Abstract
Confirming Burnet's early hypothesis, elimination of self‐reactive T cells in the thymus was demonstrated in the late 1980s, and an important question immediately arose about the nature of the self‐peptides expressed in the thymus. Many genes encoding neuroendocrine‐related and tissue‐restricted antigens (TRAs) are transcribed in thymic epithelial cells (TECs). They are then processed for presentation by proteins of the major histocompatibility complex (MHC) expressed by TECs and thymic dendritic cells. MHC presentation of self‐peptides in the thymus programs self‐tolerance by two complementary mechanisms: (1) negative selection of self‐reactive “forbidden” T cell clones starting already in fetal life, and (2) generation of self‐specific thymic regulatory T lymphocytes (tTreg cells), mainly after birth. Many studies, including the discovery of the transcription factors autoimmune regulator (AIRE) and fasciculation and elongation protein zeta family zinc finger (FEZF2), have shown that a defect in thymus central self‐tolerance is the earliest event promoting autoimmunity. AIRE and FEZF2 control the level of transcription of many neuroendocrine self‐peptides and TRAs in the thymic epithelium. Furthermore, AIRE and FEZF2 mutations are associated with the development of autoimmunity in peripheral organs. The discovery of the intrathymic presentation of self‐peptides has revolutionized our knowledge of immunology and is opening novel avenues for prevention/treatment of autoimmunity.
Keywords: self‐peptide, thymus, self‐tolerance, autoimmunity, reverse, tolerogenic, self‐vaccine
Presentation of self‐peptides in the thymus is the basic mechanism that underlies T cell differentiation, negative selection of self‐reactive T cell clones, and generation of tTreg cells. There is no doubt that thymus‐based novel strategies could alleviate in the future the weight of so many known and still unknown autoimmune diseases that remain the heavy tribute, mainly paid by mankind, for the performance and extreme diversity of its highly complex adaptive immunity.

Summary of thymus history
The word “thymus” first appeared in the manuscripts of Claudius Galen (129−around 216 A.D.) and was described as an excrescence having some morphological analogy with the leaf of the plant Thymus cunila (savory or “sarriette” in French). Galen thought that the thymus had no other function than providing protection between the sternum and superior vena cava. Galen also observed that the size of the thymus was larger in young animals and decreased with aging.1, 2
Over many centuries, the anatomy of the thymus was the essential focus of the studies on this organ until the Scottish embryologist John Beard (1858−1924), a pioneer of today's theory of cancer stem cells, wrote that he considered the thymus to be the source of some white blood cells in the body.3, 4
In 1890, the German anatomist Wilhelm Waldeyer (1836−1921) noticed that in elderly people islets of normal thymic tissue could be observed even after adipose involution of this organ. Jan‐August Hammar (Sweden, 1841−1946) also showed that, although thymic development is maximal at puberty, normal thymic tissue persists until advanced age. He observed that animal castration before puberty prevents thymic involution with age and that thymic hypoplasia is associated with pregnancy, undernourishment, and some infectious diseases. He showed that, conversely, thymic hyperplasia is associated with autoimmune Graves’ thyroid disease, Addison's adrenal insufficiency, myasthenia, and acromegaly.5 The thymus was then seen just as another glandular component of the endocrine system, and this assumption was reinforced by Hans Selye's observation of a severe thymic atrophy in stressful conditions activating the hypothalamic−pituitary−adrenal axis.6
The first immunological function of the thymus was discovered when French‐born Australian immunologist Jacques F.A.P. Miller showed that thymectomy performed in mice immediately after birth rendered them highly susceptible to infections and provoked their premature death. He also observed a marked lymphopenia in blood, spleen, and lymph nodes of these mice. These animals were also unable to reject a foreign skin graft, an essential hallmark of the immune response at that time. Miller concluded that the thymus was the organ responsible for the development of immunocompetent cells that constitute a specific cell population, thymus‐dependent (T) lymphocytes.7, 8 Despite the rightness of Miller's experiments and conclusions, the British immunologist Sir Peter Medawar (the 1960 Nobel Prize), regarded as the father of transplantation, still wrote in 1963: “We shall come to regard the presence of lymphocytes in the thymus as an evolutionary accident of no very great significance.”9
In the perspective of hematopoietic growth factors identified at that time, Miller advanced the hypothesis of one or several soluble thymic factors that would be responsible for driving T cell differentiation.10 Innumerable studies worldwide failed to demonstrate the existence of such thymus‐specific growth factor(s)/hormone(s) and it was never possible to apply the endocrine model to the communication between thymic epithelial cells (TECs) and thymocytes (T cells). The demonstration of a crucial role of the thymus during fetal life, as well as the absence of any pathogenicity resulting from thymectomy a few days after birth, reinforced the idea that the thymus was, if not a vestigial organ, at least an organ that quickly becomes useless, this being verified in human clinics: the DiGeorge congenital syndrome, which is the most common form of genetic microdeletion, associates, together with other defects, with the absence or hypoplasia of the thymus and severe immunodeficiency. On the other hand, children who have been thymectomized during surgical correction of a congenital cardiac defect do not suffer from any patent immune deficiency further in life.
Immunological self‐tolerance
Since the foundation of immunology at the end of the 19th century, prediction of autotoxicity/autoimmunity has been intimately associated with the discovery of antitoxins/antibodies. As soon as in 1900, Paul Ehrlich (1854−1915, the 1908 Nobel Prize) proposed the aphorism “horror autotoxicus” to claim the impossibility that one organism could be attacked under normal conditions by its own cells in charge for its defenses. Ehrlich thought then that either structures or mechanisms should exist to avoid autotoxicity, and this should be of the highest importance for individual health and species survival.11 In the continuation of his revolutionary theory of clonal selection, the Australian virologist and immunologist Sir Frank Macfarlane Burnet (1899−1985), who shared the 1960 Nobel Prize with Medawar, introduced the term tolerance to characterize one of the cardinal properties of the adaptive immune system with diversity and memory. In marked contrast with Medawar's statement, during a conference at the University of London in 1962, Burnet prophesized: “If, as I think, the thymus is the site where occurs proliferation of lymphocytes in clones with precise immunological functions, we have also to consider another function: elimination or inhibition of clones with reactivity to self.”
Afterward, the molecular mechanisms responsible for the stochastic recombination of gene segments encoding the variable domains of the immunoglobulin B cell receptor for the antigen (BCR)12 and T cell receptor (TCR) for the antigen were elucidated.13, 14, 15 The fantastic lottery behind the generation of diversity in the adaptive immune system may produce more than 1030 cumulated TCR and BCR combinations, the majority of which are able to recognize self‐antigens. In normal conditions, however, the adaptive immune system does not aggress self and, for a long time, immunology was defined as the science of self−nonself discrimination. Burnet was actually speaking about immunology as the science of self and nonself recognition. Indeed, without “training,” lymphocytes are not able to discriminate between self‐ and nonself‐peptides. In 1987 and 1988, the research groups of Douarin,16 Kappler and Marrack,17 MacDonald,18 and von Boehmer19 demonstrated the validity of the theory of thymic clonal negative selection proposed by Burnet many years before. These independent studies clearly evidenced that education to self and establishment of T cell self‐tolerance is imperative even before the acquisition of T cell immunocompetence. Therefore, the thymus is not only responsible for the generation of diversity of the TCR repertoire, but is also primarily a cemetery for early T cells expressing a TCR specific for self‐antigens that are presented to differentiating T cells by proteins of the major histocompatibility complex (MHC) expressed by TECs and thymic dendritic cells (DCs).
In 1972, Richard Gershon (1932−1983) from Yale identified immunosuppressive cells regulating competent lymphocytes.20 After his premature death, his studies were pursued by Shimon Sakaguchi who, in a series of experiments, identified a novel key role for thymus‐derived T cells in the regulation of autoimmune responses.21, 22, 23 Indeed, mainly after birth, the thymus is the source of a new population of tTreg cells that are able to inhibit in the peripheral self‐reactive T cells having escaped negative selection in the thymus. The Foxp3 transcription factor, through its expression in Treg cells, is essential for the prevention of autoimmunity as shown in the human immune dysregulation, polyendocrinopathy, enteropathy, X‐linked (IPEX) syndrome, as well as in scurfy and Foxp3−/− mice.24, 25, 26 The generation of tTreg cells also depends on the presentation of self‐antigens by thymic MHC proteins. How the same mechanism of MHC‐mediated self‐peptide presentation promotes CD4+ fates that are so distinct during thymic T cell differentiation (negative selection of self‐reactive T cells and generation of self‐specific tTreg cells) is still a matter of active exploration.27
A creative metaphor leading to a novel paradigm: from neuropeptides to neuroendocrine “self‐peptides”
Following these seminal studies about the powerful tolerogenic mechanisms occurring inside the thymus, the real nature of self‐peptides that are presented by thymic MHC proteins was then scrutinized. Previously, it was largely assumed that proteins circulating in the blood were captured somewhat “passively” in the thymus (by DCs and macrophages, mainly) and then presented to differentiating T lymphocytes during their transitory residence in this organ. In 1978, immunoreactive neurotensin (NT) and somatostatin were identified in the chicken thymus epithelium,28 but these observations were not followed further. In 1986, synthesis of biologically active and immunoreactive oxytocin (OT), in equimolar content with neurophysin, its binding protein derived from the same precursor, was discovered in human thymus extracts.29 In the thymus microenvironment, OT is synthesized by TECs and not by thymocytes.30 Following the metaphor of the “nursing” of newborns through OT galactagogue action, thymic “nurse” cells (TNCs) were shown to be a cortical TEC population synthesizing OT.31 Intrathymic of the oxytocin gene (OXT) transcription coincides with its expression in the hypothalamus.32 Furthermore, functional neurohypophysial receptors (OTR and V1b) are expressed by distinct thymic T cell subsets. After binding to these receptors, OT—much more than vasopressin (VP)—promotes phosphorylation of T cell tyrosine kinases closely implicated in focal adhesion, and this event could be implicated in the formation of immunological synapses between TECs and thymocytes.33 Neuropeptide Y (NPY), neurokinin A (NKA),34 and insulin‐like growth factor‐2 (IGF‐2)35, 36 were also found to be synthesized in the thymic epithelium. The repertoire of neuroendocrine‐related precursors is organized in such an economical way that one member per family is predominantly expressed in TECs: OT for the neurohypophysial family, NT for neuromedins, NKA for tachykinins, and IGF‐2 for the insulin family.37, 38 TECs were proposed to exhibit a more “promiscuous” promoter use than other peripheral tissues.39
Most importantly, in the thymus, the processing of OT precursor does not lead to classic neurosecretion as established a long time ago for the hypothalamo−neurohypophysial axis. Thymic OT is not located in classic secretory granules, but is diffuse within TEC/TNC cytosol, in large clear vacuoles, and around perimembranous space.40 Actually, OT precursor is processed for the presentation of OT peptide by thymic MHC class I (MHC‐I) molecules, and the neurophysin domain of OT precursor is associated in this presentation through an MHC‐I−neurophysin 55 kD hybrid molecule.41 The biochemical mechanism underlying formation of this hybrid protein remains to be specified but, nevertheless, these data suggest an analogy of neurophysin function between, on the one hand, the transport of the neurohormone OT along hypothalamo−neurohypophysial neurons to nerve endings in posterior pituitary and, on the other hand, the intrathymic presentation to pre‐T cells of OT as a self‐peptide of the neurohypophysial family. The selective advantage of this form of MHC‐I presentation of OT is that it would not be tightly restricted by MHC‐I alleles and would allow presentation of the OT cyclic structure. The behavior of thymic OT as a self‐antigen targeted to TEC plasma membrane was further supported by the stimulation in the production by cultured human TECs of interleukin‐6 (IL‐6) and leukemia inhibitory factor through the immunological recognition of OT by specific OT‐specific monoclonal antibodies.42 This study suggests that deadly self‐recognition in the thymus could lead to some cytokine release helpful for the survival and development of thymocytes that do not recognize self‐peptides.
The hypothesis that a thymic neuropeptide actually behaves as a self‐peptide was further investigated with NT, a 13‐amino acid linear neuropeptide. Intrathymic MHC‐I presentation of NT was demonstrated according to the biochemical rules of MHC‐I presentation as established by Hans‐Georg Rammensee's laboratory in Tübingen.43 Interestingly, the C‐terminal sequence of NT includes tyrosine, leucine, and isoleucine residues, which can be used for anchorage to most of MHC‐I proteins.44 So, NT and NT‐derived C‐terminal fragments could serve as natural ligands for a majority (if not all) of MHC‐I alleles, thus also without any tight allelic restriction that is hardly conceivable for the establishment of central immune self‐tolerance to a universal peptide. This hypothesis also concords with the high degree of conservation of NT‐related C‐terminal region during evolution. All these studies allowed us to propose a theoretical model that completely transposes at the molecular level the function of the thymus in T cell development and central self‐tolerance. This was extensively discussed in Refs. 37 and 38 (Fig. 1).
Figure 1.
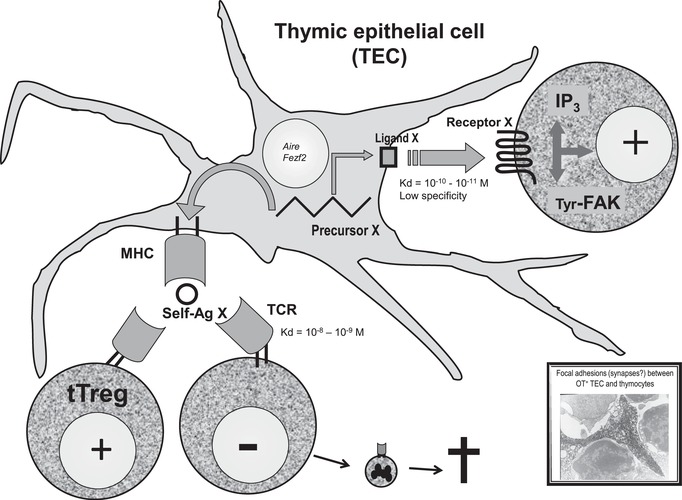
The triple role of thymic neuroendocrine self‐peptides in T cell differentiation. Following its transcription under AIRE (or FEZF2) control in TEC, a neuroendocrine precursor X is processed according to two distinct pathways. On the one hand, it is the source of a cryptocrine ligand X that is able to bind to a neuroendocrine cognate receptor expressed by T cells, to mobilize second messengers (such as IP3), and to induce intracellular events (such as phosphorylation of the focal adhesion‐related kinases p125Fak and p130Cas for thymic OT). This constitutes a positive accessory signal during T cell development. On the other hand, the same precursor X is also processed as the source of self‐peptide(s) X that is presented by MHC proteins of TECs or after transfer to thymic DCs. During fetal life, self‐presentation induces negative selection of T cells that are randomly bearing a TCR specific of this MHC−self‐peptide X complex. Mainly after birth, self‐presentation also promotes the generation of tTreg cells specific to the same complex. The electron microscopy photograph is a generous gift from Martin Wiemann, now at the University of Duisburg‐Essen.
In the early 2000s, Bruno Kyewski, Jens Derbinski, and Ludger Klein in Heidelberg further proposed that promiscuous gene expression of tissue‐restricted antigens (TRAs) in the thymus was a novel key to understand immune self‐tolerance and autoimmunity.45, 46 Contrary to neuroendocrine self‐peptides, TRAs do not behave as accessory signals binding to cognate receptors during T cell development. Also, while neuroendocrine self‐peptides are synthesized in all TECs, promiscuous gene expression of TRAs in the thymus is a unique property of medullary TECs (mTECs) subsets, and these genes are transiently transcribed in clusters along chromosomes.47 After some initial skepticism, the immunology field recognized the importance of thymus‐dependent central self‐tolerance.37, 48, 49 Kyewski's group also showed that thymic DCs are implicated in the presentation of TRAs synthesized in mTECs indicating a unidirectional transfer of self‐peptides inside the thymic cellular microenvironment.50 Patterns of promiscuous expression of insulin and casein locus‐related genes also indicated that a stochastic mechanism underlies their transcription in mTECs.51
The epigenetic regulation of promiscuous gene expression in the thymus is currently under intense investigation.52, 53 Most probably, these studies might explain in the future the hierarchy in the expression of neuroendocrine self‐peptides belonging to the same family, the absence of parental imprinting of IGF2 transcription in TECs,47 and control of the immunological mirror of self in the thymus.
A thymus defect as the earliest event promoting autoimmunity
While the unique physiological function of the thymus in programming central self‐tolerance is now well established, more and more experimental observations point to a disturbance of thymus‐dependent tolerance as a key event in the early development of organ‐specific autoimmune diseases (Fig. 2). In 1973, Burnet already predicted that “forbidden” T cell clones having mutated from a state of self‐tolerance to self‐reactivity could be a major event in the physiopathology of autoimmunity.54 In the BioBreeding (BB) rat, an animal model of type 1 diabetes mellitus (T1D), removal of the thymus at birth prevents the appearance of autoimmune diabetes.55 Transplantation of the thymus from diabetes‐resistant (DR) to diabetes‐prone (DP) BB rats also inhibits this spontaneous disease.56 In the nonobese diabetic (NOD) mouse, another animal model of T1D, grafts of NOD thymuses to DR mouse strains induce the occurrence of autoimmune diabetes in recipients.57 Similarly, transplantation of embryonic NOD thymic epithelium to C57BL/6 athymic mice induces in recipients autoimmune sialitis and insulitis similar to those observed in adult female NOD.58 Other authors also reported defects of central tolerance and apoptosis of self‐reactive T cells in the NOD thymus.59, 60
Figure 2.

Role of the thymus in programming central self‐tolerance and in the development of autoimmunity. In normal conditions, under the control of AIRE and FEZF2, TECs express numerous genes related to neuroendocrine families or encoding TRAs. MHC presentation of these self‐peptides induces T cell differentiation, negative selection of self‐reactive T cells, and generation of tTreg cells with the same specificity. In pathological conditions, the decrease in intrathymic expression and presentation of self‐peptides leads to continuous generation in the blood of self‐reactive “forbidden” T cells (Teff cells), as well as to a decrease in the generation of self‐specific tTreg cells. This is a condition, necessary but not sufficient, for the development of an autoimmune response against target antigens. For the clinical manifestations of an autoimmune disease, the intervention of environmental factors is also requested.
Several groups then addressed the fundamental question whether inhibition or a significant decrease in intrathymic self‐presentation could result in a continuous release of “forbidden” self‐reactive T cells accumulating in the peripheral repertoire, as well as to a decrease in the generation of self‐reactive tTreg cells. If this hypothesis was verified, it would be clear that a defect in thymic tolerogenic function might determine early the development of organ‐specific autoimmune diseases. Our laboratory investigated this question more particularly for the pathogenesis of autoimmune insulin‐dependent T1D. Concerning the members of the insulin gene family, IGF2 is highly expressed in TECs in the cortex and medulla of the thymus from different species. IGF1 transcripts are detected in all TECs as well as in thymic macrophages, while INS is transitorily transcribed in rare subsets of mTECs.47 This hierarchical profile implicates that IGF‐2 is highly tolerated by the T cell system since T cell tolerance is directly proportional to the intrathymic concentration of self‐peptides.61 Using fetal thymic organ cultures, we also observed that T cell proliferation and differentiation was severely inhibited after the interference of IGF‐mediated signaling between TECs and thymocytes, an effect that was absent after the blockade of proinsulin signaling.62 Igf2 transcription is detected in the thymus of BB‐DR rats but is absent in the thymus of ±85% of BB‐DP rats, in close coincidence with the incidence of autoimmune diabetes in BB‐DP rats.63 These data may explain the BB‐DP lymphopenia (including a decrease in the frequency of rat Treg cells), as well as defective programming of central tolerance to insulin‐secreting islet β cells.
A specific topography and hierarchy also exist in the expression of the two mouse insulin genes, Ins1 and Ins2. Ins2 transcription predominates in murine mTECs, while Ins1 is mostly expressed in islet β cells. These contrasted profiles explain why insulitis and autoimmune diabetes are accelerated in Ins2 −/− NOD mice,64 whereas these processes are significantly inhibited in Ins1 −/− NOD mice.65 Levels of Ins2 transcription in the thymus also modulate insulin‐specific T cell tolerance.66 Interestingly, thymic Ins2 transcription is independent of glycemia and is markedly enhanced by an anti‐lymphotoxin‐β monoclonal antibody.67 However, the insulin‐specific transactivator Mafa also promotes thymic Ins2 transcription and Mafa inactivation was shown to reduce thymic Ins2 expression and to stimulate in parallel the generation of autoantibodies against anti‐islet β cells.68 INS transcripts are quantified at a lower level in the thymus of human fetuses with the genetic marker insulin‐dependent diabetes mellitus 2 (IDDM2) of susceptibility to T1D.69, 70
The identification of the autoimmune regulator gene (AIRE), a member of the zinc‐finger gene family, played a major role in further evidencing the central role played by a thymus dysfunction in the pathogenesis of organ‐specific autoimmunity.71 AIRE mutations determine a rare recessive congenital syndrome called autoimmune polyglandular syndrome type 1 or autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy syndrome.72 Aire transcription is maximal in mTECs, and Aire−/− mice develop several autoimmune processes in parallel with a marked decrease in the intrathymic expression of numerous neuroendocrine self‐peptides (including OT, INS, IGF‐2, and NPY) and many TRAs.73 Both IDDM2 and AIRE transcription determine the level of INS transcription in the human thymus.74 The development and differentiation of murine Aire‐expressing mTECs is regulated by RANK signals from thymic CD4+CD3− lymphoid tissue inducer cells.75 Interestingly, extrathymic Aire‐expressing cells were recently identified as distinct bone marrow−derived tolerogenic cells that could anergize in secondary lymphoid organs effector self‐reactive CD4+ T cells having escaped thymic negative selection.76, 77
More recently, it has been shown that the gene Fezf2 encoding fasciculation and elongation protein zeta family zinc finger‐2 protein also controls intrathymic transcription of self‐peptides, the majority of which are not regulated by Aire. Fezf2 disruption is also associated with autoimmune processes in the periphery.78 Noteworthy, Fezf2 is also a transcription factor implicated in some developmental processes of the central nervous system (CNS).
With regard to the most frequent autoimmune endocrine disease, all major thyroid‐specific autoantigens, such as thyroperoxydase, thyroglobulin, and thyrotropin receptor (TSHR), are also transcribed in human TECs in normal conditions.79, 80, 81 It was further shown that homozygotes for an SNP allele predisposing to Graves’ disease have significantly lower intrathymic TSHR transcripts than carriers of the protective allele.82
A defect in α‐myosin expression in TECs exerts a major role in the physiopathology of autoimmune myocarditis,83 and a defect in Aire‐mediated central tolerance to myelin protein zero promotes the appearance of an autoimmune TH1 effector response toward peripheral nerves.84 There is also large supportive evidence that thymic tolerance plays an essential role in CNS autoimmune diseases.85
Coevolution of immune and neuroendocrine systems
The innate immune system evolves in parallel with the neuroendocrine system in all living species without any sign of autotoxicity/autoimmunity (Fig. 3). Toll‐like receptors, which are major mediators of innate immunity, do not react against normal or undamaged self. Primitive forms of immune diversity are present in agnathans, mediated by diverse variable lymphocyte receptors, with 4−12 leucine‐rich repeat modules that were most probably assembled by some gene conversion process.86 About 450–500 million years ago, the emergence of transposon‐like recombination–activating genes Rag1 and Rag2 in jawed fishes was responsible for the appearance of a novel and highly complex system of immune defenses, the adaptive immunity. The subsequent development of the combinatorial immune system has been sometimes regarded as the immunological “Big Bang.” Because of its high risk of autotoxicity inherent to its diversity, the emergence of adaptive immunity exerted an evolutionary pressure so strong that, according to Ehrlich's prediction of horror autotoxicus, novel structures and mechanisms appeared with the specific role of orchestrating immune self‐tolerance. The first unique thymus also appeared in sharks and rays but it was preceded by thymoid lymphoepithelial structures located in the gill baskets of lamprey larvae.87 These structures express the gene encoding forkhead box N4 (Foxn4), the paralog of Foxn1, which is responsible for the differentiation of the thymic epithelium in most vertebrates. Thus, Foxn4/Foxn1 determined the emergence of the thymic epithelium, which is an absolute requirement for T cell differentiation and programming of central immune self‐tolerance.88
Figure 3.

Integrated evolution of the immune and neuroendocrine systems. Neuroendocrine precursors did not evolve extensively except by gene duplication and differential RNA splicing. Throughout evolution, the neuroendocrine and innate immune system have evolved in parallel, and still coexist in all living species without any aggression of the innate immune system toward neuroendocrine glands. A high risk of inherent autoimmunity toward neuroendocrine tissues resulted from the appearance of recombination‐activating genes RAG1 and RAG2, and RAG‐dependent adaptive immunity in jawed cartilaginous fishes some 450 million years ago. Preceded by ancestor paralog Foxn4‐expressing thymoids in gill baskets of lamprey larvae, the first unique thymus (with Foxn1‐expressing TECs) also emerged in jawed vertebrates. The intrathymic presentation of dominant neuroendocrine self‐peptides (arrows) may be viewed a posteriori as a very efficient and economical way to instruct the adaptive T cell system in recognizing and tolerizing neuroendocrine families already during thymus‐dependent T cell differentiation in fetal life. VCBP, variable region‐containing chitin‐binding protein; VLR, variable lymphocyte receptor.
The hierarchy observed in the organization of the thymic repertoire of neuroendocrine self‐peptides has also evolutive implications. Neuroendocrine functions having been installed before adaptive immunity, they had to be protected against autoimmunity. OT is implicated at different steps of the reproductive process and is therefore fundamental for the preservation of animal and human species. Because of its predominance in the thymus, OT is much more tolerated than VP, its neurohypophysial homolog, which mainly regulates water homeostasis and vascular pressure. This lower immunological tolerance of VP may explain why rare cases of autoimmune central diabetes insipidus have been repeatedly observed.89
In the insulin family, insulin is the primary T1D autoantigen and no autoimmunity has been reported against IGF‐2, which is fundamental for fetal growth and development. Nevertheless, because of their homology, thymic neuroendocrine self‐peptides program immune cross‐tolerance to their whole family, and tolerance of insulin is indeed decreased in Igf2−/− mice.90
Conceptual translation: from immunogenic to tolerogenic vaccines
Until now, the development of anti‐infective vaccines was essentially based on immunogenic and memory properties of the adaptive immune system. Future vaccines against autoimmune diseases could be developed on the basis of the initial property of self‐tolerance programmed for this system and recent knowledge about the potent tolerogenic properties of the thymus, which have not been exploited until now. As reported above, although Ins2 is expressed at very low levels in rare mTEC subsets, proinsulin per se does not exert any tolerogenic properties that could be used to reprogram immunological tolerance toward islet β cells. With the exception of only two studies (that were not confirmed until now),91, 92 all the clinical trials based on insulin failed to protect the residual β cell mass from the diabetogenic autoimmune response. At the opposite, the potent immunogenic properties of insulin were repeatedly evidenced,93, 94 and insulin immunogenicity could actually be linked to the very low level of INS transcription in mTEC subsets. The risk of hypersensitivity or anaphylaxis following administration of an autoantigen was also reported.95
IGF‐2 could provide a more efficient basis than insulin for developing a specific “reverse/tolerogenic self‐vaccination”96 (Fig. 4) based on the following data:
Igf2 is a dominant member of the insulin family expressed in the thymus.35
Igf2 transcription is defective in the thymus of BB‐DP rats.63
IGF‐2 B11−25 and insulin B9−23 (InsB9−23) compete for binding to DQ2 and DQ8 (collaboration with K. Wücherpfennig, unpublished data), the MHC‐II alleles conferring the highest genetic susceptibility to T1D.
Contrary to InsB9−23, IGF‐2 B11−25 presentation by peripheral blood mononuclear cells isolated from DQ8+ diabetic patients induces a tolerogenic cytokine profile with marked IL‐10 induction.97
IGF‐2 mediates significant cross‐tolerance to insulin.90
Infection of a murine TEC line with the diabetogenic coxsackievirus B4‐E2 inhibits Igf2 transcription and IGF‐2 production.98
Figure 4.

Classical vaccination and the concept of “reverse” self‐vaccination. Classical vaccination essentially relies on an immunogenic response (naive T cell activation and induction of memory immune cells) elicited by administration of antigen(s) representative of pathogens. T1D pathogenesis also relies on an immunogenic response targeting several T1D antigens, such as insulin as the primary T1D autoantigen (X). The novel type of “reverse” self‐vaccination proposes to use thymus self‐peptides for promoting a tolerogenic response (deletion of self‐reactive T cells and generation of self‐reactive Treg cells). For T1D prevention and cure, the corresponding thymus self‐peptide is IGF‐2 (X′). Noteworthy, insulin is actually an “altered self” peptide of IGF‐2.101 − 103
This promising concept of reverse tolerogenic self‐vaccination is under current development with the active support of Wallonia DGO6 Win2Wal THYDIA Project.
Conclusion
Far from being a useless “vestigial” organ, numerous studies performed worldwide have definitively demonstrated that the thymus is crucial for ensuring the global homeostasis of the adaptive immune system and is unique in programming central immune self‐tolerance. Presentation of self‐peptides in the thymus is the basic mechanism that underlies T cell differentiation, negative selection of self‐reactive T cell clones, and generation of tTreg cells. There is no doubt that thymus‐based novel strategies could alleviate in the future the weight of so many known and still unknown autoimmune diseases that remain the heavy tribute, mainly paid by mankind, for the performance and extreme diversity of its highly complex adaptive immunity.
Competing interests
The authors declare no competing interests.
Acknowledgments
This paper is dedicated to the memory of my very good friend Bruno Kyewski and Harald von Boehmer who sadly left us in 2018, in recognition of their seminal work in thymology. Our gratitude is due to Anne Cooke (University of Cambridge), Pierre J. Lefèbvre (Past‐President of the International Diabetes Federation), and Pierre De Meyts (de Duve Institute, UCLouvain) for their critical reading of this manuscript. The studies summarized here have been supported by the Fonds Léon Fredericq for biomedical research (ULiège and CHU Liège), the University of Liège, SPW‐Recherche THYDIA 181013, the F.S.R.‐NFSR of Belgium, the Wallonia‐Brussels Federation, the Fonds Alphonse Rahier for research in Diabetology (Belgium), the Fondation contre le Cancer (Belgium), the European Commission (FP6 Integrated Project Euro‐Thymaide 2004−2008), the European Association for the Study of Diabetes (EASD, Germany), and the Juvenile Diabetes Research Foundation (JDRF, New York). V.G. is Research Director at F.S.R. of Belgium, professor of developmental biology and history of biomedical research at ULiège, and clinical head in endocrinology at CHU of Liège. He is a member of the Royal Academy of Medicine of Belgium.
References
- 1. Geenen, V. 2017. Histoire du thymus—D'un accident de l’évolution à la programmtion de la tolérance immunitaire. Med. Sci. (Paris) 33: 653–663. [DOI] [PubMed] [Google Scholar]
- 2. Geenen, V. & Savino W.. 2019. History of the thymus—from a vestigial organ to the programming of immunological self‐tolerance In Thymus Transcriptome and Cell Biology. Passos G.A., Ed. London: Springer‐Nature. [Google Scholar]
- 3. Beard, J. 1894. The development and probable function of the thymus. Anat. Anz. 9: 474–486. [Google Scholar]
- 4. Beard, J. 1899. The true function of the thymus. Lancet 153: 144–146. [Google Scholar]
- 5. Hammar, J. 1921. The new views at the morphology of the thymus gland and their bearing on the problem of the function of the thymus. Endocrinology 5: 543–573. [Google Scholar]
- 6. Selye, H. 1946. The general adaption syndrome and the diseases of adaptation. J. Clin. Endocrinol. Metab. 6: 117–130. [DOI] [PubMed] [Google Scholar]
- 7. Miller, J.F. 1961. The immunological function of the thymus. Lancet 2: 748–749. [DOI] [PubMed] [Google Scholar]
- 8. Miller, J.F. 1964. The thymus and the development of immunologic responsiveness. Science 144: 1544–1551. [DOI] [PubMed] [Google Scholar]
- 9. Medawar, P.B. 1963. Discussion after Miller J.F.A.P. and Osoba D. Role of the thymus in the origin of immunological competence In The Immunologically Competent Cell: Its Nature and Origin. Vol. 16. Wostenholme G.E.W. & Knight J., Eds.: 70 London: Ciba Foundation Study Group. [Google Scholar]
- 10. Osoba, D & Miller J.F.. 1963. Evidence for a humoral factor responsible for the maturation of immunological faculty. Nature 199: 653–654. [DOI] [PubMed] [Google Scholar]
- 11. Ehrlich, P. 1900. The Croonian Lecture: on immunity. Proc. Soc. Lond. Biol. 66: 424. [Google Scholar]
- 12. Tonegawa, S., Steinberg C., Dube S. & Bernardini A.. 1974. Evidence for somatic generation of antibody diversity. Proc. Natl. Acad. Sci. USA 71: 4027–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malissen, M., Minard K., Mjolsness S., et al 1984. Mouse T cell antigen receptor: structure and organization of constant and joining segments encoding the beta polypeptide. Cell 73: 1101–1110. [DOI] [PubMed] [Google Scholar]
- 14. Toyonaga, B., Yanagi Y., Suciu‐Foca N., et al 1984. Rearrangements of T‐cell receptor gene YT35 in human DNA from thymic leukemia T‐cell lines and functional T‐cell clones. Nature 311: 385–387. [DOI] [PubMed] [Google Scholar]
- 15. Davis, M.M. , Chien Y.H., Gascoigne N.R. & Hedrick S.M.. 1984. A murine T cell receptor gene complex: isolation, structure and rearrangement. Immunol. Rev. 81: 235–258. [DOI] [PubMed] [Google Scholar]
- 16. Ohki, H. , Martin C., Corbel C., et al 1987. Tolerance induced by thymic epithelial grafts in birds. Science 237: 1032–1035. [DOI] [PubMed] [Google Scholar]
- 17. Kappler, J.W. , Roehm N. & Marrack P.. 1987. T cell tolerance by clonal elimination in the thymus. Cell 49: 273–280. [DOI] [PubMed] [Google Scholar]
- 18. MacDonald, H.R. , Glasebrrok A.L., Schneider R., et al 1988. T‐cell receptor Vβ use predicts reactivity and tolerance to Mlsa‐encoded antigens. Nature 332: 40–45. [DOI] [PubMed] [Google Scholar]
- 19. Kisielow, P. , Blüthmann H., Staerz U.D., et al Tolerance in T‐cell receptor transgenic mice involves deletion of non mature CD4+8+ thymocytes. Nature 333: 742–746. [DOI] [PubMed] [Google Scholar]
- 20. Gershon, R.K. , Cohen P., Hencin R. & Liebhaler S.A.. 1972. Suppressor T cells. J. Immunol. 108: 586–590. [PubMed] [Google Scholar]
- 21. Sakaguchi, S. , Takahashi T. & Nishizuka Y.. 1982. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt‐1 effector cells for oocytes damage after adoptive transfer. J. Exp. Med. 156: 1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakaguchi, S. , Takahashi T. & Nishizuka Y.. 1982. Study on cellular events in postthymectomy autoimmune oophoritis in mice. II. Requirement of Lyt‐1 cells in normal female mice for the prevention of oophoritis. J. Exp. Med. 156: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakaguchi, S. , Sakaguchi N., Asano M., et al 1995. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chain (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J. Immunol. 155: 1151–1164. [PubMed] [Google Scholar]
- 24. Chatila, T.A. , Blaeser N., Ho N., et al 2000. JM2, encoding a fork head‐related protein, is mutated in X‐linked autoimmunity‐allergy disregulation syndrome. J. Clin. Invest. 106: R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunkow, M.E. , Jeffery E.W., Hjerrild K.A., et al 2001. Disruption of a new forkhead/winged‐helix protein, scurfin, results in fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27: 68–77. [DOI] [PubMed] [Google Scholar]
- 26. Hori, S. , Nomura T. & Sakaguchi S.. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 269: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 27. Klein, L. , Robey E.A. & Hsieh C‐S.. 2019. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 19: 7–18. [DOI] [PubMed] [Google Scholar]
- 28. Sundler, F. , Carraway R.E., Hakanson R., et al 1978. Immunoreactive neurotensin and somatostatin in the chicken thymus. A chemical and histochemical study. Cell Tissue Res. 194: 367–376. [DOI] [PubMed] [Google Scholar]
- 29. Geenen, V. , Legros J.J., Franchimont P., et al 1986. The neuroendocrine thymus: coexistence of oxytocin and neurophysin in the human thymus. Science 232: 508–511. [DOI] [PubMed] [Google Scholar]
- 30. Geenen, V. , Legros J.J., Franchimont P., et al 1987. The thymus as a neuroendocrine organ. Synthesis of vasopressin and oxytocin in human thymic epithelium. Ann. N.Y. Acad. Sci. 496: 56–66. [DOI] [PubMed] [Google Scholar]
- 31. Geenen, V. , Defresne M.P., Robert F., et al 1988. The neurohormonal thymic microenvironment: immunocytochemical evidence that thymic nurse cells are neuroendocrine cells. Neuroendocrinology 47: 365–368. [DOI] [PubMed] [Google Scholar]
- 32. Hansenne, I. , Rasier G., Péqueux C., et al 2005. Ontogenesis and functional aspects of oxytocin and vasopressin gene expression in the thymus network. J. Neuroimmunol. 158: 67–75. [DOI] [PubMed] [Google Scholar]
- 33. Martens, H. , Kecha O., Renard‐Charlet C., et al 1998. Neurohypophysial peptides activate phosphorylation of focal adhesion kinases in immature thymocytes. Neuroendocrinology 67: 282–289. [DOI] [PubMed] [Google Scholar]
- 34. Ericsson, A. , Geenen V., Vrindts‐Gevaert Y., et al 1990. Expression of preprotachykinin A and neuropeptide‐Y messenger RNA in the thymus. Mol. Endocrinol. 4: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 35. Geenen, V. , Achour I., Robert F., et al 1993. Evidence that insulin‐like growth factor 2 (IGF2) is the dominant thymic peptide of the insulin superfamily. Thymus 21: 115–127. [PubMed] [Google Scholar]
- 36. Kecha, O., Martens H., Franchimont N., et al 1999. Characterization of the insulin‐like growth factor axis in the human thymus. J. Neuroendocrinol. 11: 435–440. [DOI] [PubMed] [Google Scholar]
- 37. Martens, H. , Goxe B. & Geenen V.. 1996. The thymic repertoire of neuroendocrine self‐peptides: physiological implications in T‐cell life and death. Immunol. Today 17: 312–317. [DOI] [PubMed] [Google Scholar]
- 38. Geenen, V. , Kecha O. & Martens H.. 1998. Thymic expression of neuroendocrine self‐peptide precursors: role in T cell survival and self‐tolerance. J. Neuroendocrinol. 10: 811–822. [DOI] [PubMed] [Google Scholar]
- 39. Geenen, V. & Kroemer G.. 1993. Multiple ways to cellular immune tolerance. Immunol. Today 14: 573–575. [DOI] [PubMed] [Google Scholar]
- 40. Wiemann, M. & Ehret G.. 1993. Subcellular characterization of immunoreactive oxytocin within thymic epithelial cells of the mouse. Cell Tissue Res. 273: 573–575. [DOI] [PubMed] [Google Scholar]
- 41. Geenen, V. , Vandersmissen E., Cormann‐Goffin N., et al 1993. Membrane translocation and relationship with MHC class I of a human thymic neurophysin‐like molecule. Thymus 22: 55–66. [PubMed] [Google Scholar]
- 42. Martens, H. , Malgrange B., Robert F., et al 1996. Cytokine production by human thymic epithelial cells: control by the immune recognition of the neurohypophysial self‐antigen. Regul. Pept. 67: 39–45. [DOI] [PubMed] [Google Scholar]
- 43. Rammensee, H.G. , Falk K. & Rötschke O.. 1993. Peptides naturally presented by MHC class I molecules. Annu. Rev. Immunol. 11: 213–244. [DOI] [PubMed] [Google Scholar]
- 44. Vanneste, Y. , Ntodou‐Thome A., Vandersmissen E., et al 1997. Identification of neurotensin‐related peptides in human thymic epithelial cell membranes and relationship with major histocompatibility complex class I molecules. J. Neuroimmunol. 76: 161–166. [DOI] [PubMed] [Google Scholar]
- 45. Klein, L. & Kyewski B.. 2000. “Promiscuous” expression of tissue antigens in the thymus: a key to T‐cell tolerance and autoimmunity. J. Mol. Med. 78: 483–494. [DOI] [PubMed] [Google Scholar]
- 46. Derbinski, J. , Schulte A., Kyewski B. & Klein L.. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 47. Gotter, J. , Brors B., Hergenhahn M. & Kyewski B.. 2004. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue‐specific genes colocalized in chromosomal clusters. J. Exp. Med. 199: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mathis, D. & Benoist C.. 2004. Back to central tolerance. Immunity 20: 509–516. [DOI] [PubMed] [Google Scholar]
- 49. Kyewski, B. & Klein L.. 2006. A central role for central tolerance. Annu. Rev. Immunol. 24: 571–606. [DOI] [PubMed] [Google Scholar]
- 50. Koble, C. & Kyewski B.. 2009. The thymic medulla: a unique microenvironment for intercellular self‐antigen transfer. J. Exp. Med. 206: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Derbinski, J. , Pinto S., Rösch S., et al 2008. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc. Natl. Acad. Sci. USA 105: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tykocinski, L.O. , Sinemus A., Rezavandy E., et al 2010. Epigenetic regulation of promiscuous gene expression in thymic medullary epithelial cells. Proc. Natl. Acad. Sci. USA 107: 19426–19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Handel, A.E. , Shikama‐Dorn N., Zhanybekova S., et al 2018. Comprehensive profiling the chromatin architecture of tissue‐restricted antigen expression in thymic epithelial cells over development. Front. Immunol. 9: 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burnet, F.M. 1973. A reassessment of the forbidden clone hypothesis of autoimmune diseases. Aust. J. Exp. Biol. Med. 50: 1–9. [DOI] [PubMed] [Google Scholar]
- 55. Like, A.A. , Kslaukis E., Williams R.M. & Rossini A.A.. 1982. Neonatal thymectomy prevents spontaneous diabetes mellitus in the BB/W rat. Science 216: 644–646. [DOI] [PubMed] [Google Scholar]
- 56. Georgiou, H.M. & Bellgrau D.. 1989. Thymus transplantation and disease prevention in the diabetes‐prone Bio‐Breeding rat. J. Immunol. 142: 3400–3405. [PubMed] [Google Scholar]
- 57. Georgiou, H.M & Mandel T.E.. 1995. Induction of insulitis in athymic (nude) mice. The effect of NOD thymus and pancreas transplantation. Diabetes 44: 49–59. [DOI] [PubMed] [Google Scholar]
- 58. Thomas‐Vaslin, V. , Damotte D., Coltey M., et al 1997. Abnormal T cell selection on NOD thymic epithelium is sufficient to induce autoimmune manifestations in C57/BL/6 athymic nude mice. Proc. Natl. Acad. Sci. USA 94: 4598–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kishimoto, H. & Sprent J.. 2001. A defect in central tolerance in NOD mice. Nat. Immunol. 2: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 60. Zucchelli, S. , Holler P., Yamagata T., et al 2005. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 22: 285–396. [DOI] [PubMed] [Google Scholar]
- 61. Ashton‐Rickardt, P.G. , Bandeira A., Delaney J.R., et al 1994. Evidence for a differential avidity model of T selection in the thymus. Cell 76: 651–663. [DOI] [PubMed] [Google Scholar]
- 62. Kecha, O. , Brilot F., Martens H., et al 2000. Involvement of insulin‐like growth factors in early T cell development: a study using fetal thymic organ cultures. Endocrinology 141: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 63. Kecha‐Kamoun, O. , Achour I., Martens H., et al 2001. Thymic expression of insulin‐related genes in an animal model of type 1 diabetes. Diab. Metab. Res. Rev. 17: 146–152. [DOI] [PubMed] [Google Scholar]
- 64. Thebault‐Beaumont, K. , Dubois‐Lafargue D., Krief P., et al 2003. Acceleration of type 1 diabetes mellitus in proinsulin‐2 deficient NOD mice. J. Clin. Invest. 111: 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moriyama, H. , Abiru N., Paronen J., et al 2003. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in nonobese diabetic mouse. Proc. Natl. Acad. Sci. USA 100: 10376–10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chentoufi, A.A. & Polychronakos C.. 2002. Insulin expression levels in the thymus modulate insulin‐specific autoreactive T‐cell tolerance. Diabetes 51: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 67. Levi, D. & Polychronakos C.. 2009. Regulation of insulin gene expression by cytokines and cell–cell interactions in mouse medullary thymic epithelial cells. Diabetologia 52: 2812–2824. [DOI] [PubMed] [Google Scholar]
- 68. Noso, S. , Kataoka K., Kawabata Y., et al 2010. Insulin transactivator Mafa regulates intrathymic expression of insulin and affects susceptibility to type 1 diabetes. Diabetes 59: 2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vafiadis, P. , Bennett S.T., Todd J.A., et al 1997. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 15: 289–292. [DOI] [PubMed] [Google Scholar]
- 70. Pugliese, A. , Zeller M., A. Fernandez, Jr. , et al 1997. The insulin gene is transcribed in human thymus and transcription levels correlate with allelic variation at the INS VNTR‐IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15: 293–297. [DOI] [PubMed] [Google Scholar]
- 71. The Finnish‐German APECED Consortium . 1997. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD‐type zinc‐finger domains. Nat. Genet. 17: 399–403. [DOI] [PubMed] [Google Scholar]
- 72. Nagamine, K. , Peterson P., Scott H., et al 1997. Positional cloning of the APECED gene. Nat. Genet. 17: 393–398. [DOI] [PubMed] [Google Scholar]
- 73. Anderson, M.S. , Venanzi E.S., Klein L., et al 2002. Projection of an immunological self‐shadow within the thymus by the Aire protein. Science 298: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 74. Sabater, L. , Ferrer‐Francesch X., Sospedra M., et al Insulin alleles and autoimmune regulator (AIRE) gene both influence insulin expression in the thymus. J. Autoimmunity 25: 312–318. [DOI] [PubMed] [Google Scholar]
- 75. Rossi, S.W. , Kim M.Y., Leibbrandt A., et al 2007. RANK signals from CD4(+)3(–) inducer cells regulate development of Aire‐expressing epithelial cells in the thymic medulla. J. Exp. Med. 204: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gardner, J.M. , Devos J.J., Friedman R., et al 2008. Deletional tolerance mediated by extrathymic Aire‐expressing cells. Science 321: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gardner, J.M. , Metzger T.C., McMahon E.J., et al 2013. Extrathymic Aire‐expressing cells are a distinct bone marrow‐derived population that induces functional activation of CD4+ T cells. Immunity 39: 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takaba, H. , Morishita Y., Tomofuji Y., et al 2015. Fezf2 orchestrates a thymic program of self‐antigen expression for immune tolerance. Cell 163: 975–987. [DOI] [PubMed] [Google Scholar]
- 79. Paschke, R. & Geenen V.. 1995. Messenger RNA expression for a TSH receptor variant in the thymus of a 2‐yr old child. J. Mol. Med. 73: 577–580. [DOI] [PubMed] [Google Scholar]
- 80. Sospedra, M. , Ferrer‐Francesch X., Dominguez O., et al 1998. Transcription of a broad range of self‐antigens in human thymus suggesting a role for central mechanisms in tolerance towards peripheral antigens. J. Immunol. 161: 5918–5929. [PubMed] [Google Scholar]
- 81. Murakami, M. , Hosoi Y., Negishi T., et al 1996. Thymic hyperplasia in patients with Graves’ disease. Identification of thyrotropin receptor in human thymus. J. Clin. Invest. 98: 2228–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Colobran, R. , del Pilar Armengol M., Faner R., et al 2011. Association of an SNP with intrathymic expression of TSHR and Graves’ disease: a role for defective tolerance. Hum. Mol. Genet. 20: 3415–3423. [DOI] [PubMed] [Google Scholar]
- 83. Lv, H. , Havari E., Pinto S., et al 2011. Impaired thymic tolerance to α‐myosin directs autoimmunity to the heart in mice and humans. J. Clin. Invest. 121: 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Su, M. , Davini D., Cheng P., et al 2012. Defective autoimmune regulator‐dependent central tolerance to myelin protein zero is linked to autoimmune peripheral neuropathy. J. Immunol. 188: 4906–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Handel, A.E. , Sarosh R. & Holländer G.A.. 2018. The role a thymic tolerance in CNS autoimmune disease. Nat. Rev. Neurosci. 14: 723–734. [DOI] [PubMed] [Google Scholar]
- 86. Boehm, T. , McCurley N., Sutoh Y., et al 2012. VLR‐based adaptive immunity. Ann. Rev. Immunol. 30: 203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bajoghli, B. , Guo P., Aghaalei N., et al 2011. A thymus candidate in lampreys. Nature 470: 90–94. [DOI] [PubMed] [Google Scholar]
- 88. Zuklys, S. , Handel A., Zhanybekova S., et al 2016. Foxn1 regulates key target genes essential for T cell development in postnatal thymic epithelial cells. Nat. Immunol. 17: 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Bellis, A. , Bizzarro A. & Bellestella A.. 2004. Autoimmune diabetes insipidus In Immunoendocrinology in Health and Disease. Geenen V. & Chrousos G.P., Eds.: 461–490. New York, NY: Marcel Dekker. [Google Scholar]
- 90. Hansenne, I. , Renard‐Charlet C., Greimers R. & Geenen V.. 2006. Dendritic cell differentiation and immune tolerance to insulin‐related peptides in Igf2‐deficient mice. J. Immunol. 176: 4651–4657. [DOI] [PubMed] [Google Scholar]
- 91. Fourlanos, S. , Perry C., Gellert S., et al Evidence that intranasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 60: 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Roep, B. , Salvason N., Gottlieb P., et al 2013. Plasmid‐endcoding proinsulin preserves C‐peptide while specifically reducing proinsulin‐specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 5: 191ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blanas, E. , Carbone F.R., Allison J., et al 1996. Induction of autoimmune diabetes by oral administration of autoantigen. Science 274: 1707–1709. [DOI] [PubMed] [Google Scholar]
- 94. Liu, E. , Moriyama H., Abiru N., et al 2002. Anti‐peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self‐peptides. J. Clin. Invest. 110: 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pedotti, R. , Mitchell D., Wedemeyer J., et al 2001. An unexpected version of horror autotoxicus: anaphylactic shock to a self‐peptide. Nat. Immunol. 2: 216–222. [DOI] [PubMed] [Google Scholar]
- 96. Geenen, V. , Mottet M., Dardenne O., et al 2010. Thymic self‐antigens for the design of a negative/tolerogenic self‐vaccination against type 1 diabetes. Curr. Opin. Pharmacol. 10: 461–472. [DOI] [PubMed] [Google Scholar]
- 97. Geenen, V. , Louis C., Martens H. & The Belgian Diabetes Registry . 2004. An insulin‐like growth factor 2‐derived self antigen inducing a regulatory cytokine profile after presentation to peripheral blood mononuclear cells form DQ8+ type 1 diabetic adolescents: preliminary design of a thymus‐based tolerogenic self‐vaccination. Ann. N.Y. Acad. Sci. 1037: 59–64. [DOI] [PubMed] [Google Scholar]
- 98. Jaïdane, H. , Caloone D., Lobert P.E., et al 2012. Persistent infection of thymic epithelial cells with coxsackie virus B4 results in decreased expression of type 2 insulin‐like growth factor. J. Virol. 86: 11151–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang, G. , Geng X.R., Song J.P., et al 2014. Insulin‐like growth factor 2 enhances regulatory T‐cell functions and suppresses food allergy in an experimental model. J. Allergy Clin. Immunol. 133: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 100. Geng, X.R. , Yang G., Li M., et al 2014. Insulin‐like growth factor 2 enhances functions of antigen‐specific regulatory B cells. J. Biol. Chem. 289: 17941–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zinkernagel, R. & Doherty P.. 1974. Immune surveillance against altered self components by sensitized T lymphocytes in lymphocytic choriomeningitis. Nature 251: 547–548. [DOI] [PubMed] [Google Scholar]
- 102. Houghton, A.N. 1994. Cancer antigens: immune recognition of self and altered self. J. Exp. Med. 180: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yin, L. , Dai S., Clayton G., et al 2013. Recognition of self and altered self by T cells in autoimmunity and allergy. Protein Cell 4: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


