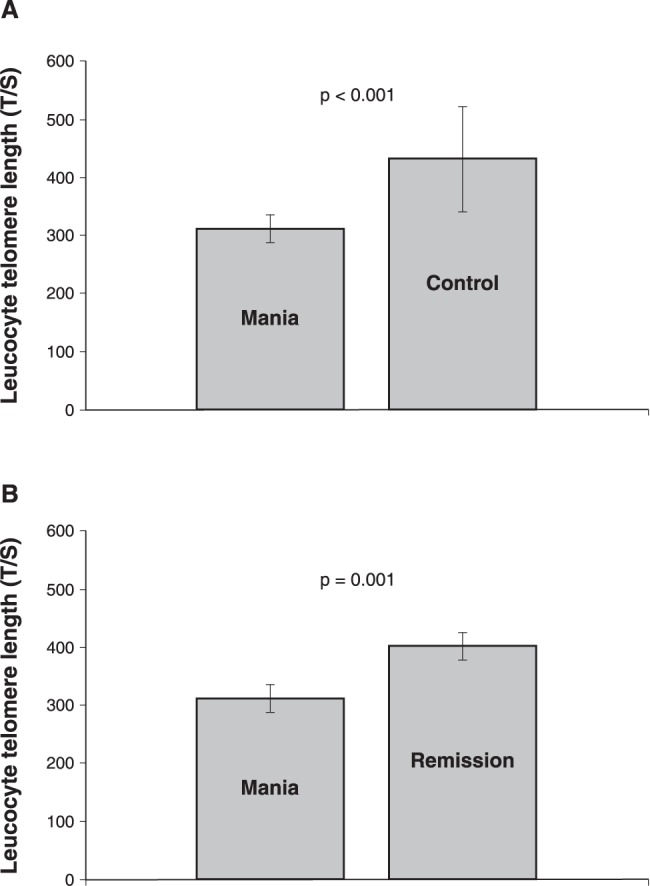
Baseline LTL was significantly shorter in the manic sample (mean ± standard error of mean [SEM] = 311.27±22.94) than in controls (430.43±91.01) (independent samples t-test: t = -3.89, p < 0.001, d = 1.79) (Figure 1A). Baseline LTL correlation with baseline YMRS scores was not significant (r = 0.33, p = 0.16), but when the staging effect was removed the negative correlation became significant (r = -0.63, p = 0.02). A significant negative relationship existed between smoking and LTL (rho = -0.39, p = 0.01). The number of depressive and manic episodes and the number of suicide attempts showed no significant relationship with LTL or other studied parameters. Age, BMI, years of education, and occupational status also showed no significant relationship with LTL or other studied parameters.
Figure 1. A) Leucocyte telomere length (T/S) comparison between mania and controls; B) leucocyte telomere length (T/S) comparison between mania and remission.
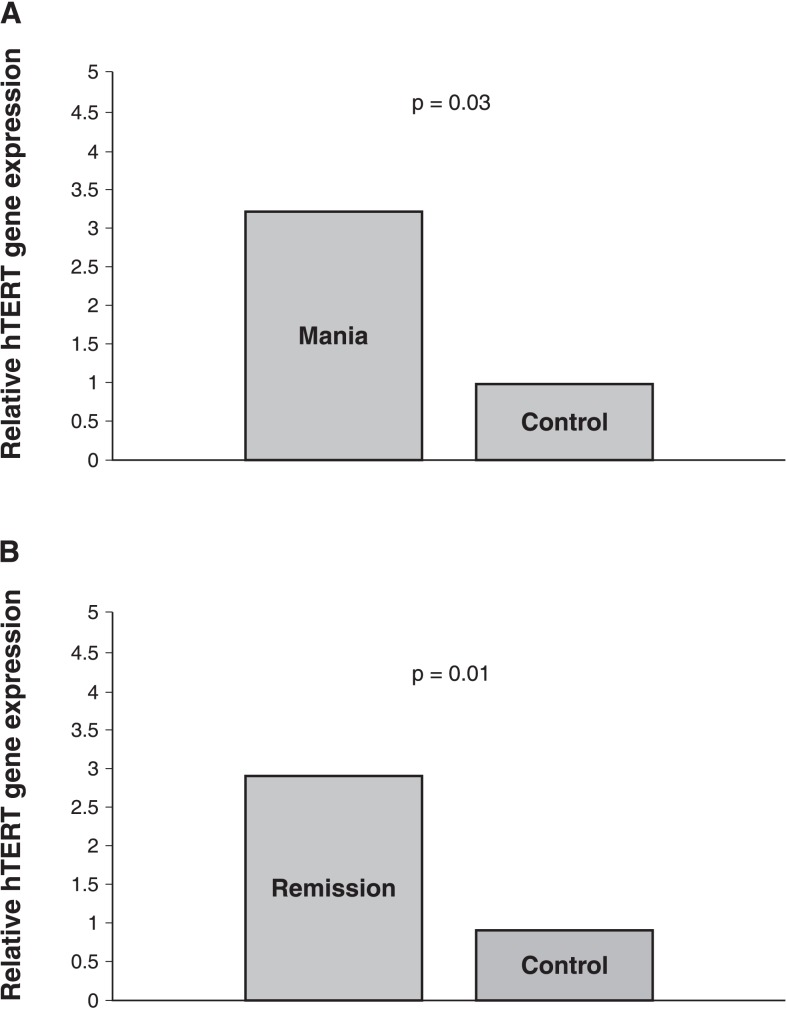
Baseline hTERT gene expression in the manic sample was 2.2 fold (120%) higher than the controls, calculated according to the 2−ΔΔCt method. This difference was significant (mean ± SEM manic sample hTERT = 10.16±0.43; control sample hTERT = 11.36±0.34; independent samples t-test: t = -2.19, p = 0.03, d = 3.09) (Figure 2).
Figure 2. A) hTERT gene expression as fold changes relative to controls in mania; B) hTERT gene expression as fold changes relative to controls in remission.
Baseline serum BDNF level (ng/ml) in the manic sample was lower (mean ± SEM = 18.25±3.17) than the controls (27.23±4.96), but this difference was not statistically significant (independent samples t-test: t = -1.52, p = 0.13). There were no significant correlations between baseline manic hTERT or BDNF and the other variables. Baseline LTL, hTERT, and BDNF levels were not significantly correlated with each other.
Treatment-associated changes
LTL was significantly longer in the remission state (mean ± SEM = 400.68±24.01) than in the baseline manic state of the same patients (311.27±22.94) (paired samples t-test: t = -3.94, p = 0.001) (Figure 1B). YMRS ratings significantly declined over the course of treatment, demonstrating an improvement (mean ± standard deviation YMRS scores at baseline = 27.57±8.62; YMRS scores at week 8 = 2.10±1.22; paired samples t-test: t = 15.20, p < 0.001). The correlation between remission state LTL and YMRS scores at week 8 was not significant (r = 0.16, p = 0.46). However, a positive relationship existed between antipsychotic use and remission state LTL (r = 0.52, p = 0.01).
hTERT gene expression in the remitted patients was 3.2 fold (220%) higher than in controls, which was statistically significant (mean ± SEM remission sample hTERT = 9.66±0.55; control sample hTERT = 11.36±0.34; independent samples t-test: t = -2.60, p = 0.01) (Figure 2).
Although serum BDNF level (ng/mL) in the remission sample was higher (mean ± SEM 22.41±4.03) than the baseline (18.25±3.17), the difference was not statistically significant (paired samples t-test: t = -0.08, p = 0.40) (Figure 2B). There were no significant correlations between remission state hTERT or BDNF and the other variables. Post-treatment LTL, hTERT, and BDNF levels were not significantly correlated with each other.
Early vs. late stage comparison with controls
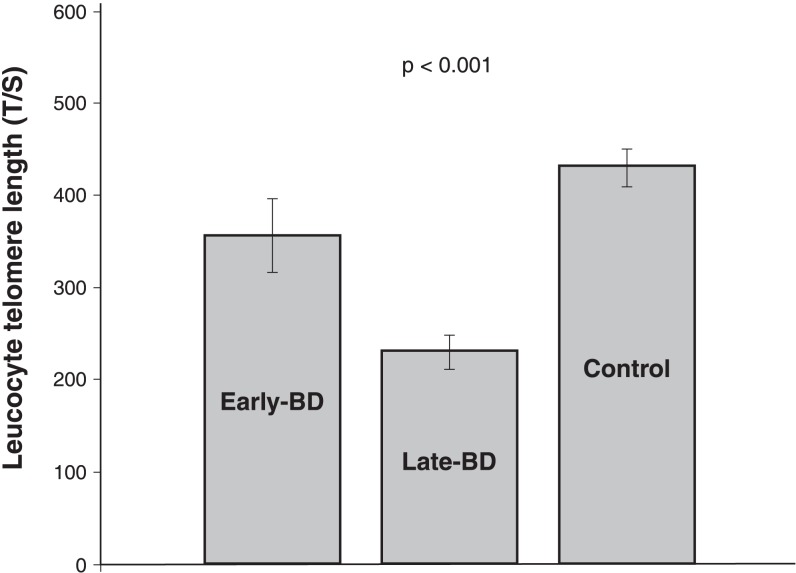
Baseline measurements from early stage (n=9) and late stage (n=12) patients and controls were compared. The LTL results were significant: late stage patients had the shortest LTL (mean ± SEM = 270.33±17.82) in comparison with early stage patients (356.76±40.14) and controls (430.43±20.35) (one way ANOVA F = 10.35, p < 0.001, ηp2 = 0.18) (Figure 3). hTERT gene expression and serum BDNF level were lower in late stage patients than in early stage patients and controls, although not statistically significant (one way ANOVA F = 3.03, p = 0.06, and F = 1.25, p = 0.29, respectively). The only significant correlation between the variables was a positive correlation between years with the disorder and baseline YMRS scores (r = 0.76, p < 0.001).
Figure 3. Leucocyte telomere length (T/S) comparison between early-stage patients, late-stage patients and controls.
Discussion
We found that LTL was significantly lower in unmedicated manic patients than in age- and gender-matched healthy controls. Late-stage patients had the shortest LTL compared to early-stage patients and controls. LTL significantly increased with treatment (lithium + antipsychotics) but did not reach healthy control levels. hTERT gene expression levels were significantly higher in manic subjects than in controls and were even higher in the remission state. These results were interpreted as increased hTERT levels in mania and remission, possibly to counteract LTL decreases. LTL and hTERT gene expression might reflect a novel aspect of BD pathophysiology, and LTL might represent a novel biomarker for BD staging.
Several studies have found evidence for accelerated cell aging in bipolar patients, as demonstrated by the shortened telomeres.22-26 These studies differed in terms of methodology and patient selection. TL was measured in DNA isolated from either leucocytes or peripheral blood mononuclear cells (PBMCs). Euthymic or depressed patients with various different disease durations, episode numbers and treatment protocols constituted the majority of the participants. BD type was not specified in some of the studies, so the association between TL and BD I is still unclear. The first study reported lower LTL in patients with chronic mood disorder (major depressive disorder and bipolar disorder-state not specified) than in controls.22 Rizzo et al. found shorter PBMC TL in 22 euthymic female BD type I patients than in 17 age-matched controls.24 Lima et al.23 found shorter PBMC TL in (moderately depressed) BD patients than in age, gender and, educational-level matched controls.
The impact of the number of BD episodes on TL has also been studied. The hypothesis is that if recurrent mood episodes constitute key stressors in the allostatic load model, patients with more episodes should present shorter telomeres. We found no significant association between the number of episodes or suicide attempts and the studied parameters. Our data is supported by the findings of previous studies investigating the effects of the number of manic/hypomanic episodes or suicide attempts on TL.23,27 Elvsåshagen et al.26 found that lifetime number of depressive episodes positively correlated with the load of short telomeres (PBMC) in BD type II (euthymic and mildly/moderately depressed patients).
In a study by Martinsson et al.,28 patients (outpatients diagnosed with BD type I or II) who had experienced more than five episodes were grouped together and compared with those who had experienced fewer episodes. The findings showed that LTL was significantly shorter in patients who had experienced a larger number of depressive episodes, and that this effect was even more pronounced for males. The results of the present study support this finding, since early-stage patients had longer LTL than late-stage patients. Barbé-Tuana et al.25 compared TL in early- and late-stage euthymic BD patients with controls in a cross-sectional study and found that TL was shorter in both the early and late stages of the disorder. These results were similar to those of the present study, and suggest that telomere shortening occurs early in the course of BD and could precede and lead to all of oxidative stress changes and inflammatory responses in BD.
Martinsson et al.28 also found that LTL was higher in lithium-treated BD patients than in healthy controls. In this study, almost all of the patients were treated with lithium, an important factor not controlled for in previous studies. LTL was positively correlated with duration of lithium treatment. After correcting for age, number of depressive episodes, and duration of lithium treatment, LTL was 10% greater in lithium responders than in non-responders. Lima et al.23 found no difference in LTL between patients treated with lithium versus patients treated with valproic acid or other medications. The present study found that post-treatment LTL and hTERT levels were greater than pre-treatment levels. All of the patients were taking lithium + antipsychotics, so it was not possible to rule out the effects of antipsychotics. Use of psychotropic drugs had been shown to have antioxidative effects and, thus, a protective effect on telomeres.29
Mechanistic support for an association between lithium treatment and TL has recently been provided. A correlation may exist between lithium, β-catenin, and telomerase activity. β-Catenin is indirectly regulated by lithium, with the main mediator between lithium and β-catenin being glycogen synthase kinase-3β.30 Lithium has also been reported to promote expression of BDNF, which, in turn, enhances TERT expression.14,31 In general, hTERT is considered to be regulated primarily on the transcriptional level. Specifically, transcription of hTERT has been found to be activated by β-catenin, resulting in increased telomerase activity and longer TL in human cancer cell lines and human embryonic kidney cells.31
In studies measuring telomerase in stressed individuals, post-treatment (approximately 3 months) telomerase activity was found to be greater than pre-treatment levels. Epel32 concluded that an increase in telomerase activity may be due to cessation of stress and/or change in circulating blood cells. Recently, Li et al.33 found a protein (telomeric zinc finger-associated protein [TZAP]) that triggers deletion of telomeric repeats and seems to be involved in TL regulation in mammalian cells. The binding of TZAP to long telomeres triggers telomere trimming, setting the upper limit for TL. In the present study, the TL difference found between BD and controls may be controlled by hTERT as well as other proteins in the telomere system, one of which is TZAP.
The present study found a significant negative relationship between smoking and LTL. Years of disorder and baseline YMRS scores also demonstrated a significant positive relationship. Factors such as BMI and smoking have an influence on TL and telomerase activity, and these factors could have hypothetically added to oxidative and inflammatory stress states, which may trigger cellular responses that ultimately lead to senescence and telomere shortening. It has thus been suggested that TL may be a response to cellular stress.34 Shorter LTL has been reported to be associated with smoking, obesity, inflammation, and several somatic diseases.35,36 The present study focused on male participants because TL is also effected by gender.37
A major strength of the present study was the selection of physically healthy, middle-aged men as participants. Although the patients were medication-free for the first measurements, it was not possible to control for the medication effect in the latter measurements. Previous treatments (before the medication-free period) were not analyzed, and these treatments could have affected LTL. It should also be noted that cognitive evaluations were not performed. The sample size was small for a study evaluating LTL using qPCR, and larger samples with prolonged follow-up periods must be considered in future studies. LTL and hTERT might even be biomarkers of treatment response. This is a question arising from our findings that could be resolved through proper study designs.
There does not appear to be any data about medication-free bipolar manic patients, their remission states, and TL. Studies postulating that decreased TL could be a marker of BD could not demonstrate whether or not this is truly the case based upon their data, since a wide variety of traumas and pathologies reduce TL. Considering that only a few studies have investigated TL in BD and even fewer have analyzed the clinical features related to it, it is important to target these aspects. Although the findings of the present study are novel and should be replicated, they still raise the possibility that LTL is involved with hTERT in BD. Whether a shorter LTL in mania reflects shorter brain tissue TL in bipolar individuals is unknown, but blood TL measurement is easy and may be useful as a state marker.
Disclosure
The author reports no conflicts of interest.
Acknowledgements
This study was supported by the Trakya University Scientific Research Project Committee (TÜBAP 2015/08).
References
- 1.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–86. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Fries GR, Pfaffenseller B, Stertz L, Paz AV, Dargel AA, Kunz M, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14:667–75. doi: 10.1007/s11920-012-0319-2. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 4.Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–92. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Kapczinski F, Magalhaes PV, Balanza-Martinez V, Dias VV, Frangou S, Gama CS, et al. Staging systems in bipolar disorder: an international society for bipolar disorders task force report. Acta Psychiatr Scand. 2014;130:354–63. doi: 10.1111/acps.12305. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo LB, Costa LG, Mansur RB, Swardfager W, Belangero SI, Grassi-Oliveira R, et al. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. Neurosci Biobehav Rev. 2014;42:157–69. doi: 10.1016/j.neubiorev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12:753–64. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, et al. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–64. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartwig FP, Nedel F, Collares TV, Tarquinio SB, Nor JE, Demarco FF. Telomeres and tissue engineering: the potential roles of TERT in VEGF-mediated angiogenesis. Stem Cell Rev. 2012;8:1275–81. doi: 10.1007/s12015-012-9414-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhao F, Qu Y, Liu H, Du B, Mu D. Umbilical cord blood mesenchymal stem cells co-modified by TERT and BDNF: a novel neuroprotective therapy for neonatal hypoxic-ischemic brain damage. Int J Dev Neurosci. 2014;38:147–54. doi: 10.1016/j.ijdevneu.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Terai M, Uyama T, Sugiki T, Li XK, Umezawa A, Kiyono T. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell. 2005;16:1491–9. doi: 10.1091/mbc.E04-07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu C, Yip HK. Neuroprotective signaling mechanisms of telomerase are regulated by brain-derived neurotrophic factor in rat spinal cord motor neurons. J Neuropathol Exp Neurol. 2011;70:634–52. doi: 10.1097/NEN.0b013e318222b97b. [DOI] [PubMed] [Google Scholar]
- 14.Fu W, Lu C, Mattson MP. Telomerase mediates the cell survival-promoting actions of brain-derived neurotrophic factor and secreted amyloid precursor protein in developing hippocampal neurons. J Neurosci. 2002;22:10710–9. doi: 10.1523/JNEUROSCI.22-24-10710.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darrow SM, Verhoeven JE, Revesz D, Lindqvist D, Penninx BW, Delucchi KL, et al. The association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom Med. 2016;78:776–87. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colpo GD, Leffa DD, Köhler CA, Kapczinski F, Quevedo J, Carvalho AF. Is bipolar disorder associated with accelerating aging? A meta-analysis of telomere length studies. J Affect Disord. 2015;186:241–8. doi: 10.1016/j.jad.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Lukasiewicz M, Gerard S, Besnard A, Falissard B, Perrin E, Sapin H, et al. Young mania rating scale: how to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22:46–58. doi: 10.1002/mpr.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berk M, Ng F, Wang WV, Calabrese JR, Mitchell PB, Malhi GS, et al. The empirical redefinition of the psychometric criteria for remission in bipolar disorder. J Affect Disord. 2008;106:153–8. doi: 10.1016/j.jad.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Magalhaes PV, Dodd S, Nierenberg AA, Berk M. Cumulative morbidity and prognostic staging of illness in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Aust N Z J Psychiatry. 2012;46:1058–67. doi: 10.1177/0004867412460593. [DOI] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Lima IM, Barros A, Rosa DV, Albuquerque M, Malloy-Diniz L, Neves FS, et al. Analysis of telomere attrition in bipolar disorder. J Affect Disord. 2015;172:43–7. doi: 10.1016/j.jad.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Rizzo LB, Do Prado CH, Grassi-Oliveira R, Wieck A, Correa BL, Teixeira AL, et al. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013;15:832–8. doi: 10.1111/bdi.12121. [DOI] [PubMed] [Google Scholar]
- 25.Barbe-Tuana FM, Parisi MM, Panizzutti BS, Fries GR, Grun LK, Guma FT, et al. Shortened telomere length in bipolar disorder: a comparison of the early and late stages of disease. Rev Bras Psiquiatr. 2016;38:281–6. doi: 10.1590/1516-4446-2016-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Squassina A, Pisanu C, Congiu D, Caria P, Frau D, Niola P, et al. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur Neuropsychopharmacol. 2016;26:1241–7. doi: 10.1016/j.euroneuro.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Martinsson L, Wei Y, Xu D, Melas PA, Mathe AA, Schalling M, et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry. 2013;3:e261. doi: 10.1038/tp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieratschker V, Lahtinen J, Meier S, Strohmaier J, Frank J, Heinrich A, et al. Longer telomere length in patients with schizophrenia. Schizophr Res. 2013;149:116–20. doi: 10.1016/j.schres.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Wei YB, Backlund L, Wegener G, Mathe AA, Lavebratt C. Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int J Neuropsychopharmacol. 2015;18:pyv002. doi: 10.1093/ijnp/pyv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Toh L, Lau P, Wang X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/beta-catenin pathway in human cancer. J Biol Chem. 2012;287:32494–511. doi: 10.1074/jbc.M112.368282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epel E. How “reversible” is telomeric aging? Cancer Prev Res (Phila). 2012;5:1163–8. doi: 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- 33.Li JS, Miralles Fuste J, Simavorian T, Bartocci C, Tsai J, Karlseder J, et al. TZAP: a telomere-associated protein involved in telomere length control. Science. 2017;355:638–41. doi: 10.1126/science.aah6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 35.Barrett JH, Iles MM, Dunning AM, Pooley KA. Telomere length and common disease: study design and analytical challenges. Hum Genet. 2015;134:679–89. doi: 10.1007/s00439-015-1563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kordinas V, Ioannidis A, Chatzipanagiotou S. The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes (Basel). 2016;7:pii: E60. doi: 10.3390/genes7090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]