Abstract
An intravenous (IV) formulation of meloxicam is being studied for moderate to severe pain management. This phase 3, randomized, multicenter, double‐blind, placebo‐controlled trial evaluated the safety of once‐daily meloxicam IV 30 mg in subjects following major elective surgery. Eligible subjects were randomized (3:1) to receive meloxicam IV 30 mg or placebo administered once daily. Safety was evaluated via adverse events, clinical laboratory tests, vital signs, wound healing, and opioid consumption. The incidence of adverse events was similar between meloxicam IV– and placebo‐treated subjects (63.0% versus 65.0%). Investigators assessed most adverse events as mild or moderate in intensity and unrelated to treatment. Adverse events of interest (injection‐site reactions, bleeding, cardiovascular, hepatic, renal, thrombotic, and wound‐healing events) were similar between groups. Over the treatment period, meloxicam IV was associated with a 23.6% (P = .0531) reduction in total opioid use (9.2 mg morphine equivalent) compared to placebo‐treated subjects. The results suggest that meloxicam IV had a safety profile similar to that of placebo with respect to numbers and frequencies of adverse events and reduced opioid consumption in subjects with moderate to severe postoperative pain following major elective surgery.
Keywords: meloxicam, nonsteroidal anti‐inflammatory drug, postoperative pain, safety, surgery
Acute pain after surgery can have a significant impact on clinical outcomes. Inadequate pain management in the postoperative period may lead to adverse events, including myocardial ischemia, myocardial infarction, impaired pulmonary function, paralytic ileus, urinary retention, thromboembolism, impaired immune function, and anxiety.1, 2 These events have the potential to produce unfavorable clinical, economic, and patient‐related outcomes. Although effective and well‐tolerated pain control is an important component of postoperative care, a large proportion of patients continue to report significant postoperative pain.3, 4 In 2 large surveys of postsurgical patients, 62% to 71% of patients experienced moderate to extreme pain after discharge, with 23% to 29% reporting severe to extreme levels of pain.3, 4
Opioids have been a mainstay of postoperative pain management, but they are associated with adverse events (eg, gastrointestinal events, pruritus, respiratory depression) and other risks (eg, dependency, overdose, and diversion).2, 5, 6, 7 Despite these concerns, the limited number of treatment alternatives has led to a reliance on opioids in pain management regimens.8 This has prompted the development of new nonopioid treatment regimens or opioid‐minimizing regimens that may provide safer and more effective management of postoperative pain,8, 9 such as the use of multimodal pain management therapy. Such regimens, which include 2 or more drugs, each with a different mechanism of action, often produce improved postoperative pain scores and greater pain relief.10, 11
Nonsteroidal anti‐inflammatory drugs (NSAIDs) have long been used for the treatment of chronic pain conditions (eg, rheumatoid arthritis, osteoarthritis), but their pharmacological profile (ie, analgesic, anti‐inflammatory) also makes them attractive agents for the management of postoperative pain.12 NSAIDs have also been shown to have an opioid‐sparing effect and to decrease opioid‐induced nausea and vomiting.13, 14, 15, 16
Meloxicam is a preferential cyclooxygenase‐2 inhibitor with analgesic, antipyretic, and anti‐inflammatory properties.17, 18, 19, 20, 21, 22 An oral dosage form of the drug has demonstrated efficacy for the treatment of pain and inflammation in patients with rheumatoid arthritis and osteoarthritis and is associated with fewer gastrointestinal adverse events compared with nonselective NSAIDs.18, 19, 23 Oral meloxicam is metabolized in the liver to 4 inactive metabolites that are excreted in urine and feces with an elimination half‐life of ∼20 hours.18, 20, 21 The cytochrome P450 (CYP) 2C subgroup of isozymes, CYP2C9 (primary) and CYP3A4 (minor), are responsible for the oxidative metabolism of oral meloxicam.24 Oral meloxicam has poor solubility that contributes to a delay in absorption and onset of action. Peak plasma concentrations are not achieved until 9 to 11 hours after oral administration of a 30‐mg dose.18, 25 Because of this, oral meloxicam is not currently indicated for the management of acute pain.
A novel nanocrystal intravenous (IV) formulation of meloxicam that is administered by bolus injection is being studied for the management of moderate to severe pain. Meloxicam IV is an investigational product that has not been approved by the US Food and Drug Administration. Although pharmacokinetic information for the nanocrystal IV meloxicam formulation has not been published, a standard population pharmacokinetic model was developed to evaluate sources of exposure variability and to assess the effect of meloxicam IV on pain intensity (PI). The results, presented at a conference, found that body weight and renal function had an effect on the clearance of meloxicam IV.26 An exposure‐response analysis found that a statistically significant exposure‐response relationship was observed, indicating that higher area under the concentration‐time curve over 24 hours for meloxicam IV was associated with improved pain control and fewer uses of rescue medication over 24 and 48 hours.26
In phase 2 trials of subjects with postoperative pain after surgical procedures including removal of impacted third molars,27 open abdominal hysterectomy,28 laparoscopic abdominal surgery,29 and bunionectomy,30 meloxicam IV exhibited onset of analgesia within 15 minutes or less postdose, with maintenance of the analgesic effect through the 24‐hour dosing period. The analgesic effect and safety of meloxicam IV were evaluated in 2 phase 3 surgical models, including soft‐tissue surgery (abdominoplasty)31 and hard‐tissue surgery (bunionectomy).32 In the abdominoplasty study, subjects who received meloxicam IV had a statistically significant reduction in PI as measured by the summed PI difference from hour 0 to hour 24 compared with placebo‐treated subjects (‒4262.1 versus ‒3535.7; P = .0145).31 Subjects randomized to meloxicam IV 30 mg in the bunionectomy study experienced a statistically significant difference in summed PI difference from hour 0 to hour 48 versus the placebo group (‒6956.0 versus ‒4829.3; P = .0034).32 In both phase 3 studies, meloxicam IV was generally well tolerated, with a safety profile that included a low incidence of adverse events that was comparable to that of placebo.
An additional phase 3 clinical trial was conducted to investigate meloxicam IV safety in a separate population of subjects experiencing postoperative pain following a range of surgical procedures. The primary objective of this study was to evaluate the safety and tolerability of meloxicam IV 30 mg following major surgery as assessed by adverse events, opioid consumption, physical examination, vital signs, clinical laboratory tests, electrocardiograms (ECGs), and wound evaluation. Mean opioid consumption was assessed in this study as a surrogate measure of analgesic efficacy.
Methods
Study Design
The protocol for this phase 3, randomized, multicenter, double‐blind, placebo‐controlled trial was reviewed and approved by a central Institutional Review Board (Copernicus Group Independent Review Board, Durham, North Carolina), and all subjects provided written informed consent. The trial was conducted at 31 centers in 4 countries (the United States, Canada, New Zealand, and Australia) during the period from March 2016 to April 2017. Clinical work was completed according to current Good Clinical Practice guidelines outlined by the International Conference on Harmonisation Guidance for Industry, E6 Good Clinical Practice: Consolidated Guidance, and, where applicable, the principles of the Declaration of Helsinki. This study was registered with ClinicalTrials.gov (NCT02720692) on March 22, 2016, with principal investigators at each site. The principal investigator at each clinical site that enrolled ≥1 subject and the ethics committee institutional review board for each study location are provided in Supplemental Table 1.
Key Eligibility Criteria
Men and nonpregnant, nonlactating women aged 18 to 80 years (inclusive) with a body mass index up to 40 kg/m2, scheduled to undergo major elective surgery and expected to require IV analgesia, to remain in an inpatient setting for at least 24 to 48 hours, and to receive at least 2 study doses were eligible for inclusion in the study.
The first dose was to be completed within 6 hours of the end of the surgery among subjects who met the following postoperative criteria: (1) the subject was able to achieve hemostasis and surgical incision closure before operating room discharge; (2) the surgical procedure did not require use of more than 2 units of packed red blood cells or platelets; (3) the surgical procedure from incision to closure was no longer than 12 hours; (4) the subject was expected to have sufficient pain to require IV analgesia; and (5) there was no evidence of respiratory insufficiency, clinically significant hypotension, bradycardia, coagulopathy, or any other abnormality during or following surgery that, in the investigator's opinion, significantly increased the risks of study participation.
Subjects were excluded if they had allergy/hypersensitivity to meloxicam or other NSAIDs or excipients; were undergoing a surgical procedure in which NSAIDs are contraindicated; had a planned/actual admission to the intensive care unit; had elevated aminotransferases, alkaline phosphatase, total bilirubin, or prothrombin time; had a history of HIV, hepatitis B, hepatitis C; or had a significant renal, hepatic, cardiovascular, metabolic, neurologic, and/or psychiatric condition. Subjects were also not eligible if they had a myocardial infarction or coronary artery bypass graft surgery within 12 months, active or recent bleeding (within 6 months), gastrointestinal ulceration, a known bleeding disorder, or a history of alcohol abuse or positive results on a drug screen.
Randomization and Study Drug Administration
Following screening, eligible subjects were randomized to treatment with either meloxicam IV 30 mg or placebo (3:1), administered intravenously every 24 hours (±1 hour). Randomization was achieved via a computer‐generated block randomization scheme (block size 4). The randomization was stratified by subjects greater than 65 years of age with mild renal failure (glomerular filtration rate 60‐89 mL/[min·1.73 m2]) versus other, and by surgery type (orthopedic versus other surgeries). All doses administered in this study were prepared and administered by a designated unblinded and appropriately qualified staff member of the healthcare team at the research center, who maintained the blind of subjects and investigators. No subject treatment assignments were unblinded during the course of this study.
Surgeries were conducted under appropriate anesthesia and analgesic regimens that were used according to the clinical practice of the surgeon based on the surgery type. Administration of the first dose (set as hour 0) was completed within 6 hours of the end of surgery for those who met the postoperative randomization key eligibility criteria. Subjects received study drug for as long as IV analgesia was deemed clinically appropriate up to a maximum of 7 doses. The final dose of study drug could be administered up to 4 hours early for subjects who were scheduled to be discharged. For subjects who no longer needed inpatient care, additional doses under continued supervision in an appropriate setting were allowed. Any subjects who had not received a dose for more than 28 hours were considered off treatment. Concomitant use of NSAIDs other than study medication was prohibited. Other concomitant medications that had not been at a stable dose for at least 14 days before the surgical procedure were also prohibited within 5 half‐lives of the medication. After discharge, subjects received routine pain management and were instructed to return to the study site to complete end‐of‐study assessments. A final safety assessment was conducted via telephone on day 28.
Rescue medication was allowed for subjects with inadequately controlled pain with sites utilizing standard‐of‐care analgesics at the discretion of the investigator, as indicated by the types of surgery at their institution and based on subject needs. Although any analgesic was acceptable (except for NSAIDs), opioid analgesics were the most commonly used rescue medications.
Primary End Points
Adverse events, including those volunteered, elicited, or noted on physical examination, were recorded and assessed by the investigators for intensity and causality. Hematology, clinical chemistry, urinalysis, and coagulation tests were performed at screening, day 1, hour 48, before discharge, and at follow‐up visit (7 days after last scheduled dose). Vital signs were measured at screening, 1 day before surgery, before the first 2 study doses, before discharge, and at follow‐up visit. A 12‐lead ECG was performed at screening, check‐in, before discharge, and at follow‐up. Surgical wound healing was evaluated by the investigator before discharge and at the follow‐up visit using an 11‐point scale (0 to 10, “completely unsatisfied” to “completely satisfied”). Total opioid consumption was measured as the IV morphine equivalent dose during day 1 (0‐24 hours), day 2 (24‐48 hours), day 3 (48‐72 hours), days 1 to 2 (0‐48 hours), days 1 to 3 (0‐72 hours), and as total consumption during treatment.
Statistical Analysis
The sample size was selected to support the required total exposure population for meloxicam IV. For a sample size of 525 subjects to be treated with meloxicam IV, the study was designed to have a 95% probability to observe at least 1 event if the occurrence rate was at least 0.57% in the meloxicam IV group. The safety analysis set included all subjects treated with the study drug. Adverse events and other safety variables (clinical laboratory values, vital signs, ECG findings) were summarized descriptively without inferential statistics. Adverse events were coded using the Medical Dictionary for Regulatory Activities (version 18.1). Differences between groups for opioid consumption were analyzed using an analysis of covariance model that included treatment and analysis site. An analysis site was defined as any individual investigator site where the total number of subjects was at least 10. For sites with fewer than 10 subjects (n = 12 sites), sites were pooled on the basis of geographical location (n = 4 pooled analysis sites). In addition, a confirmatory analysis using Cochran‐Mantel‐Haenszel analysis of variance (row mean scores) on rank, a nonparametric approach, was performed for total opioid consumption. All analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, North Carolina).
Results
Subjects
Of the 722 subjects randomized, 721 (428 female, 293 male) received at least 1 dose of study medication and were included in the safety set (Figure 1). Subject demographics and surgical characteristics (Table 1) were generally similar between treatment groups. The most common surgeries were soft‐tissue surgery, total knee replacement, gynecologic surgery, complex foot surgery, total hip replacement, and bunionectomy (Table 2). One hundred nineteen subjects (meloxicam IV [n = 88]; placebo [n = 31]) were over 65 years of age and had mild renal impairment (glomerular filtration rate of 60‐89 mL/[min·1.73 m2]).
Figure 1.
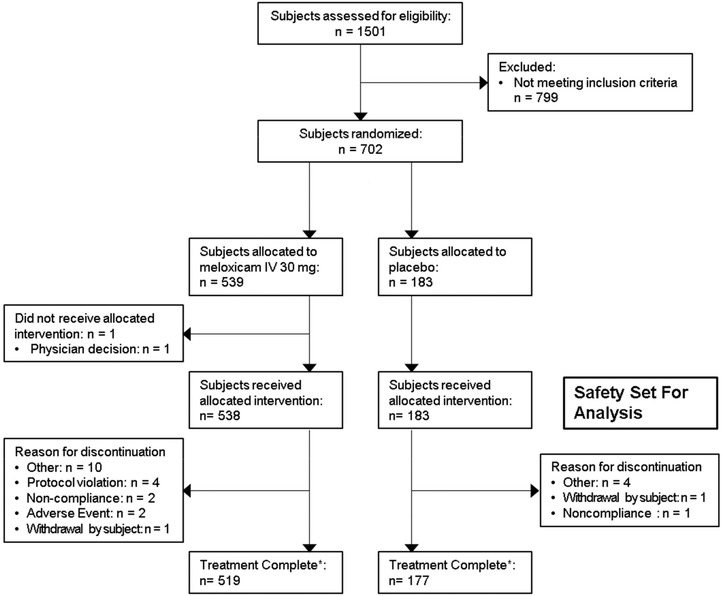
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patient disposition in study NCT02720692. IV indicates intravenous. *All treated subjects were to complete a final safety assessment by telephone 28 days following their last study dose; 8 subjects allocated to meloxicam IV 30 mg, and 3 subjects allocated to placebo were lost to follow‐up.
Table 1.
Summary of Subject Demographics
| Characteristics | Meloxicam IV 30 mg (n = 538) | Placebo (n = 183) | All Subjects (N = 721)a |
|---|---|---|---|
| Age (y), mean ± SD | 52.9 ± 13.56 | 53.0 ± 13.77 | 53.0 ± 13.60 |
| Age categories, n (%) | |||
| <65 y | 418 (77.7) | 140 (76.5) | 558 (77.4) |
| ≥65 y | 120 (22.3) | 43 (23.5) | 163 (22.6) |
| ≥75 y | 15 (2.8) | 10 (5.5) | 25 (3.5) |
| Sex, male, n (%) | 223 (41.4) | 70 (38.3) | 293 (40.6) |
| Race, n (%) | |||
| White | 459 (85.3) | 155 (84.7) | 614 (85.2) |
| Black | 68 (12.6) | 21 (11.5) | 89 (12.3) |
| Asian | 7 (1.3) | 4 (2.2) | 11 (1.5) |
| Native American or Alaskan native | 1 (0.2) | 0 | 1 (0.1) |
| Native Hawaiian or other Pacific Islander | 2 (0.4) | 0 | 2 (0.3) |
| Other/multiple | 1 (0.2) | 3 (1.6) | 4 (0.6) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 76 (14.1) | 29 (15.8) | 105 (14.6) |
| Non‐Hispanic, non‐Latino | 462 (85.9) | 154 (84.2) | 616 (85.4) |
| BMI (kg/m2), mean ± SD | 29.5 ± 4.95 | 29.1 ± 4.88 | 29.4 ± 4.93 |
| Age >65 y with mild renal impairment, n (%) | 88 (16.4) | 31 (16.9) | 119 (16.5) |
| Surgery duration (h), mean ± SD | 1.31 ± 1.01 | 1.31 ± 0.93 | 1.31 ± 0.99 |
| Time (h) from end of surgery to first dose, mean ± SD | 1.67 ± 12.5 | 1.73 ± 1.26 | 1.69 ± 1.25 |
| Incision type, n (%) | |||
| Laparoscopic | 64 (11.9) | 19 (10.4) | 83 (11.5) |
| Open | 474 (88.1) | 164 (89.6) | 638 (88.5) |
| Surgery site, n (%) | |||
| Orthopedic | 272 (50.7) | 93 (50.8) | 366 (50.8) |
| Abdominal/pelvic | 254 (47.2) | 87 (47.5) | 341 (47.3) |
| Spinal | 10 (1.9) | 3 (1.6) | 13 (1.8) |
| Other | 1 (0.2) | 0 | 1 (0.1) |
BMI indicates body mass index; IV, intravenous.
Safety set (received at least 1 dose of study drug).
Table 2.
Summary of Surgery Types
| Surgery | Meloxicam IV 30 mg (n = 538) | Placebo (n = 183) | Overall (N = 721)a |
|---|---|---|---|
| Orthopedic surgeries, n (%) | |||
| Total knee replacement | 117 (21.7) | 39 (21.3) | 156 (21.6) |
| Complex foot | 52 (9.7) | 19 (10.4) | 71 (9.8) |
| Total hip replacement | 50 (9.3) | 18 (9.8) | 68 (9.4) |
| Bunionectomy | 40 (7.4) | 13 (7.1) | 53 (7.4) |
| Spinal | 10 (1.9) | 3 (1.6) | 13 (1.8) |
| Total shoulder replacement | 7 (1.3) | 1 (0.5) | 8 (1.1) |
| Complex shoulder surgery | 6 (1.1) | 2 (1.1) | 8 (1.1) |
| Total ankle replacement | 1 (0.2) | 0 | 1 (0.1) |
| Orthopedic trauma | 0 | 1 (0.5) | 1 (0.1) |
| Other surgery types, n (%) | |||
| Soft tissue surgery | 128 (23.8) | 42 (23.0) | 170 (23.6) |
| Gynecologic surgery | 68 (12.6) | 21 (11.5) | 89 (12.3) |
| Abdominoplasty | 32 (5.9) | 11 (6.0) | 43 (6.0) |
| GI surgery | 26 (4.8) | 13 (7.1) | 39 (5.4) |
| Head and neck | 1 (0.2) | 0 | 1 (0.1) |
GI indicates gastrointestinal; IV, intravenous.
Safety set (received at least 1 dose of study drug).
Safety
The majority of subjects received a total of 2 or 3 doses (89.6% meloxicam IV and 90.7% placebo), although some subjects received up to 7 doses during treatment. The number of doses administered was similar between treatment groups and consistent across age and renal function status and surgery type. The overall incidence of adverse events for meloxicam IV–treated subjects and placebo‐treated subjects was 63.0% and 65.0%, respectively (Table 3). Across treatment groups (meloxicam IV 30 mg and placebo), study investigators assessed adverse events to be mild (49.6% and 51.4%, respectively) or moderate (27.1% and 29.0%, respectively) in intensity. The majority of events were assessed by the investigator to be either “not related” to treatment (meloxicam IV, n = 267 [49.6%]; placebo, n = 89 [48.6%]), or “only possibly related” to treatment (meloxicam IV, n = 125 [23.2%]; placebo, n = 53 [29.0%]). There were a total of 33 serious adverse events in 24 subjects, with a greater frequency observed in the placebo group: 20 events in 14 subjects (2.6%) in the meloxicam IV group and 13 events in 10 subjects (5.5%) in the placebo group. The most common serious adverse events were infections (meloxicam IV, n = 3 [0.6%]; placebo, n = 2 [1.1%]), procedural complications (meloxicam IV, n = 6 [1.1%]; placebo, n = 3 [1.6%]), and gastrointestinal events (meloxicam IV, n = 4 [0.7%]; placebo, n = 2 [1.1%]). Procedural complications in the meloxicam IV group included postprocedural embolism (n = 3 [0.6%]), femoral neck fracture (n = 1 [0.2%]), postoperative ileus (n = 1 [0.2%]), and tendon injury (n = 1 [0.2%]); in the placebo group they included anastomotic ulcer (n = 1 [0.5%]), incisional hernia (n = 1 [0.5%]), and postoperative ileus (n = 1 [0.5%]). Two subjects (both in the meloxicam IV group) discontinued treatment due to an adverse event. One subject discontinued due to localized edema, and the other subject discontinued as a result of postprocedural pulmonary embolism. There were no deaths during the study.
Table 3.
Summary of Treatment‐Emergent Adverse Events in Subjects
| Meloxicam IV (n = 538) | Placebo (n = 183) | |||
|---|---|---|---|---|
| Event | Events | No. Subjects (%) | Events | No. Subjects (%) |
| ≥1 Event | 731 | 339 (63.0) | 281 | 119 (65.0) |
| Intensity | ||||
| Mild | 493 | 267 (49.6) | 182 | 94 (51.4) |
| Moderate | 220 | 146 (27.1) | 92 | 53 (29.0) |
| Severe | 18 | 15 (2.8) | 7 | 5 (2.7) |
| Relationship | ||||
| Not related | 529 | 267 (49.6) | 186 | 89 (48.6) |
| Possibly related | 192 | 125 (23.2) | 90 | 53 (29.0) |
| Probably related | 9 | 5 (0.9) | 4 | 4 (2.2) |
| Definitely related | 1 | 1 (0.2) | 1 | 1 (0.5) |
| Adverse event‐related treatment discontinuation | 2 (0.4) | 0 | ||
| Most common events (≥3%) | ||||
| Nausea | 129 | 123 (22.9) | 58 | 51 (27.9) |
| Constipation | 51 | 51 (9.5) | 17 | 17 (9.3) |
| Vomiting | 30 | 27 (5.0) | 15 | 14 (7.7) |
| Headache | 21 | 20 (3.7) | 13 | 12 (6.6) |
| Pruritus | 21 | 21 (3.9) | 10 | 10 (5.5) |
| GGT increased | 21 | 21 (3.9) | 5 | 5 (2.7) |
| Dizziness | 15 | 15 (2.8) | 9 | 8 (4.4) |
| Anemia | 19 | 18 (3.3) | 4 | 4 (2.2) |
| ALT increased | 11 | 11 (2.0) | 7 | 7 (3.8) |
ALT indicates alanine aminotransferase; GGT, γ‐glutamyltransferase; IV, intravenous.
The most common adverse events for both meloxicam IV–treated and placebo‐treated subjects were gastrointestinal events (ie, nausea, constipation, vomiting), headache, and pruritus (Table 3). Between the meloxicam IV and placebo groups, there was a numerical reduction in many adverse events typically associated with opioid therapy, such as nausea (22.9% versus 27.9%, respectively), vomiting (5.0% versus 7.7%), and pruritus (3.9% versus 5.5%). Rates of adverse events were higher for subjects older than 65 years of age with mild renal impairment compared with the remainder of the population in both the meloxicam IV (67.0% versus 62.2%) and placebo (77.4% versus 62.5%) groups. Adverse events were also higher among subjects undergoing orthopedic surgery than among those undergoing other surgery types in both the meloxicam IV (64.7% versus 61.2%) and placebo (68.8% versus 60.9%) groups.
Adverse events of special interest included injection‐site reactions, bleeding, and cardiovascular, hepatic, renal, thrombotic, and wound‐healing events (Table 4). Bleeding events occurred in 4.8% of meloxicam IV–treated subjects and in 3.8% of placebo‐treated subjects, including anemia (the most common bleeding event [3.3% versus 2.2%]). Renal events (1.3% versus 0%), injection‐site reactions (1.5% versus 0%), wound‐healing events (5.8% versus 3.8%), and hepatic events (6.5% versus 8.2%) were reported in the meloxicam IV group and placebo group, respectively. Two of the 3 renal events (acute renal injury) were assessed as serious events that were not considered related to the study medication. One event occurred in a subject after sepsis, multiorgan failure, and a prolonged intensive care hospitalization. The other event was in a subject with a history of congestive heart failure who had high creatinine values, 2+ protein in his urine, and below‐normal hematocrit and hemoglobin values before surgery. The low hematocrit and hemoglobin values in the postoperative period ultimately required 3 units of packed red blood cells; the resultant fluid volume overload, which was treated with furosemide, resulted in prolonged hospitalization. This subject experienced an event of acute renal failure on day 18; the final dose of study medication had been given on day 4. Three subjects in the meloxicam IV group experienced postprocedural pulmonary embolisms that were considered serious adverse events unrelated to the study treatment. Two of these subjects had predisposing medical conditions (ie, previous deep vein thrombosis, hyperlipidemia, coronary artery disease).
Table 4.
Adverse Events of Special Interest
| Meloxicam IV (n = 538) | Placebo (n = 183) | |||
|---|---|---|---|---|
| Event | Events | No. Subjects (%) | Events | No. Subjects (%) |
| ≥1 Event | 144 | 100 (18.6) | 43 | 29 (15.8) |
| Bleeding | 30 | 26 (4.8) | 7 | 7 (3.8) |
| Anemia | 19 | 18 (3.3) | 4 | 4 (2.2) |
| Ecchymosis | 2 | 2 (0.4) | 0 | 0 |
| Hematochezia | 1 | 1 (0.2) | 1 | 1 (0.5) |
| Incision site hemorrhage | 1 | 1 (0.2) | 1 | 1 (0.5) |
| Wound hematoma | 3 | 3 (0.6) | 0 | 0 |
| Hepatic | 52 | 35 (6.5) | 26 | 15 (8.2) |
| ALT increased | 11 | 11 (2.0) | 7 | 7 (3.8) |
| AST increased | 5 | 5 (0.9) | 5 | 5 (2.7) |
| Alkaline phosphatase increased | 2 | 2 (0.4) | 3 | 3 (1.6) |
| Bilirubin increased | 1 | 1 (0.2) | 1 | 1 (0.5) |
| GGT increased | 21 | 21 (3.9) | 5 | 5 (2.7) |
| Hepatic enzyme increased | 0 | 0 | 2 | 2 (1.1) |
| LFT abnormal | 5 | 5 (0.9) | 2 | 2 (1.1) |
| Injection‐site reactions | 9 | 8 (1.5) | 0 | 0 |
| Renal | 7 | 7 (1.3) | 0 | 0 |
| Acute kidney injury | 3 | 3 (0.6) | 0 | 0 |
| Blood urea increased | 2 | 2 (0.4) | 0 | 0 |
| Urine output decreased | 2 | 2 (0.4) | 0 | 0 |
| Thrombotic | 4 | 4 (0.7) | 0 | 0 |
| Postprocedural PE | 3 | 3 (0.6) | 0 | 0 |
| Wound healing | 36 | 31 (5.8) | 8 | 7 (3.8) |
| Cellulitis | 5 | 5 (0.9) | 0 | 0 |
| Incision site erythema | 2 | 2 (0.4) | 1 | 1 (0.5) |
| Incision site hemorrhage | 1 | 1 (0.2) | 1 | 1 (0.5) |
| Incision site infection | 6 | 5 (0.9) | 0 | 0 |
| Incision site edema | 3 | 3 (0.6) | 0 | 0 |
| Incision site rash | 2 | 2 (0.4) | 0 | 0 |
| Procedural complication | 2 | 2 (0.4) | 1 | 1 (0.5) |
| Seroma | 2 | 2 (0.4) | 1 | 1 (0.5) |
| Wound dehiscence | 3 | 3 (0.6) | 2 | 2 (1.1) |
| Wound hematoma | 3 | 3 (0.6) | 0 | 0 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ‐glutamyltransferase; IV, intravenous; LFT, liver function test; PE, pulmonary embolism.
Changes in laboratory values were evaluated using shift plots, and Table 5 summarizes clinically significant shifts from normal at baseline. No individual laboratory parameters were identified to have a clinically meaningful shift among meloxicam IV–treated subjects during the study. Although shifts in individual laboratory parameters were observed, the shifts were similar between meloxicam IV– and placebo‐treated subjects. The incidence of clinically meaningful changes in vital signs was also similar between treatment groups. There were 2 clinically significant abnormal ECGs obtained during the study (both in the meloxicam IV group) that occurred 7 days posttreatment and were not considered related to the study medication.
Table 5.
Potentially Clinically Significant Change in Laboratory Assessments From Normal at Baseline, n (%)
| Parameter | Potential Significance Criteria | Meloxicam IV (n = 538) | Placebo (n = 183) |
|---|---|---|---|
| Hematocrit | <30% | 24 (4.5) | 8 (4.4) |
| Hemoglobin | <10 g/dL | 41 (7.6) | 12 (6.6) |
| BUN | 1.5‐3 × ULN | 1 (0.2) | 1 (0.6) |
| Creatinine | >1.5 × ULN | … | … |
| ALT | 3‐10 × ULN | 8 (1.8) | 4 (2.4) |
| ≥10 × ULN | 1 (0.2) | … | |
| AST | 3‐10 × ULN | 8 (1.7) | 5 (3.0) |
| ≥10 × ULN | … | … | |
| GGT | 3‐10 × ULN | 17 (3.7) | 8 (5.1) |
| ≥10 × ULN | 1 (0.2) | 1 (0.6) | |
| Alkaline phosphatase | 1‐3 × ULN | 63 (13.6) | 19 (11.9) |
| 3‐10 × ULN | 1 (0.2) | … | |
| Total bilirubin | 1.5‐2 × ULN | 3 (0.6) | 2 (1.2) |
| >2 × ULN | … | 1 (0.6) | |
| aPTT | ≥55 seconds | … | … |
| INR | >1.5 | 5 (0.9) | 1 (0.5) |
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; GGT, γ‐glutamyltransferase; INR, international normalized ratio; IV, intravenous; ULN, upper limit of normal.
Assessment of investigator satisfaction with wound healing was similar between meloxicam IV– and placebo‐treated subjects. Mean assessment scores were at least 9.2 out of 10, both at discharge and at the 7‐day follow‐up visit, suggesting that investigators were consistently satisfied with overall wound healing. The incidence of clinically significant findings on all wound evaluation parameters (erythema, drainage, edema, induration, hematoma) was no greater than 1.1% for all wound assessment parameters in both treatment groups.
Opioid Consumption
Mean opioid consumption, measured by converting opioid analgesic doses to the IV morphine equivalent dose, was numerically lower in the meloxicam IV group compared with the placebo group at all time points (hours 0‐24, 24‐48, 48‐72, 0‐48, and 0‐72 hours) and reached statistical significance at hours 0‐24, 0‐48, and 0‐72 (Table 6, Figure 2). Over the treatment period, the total IV morphine equivalent dose was 9.2 mg lower among meloxicam IV–treated subjects compared with those receiving placebo, although the difference was not statistically significant (29.8 versus 39.0; P = .0531). Subjects undergoing orthopedic surgery used more total opioids on average than did the overall population, and the difference in opioid use between placebo‐treated and meloxicam IV–treated patients was greater in orthopedic subjects. The mean total IV morphine equivalent dose use during treatment was 36.8 mg for meloxicam IV versus 50.3 mg for placebo (P = .0081) for orthopedic surgery compared with values of 22.1 mg (meloxicam IV) and 26.5 mg (placebo) for other surgeries. The overall conclusion per confirmatory analysis using a nonparametric approach was consistent with the planned analysis, and an additional 6 comparisons showed a significant difference in rank (Table 6).
Table 6.
Summary of Total Opioid Consumptiona by Time Interval
| All Subjects | ||||
|---|---|---|---|---|
| Interval | Meloxicam IV (n = 537)b | Placebo (n = 183) | P Valuec | P Valued |
| 0‐24 h | 17.9 ± 22.86 | 23.3 ± 27.90 | .0025 | <.0001 |
| 24‐48 he | 8.7 ± 19.20 | 11.3 ± 21.82 | .0846 | .0015 |
| 48‐72 hf | 4.1 ± 16.59 | 6.2 ± 21.83 | .2725 | .1530 |
| 0‐48 h | 26.3 ± 37.73 | 34.3 ± 44.08 | .0060 | <.0001 |
| 0‐72 h | 28.4 ± 45.49 | 37.4 ± 55.31 | .0126 | <.0001 |
| During treatment | 29.8 ± 58.02 | 39.0 ± 68.08 | .0531 | <.0001 |
| Orthopedic Surgery | ||||
|---|---|---|---|---|
| Meloxicam IV (n = 282)b | Placebo (n = 96) | P Valuec | P Valued | |
| 0‐24 h | 22.1 ± 22.75 | 31.1 ± 30.87 | .0032 | .0006 |
| 24‐48 he | 11.7 ± 18.67 | 15.7 ± 23.92 | .0362 | .0067 |
| 48‐72 hf | 5.3 ± 11.13 | 8.6 ± 22.80 | .2178 | .2768 |
| 0‐48 h | 33.5 ± 37.05 | 46.3 ± 46.51 | .0032 | <.0001 |
| 0‐72 h | 35.9 ± 40.86 | 50.0 ± 55.59 | .0037 | <.0001 |
| During treatment | 36.8 ± 42.69 | 50.3 ± 47.7 | .0081 | <.0001 |
| Other Surgery | ||||
|---|---|---|---|---|
| Meloxicam IV (n = 255) | Placebo (n = 87) | P Valuec | P Valued | |
| 0‐24 h | 13.3 ± 22.14 | 14.6 ± 21.23 | .4704 | .0409 |
| 24‐48 he | 5.3 ± 19.25 | 6.4 ± 18.21 | .7216 | .0600 |
| 48‐72 hf | 3.1 ± 20.09 | 4.3 ± 21.07 | .3507 | .2830 |
| 0‐48 h | 18.4 ± 36.96 | 20.9 ± 37.13 | .5078 | .0081 |
| 0‐72 h | 20.2 ± 48.88 | 23.5 ± 51.84 | .5308 | .0069 |
| During treatment | 22.1 ± 70.53 | 26.5 ± 77.95 | .5734 | .0072 |
IV indicates intravenous.
Intravenous morphine equivalent dose (mg, mean ± SD).
Excluded 1 subject who had erroneous data that could not be confirmed.
P value from analysis of covariance for treatment group difference.
P value from Cochran‐Mantel‐Haenszel analysis on rank controlling center.
n = 519, 275, and 244, respectively, for meloxicam IV and n = 178, 93, and 85, respectively, for placebo, in all subjects, orthopedic surgery, and other surgery.
n = 274, 126, and 148, respectively, for meloxicam IV and n = 93, 41, and 52, respectively, for placebo, in all subjects, orthopedic surgery, and other surgery.
Figure 2.
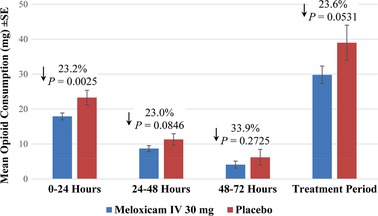
Total mean opioid consumption as measured by IV morphine equivalent dose at different time intervals during the study and over the treatment period. IV indicates intravenous.
Discussion
A novel nanocrystal formulation of meloxicam is being studied for the management of moderate to severe pain that allows for once‐daily administration as an IV bolus. Results from the current study demonstrate that meloxicam IV 30 mg was generally well tolerated and has an opioid‐reducing effect when administered to subjects with moderate to severe pain following a variety of elective surgical procedures.
Meloxicam IV had a safety profile similar to that of placebo with respect to numbers and frequencies of adverse events. This was true of adverse events of special interest (bleeding, injection‐site reactions, hepatic events, renal events, thrombotic events, and wound‐healing events). These results suggest that short‐term postoperative meloxicam IV is not associated with an increased risk of these complications, although it must be noted that subjects at highest risk of these events (eg, those with a history of significant cardiovascular, renal, hepatic, bleeding events) were not eligible for inclusion in the study.
Meloxicam IV was also associated with a statistically significant reduction in opioid use at several postoperative time intervals (hours 0‐24, 0‐48, and 0‐72). Over the treatment period, meloxicam IV was associated with a 23.6% reduction in total opioid use (9.2‐mg morphine equivalent) compared to placebo‐treated subjects. Differences in opioid use were identified when subjects were stratified by surgery type. For example, the overall use of opioids and the opioid‐sparing effect of meloxicam IV were greater in orthopedic surgeries than in other surgery types. The use of multimodal and complementary medications to reduce opioid consumption is a goal cited by recent guidelines,11, 33 and the magnitude of the opioid‐reducing effect observed in this study is clinically meaningful within this context.
Although the study was not powered to assess differences in opioid‐related adverse events, there was a numeric reduction in many adverse events typically associated with opioid therapy (eg, nausea, vomiting, pruritus) among meloxicam IV–treated subjects. Consensus guidelines on the management of postoperative nausea and vomiting have emphasized the importance of minimizing postoperative opioids as a means of reducing the risk of opioid‐induced nausea and vomiting and have noted the value of opioid‐sparing NSAIDs as a means of achieving this goal.34
A limitation of this study was that differences in adverse event rates were difficult to identify among adverse events with lower incidence rates and between study groups with limited numbers of subjects.
Conclusion
Once‐daily IV bolus doses of meloxicam IV 30 mg were generally well tolerated, as indicated by a low incidence of adverse events that was comparable to that with placebo. Safety assessments demonstrated that meloxicam IV did not cause an increase in events that are commonly associated with NSAIDs. Opioid consumption was reduced in the meloxicam IV group during all intervals versus placebo.
Conflicts of Interest Statement
Sergio D. Bergese has received grants from Recro Pharma, Inc. Keith A. Candiotti has received consultancies and grants from Recro Pharma, Inc. Randall J. Mack and Stewart W. McCallum have stock ownership and options and are employees of Recro Pharma, Inc. Wei Du receives consultancies from Recro Pharma, Inc. Alexis Gomez has stock ownership and options and was an employee of Recro Pharma, Inc at the time of this study. Timothy I. Melson, Sabry S. Ayad, and Jorge E. Marcet do not have any conflicts of interest to disclose.
Supporting information
Supplemental Table S1. Investigators, Clinical Sites Enrolling ≥1 Subject, and Ethics Committee Institutional Review Board for Each Study Location
Acknowledgments
Assistance with manuscript preparation was provided by Bret Fulton, RPh, and Susan Martin, PhD, of The Medicine Group, and funding was provided by Recro Pharma, Inc, Malvern, Pennsylvania.
Funding Statement
Funding for this research was provided by Recro Pharma, Inc, Malvern, Pennsylvania.
Protocol is available on request from the Corresponding Author.
Trial Registration Information:
Identifier: NCT02720692
Website: ClinicalTrials.gov
URL: https://clinicaltrials.gov/ct2/show/NCT02720692
Date of Registration: March 22, 2016
Prior Presentations: PAINWeek National Conference, September 5‐9, 2017; Poster 78. Encore presentation at the 43rd Annual Regional Anesthesiology and Acute Pain Medicine Meeting, cohosted by the American Society of Regional Anesthesia and Pain Medicine (ASRA), April 19‐21, 2018; Poster 5171.
References
- 1. Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23(1):21‐36. [DOI] [PubMed] [Google Scholar]
- 2. Prabhakar A, Mancuso KF, Owen CP, et al. Perioperative analgesia outcomes and strategies. Best Pract Res Clin Anaesthesiol. 2014;28(2):105‐115. [DOI] [PubMed] [Google Scholar]
- 3. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post‐surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149‐160. [DOI] [PubMed] [Google Scholar]
- 4. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534‐540. [DOI] [PubMed] [Google Scholar]
- 5. Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 suppl):S105‐S120. [PubMed] [Google Scholar]
- 6. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55‐e71. [DOI] [PubMed] [Google Scholar]
- 7. Lovich‐Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95(2):301‐318. [DOI] [PubMed] [Google Scholar]
- 8. Skolnick P, Volkow ND. Re‐energizing the development of pain therapeutics in light of the opioid epidemic. Neuron. 2016;92(2):294‐297. [DOI] [PubMed] [Google Scholar]
- 9. Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477‐487. [DOI] [PubMed] [Google Scholar]
- 10. Dahl JB, Nielsen RV, Wetterslev J, et al. Post‐operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1165‐1181. [DOI] [PubMed] [Google Scholar]
- 11. Chou R, Gordon DB, de Leon‐Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131‐157. [DOI] [PubMed] [Google Scholar]
- 12. Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non‐steroidal anti‐inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11(1):52‐64. [DOI] [PubMed] [Google Scholar]
- 13. Huang YM, Wang CM, Wang CT, et al. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskel Disord. 2008;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sutters KA, Shaw BA, Gerardi JA, Hebert D. Comparison of morphine patient‐controlled analgesia with and without ketorolac for postoperative analgesia in pediatric orthopedic surgery. Am J Orthop (Belle Mead, NJ). 1999;28(6):351‐358. [PubMed] [Google Scholar]
- 15. Gan TJ, Singla N, Daniels SE, et al. Postoperative opioid sparing with injectable hydroxypropyl‐β‐cyclodextrin‐diclofenac: pooled analysis of data from two phase III clinical trials. J Pain Res. 2017;10:15‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawlinson A, Kitchingham N, Hart C, et al. Mechanisms of reducing postoperative pain, nausea and vomiting: a systematic review of current techniques. Evid Based Med. 2012;17(3):75‐80. [DOI] [PubMed] [Google Scholar]
- 17. Degner F, Türck D, Pairet M. Pharmacological, pharmacokinetic and clinical profile of meloxicam. Drugs Today. 1997;33(10):739‐758. [Google Scholar]
- 18. Del Tacca M, Colucci R, Fornai M, Blandizzi C. Efficacy and tolerability of meloxicam, a COX‐2 preferential nonsteroidal anti‐inflammatory drug. Clin Drug Invest. 2002;22(12):799‐818. [Google Scholar]
- 19. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase‐2 selective non‐steroidal anti‐inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12(11):1‐278, iii. [DOI] [PubMed] [Google Scholar]
- 20. Noble S, Balfour JA. Meloxicam. Drugs. 1996;51(3):424‐430; discussion 431‐432. [DOI] [PubMed] [Google Scholar]
- 21. Davies NM, Skjodt NM. Clinical pharmacokinetics of meloxicam. A cyclo‐oxygenase‐2 preferential nonsteroidal anti‐inflammatory drug. Clin Pharmacokinet. 1999;36(2):115‐126. [DOI] [PubMed] [Google Scholar]
- 22. Engelhardt G, Homma D, Schlegel K, Utzmann R, Schnitzler C. Anti‐inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non‐steroidal anti‐inflammatory agent with favourable gastrointestinal tolerance. Inflamm Res. 1995;44(10):423‐433. [DOI] [PubMed] [Google Scholar]
- 23. Gates BJ, Nguyen TT, Setter SM, Davies NM. Meloxicam: a reappraisal of pharmacokinetics, efficacy and safety. Expert Opin Pharmacother. 2005;6(12):2117‐2140. [DOI] [PubMed] [Google Scholar]
- 24. Chesné C, Guyomard C, Guillouzo A, et al. Metabolism of meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica. 1998;28(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 25. Busch U, Heinzel G, Narjes H. Effect of food on pharmacokinetics of meloxicam, a new non steroidal anti‐inflammatory drug (NSAID). Agents Actions. 1991;32(1‐2):52‐53. [DOI] [PubMed] [Google Scholar]
- 26. Marier J, Offman E, Gosselin NH, et al. Meloxicam IV dose selection: results of a population pharmacokinetic and exposure‐response analysis for the management of moderate to severe pain. World Congress on Regional Anesthesia & Pain Medicine; April 19‐21, 2018; New York, NY. https://epostersonline.com/ASRAWORLD18/node/37. Accessed December 13, 2018.
- 27. Christensen SE, Cooper SA, Mack RJ, et al. A randomized double‐blind controlled trial of intravenous meloxicam in the treatment of pain following dental impaction surgery. J Clin Pharmacol. 2018;58(5):593‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rechberger T, Mack RJ, McCallum SW, Du W, Freyer A. Analgesic efficacy and safety of intravenous meloxicam in subjects with moderate‐to‐severe pain after open abdominal hysterectomy: a phase 2 randomized clinical trial. Anesth Analg. 2019;128:1309‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singla N, McCallum SW, Mack RJ, et al. Safety and efficacy of an intravenous nanocrystal formulation of meloxicam in the management of moderate to severe pain following laparoscopic abdominal surgery. J Pain Res. 2018;11:1901‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gottlieb IJ, Tunick DR, Mack RJ, et al. Evaluation of the safety and efficacy of an intravenous nanocrystal formulation of meloxicam in the management of moderate‐to‐severe pain after bunionectomy. J Pain Res. 2018;11:383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singla N, Bindewald M, Singla S, et al. Efficacy and safety of intravenous meloxicam in subjects with moderate‐to‐severe pain following abdominoplasty. Plast Reconstr Surg—Glob Open. 2018;6(6):e1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pollak R, Gottlieb IJ, Hakakian F, et al. Efficacy and safety of intravenous meloxicam in subjects with moderate‐to‐severe pain following bunionectomy: a randomized, double‐blind, placebo‐controlled trial. Clin J Pain. 2018;34(10):918‐926. [DOI] [PubMed] [Google Scholar]
- 33. Helander EM, Menard BL, Harmon CM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21(1):3. [DOI] [PubMed] [Google Scholar]
- 34. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85‐113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Investigators, Clinical Sites Enrolling ≥1 Subject, and Ethics Committee Institutional Review Board for Each Study Location


