Abstract
Molecular bacterial load assay (MBLA) rapidly quantifies viable Mycobacterium tuberculosis (Mtb) and may be useful for monitoring treatment response and treatment efficacy.
We conducted a prospective study in 56 adults with pulmonary tuberculosis from whom 244 sputum samples were collected before and during the first month of treatment. We evaluated MBLA for early monitoring of bacterial burden and investigated bactericidal activities of first-line therapy in patients infected with drug susceptible and resistant isolates.
Mtb loads measured by MBLA and culture were correlated after one-week (r = 0.56) and one-month (r = 0.73) of treatment. Correlations between culture and GeneXpert or microscopy were weaker during treatment. Mtb load by MBLA declined more rapidly than GeneXpert after one-week (2.73 Ct, P < 0.001; 0.95 Ct, P = 0.297, respectively) and one-month (8.94 Ct, P < 0.001; 6.78 Ct, P < 0.001). Mtb loads in multidrug resistant (MDR) infections were significantly greater than in both sensitive and poly/mono-resistance after one-week (P < 0.02) and one-month treatment (P = 0.001).
MBLA performed better than GeneXpert and microscopy in comparison to culture for quantifying viable Mtb during treatment. It can be used for monitoring bacterial load during TB treatment, facilitating early detection of treatment failure thus improving outcomes.
Keywords: Mycobacterium tuberculosis, Viable bacterial load, 16S rRNA, Treatment, Bactericidal activity
1. Introduction
Tuberculosis (TB), which is caused by Mycobacterium tuberculosis (Mtb), is ranked as one of the top ten causes of death globally among infectious diseases. Annually, 10.4 million people get active TB, of whom 1.3 million cases result in death [1]. Early diagnosis and proper initiation of anti-TB therapy are important in treatment and control of disease transmission.
Early monitoring of treatment through viable bacterial quantification is important for evaluating treatment efficacy and predicting outcomes. It allows for improved precision in clinical care [2,3]. Monitoring treatment response requires a rapid test to measure viable bacterial numbers. This is challenging with Mtb. Bacterial load in clinical samples from TB patients can be inferred from automatic MGIT culture or GeneXpert. However, culture-based methods are time-consuming and usually at risk of contamination. The presence of non-culturable Mtb populations also makes culture less accurate for monitoring treatment efficiency [4]. DNA-based methods are faster than culture but do not reflect the viable bacterial number accurately because the DNA from dead bacteria can persist for a long time [5,6]. Honeyborne et al. has reported that the molecular bacterial load assay (MBLA) based on 16S rRNA has potential to rapidly quantify viable bacteria thereby offering promise in the early detection of poor response to therapy [[7], [8], [9]]. No studies have reported on bactericidal activity of first-line drugs in TB patients infected with resistant Mtb.
We conducted a prospective study in a cohort of 56 pulmonary TB (PTB) patients whose 244 sputum samples were collected before and during the first month of first-line standard treatment. We evaluated the MBLA method by comparing it with Ziehl-Neelsen (ZN) microscopy, GeneXpert and MGIT culture in terms of rapid and accurate detection of viable Mtb load. We also used MBLA to investigate the changes of bacterial load during the first month of treatment among isolates having different anti-TB drug resistance phenotypes.
2. Methods
2.1. Human subject's approvals and patient enrollment
Protocols were approved by the Institutional Review Boards of the Hospital for Tropical Diseases in Vietnam and the Oxford Tropical Research Ethics Committee in the United Kingdom. We enrolled 56 adults who had symptoms of PTB and ZN smear-positive with acid-fast bacilli (AFB) from two District TB control units in Ho Chi Minh City, Vietnam between January 2015 and October 2016. Written informed consent was obtained from each participant.
All participants were HIV negative, ≥18 years old, and had new PTB with no history of previous TB treatment and were given supervised first-line anti-TB treatment regimen according to national and WHO guidelines [10,11]. In the 2-month intensive phase of therapy, a fixed-dose combination of rifampicin (10 mg/kg), isoniazid (5 mg/kg), pyrazinamide (25 mg/kg) and ethambutol (15 mg/kg) was given daily. The continuation phase consisted of daily dosages of rifampicin, isoniazid and ethambutol. Treatment regimens were changed if drug susceptibility test (DST) showed multidrug resistance. Approximately, 5 mL of sputum was collected from the participants before treatment (week 0), after week 1, week 2 and week 4 of treatment.
2.2. Sample processing and microbiological examination
Sputum samples were stored for MBLA and processed for ZN smear, MGIT culture and GeneXpert. ZN smears were prepared directly from the sputum to approximately quantify the number of acid-fast bacilli from 100 microscopic fields under 100X objectives. This number then was transformed to log10 values for statistical tests.
The remaining sputum was decontaminated by NALC-NaOH [12] and finally re-suspended in 1 mL of phosphate buffer, equivalent to 1/5 original volume. To quantify bacterial load by MGIT, 200 μL of decontaminated sputum was inoculated in a MGIT tube and incubated in MGIT system for up to 56 days to detect mycobacteria growth. The positive MGIT culture was then used for drug susceptibility testing. Time to detection of positive growth (TTP) in days was automatically recorded and used as a measure of live bacterial load. If there was no detection of Mtb growth at day 56, the MGIT culture was deemed negative, and a TTP value of 56 was assigned for data analysis.
For determining bacterial load by GeneXpert, another 200 μL of decontaminated sputum was added into 2 mL of sample reagent and transferred into a test cartridge. The cartridge then was inserted into the test platform of GeneXpert instrument. Threshold Cycle (Ct) values for each of five rpoB probes were recorded [13]. The average Ct value of five probes (excluding any delayed values due to rifampicin resistance) was used to estimate bacterial load. A Ct value of 40 was assigned if GeneXpert was negative for Mtb detection.
2.3. RNA extraction and RT-qPCR for MBLA
To store sputum for MBLA, 100 μL of decontaminated sputum, equivalent to 0.5 mL of fresh sputum, was transferred to a Rnase-free tube containing 1000 μL preservative buffer of 4 M guanidine thiocyanate, 0.1 M Tris-HCl, and 1% β-mercaptoethanol, and stored at −80 °C immediately. After collecting all samples, total RNA was extracted as previously described. Mycobacterium marinum (M. marinum) at a concentration of 4log10 CFU was used as an internal control [14]. In each extraction batch, artificial sputa spiked with 8log10 and 4log10 M. bovis BCG were used as high positive and low positive standards respectively.
Expression of 16S rRNA was measured by reverse transcriptase real-time PCR using LightCycler Multiplex RNA Virus Master (Table S1) [14]. One high and one low BCG positive standard were included in each run. Ct values of MBLA were recorded only if all BCG positive standards and internal controls were passed. According to our optimization, an M. marinum internal control at 4log10 CFU gave a Ct value between 21.0 and 27.5. Samples with internal controls which yielded Ct values outside that range were excluded from the analysis. The 16S rRNA Ct value of MBLA was used to estimated bacterial load after normalization using the equation 16S rRNA Ct - [(internal control Ct −21.00) x 0.8457] which was constructed based on our data [8] (Fig. S1). Log10 bacterial number per milliliter (log10 bacteria/mL) of sputum was converted from normalized Ct value using the standard curve in Fig. S1. To avoid results being affected by cross-reactivity of the internal control and 16S rRNA target, the limit of detection was set to 2log10 bacteria/mL. Samples with bacterial loads less than 2log10 bacteria/mL were considered negative.
2.4. Drug susceptibility testing
DST of isolates was performed for streptomycin (STR), isoniazid (INH), rifampicin (RIF), ethambutol (EMB) and pyrazinamide (PZA) using BACTEC MGIT 960 SIRE and PZA kits. The critical concentrations of drugs were 1.0 μg/mL for STR, 0.1 μg/mL for INH, 1.0 μg/mL for RIF, 5.0 μg/mL for EMB and 100 μg/mL for PZA.
2.5. Statistical analysis
Statistical analyses were performed using R version 3.3.1 [15] and Graphpad Prism version 6 (Graphpad Software Inc., San Diego, California, USA). The linear relationships of MBLA and other methods to MGIT were analyzed using the Spearman's rank correlation method. To avoid correlation coefficients being affected by a bias or skewing of the data, only samples which gave positive results for all methods were used. To examine bacterial load decline (defined as increase in Ct or TTP value) after one week or one month within a method, the Paired Sample T test was used. To pairwise compare the bacterial load among four drug susceptible and resistant Mtb strains during treatment, Wilcoxon rank sum tests were applied. P values of <0.05 were considered statistically significant.
3. Results
Sputum samples were collected from 56 PTB participants before treatment. All were AFB microscopy positive, 54/56 (96%) were positive by MGIT. Of two MGIT-negative cases, one was scanty by microscopy but negative for both MBLA and GeneXpert, suggesting a very low number of bacteria. One was AFB grade 1 and positive for both MBLA and GeneXpert, suggesting non-culturable Mtb. These two cases were excluded from this study. Baseline characteristics of the remaining 54 participants are given in Table 1.
Table 1.
Baseline characteristics of 54 participants by drug sensitivity phenotypes.
| Characteristics | Total (n = 54) | Sensitive (n = 27) | EMB-PZA R (n = 10) | INH R (n = 12) | MDR |
|---|---|---|---|---|---|
| (n = 5) | |||||
| Age (year), median [IQR] | 46.0 [35.0; 54.0] | 43.0 [33.5; 56.5] | 43.5 [36.0; 56.0] | 46.0 [41.0; 50.5] | 48.0 [43.0; 52.0] |
| Sex n (%) | |||||
| Male | 46 (85.2%) | 21 (77.8%) | 9 (90.0%) | 12 (100%) | 4 (80.0%) |
| Female | 8 (14.8%) | 6 (22.2%) | 1 (10.0%) | 0 (0.00%) | 1 (20.0%) |
| Duration of illness (days), median [IQR] | 7.00 [7.00; 10.0] | 7.00 [5.00; 7.00] | 10.0 [7.00; 10.0] | 7.00 [6.00; 8.50] | 10.0 [7.00; 10.0] |
| Weight loss n (%) | |||||
| Yes | 13 (25.0%) | 5 (18.5%) | 2 (22.2%) | 3 (27.3%) | 3 (60.0%) |
| No | 39 (75.0%) | 22 (81.5%) | 7 (77.8%) | 8 (72.7%) | 2 (40.0%) |
| Fever n (%) | |||||
| Yes | 33 (63.5%) | 19 (70.4%) | 6 (66.7%) | 4 (36.4%) | 4 (80.0%) |
| No | 19 (36.5%) | 8 (29.6%) | 3 (33.3%) | 7 (63.6%) | 1 (20.0%) |
3.1. Correlation between bacterial load measured by MBLA and culture before and during treatment
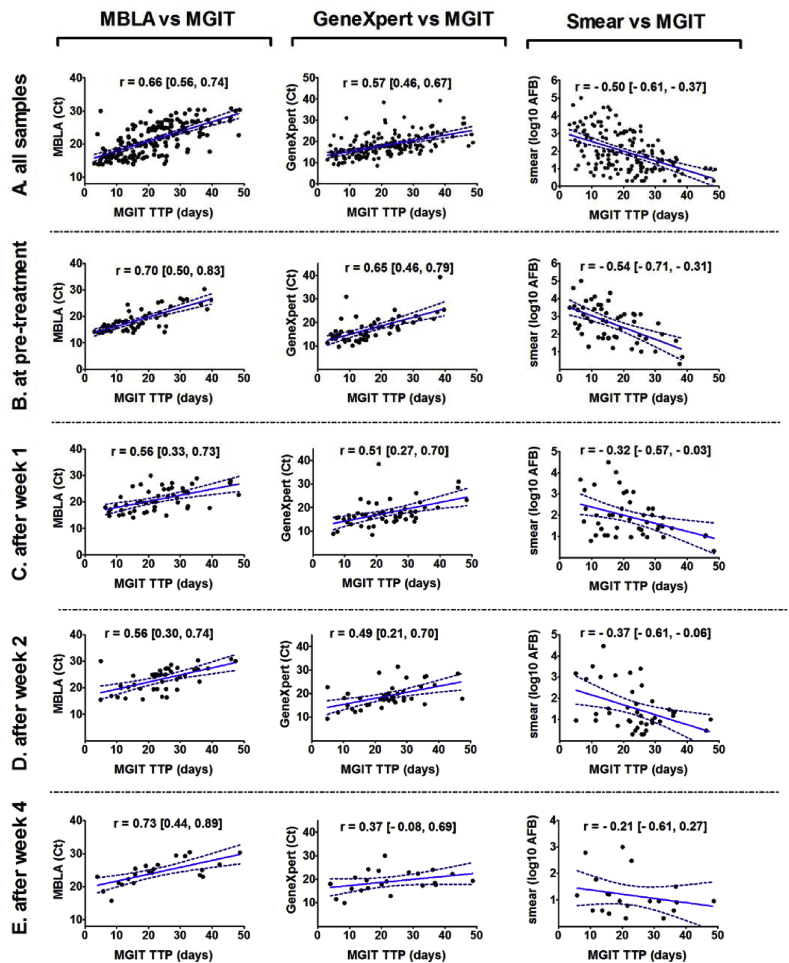
Of the 224 samples collected before and during treatment, 168 positive samples (defined as positive in all four methods) were included in correlation analysis. Spearman rank correlation revealed a relatively strong correlation of Mtb load between the MBLA and MGIT (r = 0.66; 95% CI [0.56, 0.74]). GeneXpert and microscopy were less correlated with MGIT (r = 0.57; 95% CI [0.46, 0.67]; and r = −0.50; 95% CI [-0.61, −0.37], respectively) (Fig. 1A).
Fig. 1.
Correlations of Mtb load measured by MGIT versus MBLA, GeneXpert and microscopy. Plots of MGIT TTP (days) against MBLA (Ct), GeneXpert (Ct) and microscopy (log10 AFB) in (A) all samples (n = 168), (B) before treatment (n = 54), (C) after week 1 (n = 50), (D) after week 2 (n = 42) and (E) after week 4 of treatment (n = 22). TTP, Ct and log10 AFB number were used to calculate correlations using the Spearman method. The analysis included all drug-sensitive and drug-resistant bacteria samples from patients. Only samples with detected values of bacterial load were included in analyses (n = 168). Spearman's correlation coefficients (r) and 95% confident interval are presented in the figure. Best-fit linear regression lines are shown with 95% confidence intervals. TTP: time to positivity; Ct: threshold cycle value.
In the 54 samples before anti-TB treatment, the correlation of MGIT with MBLA (r = 0.70; 95% CI [0.50, 0.83]) was stronger than that with GeneXpert (r = 0.65; 95% CI [0.46, 0.79] or microscopy (r = −0.54; 95% CI [-0.71, −0.31]) (Fig. 1B). However, after one week of treatment, the correlation between MGIT and MBLA dropped to 0.56 (95% CI [0.33, 0.73]), while it was 0.51 (95% CI [0.27, 0.70]) for GeneXpert and −0.32 (95% CI [-0.57, −0.03]) for microscopy (Fig. 1C). After two weeks of treatment, the correlations were not very different to those seen after the first week (Fig. 1D). However, after one month, the correlation of MBLA with MGIT improved and was as high as before treatment (r = 0.73; 95% CI [0.44, 0.89]), whereas correlations of MGIT with GeneXpert and microscopy remained relatively low (r = 0.37; 95% CI [-0.08, 0.69]; and r = −0.21; 95% CI [-0.61, 0.27]) (Fig. 1E). Overall, sputum bacterial loads measured by culture and MBLA showed a strong relationship both before and during treatment.
We analyzed the decline of bacterial load measured by MBLA and GeneXpert, and compared it with the load measured by MGIT during the first month of treatment. Bacterial loads measured by all three methods declined during the first month of anti-TB treatment. However, the rates of decline were different. After one week of treatment, bacterial load measured by MBLA had significantly declined at rate of 2.73 Ct per week (95% CI [1.39, 4.09]; P < 0.001), whereas GeneXpert did not show a significant change, with a rate of 0.95 Ct (95% CI [-0.86, 2.45]; P = 0.297). The bacterial load measured by MGIT had also significantly decreased, at a rate of 7 days TTP per week (95% CI [3.62, 10.39]; P < 0.001) (Fig. S2). After one month of treatment, the rate of bacterial decline for MBLA was 8.94 Ct per month (95% CI [7.38, 10.50], P < 0.001); but for GeneXpert, it was slower at 6.78 Ct per month (95% CI [4.39, 9.16], P < 0.001) (Fig. S2). These results show that unlike MGIT, GeneXpert is not sufficiently sensitive to monitor bactericidal decline, whereas MBLA rapidly measures the decline in the first week and the first month of treatment.
3.2. Mtb load changes by drug resistance during the first month of treatment
We hypothesized that patients infected by drug-resistant Mtb would show higher bacterial loads during treatment than those with drug-sensitive Mtb. The relationships between bacterial load and Mtb drug susceptibility phenotypes before and during early treatment were examined, with four phenotype groups: sensitivity to all drugs used in treatment (S, n = 27); EMB and/or PZA resistance (EMB-PZA R, n = 10); INH resistance (INH R, n = 12); and multidrug resistance (MDR, n = 5). Baseline characteristics of these groups are given in Table 1.
We used MBLA for bacterial quantification by converting the normalized Ct values into log10 bacteria/mL of sputum (Fig. S1). There was no difference in pre-treatment bacterial load by drug susceptibility (Fig. 2A). After the first week of treatment, median bacterial loads were 5.81 (IQR 4.61, 6.83), 5.94 (IQR 4.74, 7.30), 5.04 (IQR 4.05, 6.93) and 7.75 (IQR 7.21, 7.86) log10 bacteria/mL for the sensitive, EMB-PZA R, INH R and MDR groups, respectively. Bacterial load in the MDR group was significantly greater than in the sensitive, EMB-PZA R and INH R groups (P = 0.002, P = 0.019 and P = 0.005, respectively; Fig. 2B and D). There was no significant difference in bacterial loads among the sensitive, EMB-PZA R and INH R groups. After one month of intensive treatment, bacterial load in MDR remained very high, 6.67 log10 bacteria/mL (IQR 5.30, 7.69), which is still significantly higher than in the other three groups, separately or combined (P < 0.05, Fig. 2C and D). In MDR, bacterial load appears to be unchanged in the first two weeks of treatment (P = 0.054), decreases in week 2 then increases from week 2 to week 4 (P = 0.022, Fig. 2D). The dynamic responses of bacterial load quantified by MBLA concurred with that measured by MGIT TTP, particularly after one month of treatment (Fig. S3).
Fig. 2.
Mtb load quantified by MBLA in drug sensitive and resistant Mtb-infected patient groups before and during the first month of treatment. Bacteria loads were measured by MBLA in four Mtb drug-susceptibility phenotype groups: the sensitive group (black, n = 27), EMB-PZA R group (green, n = 10), INH R group (blue, n = 12) and MDR group (red, n = 5). Bacterial loads in the four groups were compared (A) before treatment, (B) after week 1 and (C) after week 4 of treatment. (D) Bacterial loads in the four groups were plotted against time during the first month of TB treatment. Error bars in plots represent medians and interquartile ranges. Statistical comparisons were made using Wilcoxon rank sum test. Only significant results are indicated with P values. Sensitive: sensitive group; EMB-PZA R: EMB and/or PZA resistant group, INH R: INH resistant group, MDR: multidrug resistant group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In our study, MBLA performed better than GeneXpert and microscopy in comparison to MGIT culture for quantifying viable bacteria during the first month of treatment. Before treatment, when bacterial loads were high, all three methods had high correlations with culture. Once treatment started, the correlations were strongly affected by the sensitivity of the methods in detecting viable bacteria. Although the correlation of MBLA and culture slightly reduced after the first and second weeks of treatment, it recovered by the fourth week; whereas GeneXpert and microscopy showed low correlations with culture throughout. The bacterial load measured by MBLA showed a significant drop after one week of treatment while GeneXpert values remained almost unchanged. MBLA based on 16S rRNA detection could more rapidly reflect the number of viable bacteria in comparison with GeneXpert based on DNA detection of Mtb [6,8]. MBLA had advantages over conventional methods in our study. It agrees with other data [4,7,8] suggesting that MBLA is comparable to liquid and solid culture but much faster, and is more accurate than DNA-based quantitative methods. As such it could be used to monitor viable bacterial load during TB treatment.
All patients in this study received first-line drug regimen following the guidelines of Vietnam's national TB program [10]. Monitoring bacterial load, together with identifying baseline drug-susceptibility phenotypes, allowed us to understand the effect of Mtb drug resistance on bactericidal activity of these first-line drugs. Drug resistance was not associated with pre-treatment bacterial load, suggesting that drug resistance did not affect the ability of bacteria to survive and multiply before treatment. INH is a key drug in TB treatment with a strong bactericidal effect in the first few days of treatment [16]. However, INH R did not influence bacterial load after the start of treatment. Our findings indicate that poly/mono-resistance to INH, EMB and PZA does not change bactericidal activity of the first-line regimen in the first month and that the regimen still works effectively in these drug resistant strains during early treatment.
MDR-TB in this study showed consistent associations with higher bacterial load in comparison with sensitive and other drug-resistant Mtb groups. Therefore early diagnosis of new MDR TB cases for precise treatment helps reduce treatment failure and MDR TB transmission.
NALC-NaOH decontamination of sputum samples can cause a loss of viable bacteria. This has been measured by MBLA up to 0.66 ± 0.21log10 bacteria/mL in high bacteria burden samples [17]. In this study, MBLA was performed on decontaminated sputum, thus the bacterial loads reported could be slightly lower than the actual numbers. However, using the same source of sputum for all our three methods gives consistency for comparisons.
In summary, MBLA can be used for early monitoring of bacterial load during TB treatment and for assessing treatment response. Resistance to drugs other than rifampicin did not influence declines in bacterial load; whereas MDR had a significant impact, indicating the urgent need for early MDR detection and appropriate treatment.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Wellcome Trust [206724/Z/17/Z, 2017] and [106680/B/14/Z, 2015].
Acknowledgments
We acknowledge all participants in the study and the physicians and nurses at the TB Units in Districts 4 and 8 who were responsible for diagnosis and examination. We would like to thank Stephen H Gillespie and Wilber Sabiiti from University of St Andrews for their training in use of the molecular bacterial load assay and provision of internal controls for the assay.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tube.2019.101864.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . WHO; Geneva, Switzerland: 2011. Global tuberculosis report 2017. [Google Scholar]
- 2.Rockwood N. Assessment of treatment response in tuberculosis. Expert Rev Respir Med. 2016;10(6):643–654. doi: 10.1586/17476348.2016.1166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaikwad U.N., Gaikwad N.R. Modalities to monitor the treatment response in tuberculosis. Indian J Tuberc. 2018;65(2):109–117. doi: 10.1016/j.ijtb.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 4.de Knegt G.J. Assessment of treatment response by colony forming units, time to culture positivity and the molecular bacterial load assay compared in a mouse tuberculosis model. Tuberculosis. 2017;105:113–118. doi: 10.1016/j.tube.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Theron G. Xpert MTB/RIF results in patients with previous tuberculosis: can we distinguish true from false positive results? Clin Infect Dis. 2016;62(8):995–1001. doi: 10.1093/cid/civ1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicol M.P. Xpert MTB/RIF: monitoring response to tuberculosis treatment. Lancet Respir Med. 2013;1(6):427–428. doi: 10.1016/S2213-2600(13)70133-4. [DOI] [PubMed] [Google Scholar]
- 7.Honeyborne I. The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol. 2014;52(8):3064–3067. doi: 10.1128/JCM.01128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honeyborne I. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol. 2011;49(11):3905–3911. doi: 10.1128/JCM.00547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evangelopoulos D. Pediatric tuberculosis-human immunodeficiency virus co-infection in the United Kingdom highlights the need for better therapy monitoring tools: a case report. J Med Case Rep. 2017;11(1):52. doi: 10.1186/s13256-017-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viet Nam National Tuberculosis Control Programe . 2009. Guidelines for the management and treatment of Tuberculosis. [Viet Nam NTP: Hanoi, Viet Nam] [Google Scholar]
- 11.World Health Organization . WHO; Geneva, Switzerland: 2009. Treatment of tuberculosis guidelines. [Google Scholar]
- 12.World Health Organization . first ed. WHO; Geneva, Switzerland: 2014. Mycobacteriology laboratory manual. [Google Scholar]
- 13.Lawn S.D., Nicol M.P. Xpert(R) MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephen H., Gillespie W.S. Katarina Oravcova, mycobacterial load assay. In: Bishop-Lilly K.A., editor. Diagnostic bacteriology. Humana Press; New York: 2017. pp. 89–105. [Google Scholar]
- 15.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: a language and environment for statistical computing. R foundation for statistical computing. [Google Scholar]
- 16.Jindani A., Dore C.J., Mitchison D.A. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167(10):1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 17.Mtafya B. Molecular bacterial load assay concurs with culture on NaOH-induced loss of Mycobacterium tuberculosis viability. J Clin Microbiol. 2019;57(7) doi: 10.1128/JCM.01992-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.