Abstract
Objective:
To increase understanding of the influence of photoperiod variation in patients with bipolar disorders.
Methods:
We followed a sample of Italian bipolar patients over a period of 24 months, focusing on inpatients. All patients admitted to the Psychiatric Inpatient Unit of San Luigi Gonzaga Hospital in Orbassano (Turin, Italy) between September 1, 2013 and August 31, 2015 were recruited. Sociodemographic and clinical data were collected.
Results:
Seven hundred and thirty patients were included. The admission rate for bipolar patients was significantly higher during May, June and July, when there was maximum sunlight exposure, although no seasonal pattern was found. Patients with (hypo)manic episodes were admitted more frequently during the spring and during longer photoperiods than those with major depressive episodes.
Conclusions:
Photoperiod is a key element in bipolar disorder, not only as an environmental factor but also as an important clinical parameter that should be considered during treatment.
Keywords: Bipolar disorder, seasonality, photoperiod, sunlight
Introduction
It is well known that climatic variations affect human behavior, and over the last decade a number of researchers have thoroughly studied the influence of environmental factors on the onset and course of major psychiatric disorders, as well as on their treatment and prognosis.1
The main focus of these studies has been circadian rhythm, since a strong correlation has been observed between seasonal variation and impaired adaptation. Circadian rhythms are, above all, variations in physiological and behavioral processes, temperature, hormone secretion, food intake, sleep, and mood2,3 and are closely related to chronotype preference. Chronotype, or morningness-eveningness, is an individual’s preferred period of activity,4 reflecting a circadian or ultradian propensity for alertness or somnolence. Three different chronotypes have been identified: morning, evening and neither (indifferent).5 These rhythms are generated by endogenous processes and are regulated by external stimuli, such as daylight, in a 24-hour interval.
Numerous studies agree that bipolar patients display biological clock abnormalities, which lead to circadian rhythm alterations, impaired adaptation to environmental stimuli, and an unstable and hypersensitive mood.1,6,7 These features not only present serious clinical implications, such as an higher suicide rates and lower response to treatment, but also imply the presence of a seasonal pattern.8
Bipolar disorder has long been suspected to involve sensitivity to the effects of seasons and climate, especially luminosity. Seasonality and sunlight exposure have been demonstrated to play a role in the onset of affective recurrences in bipolar patients and could be considered core symptoms of bipolar disorder.9 Specifically, patients with (hypo)manic episodes have higher rates of hospitalization during spring and summer, when sunlight exposure (i.e., the photoperiod) is longer,10-14 while patients with major depressive episodes are mainly admitted during the winter.10,15-17
Photoperiod is defined as the number of hours of daylight, which can influence an individual’s physiology and metabolic cycles. Ideal photoperiods are approximately 14 hours in summer and 8 to 9 hours in winter. Studies have highlighted how sunlight exposure, which varies by season and latitude, has a positive correlation with peak admission rates for (hypo)manic and major depressive episodes. Amr & Volpe conducted a study comparing bipolar and schizophrenic patients in terms of monthly hospitalization rates: the authors reported that sunlight exposure had no influence on schizophrenic patients, whereas different admission patterns were observed for affective recurrences (manic or depressive episode) in bipolar patients.10
More recent studies, however, suggest that the seasonal variation in sunlight exposure is insufficient to explain circadian rhythm disruption and that other climatic variables, such as ultraviolet exposure levels, temperature, snow, rain, and light exposure during early life should be taken into consideration.18-21
Thus, the aim of our study was to increase understanding about the influence of photoperiod variation by following a sample of Italian bipolar patients over a period of 24 months, focusing on inpatients.
Methods
Sample
All patients consecutively admitted to the Psychiatric Inpatient Unit of San Luigi Gonzaga Hospital in Orbassano (University of Turin), Italy, over a 24-month period (September 1, 2013 until August 31, 2015) were included. All of these patients were from northwest Italy. To avoid the inclusion of patients who were readmitted during the same episode, hospitalizations occurring less than 8 weeks after a previous admission were not considered.
In Italy, the type of psychiatric admission is based on the subject’s clinical conditions and is regulated by a mental health law passed in 1980: involuntary treatment occurs when a patient refuses treatment and immediate non-hospital treatment cannot be considered under the circumstances. For this reason, the sample included patients both voluntarily and involuntarily admitted. During hospitalization, the status of an inpatient can shift from involuntary to voluntary, and after 7 days a reassessment is required to maintain involuntary hospitalization status.22
After the study’s aim and procedures had been thoroughly explained to them, recruited patients expressed their willingness to participate by signing a written consent form. The study design was reviewed by the local Ethics Committee.
Assessment
Psychiatric diagnoses were made in accordance with the DSM-523 at discharge. All diagnoses were made by clinicians with at least 5 years of postgraduate clinical experience and were carefully reviewed by a senior psychiatrist.
Sociodemographic characteristics were collected in a semi-structured interview and consisted of the patient’s gender, age, education level, and marital and employment status, while the clinical characteristics included age of onset, total length of voluntary and involuntary hospitalization, diagnoses, current suicide ideation and/or attempts, month and season of admission to the psychiatric ward (autumn was defined as September 21st to December 20th, winter as December 21st to March 20th, spring as March 21st to June 20th, and summer as June 21st to September 20th). The hospitalization periods were classified according to sunlight exposure: spring-summer (s-s) (highest solar intensity) and autumn-winter (a-w) (lowest solar intensity).
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL); statistical significance was set at p < 0.05.
The subjects’ sociodemographic and clinical characteristics were represented as mean and standard deviation (SD) for continuous variables and as frequency and percentage for categorical variables.
The total sample was divided in two groups, one of patients with bipolar disorder, the other of patients with any other diagnosis.
Since the Kolmogorov-Smirnov test, which determines whether parametric or non-parametric tests are required, indicated normal sample distribution, intergroup differences were analyzed using the Pearson chi-square test with Yates correction for categorical variables, while the t-test for independent samples was used for continuous variables. Comparative analyses of the number of admissions were adjusted for age and gender.
Results
During the 24-month study period, 730 patients were included, with a mean age of 43.4±13.9 years. Of this total, 311 subjects (42.6%) were female; slightly more than half of the patients (55.6%) were single, and 33.6% were employed.
Regarding clinical characteristics, the mean age of onset was 28.5±13.3 years, while the mean duration of hospitalization was 11.4±8.9 days. A total of 112 (15.3%) patients were involuntarily admitted.
The longitudinal diagnoses were distributed as follows: 251 (34.4%) patients had bipolar or related disorders, 192 (26.3%) had schizophrenia, 134 (18.3%) had depressive disorders and 153 (21.0%) had other diagnoses, such as personality or substance-related disorders.
Further details about the clinical and sociodemographic characteristics are summarized in Table 1.
Table 1. Sociodemographic and clinical characteristics of the total sample.
| Total sample (n=730) | |
|---|---|
| Gender (female) | 311 (42.6) |
| Age (years), mean ± SD | 43.42±13.91 |
| Education level | |
| Elementary school | 67 (9.2) |
| Middle school | 352 (48.2) |
| High school | 257 (35.2) |
| Higher education | 54 (7.4) |
| Marital status | |
| Single | 406 (55.6) |
| Married | 190 (26.0) |
| Divorced | 106 (14.5) |
| Widowed | 28 (3.9) |
| Employment status: currently employed | 245 (33.6) |
| Age at onset (years), mean ± SD | 28.47±13.30 |
| Suicide | |
| Ideation | 122 (16.7) |
| Attempt | 77 (10.5) |
| Admission | |
| Involuntary | 112 (15.3) |
| Voluntary | 618 (84.7) |
| Duration of hospitalization, mean ± SD | 11.42±8.91 |
| Diagnosis | |
| Bipolar and related disorders | 251 (34.4) |
| Schizophrenia and related disorders | 192 (26.3) |
| Depressive disorders | 134 (18.3) |
| Others | 153 (21.0) |
Data presented as n (%), unless otherwise specified.
SD = standard deviation.
As previously mentioned, the sample was divided into two subgroups: patients with bipolar disorder (n=251, 34.4%), and patients with other diagnoses (n=479, 65.6%). When these two groups were compared, as shown in Table 2, patients with bipolar disorder included more females (49.4 vs. 39.0%, p = 0.007) and had a significantly higher mean age (46.1±12.6 vs. 42.0±14.4 years, p < 0.001). Additionally, patients with bipolar disorder had a higher education level (41.8 vs. 31.7%, p < 0.001) and were more likely to be employed (39.0 vs. 30.7%, p = 0.023) and married (31.5 vs. 23.2%, p < 0.001).
Table 2. Sociodemographic and clinical characteristics in the two subgroups.
| Bipolar disorder (n=251) | Other diagnoses (n=479) | t/χ2 | df | p-value | |
|---|---|---|---|---|---|
| Gender (female) | 124 (49.4) | 187 (39.0) | 7.23 | 1 | 0.007 |
| Age (years), mean ± SD | 46.11±12.57 | 42.01±14.37 | -3.82 | 728 | < 0.001 |
| Education level | |||||
| Elementary | 14 (5.6) | 53 (11.1) | 25.46 | 3 | < 0.001 |
| Middle school | 102 (40.6) | 250 (52.2) | |||
| High school | 105 (41.8) | 152 (31.7) | |||
| Higher education | 30 (12.0) | 24 (5.0) | |||
| Marital status | |||||
| Single | 113 (45.0) | 293 (61.2) | 17.96 | 3 | < 0.001 |
| Married | 79 (31.5) | 111 (23.2) | |||
| Divorced | 48 (19.1) | 58 (12.1) | |||
| Widowed | 11 (4.4) | 17 (3.5) | |||
| Employment status: currently employed | 98 (39.0) | 147 (30.7) | 5.16 | 1 | 0.023 |
| Age at onset (years), mean ± SD | 28.76±11.89 | 28.32±13.99 | -0.42 | 728 | 0.676 |
| Suicide | |||||
| Ideation | 44 (17.5) | 78 (16.3) | 0.18 | 1 | 0.668 |
| Attempt | 31 (12.4) | 46 (9.6) | 1.32 | 1 | 0.251 |
| Admission | |||||
| Involuntary | 57 (22.7) | 55 (11.5) | 15.98 | 1 | < 0.001 |
| Voluntary | 194 (77.3) | 424 (88.5) | |||
| Duration of hospitalization (days), mean ± SD | 14.72±8.65 | 9.68±8.56 | -7.52 | 728 | < 0.001 |
Data presented as n (%), unless otherwise specified.
df = degrees of freedom; SD = standard deviation.
Regarding the clinical characteristics, the bipolar group was admitted involuntarily more frequently (22.7 vs. 11.5%, p < 0.001) and for significantly longer durations (14.7±8.6 vs. 9.7±8.6 days, p < 0.001). No significant diferences were found with respect to suicide ideation and/or attempts.
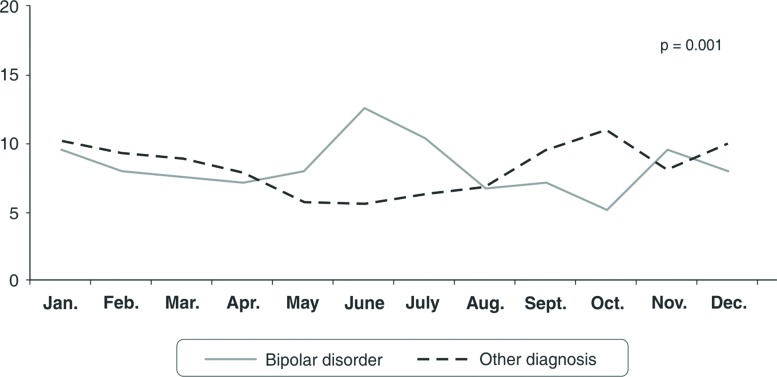
Figure 1 shows the monthly admission prevalence according to longitudinal diagnosis. The hospitalization of bipolar patients showed significant peaks during the months with more sunlight: the greatest differences with the mixed control group were in May (8.0 vs. 5.8%), June (12.7 vs. 5.6%), and July (10.4 vs. 6.3%).
Figure 1. Inpatient admission rates by month: comparison between bipolar and other mental disorders.
No significant differences were found for seasonality, despite the slightly higher admission prevalence in spring for patients with bipolar disorder, as shown in Table 3. However, patients with bipolar disorder reported a significantly higher prevalence of (hypo)manic episodes during spring-summer (i.e., the greatest period of solar intensity) than the mixed control group (51.4 vs. 42.4%, p = 0.020).
Table 3. Number of admissions divided by season and photoperiod.
| Bipolar disorder (n=251) | Other diagnoses (n=479) | t/χ2 | df | p-value | |
|---|---|---|---|---|---|
| Season | |||||
| Spring | 67 (26.7) | 99 (20.7) | 5.74 | 3 | 0.125 |
| Summer | 62 (24.7) | 104 (21.7) | |||
| Autumn | 61 (24.3) | 139 (29.0) | |||
| Winter | 61 (24.3) | 137 (28.6) | |||
| Photoperiod | |||||
| Spring-summer | 129 (51.4) | 203 (42.4) | 5.40 | 1 | 0.020 |
| Autumn-winter | 122 (48.6) | 276 (57.6) | |||
| Manic episode (n=140) | MDE (n=111) | ||||
| Season | |||||
| Spring | 46 (32.8) | 21 (18.9) | 7.73 | 1 | 0.049 |
| Summer | 36 (25.7) | 26 (23.4) | |||
| Autumn | 28 (20.0) | 33 (29.7) | |||
| Winter | 30 (21.4) | 31 (27.9) | |||
| Photoperiod | |||||
| Spring-summer | 82 (58.5) | 47 (42.3) | 6.27 | 1 | 0.012 |
| Autumn-winter | 58 (41.5) | 64 (57.7) |
Data presented as n (%).
df = degrees of freedom; MDE = major depressive episode.
When the analyses were restricted to the bipolar group, patients undergoing a (hypo)manic episode were more frequently hospitalized during spring (32.8 vs. 18.9%, p = 0.049) and during longer daylight periods (58.5 vs. 42.3%, p = 0.012) than those undergoing a major depressive episode.
Analyses adjusted for age and gender confirmed these findings.
Discussion
The primary aim of our study was to increase understanding about the influence of photoperiod variation by following a sample of Italian bipolar patients over a period of 24 months, focusing on inpatients.
The first difference that should be highlighted was gender: bipolar patients were more frequently female than patients in the mixed control group. This result agrees with a recent epidemiological study24 and is likely due to a strong link between the female reproductive hormonal axis and regulatory mechanisms of sunlight sensitivity.17
Second, a difference in mean age was found. Although substance intoxication usually involves adolescent patients, the greater age observed for the bipolar group could be attributed to the peculiar course of the disorder, with long free intervals and a progressive worsening of the symptoms after every affective recurrence; thus, treatment is often delayed. During the first (hypo)manic and/or major depressive episode, for example, patients with bipolar disorder usually do not feel the need to be treated. Moreover, bipolar patients usually achieve better social and work functioning than patients with other diagnoses (such as schizophrenia, personality disorder or substance abuse disorder), which is corroborated by the overall higher education level and prevalence of married and working subjects.25-27
Regarding suicide attempts in the bipolar group, our results were similar to international averages, which range from 11 to 19%.28-30 However, we found no significant difference between bipolar patients and those with other diagnoses, in contrast with the literature.30,31 This might be explained by the different suicide risk rates of the psychiatric disorders included in the mixed control group.
Finally, higher involuntarily admission rates were observed for bipolar patients. This also agrees with the international data, which indicates that bipolar disorder is one of the main causes of involuntary admission.10,15,32
The focus of our study, however, was on analyzing admission rates during different months and seasons among the two subgroups. Seasonality has been defined as “a driving force that has a major effect on the spatio-temporal dynamics of natural systems and their populations.”33 The fact that our results showed a slightly higher prevalence in admission rates during autumn and winter months could be explained by two factors: on the one hand, a slightly higher rate of admission in autumn-winter for unipolar major depression (17.2 vs. 19.3%) and for schizophrenic and other psychoses (23.8 vs. 28.4%) and, on the other hand, the greater length of admissions for manic episodes during spring-summer, which reduced hospital bed turnover and, consequently, the number of admissions in these months.
Numerous studies have indicated that sunlight intensity is positively correlated with the number of mania admissions and negatively correlated with bipolar depression admissions.8,14,21 Considering monthly admission rates, our results agreed with the international literature: the most interesting finding was the peak of admissions during May, June and July for patients with bipolar disorder. This result underscores the higher probability of admission during the pronounced photoperiod change between spring and summer, which agrees with numerous recent studies confirming a strong seasonal pattern in bipolar disorder.9-16,21,34-37
This finding, in light the DSM-5’s focus on seasonal pattern in bipolar disorder, prompted the following question: is seasonality really the most incisive clinical parameter for assessing the onset of bipolar disorder, or is it, rather, greater exposure to sunlight?
The answer is both, if we consider the current affective recurrence in bipolar disorder, namely subdividing the bipolar group into (hypo)manic and major depressive episodes. Our study showed that (hypo)manic episodes were significantly more sensitive to photoperiod variation, since admissions peaked during maximum sunlight exposure (i.e., spring-summer). When we assessed the effect of seasonality (admission rates by single season), the results showed that patients with (hypo)manic episodes were admitted more frequently in spring than bipolar patients with depressive recurrences.
This result agrees with recent reviews9,38 and clinical trials.8,14,21 Manic episodes peak during spring/summer and, to a lesser extent, in the autumn, depending on climatic variations, whereas depressive episodes peak during early winter and, to a lesser extent, in summer, with mixed episodes peaking in early spring or mid/late summer.9 Furthermore, Yang et al. conducted a population-based study showing that young adults presented a higher degree of seasonality in acute admissions than middle-aged adults, and the polarity of a patient’s admission index predicted the seasonality of relapse admissions.16 Symptom dimensions, such as psychosis, suicidality/aggression, or sex differences follow seasonal variations and are also influenced by climatic conditions.9 Finally, a recent study conducted on 148 bipolar I patients found that a seasonal pattern of manic episode admissions was associated with male gender and psychotic features.13
However, when we compared the bipolar group with the mixed-diagnosis control group, we found no differences in seasonality, which was probably due to the counterbalancing effect of depressive recurrences in bipolar disorder, whose admissions particularly increased in autumn.
We could conclude that if bipolar disorder is considered as single affective recurrences, it shows both seasonality and photoperiodic patterns. When we consider the diagnosis of bipolar disorder as a whole, the significant clinical variable for the onset of an affective episode is not seasonal pattern but, rather, greater sunlight exposure, which has also been shown in recent reviews9,36 and clinical trials.14,21,37
In a clinical study assessing climatic variables and admissions for mania, Volpe et al., concluded that the most frequent association is with luminosity: higher temperatures were only significantly involved in regions where the hottest months coincide with the more daylight.39 So, it is important to point out that exposure to sunlight could be considered a useful and significant clinical parameter for evaluating the course of the illness and affective recurrences in patients with bipolar disorder rather than seasonality.
Furthermore, there is emerging evidence that seasonal effects may vary with latitude, varying more strongly in the northern hemisphere than in the southern hemisphere.40 Therefore, photoperiod length has a primary mood-altering role: its variation during different seasons leads to biorhythm adaptation in humans and animals. The photoperiod reaches its maximum extension during summer and its minimum during winter; this environmental pattern reveals biological and clinical implications for human beings, since the light stimulus is received by the retina and transformed into an electrical signal that interacts with the suprachiasmatic nucleus of the hypothalamus (SCN), known as the main endogenous pacemaker. The SCN regulates the activity of many organs, the pineal gland above all, in order to modify biorhythms to better fit seasonal variations.41,42 Bipolar subjects present circadian-related gene mutations that compromise normal synchronization to environmental stimuli, such as sunlight, which ultimately leads to neurotransmitter dysregulation that mainly affects the noradrenergic, serotoninergic, dopaminergic and melatoninergic systems. The timing of melatonin secretion interacts with gene transcription in the pituitary pars tuberalis to modulate the production of TSH (thyrotropin), hypothalamic T3 (triiodothyronine), and tuberalin peptides, which modulate the production of regulatory gonadotropins and other hormones in the pituitary gland. Pituitary hormones largely mediate seasonal physiological and behavioral variations. Thus, altered interaction between the SCN and the rest of the body could have repercussions on every level, from metabolic, circadian and sleep-wake rhythm dysregulations (due to hypothalamus-pituitary-adrenal gland axis malfunction), to altered and compromised immune response, to increased oxidative stress on a cellular level.1,43
Furthermore, certain authors have indicated other climatic factors, such as humidity, day length, ultraviolet radiation and temperature, which represent a significant role in the admission of patients with bipolar disorder, particularly for (hypo)manic episodes.21,35
Our study has several limitations: first, seasonal, environmental and/or psychological factors that could contribute to the onset of an acute clinical event (e.g., holidays, stressful life events, general medical condition, poor adherence to treatment, humidity, temperature, ultraviolet radiation) have not been taken into consideration and could not be ruled out as contributing factors. Second, our findings on seasonality are based on hospital admission date, rather than actual onset of the acute episode. Third, our data are limited to a single hospital, and the control group was a mixed sample of psychiatric diagnoses. Fourth, clinical variables, such as the number of previous hospitalizations, predominant polarity and a structured interview for diagnoses are lacking.
In conclusion, bipolar disorder seems to be strongly linked to compromised communication between biorhythm pathways, since these patients exhibited an increased probability of (hypo)manic episodes during maximum sunlight exposure. Infradian rhythm abnormalities, such as seasonal aspects in bipolar patients, are well documented. However, the mechanisms underlying the effects of the natural seasonal environment on bipolar symptoms remain unclear. Various aspects, such as the nature and intensity of environmental factors or the temporal relationship between symptoms and these environmental factors, need to be investigated.
Identifying patients with such susceptibilities would enable the development of personalized therapeutic strategies to prevent affective recurrences38 or of chronotherapeutic interventions such as sleep deprivation, the blue-blocking regime in mania (“a virtual darkness therapy”), or interpersonal or social rhythm therapy.6,38
Disclosure
The authors report no conflicts of interest.
References
- 1.Muneer A. The neurobiology of bipolar disorder: an integrated approach. Chonnam Med J. 2016;52:18–37. doi: 10.4068/cmj.2016.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer M, Glenn T, Grof P, Rasgon NL, Marsh W, Sagduyu K, et al. Relationship among latitude, climate, season and self-reported mood in bipolar disorder. J Affect Disord. 2009;116:152–7. doi: 10.1016/j.jad.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–42. doi: 10.1016/j.psyneuen.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Vitale JA, Roveda E, Montaruli A, Galasso L, Weydahl A, Caumo A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32:405–15. doi: 10.3109/07420528.2014.986273. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Morales JF, Escribano C, Jankowski KS. Chronotype and time-of-day effects on mood during school day. Chronobiol Int. 2015;32:37–42. doi: 10.3109/07420528.2014.949736. [DOI] [PubMed] [Google Scholar]
- 6.Melo MC, Abreu RL, Linhares VB, Neto, de Bruin PF, de Bruin VM. Chronotype and circadian rhythm, in bipolar disorder: a systematic review. Sleep Med Rev. 2016 Jul 1 doi: 10.1016/j.smrv.2016.06.007. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Moreira J, Geoffroy PA. Lithium and bipolar disorder: impacts from molecular to behavioural circadian rhythms. Chronobiol Int. 2016;33:351–73. doi: 10.3109/07420528.2016.1151026. [DOI] [PubMed] [Google Scholar]
- 8.Parker GB, Hadzi-Pavlovic D, Graham RK. Examining for any impact of climate change on the association between seasonality and hospitalization for mania. J Affect Disord. 2017;208:431–5. doi: 10.1016/j.jad.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Geoffroy PA, Bellivier F, Scott J, Etain B. Seasonality and bipolar disorder: a systematic review, from admission rates to seasonality of symptoms. J Affect Disord. 2014;168:210–23. doi: 10.1016/j.jad.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Amr M, Volpe FM. Seasonal influences on admissions for mood disorders and schizophrenia in a teaching psychiatric hospital in Egypt. J Affect Disord. 2012;137:56–60. doi: 10.1016/j.jad.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Akther A, Fiedorowicz JG, Zhang T, Potash JB, Cavanaugh J, Solomon DA, et al. Seasonal variation of manic and depressive symptoms in bipolar disorder. Bipolar Disord. 2013;15:377–84. doi: 10.1111/bdi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Chen D. Evidence for seasonal mania: a review. J Psychiatr Pract. 2013;19:301–8. doi: 10.1097/01.pra.0000432600.32384.c5. [DOI] [PubMed] [Google Scholar]
- 13.Hochman E, Valevski A, Onn R, Weizman A, Krivoy A. Seasonal pattern of manic episode admissions among bipolar I disorder patients is associated with male gender and presence of psychotic features. J Affect Disord. 2016;190:123–7. doi: 10.1016/j.jad.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Parker GB, Graham RK. Seasonal variations in rates of hospitalisation for mania and hypomania in psychiatric hospitals in NSW. J Affect Disord. 2016;191:289–91. doi: 10.1016/j.jad.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 15.Lee HC, Tsai SY, Lin HC. Seasonal variations in bipolar disorder admissions and the association with climate: a population-based study. J Affect Disord. 2007;97:61–9. doi: 10.1016/j.jad.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Yang AC, Yang CH, Hong CJ, Liou YJ, Shia BC, Peng CK, et al. Effects of age, sex, index admission, and predominant polarity on the seasonality of acute admissions for bipolar disorder: a population-based study. Chronobiol Int. 2013;30:478–85. doi: 10.3109/07420528.2012.741172. [DOI] [PubMed] [Google Scholar]
- 17.Dominiak M, Swiecicki L, Rybakowski J. Psychiatric hospitalizations for affective disorders in Warsaw, Poland: effect of season and intensity of sunlight. Psychiatry Res. 2015;229:287–94. doi: 10.1016/j.psychres.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Bauer M, Glenn T, Alda M, Andreassen OA, Ardau R, Bellivier F, et al. Impact of sunlight on the age of onset of bipolar disorder. Bipolar Disord. 2012;14:654–63. doi: 10.1111/j.1399-5618.2012.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer M, Glenn T, Alda M, Andreassen OA, Angelopoulos E, Ardau R, et al. Relationship between sunlight and the age of onset of bipolar disorder: an international multisite study. J Affect Disord. 2014;167:104–11. doi: 10.1016/j.jad.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Bauer M, Glenn T, Alda M, Andreassen OA, Angelopoulos E, Ardau R, et al. Influence of light exposure during early life on the age of onset of bipolar disorder. J Psychiatr Res. 2015;64:1–8. doi: 10.1016/j.jpsychires.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Medici CR, Vestergaard CH, Hadzi-Panlovic D, Munk-Jorgensen P, Parker G. Seasonal variation in hospital admissions for mania: examining for associations with weather variables over time. J Affect Disord. 2016;205:81–6. doi: 10.1016/j.jad.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Mellsop G, Brink J, Wang X. Involuntary admission and treatment of patients with mental disorder. Neurosci Bull. 2015;31:99–112. doi: 10.1007/s12264-014-1493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 24.Ferrari AJ, Stockings E, Khoo JP, Erskine HE, Degenhardt L, Vos T, et al. the prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18:440–50. doi: 10.1111/bdi.12423. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita C, Mizuno M, Nemoto T, Kashima H. Social cognitive problem-solving in schizophrenia: associations with fluency and verbal memory. Psychiatry Res. 2005;134:123–9. doi: 10.1016/j.psychres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Zanello A, Perrig L, Huguelet P. Cognitive functions related to interpersonal problem-solving skills in schizophrenic patients compared with healthy subjects. Psychiatry Res. 2006;142:67–78. doi: 10.1016/j.psychres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Mueser KT, Pratt SI, Bartels SJ, Forester B, Wolfe R, Cather C. Neurocognition and social skill in older persons with schizophrenia and major mood disorders: an analysis of gender and diagnosis effects. J Neurolinguistics. 2010;23:297–317. doi: 10.1016/j.jneuroling.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abreu LN, Lafer B, Baca-Garcia E, Oquendo MA. Suicidal ideation and suicide attempts in bipolar disorder type I: an update for the clinician. Rev Bras Psiquiatr. 2009;31:271–80. doi: 10.1590/s1516-44462009005000003. [DOI] [PubMed] [Google Scholar]
- 29.Parmentier C, Etain B, Yon L, Misson H, Mathieu F, Lajnef M, et al. Clinical and dimensional characteristics of euthymic bipolar patients with or without suicidal behavior. Eur Psychiatry. 2012;27:570–6. doi: 10.1016/j.eurpsy.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Costa Lda S, Alencar AP, Nascimento PJ, Neto, dos Santo Mdo S, da Silva CG, Pinheiro Sde F, et al. Risk factors for suicide in bipolar disorder: a systematic review. J Affect Disord. 2015;170:237–54. doi: 10.1016/j.jad.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein TR, Ha W, Axelson DA, Goldstein BI, Liao F, Gill MK, et al. Predictors of prospectively examined suicide attempts among youth with bipolar disorder. Arch Gen Psychiatry. 2012;69:1113–22. doi: 10.1001/archgenpsychiatry.2012.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuepbach D, Novice D, Haro JM, Reed C, Booker H, Noda S, et al. Determinants of voluntary vs. involuntary admission in bipolar disorder and the impact of adherence. Pharmacopsychiatry. 2008;41:29–36. doi: 10.1055/s-2007-993213. [DOI] [PubMed] [Google Scholar]
- 33.Stone L, Olinky R, Huppert A. Seasonal dynamics of recurrent epidemics. Nature. 2007;446:533–6. doi: 10.1038/nature05638. [DOI] [PubMed] [Google Scholar]
- 34.Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10:256–65. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 35.Volpe FM, Tavares A, Del Porto JA. Seasonality of three dimensions of mania: psychosis, aggression and suicidality. J Affect Disord. 2008;108:95–100. doi: 10.1016/j.jad.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Abreu T, Bragança M. The bipolarity of light and dark: a review on bipolar disorder and circadian cycles. J Affect Disord. 2015;185:219–29. doi: 10.1016/j.jad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Rajkumar PR, Sarkar S. Seasonality of admissions for mania: results from a general hospital psychiatric unit in Pondicherry, India. Prim Care Companion CNS Disord. 2015;17(3) doi: 10.4088/PCC.15m01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young JW, Dulcis D. Investigating the mechanism(s) underlying switching between states in bipolar disorder. Eur J Pharmacol. 2015;759:151–62. doi: 10.1016/j.ejphar.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpe FM, da Silva EM, dos Santos TN, de Freitas DE. Further evidence of seasonality of mania in the tropics. J Affect Disord. 2010;124:178–82. doi: 10.1016/j.jad.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Friedman E, Gyulai L, Bhargava M, Landen M, Wisniewski S, Foris J, et al. Seasonal changes in clinical status in bipolar disorder: a prospective study in 1000 STEP-BD patients. Acta Psychiatr Scand. 2006;113:510–7. doi: 10.1111/j.1600-0447.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 41.Maronde E, Stehle JH. The mammalian pineal gland: known facts, un-known facets. Trends Endocrinol Metab. 2007;18:142–9. doi: 10.1016/j.tem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Dibner C, Schlibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Ann Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 43.Nathan PJ, Burrows GD, Norman TR. Melatonin sensitivity to dim white light in affective disorders. Neuropsychopharmacology. 1999;21:408–13. doi: 10.1016/S0893-133X(99)00018-4. [DOI] [PubMed] [Google Scholar]