Abstract
Objective
To compare effectiveness of onabotulinumtoxinA and topiramate for chronic migraine (CM) prevention.
Background
The efficacy* of onabotulinumtoxinA and topiramate has been established in placebo‐controlled randomized clinical trials (*defined as the benefit of treatment under ideal conditions). The effectiveness* of the 2 preventive treatments, however, has not been established (*the benefit of treatment under real‐world conditions, representing a blend of efficacy and tolerability).
Methods
In this multicenter, randomized, parallel‐group, post‐authorization, open‐label prospective study (FORWARD; http://ClinicalTrials.gov, NCT02191579), we randomized adults with CM (1:1) to onabotulinumtoxinA 155 U every 12 weeks for 3 cycles or topiramate “immediate release” 50‐100 mg/day to week 36. Primary outcome measure was proportion of patients achieving ≥50% reduction in headache days (weeks 29‐32). Missing values were imputed using baseline observation carried forward (BOCF) methodology. After 12 weeks, patients initially randomized to topiramate could cross over to onabotulinumtoxinA treatment. We monitored and recorded all adverse events (AEs).
Results
We enrolled 282 patients (onabotulinumtoxinA, n = 140; topiramate, n = 142) and 148 patients completed randomized treatment (onabotulinumtoxinA, n = 120 [86%]; topiramate, n = 28 [20%]). Primary reasons for withdrawal were ineffective treatment (onabotulinumtoxinA, n = 7 [5%]; topiramate, n = 27 [19%]) and AEs (onabotulinumtoxinA, n = 5 [4%]; topiramate, n = 72 [51%]). Eighty topiramate patients crossed over to onabotulinumtoxinA.
In the BOCF analysis, a significantly higher proportion of patients randomized to onabotulinumtoxinA experienced ≥50% reduction in headache frequency compared with those randomized to topiramate (40% [56/140] vs 12% [17/142], respectively; adjusted OR, 4.9 [95% CI, 2.7‐9.1]; P < .001). OnabotulinumtoxinA was superior to topiramate in meeting secondary endpoints. In a post hoc analysis using observed data, the 50% responder rates at week 12 were 45.6% for onabotulinumtoxinA (n = 125) and 29.4% for topiramate (n = 109) (P = .015). AEs were reported by 48% (105/220) of onabotulinumtoxinA and 79% (112/142) of topiramate patients. Results were similar in those who crossed over to onabotulinumtoxinA.
Conclusions
While using imputation methods of accounting for differences in discontinuation rates, we found onabotulinumtoxinA to have greater clinical utility than topiramate, largely because of tolerability issues associated with the latter and a relatively higher number of onabotulinumtoxinA patients remaining on treatment.
Keywords: botulinum toxin, topiramate, chronic migraine prevention, safety, clinical utility
Abbreviations
- AE
adverse event
- ANCOVA
analysis of covariance
- BOCF
baseline observation carried forward
- C‐SSRS
Columbia‐Suicide Severity Rating Scale
- CM
chronic migraine
- EM
episodic migraine
- HIT‐6
6‐item Headache Impact Test
- ITT
intent‐to‐treat
- mLOCF
modified last observation carried forward
- OR
odds ratio
- RCT
randomized controlled trial
Introduction
OnabotulinumtoxinA (Botox®, Allergan plc, Dublin, Ireland) was the first drug approved specifically for chronic migraine (CM) and is currently approved worldwide.1, 2, 3, 4, 5 In the pivotal phase 3 studies, onabotulinumtoxinA was well tolerated and few discontinued treatment because of adverse events (AEs).1, 6, 7, 8 The favorable benefit:risk profile of onabotulinumtoxinA has been confirmed in a large 2‐year open‐label study9 and a real‐world study.10
Topiramate “immediate release” (Topamax®, Janssen Pharmaceuticals, Inc., Titusville, NJ) is approved for prevention of migraine in adults and is commonly prescribed.11, 12 A first‐line preventive treatment according to practice guidelines, topiramate is associated with systemic AEs such as paresthesia, anorexia, fatigue, nausea, diarrhea, weight decrease, cognitive issues, and mood problems; AE‐associated discontinuation rates can be substantial.11, 13 For example, in an episodic migraine (EM) trial, the discontinuation rate in topiramate‐treated patients was 27% (at the recommended daily dose of 100 mg) compared with 12% in placebo‐treated patients.11
Both onabotulinumtoxinA and topiramate have been demonstrated to be effective in CM in randomized controlled trials (RCTs).6, 7, 8, 12 Results from 2 pivotal phase 3 trials (PREEMPT 1/2) indicated that onabotulinumtoxinA significantly reduces frequency of headache and migraine days compared with placebo.6, 8 Investigators also found that topiramate significantly reduced the mean number of migraine/migrainous days per month compared with placebo.7, 12 In a pilot study comparing onabotulinumtoxinA and topiramate in CM patients, efficacy was similar but AE profiles differed qualitatively and quantitatively.14 In summary, these 2 preventive medications appear to have established efficacy for prevention of CM but different tolerability profiles.
In clinical practice, tolerability can be a major contributor to medication adherence and persistence.15, 16 Efficacy, addressing the benefit of a treatment under ideal conditions, is best measured in RCTs,17 which already have been undertaken for onabotulinumtoxinA and topiramate. Effectiveness, a blend of efficacy and tolerability, refers to the benefit of a treatment under “real‐world” conditions and is typically assessed in studies with broader entry criteria and designs that prioritize generalizability once efficacy has been established. In 1967, Schwartz and Lellouch first used the term “pragmatic” to describe trials that assess an intervention in clinical practice to determine which of 2 treatments should be preferred.18 Pragmatic trials are useful for accelerating integration of research outcomes into policy and clinical practice19 and can serve to complement RCTs.20 The National Institutes of Health and the Patient‐Centered Outcomes Research Initiative support and promote pragmatic clinical trial methodology to obtain information directly relevant for the needs of healthcare providers.21 Such trials have been conducted in chronic pain, schizophrenia, depression, multiple sclerosis, headache, and migraine.22, 23, 24, 25, 26, 27, 28, 29, 30
The primary goal of the FORWARD study was to compare the effectiveness of onabotulinumtoxinA and topiramate in preventive treatment of CM via a pragmatic design and to explore the clinical utility of onabotulinumtoxinA in those who discontinued topiramate.
Methods
Study Design
The FORWARD Study (http://www.clinicaltrials.gov, NCT02191579) was a multicenter, randomized, parallel‐group, post‐authorization, open‐label, prospective study designed to reflect clinical practice. The study was conducted from August 2014 to September 2017, and the protocol and statistical analysis plan are available on http://www.clinicaltrials.gov under the trial registration number. Prospective patients were recruited through a number of channels, including identification by study staff, flyers and posters in physicians' offices, advertising, physician‐to‐physician referral, and by an email campaign. We used an interactive web response system to obtain unique patient numbers, which were used to identify patient electronic diaries (eDiaries, electronic case report forms, and electronic clinical outcome assessments), and to randomize (1:1) adult patients with CM to receive either onabotulinumtoxinA 155 U at day 1, week 12 ±7 days, and week 24 ±7 days or topiramate titrated to 50‐100 mg/day (Fig. 1A). We administered onabotulinumtoxinA 155 U into 31 sites including 7 specific head/neck muscle groups using the fixed‐site, fixed‐dose treatment paradigm approved by the US Food and Drug Administration. Similarly, the treatment paradigm we used for topiramate followed the approved label.13 We instructed patients to sequentially escalate their dose of topiramate by 25 mg/day weekly from an initial dose of 25 mg/day up to 100 mg/day or the maximum tolerable dose. Beginning at week 2, topiramate 50 mg/day was administered as 25 mg twice a day. Between weeks 4 and 12, a dose of topiramate of ≥50 mg/day was required, and at week 12 the investigator could adjust the topiramate dose within the 50‐100 mg/day dose range as tolerated, with the resulting dose to be maintained for the remainder of the study. If topiramate was discontinued, a taper period of 2 weeks was recommended.
Figure 1.

Study design. (A) The study comprised a 28‐day pretreatment (run‐in) period followed by randomization to either onabotulinumtoxinA or topiramate treatment that lasted up to 36 weeks. (B) Patients who discontinued topiramate treatment between weeks 12 and 36 could cross over to onabotulinumtoxinA and remain in the study until week 48.
The study comprised a screening visit, a 28‐day baseline screening period, the treatment period, and post‐treatment follow‐up lasting 12 weeks for those receiving onabotulinumtoxinA. For those completing study treatment as randomized, the final exit visit was at week 36.
Patients discontinuing topiramate treatment for any reason on or before week 36 could cross over to receive onabotulinumtoxinA treatment at their next scheduled study visit (ie, week 12, 24, or 36). These patients received onabotulinumtoxinA every 12 weeks up to and including the week 36 visit, for a maximum of 3 treatment sessions. OnabotulinumtoxinA treatment could be initiated during the topiramate taper period. For patients crossing over from topiramate to onabotulinumtoxinA, the final exit visit was 12 weeks after the last onabotulinumtoxinA treatment; patients who received onabotulinumtoxinA treatment at week 36 had their final visit at week 48 (Fig. 1B).
The study was conducted in accordance with Good Clinical Practice regulations and guidelines and was approved by participating sites' boards for review of clinical research.
Patients
Adult patients (aged 18‐65 years) with a diagnosis of CM according to the International Classification of Headache Disorders 3β criteria were eligible for inclusion.31 During the 28‐day baseline screening period, patients had to record ≥20 diary days indicating headache frequency, duration, and intensity; acute headache pain medication use; and Migraine Interictal Burden Scale score for days that headache was not reported. Patients were included if they reported ≥15 headache days. Patients taking other preventive treatments were eligible for enrollment if the dose had been stable and well tolerated for ≥12 weeks before screening and the patient was willing to maintain a stable dose. Patients were permitted to take prescription or over‐the‐counter acute headache pain medication, recording use in their daily diary. Patients who had previously received botulinum toxin or topiramate for any reason, pregnant patients, and those with a significant risk of suicide, assessed using the Columbia‐Suicide Severity Rating Scale (C‐SSRS), were excluded. Patients provided written informed consent prior to enrollment. Informed consent materials contained details of potential AEs of both treatments.
Outcome Measures
Patients were required to maintain daily eDiaries throughout the study. Other data were collected via questionnaires at follow‐up office visits. Headache days were assessed every day based on eDiary entries. Patient‐reported outcome evaluations were conducted both at office visits and via eDiary, but ample time was provided between these evaluations so as not to overburden patients with a need to enter long eDiary assessments at any one time point.
Primary Outcome
The primary outcome was the proportion of patients with a ≥50% decrease in headache days from baseline in the 4‐week period before week 32 (ie, weeks 29‐32). A headache day was defined as a calendar day with a headache of ≥4 hours' duration and/or a headache of any duration if acute migraine medication was taken. The primary time point was selected to assess effectiveness when topiramate had been fully titrated and after 3 cycles of onabotulinumtoxinA had been administered.
Secondary Outcomes
Secondary outcomes were change in headache days from baseline in the 4‐week period ending at week 32, change in 6‐item Headache Impact Test (HIT‐6) score from baseline for the 4 weeks ending at week 30 (ie, weeks 27‐30), and proportion of patients with a ≥70% decrease in headache days from baseline in the 4‐week period ending at week 32.
Exploratory Outcome Measure
For patients who discontinued topiramate for any reason and crossed over to onabotulinumtoxinA, effectiveness was assessed as the proportion of patients with a ≥50% decrease in headache days from baseline for each 28‐day interval of the study.
Other Efficacy Outcome Measure
The change from baseline in the number of acute headache pain medication usage days was evaluated based on patient diary records.
Safety and Tolerability
We monitored AEs, including relationship to study treatment, severity, and seriousness. At each office visit, C‐SSRS and pregnancy testing was completed. Because both medications are FDA‐approved for migraine, a data safety monitoring board was not convened, and the study was designed in accordance with FDA labeling.
If an AE occurred before treatment with onabotulinumtoxinA in patients crossing over from topiramate, we assigned the AE to topiramate. We assigned AEs occurring after treatment with onabotulinumtoxinA to either topiramate or onabotulinumtoxinA depending on the AE's apparent relationship to study treatment (“related,” “not related,” or “unknown”), as determined by the site investigator. If the investigator determined the AE was related to one of the treatments, that AE was attributed to that treatment only. If the relationship of the AE to a study treatment was unknown or considered to be related to both study treatments, that AE was included in both treatment groups, but only once in the total number of AEs for the treatments when combined.
Statistical Analyses
All statistical analyses were conducted using SAS software (SAS Institute, Inc, Cary, NC), version 9.3. Baseline demographics, characteristics, and AEs are reported descriptively. No interim analysis was planned or conducted.
A sample size of approximately 400 patients (200 patients per group) was required to provide 90% power to detect a treatment difference of 16%; 280 patients provided 80% power. The enrollment target was not met despite a 6‐month extension to 24 months; therefore, 80% power was deemed sufficient. The FDA was notified that enrollment was stopped because of recruitment challenges and not safety issues and that there would be a reduced sample size with reduced power to detect significant between‐group differences.
The intent‐to‐treat (ITT) population, including all randomized patients, was used to assess primary and secondary endpoints. Any patient receiving ≥1 dose of study treatment was included in the safety population. We included patients who crossed over to onabotulinumtoxinA in the safety population for both topiramate and onabotulinumtoxinA.
The primary outcome was a dichotomous variable (responder/nonresponder), defined as the proportion of patients with ≥50% reduction in headache days during the 28‐day period before week 32. The primary comparison between treatment groups was performed using a logistic regression model, adjusted by the baseline number of headache days as a covariate. The treatment effect was summarized using an adjusted odds ratio and 95% Wald confidence limits. The P value from the Wald Chi‐square test was also reported.
If a patient recorded ≥20 days of diary data for any 28‐day period, the headache day counts were prorated accordingly and rounded to the nearest whole number; patients with <20 days of data were set to missing for the time period. A baseline observation carried forward (BOCF) imputation method was used to impute missing values for all primary and secondary outcomes; missing values were replaced with the baseline value (eg, frequency of headache days during the 28‐day baseline period). If a patient had a missing value for any reason (eg, discontinuation due to AEs, lost to follow‐up, lack of efficacy), baseline data were used and the patient was considered a nonresponder. Sensitivity analyses for the primary outcome were undertaken using pro‐rated observed data (for patients with <28 and ≥20 days' diary data) and modified last observation carried forward (mLOCF) (see Appendix I in Supporting Information).
We used analysis of covariance (ANCOVA) to compare the change in headache days from baseline between treatment groups, with missing data handled as described for the primary outcome. Differences in HIT‐6 scores between treatment groups were compared using nonparametric rank ANCOVA with treatment as a factor and adjusting for the baseline HIT‐6 score. The ≥70% decrease in headache days was analyzed similar to the approach for the primary endpoint.
For all 2‐sided tests, P ≤ .05 was considered statistically significant. We used a hierarchical testing gatekeeping procedure to control for Type I error for multiple secondary endpoints. We ranked secondary outcomes in hierarchical order of clinical importance: (1) change in headache days, (2) change in HIT‐6 score, and (3) ≥70% headache day responder rates.
Results
Patient Disposition and Demographics
We enrolled a total of 282 patients at 35 sites (onabotulinumtoxinA, n = 140; topiramate, n = 142). Across both treatment groups, patients were primarily female (n = 239 [85%]) and white (n = 229 [81%]; Table 1). Baseline demographics and headache characteristics were similar across treatment groups, including concurrent use of other preventive treatments as well as common comorbidities of patients with CM such as anxiety, depression, and insomnia.
Table 1.
Patient Demographics and Medical History at Baseline
| OnabotulinumtoxinA (n = 140) | Topiramate (n = 142) | Total (N = 282) | Switched to OnabotulinumtoxinA (n = 80) | |
| Age, years, mean (SD) | 40.2 (11.7) | 39.4 (12.6) | 39.8 (12.1) | 39.0 (12.2) |
| Female, n (%) | 117 (84) | 122 (86) | 239 (85) | 68 (85) |
| Race, n (%) | ||||
| White | 111 (79) | 118 (83) | 229 (81) | 72 (90) |
| Black | 13 (9) | 8 (6) | 21 (7) | 3 (4) |
| Asian | 4 (3) | 2 (1) | 6 (2) | 2 (3) |
| Hispanic | 11 (8) | 12 (8) | 23 (8) | 3 (4) |
| Other | 1 (1) | 2 (1) | 3 (1) | 0 (0) |
| Use of headache preventive treatments, n (%) | 26 (18.6) | 25 (17.6) | 51 (18.1) | 16 (20.0) |
| BMI, kg/m2, mean (SD) | 28.9 (7.1) | 28.8 (6.5) | 28.8 (6.8) | 27.7 (6.5) |
| Patients with any medical history other than migraine* | 122 (87) | 127 (89.4) | 249 (88) | 77 (96) |
| Anxiety | 26 (19) | 33 (23) | 59 (21) | 21 (26) |
| Depression | 36 (26) | 30 (21) | 66 (23) | 19 (24) |
| Insomnia | 23 (16) | 25 (18) | 48 (17) | 15 (19) |
| Drug hypersensitivity | 19 (14) | 27 (19) | 46 (16) | 17 (21) |
| Seasonal allergy | 26 (19) | 37 (26) | 63 (22) | 19 (24) |
| Back pain | 17 (12) | 11 (8) | 28 (10) | 6 (8) |
| Neck pain | 6 (4) | 10 (7) | 16 (6) | 8 (10) |
| Gastroesophageal reflux disease | 15 (11) | 19 (13) | 34 (12) | 13 (16) |
| Asthma | 18 (13) | 12 (8) | 30 (11) | 7 (9) |
| Hypertension | 19 (14) | 19 (13) | 38 (13) | 10 (13) |
| Hypothyroidism | 17 (12) | 7 (5) | 24 (9) | 3 (4) |
Medical history as reported in ≥10% of patients in either treatment group.
BMI = body mass index.
A greater proportion (n = 120, 86%) of patients randomized to onabotulinumtoxinA completed study treatment versus those randomized to the topiramate treatment group (n = 28, 20%; (Fig. 2). The mean (SD) highest dose of topiramate achieved was 90.8 (36.4) mg/day. The mean (SD) dose for patients who remained on topiramate was 70 (30.1) mg/day; for those who switched, the mean (SD) dose was 56.1 (22.2) mg/day, which could include the recommended 2‐week taper. Of the topiramate patients who did not complete their randomized treatment regimen, 80 patients (56%) crossed over to onabotulinumtoxinA treatment; 71 of these 80 patients received their first onabotulinumtoxinA treatment at week 12. The mean (SD) time interval for topiramate administration was 156.8 (107.9) days for those who remained on topiramate and 60.1 (45.5) days for those who switched to onabotulinumtoxinA.
Figure 2.
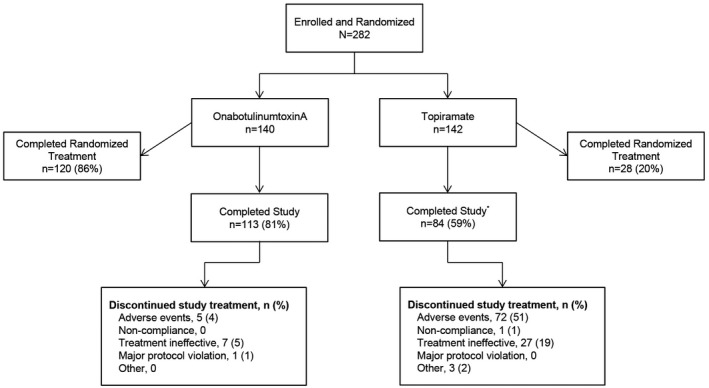
Patient disposition. *80 patients randomized to topiramate discontinued and switched to onabotulinumtoxinA treatment; 55 of the 80 (69%) completed the study and 6 (8%) discontinued: treatment ineffective, n = 3 (4%) and other reasons, n = 3 (4%).
Treatment discontinuations occurred in 11 (8%) patients who were initially randomized to onabotulinumtoxinA and 89 (63%) patients were randomized to topiramate. Lack of efficacy and AEs were the most frequently reported reasons for discontinuation (Fig. 2).
Primary and Secondary Outcomes
In the BOCF analysis, the proportion of the onabotulinumtoxinA treatment group achieving the primary endpoint at week 32 (n = 56/140, 40%) was significantly greater than that in the topiramate group (n = 17/142, 12%; Fig. 3A). The odds of being a 50% responder were 4.9 times greater for onabotulinumtoxinA than for topiramate (OR = 4.9 [95% CI: 2.7, 9.1]; P < .001, Fig. 3B). In this BOCF analysis, the 50% responder rates at week 12 were 40.7% (57/140) for the onabotulinumtoxinA treatment group and 22.5% (32/142) for the topiramate group, a statistically significant difference (P < .001). Based on a post hoc analysis of observed data at week 12, the respective onabotulinumtoxinA and topiramate 50% responder rates were 45.6% (n = 125) and 29.4% (n = 109), which differed significantly (P = .015).
Figure 3.
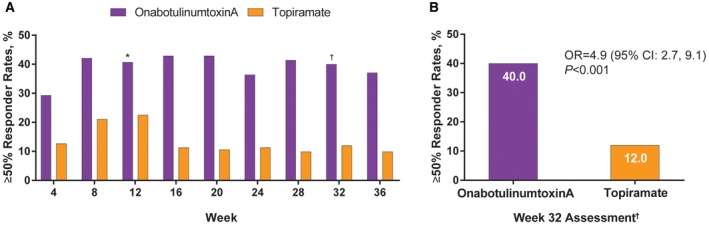
(A) Responder rates (≥50% decrease in frequency of headache from baseline) in patients receiving onabotulinumtoxinA and topiramate and (B) detailed at week 32. *Odds ratio = 2.4 (95% CI: 1.4‐4.1), P < .001; estimated using Fisher's exact test adjusted by baseline headache days for the 28‐day period between weeks 9‐12. Missing data imputed using baseline last observation carried forward imputation method. †Odds ratio = 4.9 (95% CI: 2.7‐9.1), P < .001; estimated using a logistic regression model adjusted by baseline headache days for the 28‐day period between weeks 29‐32. Missing data imputed using baseline last observation carried forward imputation method.
The frequency of headache days per 28‐day period was lower with onabotulinumtoxinA than with topiramate at weeks 12, 24, and 32. At week 32, mean (SD) reduction from baseline in headache days was 8.3 (8.9) compared with 2.1 (5.6) for topiramate, with a significant between‐group difference favoring onabotulinumtoxinA (mean difference: −6.2, 95% CI: −7.9 to −4.5; P < .001; Fig. 4A).
Figure 4.

(A) Headache day frequency per 28‐day period for onabotulinumtoxinA and topiramate at weeks 12, 24, and 32 and (B) HIT‐6 scores for onabotulinumtoxinA and topiramate at week 30. HIT‐6 = 6‐item Headache Impact Test. *Change from baseline at week 32 assessment (weeks 29‐32); P value compares the change from baseline, assessed using analysis of covariance and adjusting for baseline headache days. Other time points were not tested for statistical significance. †Change from baseline for onabotulinumtoxinA vs topiramate at week 30; P value compares the change from baseline, assessed using analysis of covariance and adjusting for baseline headache days.
At week 30, onabotulinumtoxinA resulted in a mean (SD) reduction in HIT‐6 scores from baseline of 5.6 (7.2) compared with 1.3 (3.9) for topiramate, with a significant between‐group difference favoring onabotulinumtoxinA (estimated between‐treatment difference, −4.2 [95% CI: −5.8 to −2.7]; P < .001; Fig. 4B).
OnabotulinumtoxinA treatment resulted in a higher proportion of patients (27%) with a ≥70% responder rate at week 32 compared with 8% of patients in the topiramate group (Fig. 5A). The odds of being a 70% responder were 4.1‐fold greater for onabotulinumtoxinA (OR = 4.1 [95% CI: 2.0, 8.2]; P < .001; Fig. 5B).
Figure 5.

Responder rates (≥70% decrease in frequency of headache from baseline) in patients receiving onabotulinumtoxinA and topiramate (A) for the duration of the study and (B) detailed at 32 weeks. *Odds ratio = 4.1 (95% CI: 2.0‐8.2), P < .001; estimated using a logistic regression model adjusted by baseline headache days for the 28‐day period between weeks 29‐32. Other time points were not tested for statistical significance.
In the exploratory analysis of patients who crossed over to onabotulinumtoxinA from topiramate, 31 (39%) patients were ≥50% headache day responders at week 32 compared with baseline (Fig. 6).
Figure 6.

Responder rates (≥50% decrease in frequency of headache from baseline) in patients who crossed over to onabotulinumtoxinA. Missing data were imputed using baseline last observation carried forward imputation method.
Other Efficacy Outcome Measure
During weeks 29 to 32, the mean (SD) change from baseline in the number of acute headache pain medication usage days was −5.5 (6.7) for onabotulinumtoxinA‐treated patients and −1.7 (5.2) for patients treated with topiramate, a statistically significant mean treatment difference of −4.039 (95% CI, −5.387 to −2.691; P < .001). For all other study intervals, mean reductions from baseline in acute headache pain medication usage days were greater with onabotulinumtoxinA treatment than with topiramate.
Safety and Tolerability
AEs were experienced by 48% of onabotulinumtoxinA patients (randomized or cross‐over populations, n = 220 patients) and 79% of topiramate patients (Table 2). The most common AE among onabotulinumtoxinA patients was sinusitis (6%), whereas for topiramate patients it was paresthesia (31%). One percent of onabotulinumtoxinA patients and 42% of topiramate patients discontinued treatment due to an AE. One patient (receiving topiramate) discontinued because of pregnancy. No patients who crossed over to onabotulinumtoxinA discontinued treatment due to an AE.
Table 2.
Overview of Adverse Events
| Patients with AE, n (%)* | OnabotulinumtoxinA (n = 220) | Topiramate (n = 142) | Total (N = 282) | Switched to OnabotulinumtoxinA (n = 80)† |
| Any AE | 105 (48) | 112 (79) | 179 (63) | 38 (48) |
| Serious AE | 4 (2) | 6 (4) | 8 (3) | 2 (3) |
| AE leading to discontinuation of treatment | 3 (1) | 60 (42) | 63 (22) | 0 |
| AE leading to withdrawal from study | 3 (1) | 7 (5) | 10 (4) | 0 |
| AE leading to death | 0 | 0 | 0 | 0 |
| Treatment‐related AE | 38 (17) | 99 (70) | 126 (45) | 12 (15) |
| Serious treatment‐related AE | 0 | 1 (1) | 1 (<1) | 0 |
| AEs occurring in ≥5% in any treatment group | ||||
| Cognitive disorder | 1 (<1) | 18 (13) | 18 (6) | 1 (1) |
| Disturbance in attention | 0 | 12 (8) | 12 (4) | 0 |
| Dizziness | 6 (3) | 18 (13) | 23 (8) | 1 (1) |
| Migraine | 6 (3) | 2 (2) | 7 (2) | 4 (5) |
| Paresthesia | 1 (<1) | 44 (31) | 45 (16) | 0 |
| Sinusitis | 13 (6) | 10 (7) | 17 (6) | 6 (8) |
| Nausea | 1 (<1) | 19 (13) | 20 (7) | 0 |
| Neck pain | 10 (4) | 3 (2) | 12 (4) | 5 (6) |
| Fatigue | 1 (<1) | 19 (13) | 20 (7) | 0 |
| Depression | 4 (2) | 8 (6) | 10 (4) | 2 (3) |
| Vision blurred | 6 (3) | 11 (8) | 15 (5) | 4 (4) |
| Decreased appetite | 0 | 15 (11) | 15 (5) | 0 |
Percentages based on the number of patients that received each treatment as the denominator. A patient that received both onabotulinumtoxinA and topiramate was included in the denominator for all treatment arms and overall. At each level of summarization, a patient was counted once within a treatment arm and overall.
AEs that started on or after the first onabotulinumtoxinA injection were counted in the treatment arm related to the AE as determined by the physician; if the AE was determined to be related to both treatments or the relationship to a treatment group was unknown, the AE was counted in both treatment arms but only once in the total.
AE = adverse event.
Treatment‐related AEs were reported in 17% of onabotulinumtoxinA patients and 70% of topiramate patients; 15% of patients who crossed over to onabotulinumtoxinA reported a treatment‐related AE. The most common treatment‐related AEs in patients receiving onabotulinumtoxinA were neck pain (4%), musculoskeletal pain (2%), migraine (1%), and blurred vision (1%). The most common treatment‐related AEs in patients receiving topiramate were paresthesia (29%), cognitive disorder, fatigue, nausea (all 12%), decreased appetite, dizziness (both 11%), and attention disturbance (8%). The only serious treatment‐related AE was nephrolithiasis in a patient receiving topiramate; no deaths occurred.
Discussion
The results of this study provide new data on the comparative effectiveness of onabotulinumtoxinA and topiramate in the management of CM. Using a pragmatic approach and prespecified analytical methods, we found differences that were statistically significant in favor of onabotulinumtoxinA for the primary and secondary outcomes. In particular, our results from the observed data conducted as sensitivity analyses (Appendix I) suggest that although topiramate may be beneficial in reducing headache burden for patients who can tolerate the medication, patients receiving onabotulinumtoxinA are more likely to remain on treatment, an effect that yields greater overall clinical utility.
For a given treatment to be effective, patients obviously must adhere to their treatment regimens. Overall adherence rates associated with traditional oral migraine preventive treatments are relatively low, with more than two‐thirds of individuals in one large claims database analysis designated non‐adherent.32 The sensitivity analyses we used in the FORWARD study support our conclusion that the 2 treatments evaluated have similar efficacy, as has been demonstrated in other published clinical trials, but have a marked difference in effectiveness that is largely a function of tolerability. This difference is likely to result in a high real‐world discontinuation rate for topiramate.
Patients in the FORWARD study who discontinued topiramate and crossed over to onabotulinumtoxinA experienced effectiveness comparable to that for those initially randomized to onabotulinumtoxinA, a finding that suggests treatment failure with topiramate does not predict treatment failure with onabotulinumtoxinA. Because patients could receive onabotulinumtoxinA during the topiramate taper period, it is possible that some portion of the efficacy results we observed in this cross‐over group were due to topiramate. Despite this limitation, our results may be relevant for clinicians whose patients wish to discontinue topiramate in favor of treatment with onabotulinumtoxinA. The incidence and nature of AEs were similar in those who crossed over to onabotulinumtoxinA and the overall onabotulinumtoxinA group. There were no apparent AEs associated with switching a patient from topiramate to onabotulinumtoxinA, even when onabotulinumtoxinA was initiated during the topiramate taper period.
The demographics, headache characteristics, and common comorbidities in the FORWARD patient population were consistent with those in other clinical trials and epidemiological studies of CM.33, 34, 35 Additionally, 18.1% of patients (onabotulinumtoxinA, 18.6%; topiramate, 17.6%) was receiving a stable dose of preventive treatment prior to enrolling, reflecting current management of CM in the clinical setting. Given the similarities between the 2 treatment groups in this study, it is unlikely that the use of concomitant preventive medications influenced our results in a manner detracting from their clinical relevance.
The FORWARD study has its limitations. One was the possible bias introduced by awareness among patients who were randomized to topiramate that they could cross over to onabotulinumtoxinA, potentially inflating topiramate discontinuation rates. Awareness of the potential AEs could also have influenced results. While such biases cannot be excluded, topiramate patients could not cross over to onabotulinumtoxinA until week 12 (16 weeks after enrollment). Given this time restriction, the inherent burden imposed by CM, and the sufficient opportunity for patients and their physicians to determine the dose of topiramate that was optimal for them, it seems plausible that patients would have been reasonably motivated to give topiramate an adequate trial. Indeed, the mean highest dose of topiramate achieved during the study was 90.8 mg/day, suggesting that most patients did, in fact, give topiramate a reasonable trial. The discontinuation rates reported in RCTs with topiramate were lower than those rates in our study.7, 12 At a dose of approximately 100 mg per day, the discontinuation rate with topiramate was 33.6% in the larger of the 2 trials and 25% in the smaller trial.7, 12 Our data, however, are consistent with previously reported real‐world discontinuation rates for antiepilepsy medications.15 It should be noted that the discontinuation rate for onabotulinumtoxinA in this trial was also higher than that in prior RCTs.9
In this study, when patients discontinued treatment, we carried the baseline observation forward. This approach assumes that a treatment that cannot be tolerated is unlikely to be effective. For a real‐world study, this assumption is justified although it arguably conflates lack of efficacy with tolerability.
Other limitations of this study are the lack of a placebo arm and lack of blinding of both the investigator and the patients. A number of alternative design options were considered, including a 3‐arm double‐dummy design, the addition of 2 placebo arms, and blinding of the investigator by having a third party allocate and administer treatments. We felt it unlikely, however, that a placebo control would be approvable by an IRB given the availability of 2 evidence‐based, FDA‐approved treatments. The design, however, was intended to approximate clinical practice, which precluded these alternatives.
The FORWARD study was designed on the premise that the efficacy of both treatments already had been established in double‐blind placebo‐controlled trials and that FORWARD's purpose was to extend knowledge involving the relative clinical utility of the treatments. Thus, in this study, patients randomized to topiramate could cross over to the comparator. This unidirectional cross‐over, which prohibited a patient switching from onabotulinumtoxinA to topiramate, was chosen so as to reflect current clinical practice, and we cannot exclude the possibility that this may have influenced the discontinuation rate for patients randomized to topiramate.
Conclusions
Although in those few patients who were randomized to the oral medication and completed the treatment phase, topiramate was at least as efficacious as onabotulinumtoxinA. The wide difference in discontinuation rates limits our ability to offer an assessment of the relative efficacy of topiramate “immediate release” and onabotulinumtoxinA. In the context of effectiveness, however, the high discontinuation rate associated with topiramate appears to diminish its clinical value significantly. Compared to only 4% of those randomized to onabotulinumtoxinA, the majority (51%) of patients randomized to topiramate discontinued treatment because of AEs.
Our results also demonstrate that onabotulinumtoxinA is a safe and often effective alternative for patients with CM who discontinue treatment with topiramate, and treatment with onabotulinumtoxinA can be initiated safely during the topiramate taper period.
In current practice, topiramate is often considered first‐line treatment. Given the marked difference in effectiveness between the 2 therapies indicated by our results, the logic of delaying treatment with onabotulinumtoxinA in favor of first prescribing topiramate “immediate release” can be questioned. Further research is needed to quantify the clinical impact and societal cost of delaying treatment that is associated with comparatively greater clinical utility.
Statement of Authorship
Category 1
(a) Conception and Design
Aubrey Manack Adams, Esther Jo, Xiang Zhao, Andrew M. Blumenfeld
(b) Acquisition of Data
John F. Rothrock, Andrew M. Blumenfeld
(c) Analysis and Interpretation of Data
John F. Rothrock, Aubrey Manack Adams, Richard B. Lipton, Stephen D. Silberstein, Esther Jo, Xiang Zhao, Andrew M. Blumenfeld
Category 2
(a) Drafting the Manuscript
John F. Rothrock, Aubrey Manack Adams, Richard B. Lipton, Stephen D. Silberstein, Esther Jo, Xiang Zhao, Andrew M. Blumenfeld
(b) Revising It for Intellectual Content
John F. Rothrock, Aubrey Manack Adams, Richard B. Lipton, Stephen D. Silberstein, Esther Jo, Xiang Zhao, Andrew M. Blumenfeld
Category 3
(a) Final Approval of the Completed Manuscript
John F. Rothrock, Aubrey Manack Adams, Richard B. Lipton, Stephen D. Silberstein, Esther Jo, Xiang Zhao, Andrew M. Blumenfeld
Supporting information
Acknowledgments
Writing and editorial assistance was provided to the authors by Lisa Feder, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and Lee B. Hohaia, PharmD, at Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Allergan plc. The opinions expressed in this article are those of the authors. The authors received no honorarium/fee or other form of financial support related to the development of this article. [Corrections added on October 30, 2019 after first online publication: Lee B. Hohaia was added to the Acknowledgments section.]
Conflict of Interest: John F. Rothrock, MD, serves as a senior editorial advisor for Headache and as an associate editor for Headache Currents. He has served on advisory boards and/or has consulted for Allergan, Lilly, Amgen, and Supernus. He also has received funding for travel and speaking from Supernus and has received honoraria from Allergan plc for participating as a speaker and preceptor at Allergan‐sponsored educational programs. His parent institution has received funding from Allergan plc, Amgen, and Dr. Reddy's for clinical research he has conducted. Aubrey Manack Adams, PhD, and Esther Jo, MPH, are full‐time employees of Allergan plc and own stock in the company. Richard B. Lipton, MD, serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache. He has received research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He has reviewed for the NIA and NINDS, serves as consultant or advisory board member or has received honoraria from: Alder, Allergan, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy's Laboratories, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta. He receives royalties from Wolff's Headache (8th Edition, Oxford University Press), Informa, and Wiley. He holds stock options in eNeura Therapeutics and Biohaven. Stephen D. Silberstein, MD, as a consultant and advisory panel member receives honoraria from Alder Biopharmaceuticals, Allergan, Inc., Amgen, Avanir Pharmaceuticals, Inc., eNeura, ElectroCore Medical, LLC, Labrys Biologics, Medscape, LLC, Medtronic, Inc., Neuralieve, NINDS, Pfizer, Inc., and Teva Pharmaceuticals. His employer receives research support from Allergan, Inc.; Amgen; Cumberland Pharmaceuticals, Inc.; ElectroCore Medical, Inc.; Labrys Biologics; Eli Lilly and Company; Merz Pharmaceuticals; and Troy Healthcare. Xiang Zhao, PhD, is an employee of Pharmaceutical Product Development LLC, a company that has contracts with Allergan plc and other companies. Andrew M. Blumenfeld, MD, has served on advisory boards for Allergan, Amgen, Alder, Teva, Supernus, Promius, Eaglet, and Lilly; and has received funding for speaking from Allergan, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, and Eli Lilly and Company.
Funding: This work was supported by Allergan plc, Dublin, Ireland.
Clinical Trial Registration: http://www.clinicaltrials.gov: NCT02191579
Data Availability Statement
Data reported in this manuscript are available within the article. Additional data from the FORWARD Study (http://ClinicalTrials.gov, NCT02191579) may be requested at https://www.allerganclinicaltrials.com/PatientDataRequest.htm.
References
- 1. Botox [package insert] Dublin, Ireland: Allergan Ltd. plc; 2017. [Google Scholar]
- 2. Botox [summary of product characteristics] Marlow, Buckinghamshire, UK: Allergan Ltd.; 2015. [Google Scholar]
- 3. Botox [product monograph] Markham, Ontario, Canada: Allergan plc; 2014. [Google Scholar]
- 4. National Institute for Health and Care Excellence . Botulinum toxin type A for the prevention of headaches in adults with chronic migraine. https://www.nice.org.uk/guidance/ta260/resources/botulinum-toxin-typea-for-the-prevention-of-headaches-in-adults-with-chronic-migraine-pdf-82600545273541. Published 27 June 2012. Accessed September 20, 2019. [Google Scholar]
- 5. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double‐blind, randomized, placebo‐controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793‐803. [DOI] [PubMed] [Google Scholar]
- 7. Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ. Topiramate reduces headache days in chronic migraine: A randomized, double‐blind, placebo‐controlled study. Cephalalgia. 2007;27:814‐823. [DOI] [PubMed] [Google Scholar]
- 8. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double‐blind, randomized, placebo‐controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804‐814. [DOI] [PubMed] [Google Scholar]
- 9. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56‐week PREEMPT clinical program. Headache. 2011;51:1358‐1373. [DOI] [PubMed] [Google Scholar]
- 10. Matharu M, Pascual J, Nilsson Remahl I, et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia. 2017;37:1384‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: A randomized controlled trial. JAMA. 2004;291:965‐973. [DOI] [PubMed] [Google Scholar]
- 12. Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: A randomized, double‐blind, placebo‐controlled trial. Headache. 2007;47:170‐180. [DOI] [PubMed] [Google Scholar]
- 13. Topamax [package insert] Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2017. [Google Scholar]
- 14. Mathew NT, Jaffri SF. A double‐blind comparison of onabotulinumtoxinA (Botox) and topiramate (Topamax) for the prophylactic treatment of chronic migraine: A pilot study. Headache. 2009;49:1466‐1478. [DOI] [PubMed] [Google Scholar]
- 15. Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia. 2017;37:470‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, US Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research . E9 Statistical principles for clinical trials. https://www.fda.gov/media/71336/download. Published September 1998. Accessed September 20, 2019.
- 17. Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637‐648. [DOI] [PubMed] [Google Scholar]
- 19. Glasgow RE. What does it mean to be pragmatic? Pragmatic methods, measures, and models to facilitate research translation. Health Educ Behav. 2013;40:257‐265. [DOI] [PubMed] [Google Scholar]
- 20. Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13:217‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cycle 1 2018 funding cycle . PCORI funding announcement: pragmatic clinical studies to evaluate patient‐centered outcomes Washington, DC: Patient‐Centered Outcomes Research Institute (PCORI); 2018. [Google Scholar]
- 22. Blodt S, Pach D, Roll S, Witt CM. Effectiveness of app‐based relaxation for patients with chronic low back pain (Relaxback) and chronic neck pain (Relaxneck): Study protocol for two randomized pragmatic trials. Trials. 2014;15:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parabiaghi A, D'Avanzo B, Tettamanti M, Barbato A, Gi SASSG. The GiSAS study: Rationale and design of a pragmatic randomized controlled trial on aripiprazole, olanzapine and haloperidol in the long‐term treatment of schizophrenia. Contemp Clin Trials. 2011;32:675‐684. [DOI] [PubMed] [Google Scholar]
- 24. Kato T, Furukawa TA, Mantani A, et al. Optimising first‐ and second‐line treatment strategies for untreated major depressive disorder – the SUND study: A pragmatic, multi‐centre, assessor‐blinded randomised controlled trial. BMC Med. 2018;16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boesen F, Norgaard M, Trenel P, et al. Longer term effectiveness of inpatient multidisciplinary rehabilitation on health‐related quality of life in MS patients: A pragmatic randomized controlled trial – The Danish MS Hospitals Rehabilitation Study. Mult Scler. 2018;24:340‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kristoffersen ES, Straand J, Vetvik KG, Benth JS, Russell MB, Lundqvist C. Brief intervention by general practitioners for medication‐overuse headache, follow‐up after 6 months: A pragmatic cluster‐randomised controlled trial. J Neurol. 2016;263:344‐353. [DOI] [PubMed] [Google Scholar]
- 27. Castien RF, van der Windt DA, Grooten A, Dekker J. Effectiveness of manual therapy for chronic tension‐type headache: A pragmatic, randomised, clinical trial. Cephalalgia. 2011;31:133‐143. [DOI] [PubMed] [Google Scholar]
- 28. Georgoudis G, Felah B, Nikolaidis P, Damigos D. The effect of myofascial release and microwave diathermy combined with acupuncture versus acupuncture therapy in tension‐type headache patients: A pragmatic randomized controlled trial. Physiother Res Int. 2018;23:e1700. [DOI] [PubMed] [Google Scholar]
- 29. Powers SW, Coffey CS, Chamberlin LA, et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smelt AF, Blom JW, Dekker F, et al. A proactive approach to migraine in primary care: A pragmatic randomized controlled trial. CMAJ. 2012;184:E224‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 32. Berger A, Bloudek LM, Varon SF, Oster G. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12:541‐549. [DOI] [PubMed] [Google Scholar]
- 33. Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428‐432. [DOI] [PubMed] [Google Scholar]
- 34. Blumenfeld AM, Tepper SJ, Robbins LD, et al. Effects of onabotulinumtoxinA treatment for chronic migraine on common comorbidities including depression and anxiety. J Neurol Neurosurg Psychiatry. 2019;. 90:353‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lipton RB, Fanning KM, Buse DC, et al. Identifying natural subgroups of migraine based on comorbidity and concomitant condition profiles: Results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache. 2018;58:933‐947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this manuscript are available within the article. Additional data from the FORWARD Study (http://ClinicalTrials.gov, NCT02191579) may be requested at https://www.allerganclinicaltrials.com/PatientDataRequest.htm.


