Abstract
Background:
At the end of life, cancer survivors often experience exacerbations of complex comorbidities requiring acute hospital care. Few studies consider comorbidity patterns in cancer survivors receiving palliative care.
Aim:
To identify patterns of comorbidities in cancer patients receiving palliative care and factors associated with in-hospital mortality risk.
Design, Setting/Participants:
New South Wales Admitted Patient Data Collection data were used for this retrospective cohort study with 47,265 cancer patients receiving palliative care during the period financial year 2001–2013. A latent class analysis was used to identify complex comorbidity patterns. A regression mixture model was used to identify risk factors in relation to in-hospital mortality in different latent classes.
Results:
Five comorbidity patterns were identified: ‘multiple comorbidities and symptoms’ (comprising 9.1% of the study population), ‘more symptoms’ (27.1%), ‘few comorbidities’ (39.4%), ‘genitourinary and infection’ (8.7%), and ‘circulatory and endocrine’ (15.6%). In-hospital mortality was the highest for ‘few comorbidities’ group and the lowest for ‘more symptoms’ group. Severe comorbidities were associated with elevated mortality in patients from ‘multiple comorbidities and symptoms’, ‘more symptoms’, and ‘genitourinary and infection’ groups. Intensive care was associated with a 37% increased risk of in-hospital deaths in those presenting with more ‘multiple comorbidities and symptoms’, but with a 22% risk reduction in those presenting with ‘more symptoms’.
Conclusion:
Identification of comorbidity patterns and risk factors for in-hospital deaths in cancer patients provides an avenue to further develop appropriate palliative care strategies aimed at improving outcomes in cancer survivors.
Keywords: Complex comorbidity patterns, palliative care, cancer, end of life
What is already known about the topic?
Palliative care seeks to eliminate or manage distressing symptoms and may support cancer patients at their end lives still receiving care with curative intent.
Co-existing morbidities may limit the choice of treatment and care in cancer patients, which complicates the overall care and in turn further increases the risk of death.
What this paper adds?
This study reveals five major patterns of complex comorbidities in palliative cancer patients receiving acute hospital care, that is, ‘multiple comorbidities and symptoms’, ‘more symptoms’, ‘few comorbidities’, ‘genitourinary and infection’, or ‘circulatory and endocrine’ group.
Cancer patients with ‘few comorbidities’ experienced the highest in-hospital mortality rate, and therefore their care was less complicated by comorbidities than other patients, and probably mainly related to cancer itself.
The association between intensive care unit (ICU) care and in-hospital death varies across the identified five classes, with elevated risk in patients with ‘multiple comorbidities and symptoms’ and reduced risk in patients with ‘more symptoms’.
Implications for practice, theory or policy
Clinicians should carefully evaluate the benefits and harms of various interventions for palliative cancer patients with different comorbidity profiles to avoid adopting ineffective interventions. Enhanced understanding of comorbidity patterns and how these vary across patient groups has potential for optimising comorbidity care by developing patient-centred policies and resource allocation during episodes of acute hospital care.
Introduction
Cancer is a disease requiring continuing care and treatment.1 Studies have shown that nearly half of people with cancer experience uncontrollable pain at the end of life and ultimately die in hospital. This phenomenon is particularly evident in high-income countries.2,3 Specifically, most advanced cancer patients have exacerbations of symptoms requiring urgent hospitalisation several times in the last year of their lives and many receive aggressive medical interventions, including extensive stays in intensive care units (ICUs),3–6 a trend that has increased over time.7
Palliative care focuses on whole person care. It is concerned with patients’ quality of life, comfort and support and places emphasis on both individual and family/carer needs. It may support the care of people still receiving care with curative intent.8 It seeks to eliminate or manage distressing symptoms1 and neither attempts to delay nor to accelerate death.1,2,9 Palliative care is appropriately delivered to patients far earlier than just the last days of life. According to their unique circumstances, patients may receive palliative care for a few days, weeks, months or even years.1,2,9
Cancer patients often have complex comorbidities.10–12 More than half of cancer patients aged over 65 years will have at least one significant co-existing disease.10 Concurrent diseases are closely related to mortality in cancer patients and frequently contribute as underlying causes of death.12–15 The presence of comorbidities may limit the choice of treatment and care, which in turn further increases the risk of death.11,16 Delivering optimal whole person palliative care to cancer patients requires careful attention to the management symptoms arising from the cancer and all comorbidities.
There is limited published research from administrative data sets regarding comorbidity patterns in cancer patients receiving palliative care.10,11 Defining common patterns of comorbidity in cancer patients receiving palliative care may assist clinicians to optimise whole-patient care; communicate with patients, their families and/or carers; and illuminate different progression trajectories for people in different clusters, which could in turn optimise the deployment of medical resources.
Latent class analysis is an effective method for identifying patterns in heterogeneous populations and is thus useful for filling the gap.17,18 Instead of generating all possible groupings, it classifies the data to the simplest clusters or classes. Researchers have identified subgroups of cancer patients using different measurements and have then distinguished the demographic and clinical characteristics of these subgroups.5,19,20 A retrospective study in Taiwan identified three subgroups among aggressive palliative care patients, with the ‘symptom crisis’ group accounting for more deaths in hospital.5 A Swedish retrospective study assessed differences in the causes of death among various subgroups and reported that 37.0% of patients with relieved pain died of comorbid circulatory conditions and 18.8% died of comorbid dementia.19 Furthermore, a collaborative study between Australia and the United States identified four subgroups of distinct symptoms and reported their differences in relation to quality of life according to demographic and clinical characteristics.20 However, the current literature review indicates a dearth of studies attempting to clarify comprehensive comorbidity patterns in the context of palliative care.
Therefore, using latent class modelling techniques, this study aims to identify patterns of complex comorbidities in cancer patients receiving palliative care, characterise demographic and clinical factors of patients in each identified subgroup and evaluate the association between subgroup characteristics and in-hospital deaths.
Methods
Data source
The New South Wales (NSW) Admitted Patient Data Collection (APDC) maintained by the NSW Health Department is a complete census of inpatient data from NSW hospitals. It covers all NSW public, private and repatriation hospitals in addition to private outpatient centres. The APDC data sets comprise inpatients’ demographic and clinical information. Clinical reasons for hospital care were coded at separation using the Australian Modification of 10th version of International Statistical Classification of Diseases and Related Health Problems (ICD-10 AM).21
In accordance with the Data Use Agreement established with the NSW Health Department, we selected cancer patients (ICD10-AM C codes) hospitalised for palliative care (Z51.5). There were multiple updates to the ICD-10 AM during the study period, which did not affect these codes. The study only considered cases for acute care (n = 48,553) to reduce the impact of multiple counting. Of the study population, approximately 2.7% (n = 1288) of study subjects were excluded on account of unknown age, gender, marital status, or postal residential address. Ethical approval was granted by the Australian National University Science & Medical Delegated Ethics Review Committee (#2016/030).
Measures
Latent indicator variables were comorbid disease groups (based on ICD-10 AM diagnosis codes). To ensure the presence within each latent class of sufficient numbers of patients with each disease group and make the comorbidity characteristics of patients in each subgroup more prominent, it was necessary to reject those comorbidities within the data set with a prevalence rate <10% (e.g. skin disorders and sensory impairment) or >85% (e.g. the commonly observed osteoporosis among the study population). Finally, 11 concurrent disease groups were considered for latent class analysis. These were infectious diseases, blood immune diseases, endocrine diseases, mental and behavioural disorders, nervous diseases, circulatory diseases, respiratory diseases, digestive diseases, genitourinary diseases, injury and poisoning and symptoms and signs. The presence of each comorbidity group was categorised as ‘yes’ or ‘no’.
Distal outcome variable was in-hospital deaths, categorised as alive or death at separation. Patients’ demographic and clinical characteristics were considered for latent mixture modelling, that is, sex as male or female; age group as under 65, 65–84, or 85+ years; marital status as partnered or single; rurality of residence as urban or rural; private insurance as yes or no; ICU use as yes or no; and severity of comorbidities as minor (Charlson Index score = 0), moderate (Charlson Index score = 1 or 2), or severe (Charlson Index score ⩾ 3) using the modified Charlson Index.22 Financial year was treated as a continuous variable.
Statistical analysis
We used Stata 15.1 to calculate frequencies and proportions. We used Mplus 7.4 to run a series of latent class models to examine clusters of complex comorbidities in the study population. Stage 1 – latent class analysis: the five-class model was selected through a sequential comparison of k-class and (k − 1)-class model parameters (k starts from 2) (Table 1), with number of groups chosen based on interpretability, smaller value of the Akaike information criterion (AIC), the Bayesian information criterion (BIC), and the sample size–adjusted Bayesian information criterion (SABIC), as well as no significant improvement as assessed by the Lo–Mendell–Rubin (LMR) likelihood ratio test, and a relatively higher entropy score.23–25 These model-based criteria primarily utilising the log-likelihood value, penalising model complexity and considering sample size, are the most recommended to select the number of classes. We compared all models using the BIC difference between k- and (k − 1)-class models and observed a substantial decrease in BIC difference from 18,925.6 (between the two-class and one-class models) to 323.1 (between the six-class and five-class models). Although the six-class model had the smallest BIC, the BIC difference in comparison with the five-class model was relative small. Given a non-statistically significant result of LMR likelihood ratio test when comparing the six- and five-class models, we further plotted conditional probability for each latent indicator in the specific class, visualised the difference of class profile between models, and observed little material change in terms of class interpretation. Based on a non-statistically significant difference in the LMR likelihood ratio test comparing the five- and six-class models, as well as interpretable classes and maximum membership probability to make a final class assignment,18 the parsimonious five-class model was selected as the final model. To encapsulate the profile of each class, the conditional response probabilities of each latent indicator were plotted for each latent class. Stage 2 – latent class with the distal outcome: the identified latent class membership in relation to the distal outcome (in-hospital death) was further quantified in terms of odds ratio (OR) and 95% confidence interval (CI), with statistical significance set as a p value < 0.05.23,26,27 Considering underestimation error,18,23,26 we used the 3-STEP approach in all subsequent regression analyses to correct the estimation error.26 Stage 3 – regression mixture modelling: the identified latent class membership acting as a moderator was added to the regression model of independent variables (demographic and clinical characteristics) and the dependent variable (death outcome).26
Table 1.
Model fit statistics for latent class model.
| Number of classes | Akaike information criterion | Bayesian information criterion | Sample size–adjusted Bayesian information criterion | p Value for Lo–Mendell–Rubin likelihood ratio test | p Value for Bootstrap likelihood ratio test | Entropy |
|---|---|---|---|---|---|---|
| 1 | 498,902.178 | 498,998.577 | 498,963.619 | – | – | – |
| 2 | 479,871.445 | 480,073.006 | 479,999.912 | 0.0000 | 0.0000 | 0.558 |
| 3 | 478,229.563 | 478,536.287 | 478,425.056 | 0.0000 | 0.0000 | 0.503 |
| 4 | 477,056.045 | 477,467.930 | 477,318.564 | 0.0000 | 0.0000 | 0.636 |
| 5 | 476,068.077 | 476,585.125 | 476,397.623 | 0.0000 | 0.0000 | 0.604 |
| 6 | 475,639.791 | 476,262.001 | 476,036.362 | 0.1216 | 0.0000 | 0.527 |
Results
Of the study population (47,265 cancer patients receiving palliative care), there were more males (56.2%), more aged 65–84 years (56.5%), more with partners (59.7%), more living in urban areas (91.5%), and more without private health insurance (73.1%) (Table 2). Approximately 43.5% (n = 20,573) patients died in hospitals.
Table 2.
Study population demographic and clinical characteristics during July 2001 to June 2014 (n = 47,265).
| Total | Class 1: Multiple comorbidities and symptoms | Class 2: More symptoms | Class 3: Few comorbidities | Class 4: Genitourinary and infection | Class 5: Circulatory and endocrine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Gender | ||||||||||||
| Male | 26,552 | 56.2 | 2460 | 56.9 | 7379 | 57.6 | 10,457 | 56.2 | 2052 | 49.8 | 4204 | 57.0 |
| Female | 20,713 | 43.8 | 1862 | 43.1 | 5440 | 42.4 | 8164 | 43.8 | 2072 | 50.2 | 3175 | 43.0 |
| Age group (years) | ||||||||||||
| <65 | 15,217 | 32.2 | 1127 | 26.1 | 4597 | 35.9 | 6719 | 36.1 | 1120 | 27.2 | 1654 | 22.4 |
| 65–84 | 26,683 | 56.4 | 2622 | 60.7 | 6921 | 54.0 | 10,054 | 54.0 | 2352 | 57.0 | 4734 | 64.2 |
| 85+ | 5365 | 11.4 | 573 | 13.2 | 1301 | 10.1 | 1848 | 9.9 | 652 | 15.8 | 991 | 13.4 |
| Marital status | ||||||||||||
| Partnered | 28,198 | 59.7 | 2483 | 57.4 | 7605 | 59.3 | 11,558 | 62.1 | 2364 | 57.3 | 4188 | 56.8 |
| Single | 19,067 | 40.3 | 1839 | 42.6 | 5214 | 40.7 | 7063 | 37.9 | 1760 | 42.7 | 3191 | 43.2 |
| Private insurance | ||||||||||||
| Yes | 12,731 | 26.9 | 1113 | 25.8 | 3549 | 27.7 | 5176 | 27.8 | 1144 | 27.7 | 1749 | 23.7 |
| No | 34,534 | 73.1 | 3209 | 74.2 | 9270 | 72.3 | 13,445 | 72.2 | 2980 | 72.3 | 5630 | 76.3 |
| Rurality of residence | ||||||||||||
| Rural | 4031 | 8.5 | 129 | 3.0 | 747 | 5.8 | 2409 | 12.9 | 231 | 5.6 | 515 | 7.0 |
| Urban | 43,234 | 91.5 | 4193 | 97.0 | 12,072 | 94.2 | 16,212 | 87.1 | 3893 | 94.4 | 6864 | 93.0 |
| Severity of comorbidities | ||||||||||||
| Minor | 8765 | 18.5 | 499 | 11.5 | 2384 | 18.6 | 4315 | 23.2 | 673 | 16.3 | 894 | 12.1 |
| Moderate | 6741 | 14.3 | 664 | 15.4 | 2053 | 16.0 | 2147 | 11.5 | 644 | 15.6 | 1233 | 16.7 |
| Severe | 31,759 | 67.2 | 3159 | 73.1 | 8382 | 65.4 | 12,159 | 65.3 | 2807 | 68.1 | 5252 | 71.2 |
| Intensive care | ||||||||||||
| Yes | 706 | 1.5 | 307 | 7.1 | 170 | 1.3 | 50 | 0.3 | 59 | 1.4 | 120 | 1.6 |
| No | 46,559 | 98.5 | 4015 | 92.9 | 12,649 | 98.7 | 18,571 | 99.7 | 4065 | 98.6 | 7259 | 98.4 |
Stage 1
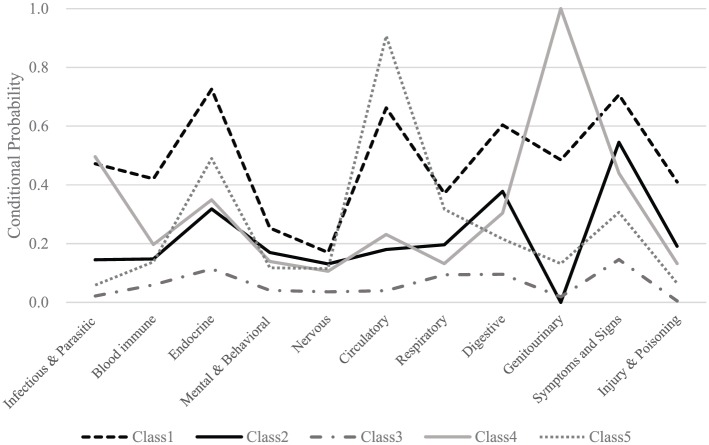
Figure 1 illustrates the conditional probabilities for comorbidity groups in relation to five latent classes, based on multiple model selection criteria. The univariate entropy ranged from 0.12 to 0.28 for these comorbidity groups (Table 3), reflecting a less clear separation of the classes based on their respective contribution alone, but a more realistic complex palliative care setting. Nevertheless, as indicated by the overall larger entropy (0.604), some of the classes have been very clearly distinguished. Class 1 consisted of 9.1% of the patients with a consistently high presence of all comorbidities, comprising a ‘typical’ patient with diabetes, hypertension, chronic kidney disease, functional intestinal disorders, urinary incontinence, nausea, vomiting, and cognitive impairment, referred to here as the ‘multiple comorbidities and symptoms’ group (Table 3). Approximately 27.1% of the patients were allocated membership of Class 2, referred to here as the ‘more symptoms’ group, comprising a ‘typical’ patient with functional intestinal disorders and volume depletion symptoms. While more than half Class 2 patients (54.5%) had clinical symptoms and signs such as nausea, vomiting, cognitive impairment, dysphagia, and oedema, there were no diagnoses of genitourinary diseases. Class 3 represented the largest latent subgroup (39.4%) and was classified as the ‘few comorbidities’ group, comprising a ‘typical’ patient with very few comorbid conditions, particularly in contrast to Class 1 patients with apparent presence of multiple comorbidities. The smallest group was Class 4 (8.7%), referred to here as the ‘genitourinary and infection’ group comprising a ‘typical’ patient with urinary disorders, acute kidney failure, and bacterial infections. Class 5 (15.6% of the study population) represented the ‘circulatory and endocrine’ group comprising a ‘typical’ patient with hypertension, chronic ischaemic heart disease, and diabetes. Cancer patients with chronic obstructive pulmonary disease (COPD) were also observed in the Class 5 group.
Figure 1.
Conditional probability for each comorbidity group indicator by class.
Table 3.
Five-class model: prevalence of latent classes and conditional probabilities.a
| Univariate entropy | Total (%) | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |
|---|---|---|---|---|---|---|---|
| 9.14% | 27.12% | 39.40% | 8.73% | 15.61% | |||
| N = 4322 | N = 12,819 | N = 18,621 | N = 4124 | N = 7379 | |||
| Multiple comorbidities and symptoms | More symptoms | Few comorbidities | Genitourinary and infection | Circulatory and endocrine | |||
| Infectious and parasitic | 0.171 | 15.2 | 0.472 | 0.145 | 0.022 | 0.496 | 0.059 |
| Blood immune | 0.134 | 14.8 | 0.421 | 0.148 | 0.060 | 0.197 | 0.138 |
| Endocrine | 0.166 | 31.1 | 0.726 | 0.318 | 0.114 | 0.349 | 0.490 |
| Mental and behavioural | 0.126 | 12.2 | 0.253 | 0.170 | 0.041 | 0.139 | 0.118 |
| Nervous | 0.119 | 10.0 | 0.170 | 0.131 | 0.036 | 0.106 | 0.115 |
| Circulatory | 0.251 | 27.5 | 0.662 | 0.180 | 0.040 | 0.231 | 0.907 |
| Respiratory | 0.128 | 18.7 | 0.371 | 0.196 | 0.094 | 0.132 | 0.317 |
| Digestive | 0.155 | 27.2 | 0.604 | 0.378 | 0.096 | 0.304 | 0.216 |
| Genitourinary | 0.279 | 15.5 | 0.485 | 0.000 | 0.019 | 1.000 | 0.132 |
| Symptoms and signs | 0.168 | 37.6 | 0.707 | 0.545 | 0.146 | 0.440 | 0.307 |
| Injury and poisoning | 0.158 | 12.5 | 0.410 | 0.191 | 0.005 | 0.132 | 0.065 |
Conditional probability value greater than and approximating 0.5 are given in bold.
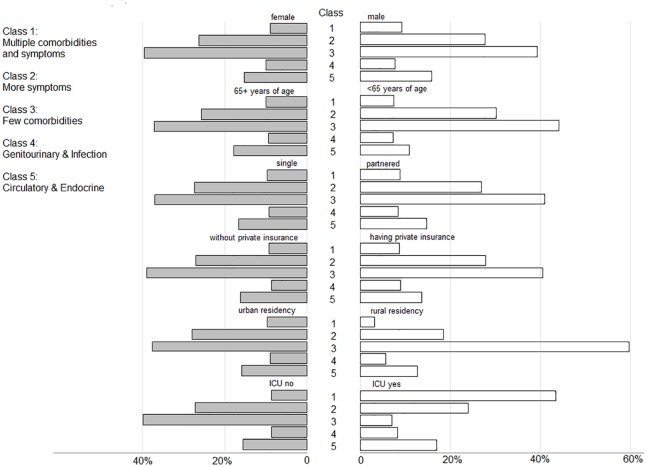
In contrast to the ‘few comorbidity’ group, rural residency was under-represented in the other groups, in particular, the ‘multiple comorbidities and symptoms’ group (Figure 2). Relative to those patients aged 65 years and above, younger patients appeared to be over-represented with ‘more symptoms’ and ‘few comorbidities’. Patients in the ‘multi-comorbidities and symptoms’, ‘more symptoms’ or ‘circulatory and endocrine’ groups accounted for the majority of ICU care, and female patients were over-represented in the ‘genitourinary and infection’ group.
Figure 2.
Selected characteristics, proportion by class.
Stage 2
In-hospital mortality is the highest for the ‘few comorbidities’ group (modelled probability: 0.55), followed by ‘circulatory and endocrine’ group (0.47), ‘multiple comorbidities and symptoms’ group (0.41), ‘genitourinary and infection’ group (0.40) and ‘more symptoms’ group (0.32). In comparison with those in the ‘few comorbidities’ group, patients in the ‘circulatory and endocrine’, ‘multiple comorbidities and symptoms’, ‘genitourinary and infection’ and ‘more symptoms’ groups were 29% (95% CI: 16%–40%), 44% (36%–50%), 46% (39%–52%) and 63% (59%–66%), respectively, less likely to die in hospitals.
Stage 3
Female patients with any complex comorbidity patterns were less likely to die in hospital (Table 4). While older age seemed protective in the ‘multiple comorbidities and symptoms’ group, it was associated with elevated risk of in-hospital deaths in other groups. Having private health insurance were associated with elevated mortality in patients from the ‘multiple comorbidities and symptoms’, ‘more symptoms’ and ‘few comorbidities’ groups. ICU care was associated with a reduced risk of in-hospital deaths for those presenting with more symptoms (Class 2) or few comorbidities (Class 3). The likelihood of in-hospital death increased over years in the ‘more symptoms’, ‘few comorbidities’, ‘genitourinary and infection’ and ‘circulatory and endocrine’ groups.
Table 4.
Regression mixture model for death outcome.
| Class 1: Multiple comorbidities and symptoms | Class 2: More symptoms | Class 3: Few comorbidities | Class 4: Genitourinary and infection | Class 5: Circulatory and endocrine | |
|---|---|---|---|---|---|
| Odds ratio | Odds ratio | Odds ratio | Odds ratio | Odds ratio | |
| Female | 0.87a | 0.92a | 0.92a | 0.76a | 0.91b |
| Age | |||||
| 65–84 | 0.95 | 1.22a | 1.34a | 1.11 | 1.14a |
| 85+ | 0.83a | 1.23a | 1.34a | 1.16 | 1.18b |
| Single | 1.00 | 0.88a | 0.89a | 0.99 | 0.87a |
| Private insurance | 1.20a | 1.17a | 1.14a | 1.11 | 1.02 |
| Financial year | 1.00 | 1.02a | 1.06a | 1.02b | 1.02a |
| Rural residence | 1.16 | 1.46a | 1.21a | 1.33b | 1.49a |
| Severity of comorbidities | |||||
| Moderate | 0.97 | 0.75a | 0.57a | 0.84 | 0.86b |
| Severe | 1.20a | 1.15a | 1.06 | 1.23b | 1.05 |
| ICU | 1.37a | 0.78a | 0.34a | 0.79 | 0.98 |
ICU: intensive care unit.
p value < 0.01.
p value < 0.05.
Discussion
This population-based study focusing on palliative cancer care in hospitals describes clusters of comorbidity burden variation in patients and suggests there may be challenges in the allocation of limited medical resources to these patients in the Australian acute hospital care setting. Although palliative cancer care is a high-priority research area in Australia and internationally, there is limited understanding of comorbidity patterns and how these vary across patient groups. To the best of our knowledge, this research constitutes the first latent class analysis of complex comorbidities in cancer patients for palliative care. The results indicate that multiple comorbidities cluster in unique ways. It was noted that females were less likely to die in hospital than males across all groups, which is consistent with previous research.28 Similarly, all rural patients experienced a higher probability of death in hospitals, perhaps implying a general lack of acute medical resources in rural areas. Other than these, the probability of in-hospital deaths and associated risk factor profiles varies across these clusters. These findings point to the potential for optimising appropriate comorbidity care by focusing on these different patterns, which could assist hospital professionals to improve palliative care for cancer patients as well as to develop countermeasures and reduce in-hospital deaths.
The ‘few comorbidities’ group accounted for 40% of the study population and demonstrated the highest in-hospital mortality. There are two possible explanations of the observed high hospital mortality in this group. First, while in general ICU use may reduce risks of in-hospital deaths, in these patients ICU admission may not be consistent with the goals of care and this could explain the lowest ICU admission rates observed in this group, and, in turn, explain the higher observed mortality rates. Second, most patients had partners and families and possessed private health insurance. Both factors might positively correlate with the adoption of more aggressive medical care in an attempt to extend life without full comprehension of the patients’ prognosis. However, both propositions are speculative and require further research to confirm their validity.
The in-hospital mortality among patients in the ‘circulatory and endocrine’ group was the second highest. No evidence was found that high use of ICU in this group significantly reduced the in-hospital deaths of these patients. Furthermore, patients with partners were associated with elevated risk of in-hospital deaths. One possible explanation would be again related to excessive use of more aggressive care. It is worth noting, therefore, that the association between family involvement in clinical decisions and the extensive use of aggressive palliative care is an area warranting further investigation. For cancer patients presenting with ‘circulatory and endocrine’ comorbidities, clinical decisions should involve proper selection of appropriate care pathways to avoid excessive and ineffective aggressive end-of-life care.
Patients in the ‘multiple comorbidities and symptoms’ group had the third highest probability of in-hospital deaths. Because the comorbidity pattern of this group demonstrated the most complex profile, comprehensive care plans for this group would become more complex. ICU care in this group was commonplace, which somehow demonstrated a risk proxy for in-hospital death. This finding raises a question as to whether patients in this group have had their end-of-life care preferences explored and documented, including whether admission to an ICU is necessary, given that previous studies have reported aggressive ICU care in similar patients did not enhance lifespan.29 In addition, holding private health insurance may make adoption of aggressive care more affordable in this group and, in turn, affect patients’ survival. When patients access palliative care with complex comorbidity profiles, there is a need for clinicians to carefully discuss with them the potential benefits and harms of different interventions, including admission to ICU, and document these to mitigate the risk of adopting ineffective interventions during their hospital stay.
The in-hospital mortality rate for the ‘genitourinary and infection’ group was lower than the previous three groups, possibly because the majority of these patients were females in whom the infections might be diagnosed then treated at an early stage. Considering infection was one of the leading causes of death in cancer patients,16 the synergetic effect of infection and other comorbid conditions may affect a patient’s chance of survival. For cancer patients with a ‘genitourinary and infection’ comorbidity pattern, palliative care would focus on the provision of appropriate management and control of infections.
The ‘more symptoms’ group had the lowest hospital mortality rate. These patients were the youngest of all the groups, which may contribute to the relatively low mortality rate for the entire group on average. ICU care reduced the risk of in-hospital death in these patients, which indicates that these patients were in a relatively ICU appropriate condition. Treatment focused on symptom relief would potentially meet their acute care needs.30–32 Therefore, palliative care delivery for them may primarily involve multiple symptom relief treatment plans and consider the use of ICU care to alleviate symptoms and improve survival.
Future directions and implications
Palliative care often occurs in acute hospital care settings and palliative patients may not receive appropriate end-of-life care in hospitals. Using an all-inclusive inpatient data collection, this study shows how comorbidity burden varies and clusters in cancer patients during acute hospital care. Variation among the five groups identified in the study suggests person-centred care should include comorbidity care that is informed by these patterns. This study further demonstrates apparent heterogeneity of in-hospital mortality in cancer patients with various comorbidity burdens. Combining the comorbidity patterns with the demographic and clinical characteristics of cancer patients may assist clinicians optimise care and plan resource allocation. Training in comorbidity pattern identification and person-centred adverse outcome evaluation for clinicians is recommended in different palliative care contexts.
Given the globally recognised right to high-quality care for all at the end of life,33 it is important to tailor personalised palliative care programmes for all cancer patients that target the most relevant comorbidity patterns. Currently, there are multiple programmes aiming to improve the quality and safety of palliative care.34–36 This study adds to the supportive evidence related to these programmes and emphasises the importance of identification of comorbidity patterns to improve care outcomes, which in turn facilitates the establishment of appropriate palliative care programmes, thereby not only avoiding deaths in hospital but also improving patient quality of life as a global achievement.
Limitations
Although model selection criteria based on model-derived statistics are recommended to determine the number of latent classes, trade-offs exist whether these statistical criteria act in concert or not. Clinical knowledge of the subject area is also important and required to guide model selection. In this study, we carried out a careful model comparison strategy considering the plausibility, interpretability, and parsimony, in addition to the statistical approach, and therefore our results are somewhat robust explaining the likely patterns of comorbidities in cancer patients receiving palliative care in NSW hospitals. Nonetheless, appropriate care should be given to interpret the identified number of classes when comparing results from other study settings. There are several limitations in this study. First, it is a descriptive study of observed patterns in an administrative data set and does not explain the underlying reasons for those observed patterns. It is possible that some cancer patients received appropriate palliative care and opted to die in hospitals as their preferred place of death.37,38 Second, the use of administrative APDC data limited the ability to comprehensively consider palliative care scenarios for risk adjustment. Based on a limited range of predefined data fields, the regression analysis was unable to differentiate effects from unmeasured factors or by chance alone. It would be prudent to be cautious extrapolating beyond the current study setting. Third, the entropy value (0.604) of the five-class model indicated errors in patient group assignment, and therefore association results should be interpreted with caution. Nonetheless, the identified patterns of comorbidities seem clinically relevant and thus an informative guide for those seeking to improve palliative care for cancer patients.
Conclusion
This study confirmed the heterogeneity and complexity of comorbidity profiles as well as their relationship to in-hospital deaths in cancer patients during their palliative care. Recognition of various patterns of comorbidities and identification of risk factors for in-hospital deaths provide an avenue to develop appropriate palliative care strategies with attention to improve care these patients deserve at the end of their lives, passing on in peace and dignity instead of uneasiness and fear.
Footnotes
Author contributions: W.D. made substantial contributions to study conception, design, and analysis. N.G. made a substantial contribution to data acquisition and interpretation. L.L. was a major contributor in the study design, data analysis, result interpretation, and the first draft. W.D., S.C., H.J. and N.G. contributed to design, analysis, and interpretation of data and revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Wei Du  https://orcid.org/0000-0003-3622-2265
https://orcid.org/0000-0003-3622-2265
References
- 1. Rome RB, Luminais HH, Bourgeois DA, et al. The role of palliative care at the end of life. Ochsner J 2011; 11(4): 348–352. [PMC free article] [PubMed] [Google Scholar]
- 2. Chan R, Webster J. End-of-life care pathways for improving outcomes in caring for the dying. Cochrane Database Syst Rev 2010; 1: CD008006. [DOI] [PubMed] [Google Scholar]
- 3. Cotogni P, Saini A, De Luca A. In-hospital palliative care: should we need to reconsider what role hospitals should have in patients with end-stage disease or advanced cancer? J Clin Med 2018; 7(2): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henson LA, Gomes B, Koffman J, et al. Factors associated with aggressive end of life cancer care. Support Care Cancer 2016; 24(3): 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen ML, Chen YY, Tang ST. Latent class analysis identifies three subtypes of aggressive end-of-life care: a population-based study in Taiwan. Eur J Cancer 2013; 49(15): 3284–3291. [DOI] [PubMed] [Google Scholar]
- 6. Cooke CR, Feemster LC, Wiener RS, et al. Aggressiveness of intensive care use among patients with lung cancer in the surveillance, epidemiology, and end results-Medicare registry. Chest 2014; 146(4): 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 2011; 29(12): 1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Definition of palliative care. http://www.who.int/cancer/palliative/definition/en/ (accessed 5 March 2019).
- 9. Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 2018; 19(11): e588–e653. [DOI] [PubMed] [Google Scholar]
- 10. Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract 2011; 7(6): 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 2016; 66: 337–350. [DOI] [PubMed] [Google Scholar]
- 12. Sogaard SM, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol 2013; 5(Suppl. 1): 3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol 2011; 29(10): 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014; 120(9): 1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iversen LH, Nørgaard M, Jacobsen J, et al. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006 – a population-based cohort study. Dis Colon Rectum 2009; 52(1): 71–78. [DOI] [PubMed] [Google Scholar]
- 16. Nagy-Agren S, Haley H. Management of infections in palliative care patients with advanced cancer. J Pain Symptom Manage 2002; 24(1): 64–70. [DOI] [PubMed] [Google Scholar]
- 17. Lanza ST, Rhoades BL. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci 2013; 14(2): 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohlen J, Russell L, Hakanson C, et al. Variations in care quality outcomes of dying people: latent class analysis of an adult national register population. J Pain Symptom Manage 2017; 53(1): 13–24. [DOI] [PubMed] [Google Scholar]
- 19. Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage 2015; 50(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the social, behavioral, and health sciences. Hoboken, NJ: John Wiley & Sons, 2010. [Google Scholar]
- 21. National Centre for Classification in Health. International statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM). 7th ed. Sydney, NSW, Australia: NCCH, 2010. [PubMed] [Google Scholar]
- 22. Quan H, Li B, Couris C, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173(6): 676–682. [DOI] [PubMed] [Google Scholar]
- 23. VanHorn ML, Jaki T, Masyn K, et al. Assessing differential effects: applying regression mixture models to identify variations in the influence of family resources on academic achievement. Dev Psychol 2009; 45(5): 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling 2007; 14(4): 535–569. [Google Scholar]
- 25. Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Soc Person Psychol Compass 2008; 2(1): 302–317. [Google Scholar]
- 26. Bakk Z, Tekle FB, Vermunt JK. Estimating the association between latent class membership and external variables using bias-adjusted three-step approaches. Sociol Methodol 2013; 43(1): 272–311. [Google Scholar]
- 27. Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: three-step approaches using Mplus. Struct Equ Modeling 2014; 21(3): 329–341. [Google Scholar]
- 28. Houttekier D, Cohen J, Pepersack T, et al. Dying in hospital: a study of incidence and factors related to hospital death using death certificate data. Eur J Public Health 2013; 24(5): 751–756. [DOI] [PubMed] [Google Scholar]
- 29. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008; 300(14): 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin 2018; 68: 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baltenberger EP, Schmitt G, Thomas CJ. Treatment of depressive symptoms in patients with cancer. Ment Health Clin 2014; 4(3): 114–117. [Google Scholar]
- 32. Wilkinson S, Lockhart K, Gambles M, et al. Reflexology for symptom relief in patients with cancer. Cancer Nurs 2008; 31(5): 354–360, quiz 361. [DOI] [PubMed] [Google Scholar]
- 33. Junger S, Payne S, Brearley S, et al. Consensus building in palliative care: a Europe-wide Delphi study on common understandings and conceptual differences. J Pain Symptom Manage 2012; 44(2): 192–205. [DOI] [PubMed] [Google Scholar]
- 34. Department of Health. National palliative care projects. Canberra, ACT, Australia: Australia Government Department of Health, 2018. [Google Scholar]
- 35. Clinical Excellence Commission. End of life program. Sydney, NSW, Australia: NSW Clinical Excellence Commission, 2018. [Google Scholar]
- 36. Agency for Clinical Innovation. NSW ambulance authorised palliative care plans. Sydney, NSW, Australia: NSW Agency for Clinical Innovation, 2015. [Google Scholar]
- 37. Choi J, Miyashita M, Hirai K, et al. Preference of place for end-of-life cancer care and death among bereaved Japanese families who experienced home hospice care and death of a loved one. Support Care Cancer 2010; 18(11): 1445–1453. [DOI] [PubMed] [Google Scholar]
- 38. Virdun C, Luckett T, Davidson PM, et al. Dying in the hospital setting: a systematic review of quantitative studies identifying the elements of end-of-life care that patients and their families rank as being most important. Palliat Med 2015; 29(9): 774–796. [DOI] [PMC free article] [PubMed] [Google Scholar]