Graphical abstract
Keywords: MERS-CoV, Dromedary camels, Seroprevalence, Prevalence of infection
Highlights
-
•
Most adult dromedaries in Africa and the Middle East have been infected with MERS-CoV.
-
•
Seroprevalence increases with age, while active infection is more common in calves.
-
•
Prevalence is higher at sites where different dromedary populations mix.
-
•
Further study is needed to determine if prevalence of infection varies seasonally.
Abstract
Human infection with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is driven by recurring dromedary-to-human spill-over events, leading decision-makers to consider dromedary vaccination. Dromedary vaccine candidates in the development pipeline are showing hopeful results, but gaps in our understanding of the epidemiology of MERS-CoV in dromedaries must be addressed to design and evaluate potential vaccination strategies. We aim to bring together existing measures of MERS-CoV infection in dromedary camels to assess the distribution of infection, highlighting knowledge gaps and implications for animal vaccination. We systematically reviewed the published literature on MEDLINE, EMBASE and Web of Science that reported seroprevalence and/or prevalence of active MERS-CoV infection in dromedary camels from both cross-sectional and longitudinal studies. 60 studies met our eligibility criteria. Qualitative syntheses determined that MERS-CoV seroprevalence increased with age up to 80–100% in adult dromedaries supporting geographically widespread endemicity of MERS-CoV in dromedaries in both the Arabian Peninsula and countries exporting dromedaries from Africa. The high prevalence of active infection measured in juveniles and at sites where dromedary populations mix should guide further investigation – particularly of dromedary movement – and inform vaccination strategy design and evaluation through mathematical modelling.
1. Introduction
Since the first human case of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection was detected in 2012 (Zaki et al., 2012), a substantial evidence base has built up showing dromedary camels to be the reservoir host of the virus, from which spill over to humans can occur (FAO-OIE-WHO MERS Technical Working Group, 2018). MERS-CoV circulates extensively in dromedary populations causing no impactful disease. Human infection, however, is associated with a measured case fatality ratio of around 35% (World Health Organisation, 2018a). Following spill-over events, human-to-human transmission of MERS-CoV is relatively inefficient and limited to close, unprotected contact environments such as hospitals (Breban et al., 2013; Cauchemez et al., 2013). Phylogenetic analysis of viral sequences isolated from dromedaries and humans indicates that hundreds of camel-to-human spill-over events are likely to have occurred since 2012 (Dudas et al. 2018). Taken together, recurring dromedary-to-human transmission is driving ongoing human infection.
The key role of dromedaries in human MERS-CoV infection has led decision-makers to consider dromedary vaccination as part of MERS-CoV prevention interventions (FAO-OIE-WHO MERS Technical Working Group, 2018). Dromedary-targeted vaccine candidates in the development pipeline are showing promising results and include an orthopox-virus based vaccine capable of greatly reducing viral shedding in dromedary challenge studies (Haagmans et al., 2016).
However, vaccine strategy evaluation is currently precluded by gaps in the understanding of the epidemiology of MERS-CoV in dromedaries. The dromedary population is highly heterogeneous and spans a wide geographic area stretching from West Africa through to the Middle East and parts of Asia. Knowing how infection is distributed within the population, when and where dromedaries would need to be targeted, and the likely impact of vaccination, is necessary before further consideration of dromedary vaccination in the wider socioeconomic and cultural context.
Here, we systematically reviewed published studies that measured MERS-CoV antibody seroprevalence in dromedaries and/or prevalence of viral RNA in dromedaries. Assuming assay specificity and long-term presence of antibodies after infection, seroprevalence can be used to estimate what proportion of a dromedary population has ever been infected with MERS-CoV. Broken down by age class, this can tell us when most animals encounter the infection for the first time. Additionally, although whole-virus isolation and culture is necessary to confirm whether the shedding is infectious, detection of viral RNA through Reverse Transcription Polymerase Chain Reaction (RT-PCR) can be used as a proxy for the prevalence and distribution of infectious dromedaries (Corman et al., 2012; OIE, World Organisation for Animal Health, 2017; World Health Organisation, 2018b).
By conducting a qualitative synthesis of the study findings, considering reported heterogeneities, and summarising the results of longitudinal studies of infection and immunity, we aim to assess the extent of current understanding of MERS-CoV epidemiology in dromedaries, implications for control, and gaps to be addressed going forward. We note that a similar systematic review of the literature up until May 2018 was unknowingly carried out in parallel to our own (Sikkema et al., 2019), with no discussion between the two groups. Here, we validate and update the results of the parallel review, discussing our results in the context of potential animal vaccination and mathematical modelling of MERS-CoV in dromedary camels.
2. Methods
We conducted a systematic review of studies published prior to 31st December 2018 reporting measures of seroprevalence or prevalence of hCoV-EMC or MERS-CoV RNA in dromedary populations by searching EMBASE (Elsevier, 2018), MEDLINE (National Library of Medicine, 2018) and Web of Science (Clarivate Analytics, 2018b) using the search strategy in Fig. 1 . The search and the data extraction were conducted by a single author and no publication date restrictions were imposed. For a full list of search terms used, corresponding PRISMA flowchart and checklist see supplementary material S1–3 respectively.
Fig. 1.
Review strategy. Published studies found with all three of our selected search term groups were then assessed against the exclusion criteria resulting in a final selection of 60 publications.
Records were excluded if they met the following criteria established prior to the search: opinion pieces, or reviews reporting no new data, studies investigating an aspect of MERS-CoV or another pathogen that did not involve dromedary samples or involved experimentally infecting dromedaries with MERS-CoV, and studies not available in English. Remaining studies were categorised as longitudinal or cross-sectional for qualitative synthesis. To address potential bias, we consider studies investigating dromedary populations epidemiologically linked to human MERS-CoV infection separately from those aiming for random sampling.
When available, we took the results of neutralisation-based testing over methods that determined seropositivity based on antibody screening tests alone. Neutralising antibody tests are more specific and are the World Organisation for Animal Health (OIE) recommended method for confirming MERS-CoV seropositivity in dromedaries (OIE, World Organisation for Animal Health, 2017).
We extracted RNA prevalence values determined by RT-PCR of nasal swabs, ignoring any additional samples taken. Use of RT-PCR to test for the presence of at least two of the established genomic regions unique to MERS-CoV is the OIE standard for detection of active MERS-CoV infection in dromedaries (OIE, World Organisation for Animal Health, 2017) and viral RNA is most frequently and abundantly present in nasal swabs compared to other non-invasive samples (Mohran et al., 2016).
Throughout this review, we use ‘calf’ to refer to animals under one-year-old, ‘juvenile’ ≤2 years old, and ‘adult’ >2 years old.
3. Results
Our search retrieved 802 publications. Duplicates were detected and removed in EndNote X8.2 (Clarivate Analytics, 2018a), leaving 414 unique publications. A further 322 records were excluded during abstract screening using the criteria given above. Of the remaining 92, full text screening proved that a further 30 records met the exclusion criteria. Two records sampled Bactrian camels (largely restricted to Central Asia, they have not yet been found to have been infected with MERS-CoV) (Chan et al., 2015; Liu et al., 2015; Miguel et al., 2016), leaving 60 records pertaining to MERS-CoV seropositivity and/or RNA positivity in dromedaries (Fig. 1). 55 of these described cross-sectional studies of dromedary populations, sampling each animal at a single time-point only (40 measured seroprevalence and 32 measured RNA prevalence). Note that 6 studies were designed to investigate groups of dromedaries that had been epidemiologically linked to human cases of MERS-CoV infection rather than conducting a systematic/random survey. Longitudinal studies that measured seropositivity and viral RNA shedding in the same animals at multiple time-points, featured in 11 publications.
3.1. Seroprevalence – cross-sectional studies
Variously, studies conducted dedicated MERS-CoV sero-surveys, opportunistically tested samples taken for other means, tested stored sera or sampled dromedaries during human outbreak investigations. Not all studies used neutralisation-based testing to determine or confirm seropositivity, and, between those that did, cut-off positivity titres varied (Table 1 ).
Table 1.
Cross-sectional surveys of MERS-CoV seroprevalence and RNA prevalence in camels.
| Ref | Country | Year | Seroprevalence |
RNA prevalence |
Stratifications | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | na | Rangeb | NTc | % | na | Rangeb | ||||
| (Crameri et al., 2015) | Australia | 2013-14 | 0% | 307 | – | 1:10 | – | – | – | – |
| (Hemida et al., 2014b) | 2014 | 0% | 25 | – | 1:40 | – | – | – | – | |
| (Islam et al., 2018) | Bangladesh | 2015 | 31% | 55 | – | 1:20 | 0% | 55 | – | age, site type, origin, sex, body condition |
| (Miguel et al., 2017) | Burkina Faso | 2015 | 80%d | 525 | 73-85% | 1:20 | 5%d | 525 | 0-12% | Region. Further factors assessed in GLMM |
| (Reusken et al., 2013a) | Canary Islands | 2012-13 | 9% | 105 | – | 1:20 | – | – | – | origin |
| (Gutierrez et al., 2015) | 2015 | 4% | 170 | – | – | – | – | – | origin | |
| (Muller et al., 2014) | Egypt | 1997 | 79% | 43 | – | 1:80 | – | – | – | – |
| (Chu et al., 2014) | 2013 | 92% | 52 | – | 1:20 | 4% | 110 | 3-30% | sex | |
| (Perera et al., 2013) | 2013 | 94% | 110 | – | 1:20 | – | – | – | – | |
| (Hemida et al., 2014b) | 2014 | 100% | 8 | – | 1:40 | – | – | – | – | |
| (Ali et al., 2017b) | 2014-16 | 71% | 2541 | 59-95%e | 1:20 | 15% | 2825 | 1-36%e | origin, site type, sex, month | |
| (Ali et al., 2017a) | 2015-16 | 85% | 1031 | 77-96%e | 1:20 | 4% | 1078 | 1-9%e | origin, site type, sex | |
| (Reusken et al., 2014b) | Ethiopia | 2010-11 | 96%d | 188 | 95-100% | • | – | – | – | region |
| (Fukushi et al., 2018) | 2013 | 96% | 66 | – | NA | – | – | – | – | |
| (Miguel et al., 2017) | 2015 | 96%d | 632 | 85-99% | 1:20 | 10%d | 632 | 0-16% | Region. Further factors assessed in GLMM | |
| (Thwiny et al., 2018) | Iraq | 2014-15 | 85% | 180 | 85-86% | – | – | – | – | age, region, sex |
| (Al Salihi and Alrodhan, 2017) | 2015-16 | – | – | – | – | 15% | 100 | 0-35% | age, region, month | |
| (David et al., 2018) | Israel | 2012-17 | 62% | 411 | – | 1:20 | 0% | 540 | – | – |
| (Harcourt et al., 2018) | 2013 | 72% | 71 | – | 1:20 | – | – | – | sex | |
| (Shirato et al., 2015) | Japan | <2015 | 0% | 5 | – | 1:20 | 0% | 4 | – | – |
| (Reusken et al., 2013b) | Jordan | 2013 | 100% | 11 | – | 1:20 | – | – | – | |
| (van Doremalen et al., 2017) | 2016 | 82%d | 45 | 77-87% | – | 62% | 45 | 48-77% | age, region, lifestyle | |
| (Miguel et al., 2016) | Kazakhstan | 2015 | 0% | 455 | – | 1:20 | – | – | – | – |
| (Corman et al., 2014) | Kenya | 1992-2013 | 30%f | 228 | 0-100% | – | – | – | – | region, year |
| (Deem et al., 2015) | 2013 | 47%d | 335 | 14-83% | – | – | – | – | age, herd, lifestyle, isolation | |
| (Munyua et al., 2017) | 2013 | 90% | NA | – | – | – | – | – | age, region, sex | |
| (Kiambi et al., 2018) | 2016-17 | – | – | – | – | 0.35%d | 1421 | 0-1.2%d | region, | |
| (Ommeh et al., 2018) | 2016-18 | 68% | 1163 | 17-87% | 1:20 | 0.95%d | 1163 | – | age, region, sex | |
| (Alagaili et al., 2014) | KSAk | 1992-2010 | 87%d | 264 | 77-100% | – | – | – | – | age, region, year |
| (Alagaili et al., 2014) | 2013 | 74% | 150 | 66-100% | – | 25% | 202 | 0-66% | age, region | |
| (Alfuwaires et al., 2017) | 2015-16 | – | – | – | – | 14% | 44 | 0-23% | yearg | |
| (Harrath and Abu Duhier, 2018) | 2016 | 84% | 171 | – | – | – | – | – | age, sex | |
| (Hemida et al., 2014b) | 1993 | 90% | 131 | 73-96% | 1:40 | – | – | – | region | |
| (Hemida et al., 2013) | 2012-13 | 90% | 310 | 85-94% | 1:20 | – | – | – | age, region | |
| (Kasem et al., 2018a) | 2015-17 | – | – | – | – | 56% | 698 | 5-85% | region, site-type, month, year | |
| (Khalafalla et al., 2015) | 2013-14 | – | – | – | – | 29% | 96 | – | age, site, month | |
| (Sabir et al., 2016) | 2014-15 | – | – | – | – | 0.12% | 1309 | – | – | |
| (Falzarano et al., 2017) | Mali | 2009-10 | 88% | 562 | 0-91% | • | – | – | – | region |
| (Miguel et al., 2017) | Morocco | 2015 | 77%d | 343 | 48-100% | 1:20 | 2%d | 343 | 0-8% | region. Further factors assessed in GLMM |
| (Chu et al., 2015) | Nigeria | 2015 | 96% | 131 | – | 1:20 | 11% | 132 | – | – |
| (Reusken et al., 2014b) | 2010-11 | 94% | 358 | 82-96% | • | – | – | – | region | |
| (So et al., 2018) | 2016 | – | – | – | – | 3%d | 2529 | 0-8.4%h | age, week tested | |
| (Nowotny and Kolodziejek, 2014) | Oman | 2013 | – | – | – | – | 7% | 76 | – | – |
| (Reusken et al., 2013a) | 2013 | 100% | 50 | – | 1:20 | – | – | – | – | |
| (Saqib et al., 2017) | Pakistan | 2012-15 | 40% | 565 | 0-83% | 1:80 | – | – | – | region |
| (Zohaib et al., 2018) | 2015-18 | 76% | 1050 | 72-80% | • | 3%d | 776 | – | age, region, sex, lifestyle | |
| (Mohran et al., 2016) | Qatar | 2014 | – | – | – | – | 79% | 53 | 67-92% | – |
| (Reusken et al., 2014a) | 2014 | 100% | 33 | – | 1:** | 21%d | 33 | 0-58% | region | |
| (Raj et al., 2014) | 2014 | – | – | – | – | 2% | 53 | – | – | |
| (Muller et al., 2014) | Somalia | 1983-4 | 81%d | 86 | – | 1:80 | – | – | – | year |
| (Muller et al., 2014) | Sudan | 1984 | 82% | 60 | – | 1:80 | – | – | – | year |
| (Reusken et al., 2014b) | Tunisia | 2009 | 49%d | 204 | 36-100% | • | – | – | – | region |
| (Meyer et al., 2014) | UAEl | 2003 & 13 | 97% | 651 | – | • | – | – | – | year |
| (Alexandersen et al., 2014) | 2005 | 82% | 11 | 0-100%e | 1:12 | – | – | – | site | |
| (Wernery et al., 2015a) | 2014 | 93% | 853 | – | – | 5% | 871 | – | age | |
| (Wernery et al., 2015b) | <2015 | 95% | 254 | – | – | 0% | 254 | – | age | |
| (Yusof et al., 2015) | 2014 | – | – | – | – | 1.6% | 7803 | – | site-type | |
| (Li et al., 2017; Yusof et al., 2017) | 2015 | – | – | – | – | 29%i | 376i | – | – | |
| (Alexandersen et al., 2014) | USAm & Canada | 2000-1 | 0% | 6 | – | 1:12 | – | – | – | – |
| Studies investigating dromedary camel populations linked to human MERS-CoV infection | ||||||||||
| (Muhairi et al., 2016) | UAE | 2012 | – | – | – | – | 4% | 1113 | farm | |
| (Paden et al., 2018) | UAE | 2014 | – | – | – | – | 100% j | 6j | – | – |
| (Al Hammadi et al., 2015) | UAE | 2015 | 100% | 8 | – | 1:40 | 100% | 8 | – | – |
| (Kasem et al., 2018b) | KSA | 2014-16 | 71% | 595 | 37-100% | • | 13% | 584 | 0-56% | region, age, sex |
| (Haagmans et al., 2014) | Qatar | 2013 | 100% | 14 | – | 1:20 | 21% | 14 | – | – |
| (Farag et al., 2015) | Qatar | 2014 | 97% | 103 | – | • | 59% | 105 | – | – |
| Bactrian camels | ||||||||||
| (Chan et al., 2015) | Mongolia | 2014 | 0% | 190 | – | 1:2 | 0% | 190 | – | |
| (Liu et al., 2015) | Mongolia | 2015 | 0% | 200 | – | 1:2 | 0% | 200 | – | |
| (Miguel et al., 2016) | Kazakhstan | 2015 | 0% | 95 | – | – | – | – | – | |
a. total number of camels sampled.
b. range across sub-national locations surveyed.
c. cut off titre to determine positivity if neutralisation test used.
d. we calculated this value from disaggregated values presented by the authors.
e. range given is across site types rather than geographical locations.
f. neutralisation at dilution >1:80 gave 15% seropositivity but regional range only available for ELISA (Enzyme Linked Immunosorbent Assay) results – reported accordingly.
g. study also tested different site-types but measured RNA in serum sample rather than nasal swab so this was not included.
h. range is across weeks rather than regions.
i. both (Li et al., 2017) and (Yusof et al., 2017) report RNA prevalence from the same study.
j. unclear whether study found negative camels – it only mentions that 6 camels were tested, found positive and viral genomes were isolated.
k. Kingdom of Saudi Arabia.
l. United Arab Emirates.
m. United States of America.
•neutralisation test limited to a subset of samples or only used to detect presence of high titres.
** No positivity cut-off titre given but all samples had incredibly high titres, and were able to neutralise at dilution >1:1280.
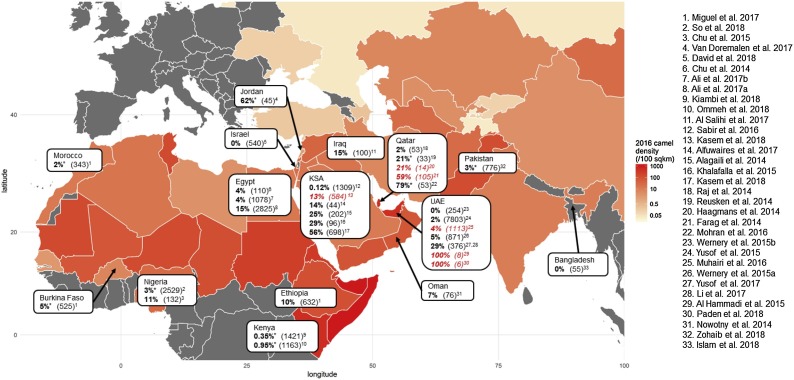
Seropositive dromedaries have been found in 20 of the 24 countries studied. See Table 1 and Fig. 2 for a geographical map of seropositivity. MERS-CoV seroprevalence was between 71–100% in most country-level, age-aggregated study populations across West, North and East Africa and the Middle East. Exceptions included one of the two studies in Israel (62%), and 4–49% in Bangladesh, the Canary Islands, and Tunisia as well as one of the two studies in Pakistan and two of the four studies in Kenya. Samples taken from the large feral camel population in Australia were seronegative, along with dromedaries in Kazakhstan and in zoos in Japan, and North America (Reusken et al., 2013a; Alexandersen et al., 2014; Hemida et al., 2014b; Crameri et al., 2015; Shirato et al., 2015; Miguel et al., 2016). No other species tested alongside dromedaries had neutralising antibodies except a small number of alpacas and llamas living in close quarters with dromedaries in Israel (David et al., 2018), and 1 sheep in Egypt (Ali et al., 2017a). Seroprevalence was >71% in all 4 studies conducted in dromedary populations epidemiologically linked with human MERS-CoV infection.
Fig. 2.
Map of MERS-CoV seroprevalence in dromedaries. Measures of MERS-CoV seroprevalence in dromedaries, aggregated at the country level. Total sample size tested is given in parenthesis. Camel density is calculated using FAOSTAT country-level camel population data (FAO, 2016) and World Bank data on country surface area (World Bank, 2016) *value calculated by us from disaggregated sub-national measures of seroprevalence. Red text highlights studies conducted in dromedary populations in response to an epidemiologically linked human MERS-CoV infection. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Factors effecting seroprevalence
3.2.1. Age
MERS-CoV seroprevalence was found to increase with dromedary age in 13 studies (Table 2 , Fig. 3 ). Although seroprevalence was 80–100% in adult dromedaries in most populations across the Middle East and the horn of Africa, juveniles in the same populations were repeatedly found to have lower and more variable seroprevalence (˜40-90%). One study disaggregated calf age through the first year of life. In this setting (UAE) calves had high seroprevalence increasing with age to 90% in 7–12-month-olds (Wernery et al., 2015b).
Table 2.
Studies reporting seroprevalence stratified by age of dromedary.
| Country | Stratified seroprevalencec | Reported trend | Reported significance | Ref. |
|---|---|---|---|---|
| Bangladesh | <2yrs 9% (n = 11) ≥2yrs 36% (n = 44) |
Higher in camels >2yrs | Not significant | (Islam et al., 2018) |
| Burkina Faso, Ethiopia and Morocco |
Generalised Linear Mixed Model (n = 1500) | Increase with age | p = 0.032 | (Miguel et al., 2017) |
| Egypt | <2yrs 52% (n = 81) ≥2yrs 87% (n = 950) |
Higher in camels >2yrs | p < 0.0001 | (Ali et al., 2017a) |
| Ethiopia | 1- ≤2yrs 93% (n = 31) 2-13yrs 97% (n = 157) |
None | Not significant | (Reusken et al., 2014b) |
| Iraq | <2yrs 89% (n = 44) >2yrs 84% (n = 136) 2-4yrs 81% (n = 58) >4yrs 86% (n = 78) |
Lower in camels 2-4yrs compared with <2yrs | Not significant | (Thwiny et al., 2018) |
| Jordan | ≤2yrs 74%a (n = 31a) >2yrs 100%a (n = 14a) |
ELISA ratio higher in camels >3yrs | Significant p = NA | (van Doremalen et al., 2017) |
| Kenya | <2yrs 29%a (n = 141a) >2yrs 61% (n = 194) <6 m 39% (n = 61) 6 m-2yrs 21% (n = 80) |
Higher in camels >2yrs than <6 m | p < 0.05 | (Deem et al., 2015) |
| Kenya | 1-4yrs 73% (n = 285) >4yrs 98% (n = 592) 4-6yrs 98% (n = 116) 6yrs 98% (n = 476) |
Higher in camels >4yrs | p < 0.05 | (Munyua et al., 2017) |
| Kenya | <4yrs 36%(n = 319) >4yrs 80% (n = 830) >4 < 7yrs 59% (n = 70) >7yrs 82% (n = 760) |
Higher in camels >7yrs | p < 0.001 | (Ommeh et al., 2018) |
| KSA | ≤2yrs 55% (n = 104) >2yrs 95% (n = 98) |
Higher in camels >2yrs | p < 0.0001 | (Alagaili et al., 2014) |
| KSA | ≤2yrs 73% (n = 77) >2yrs 93% (n = 187) |
Higher in older animals | Not presented | (Alagaili et al., 2014) |
| KSA | 1-2yrs 93% (n = 71) 3-5yrs 78% (n = 100) |
Lower in camels >2yrs | p = 0.03 | (Harrath and Abu Duhier, 2018) |
| KSA | <1 yr 72% (n = 65) >1 yr 95%a (n = 245) 1-3yrs 95% (n = 106) 4-5yrs 97% (n = 76) >5yrs 92% (n = 63) |
Higher in camels >1 yr | p < 0.01 | (Hemida et al., 2013) |
| Mali | <2yrs 83% (n = NAb) >2yrs ∼90%d (n = NAb) 3-8yrs 91% (n = NAb) 9-16yrs 88% (n = NAb) |
None | Not significant | (Falzarano et al., 2017) |
| Pakistan | ≤3yrs 58% (n = 177) >3yrs 79% (n = 873) 3.1-10yrs 79% (n = 712) >10yrs 81% (n = 161) |
Lower in animals ≤3yrs | p < 0.001 | (Zohaib et al., 2018) |
| Pakistan | ≤2yrs 29% (n = 89) >2yrs 41%(n = 476) 2.1-5yrs 30% (n = 208) 5.1-10yrs 51% (n = 180) >10yrs 49%(n = 88) |
Higher in older animals | p < 0.001 | (Saqib et al., 2017) |
| UAE | <1 yr 85% (n = 108) >1 yr 96%a (n = 650) 2-4yrs 97% (n = 340) >4yrs 96% (n = 310) |
Lower in calves <1 yr | p < 0.05 | (Wernery et al., 2015a) |
| UAE | <1 yrs 84% (n = 121) >1 yrs 99% (n = 133) 0-3 m 75% n = 32) 4 m 79% (n = 14) 5-6 m 89% (n = 46) 7-12 90% (n = 29) |
Increase with age | Not tested | (Wernery et al., 2015b) |
| Studies investigating dromedary camel populations linked to human MRS-CoV infection | ||||
| KSA |
≤2yrs58%(n = 25) >2yrs81%(n = 344) 2.1-4yrs 77%c(n = 156) 4.1-6yrs 81%c (n = 98) >6yrs 87%c (n = 90) |
Higher in camels >2yrs | p = 0.003 | (Kasem et al., 2018b) |
The bold type highlights the aggregated seroprevalence values presented graphically in figure 3.
Age stratified results were calculated by us, using disaggregated results presented by authors.
Number of animals in each age class not supplied.
n = the total number of dromedaries sampled.
Seroprevalence in >2yrs estimated under the assumption that age classes 3-8yrs and 9-16yrs are the same size.
Fig. 3.
Age stratified seroprevalence. Measures grouped by available stratification and arranged in order of increasing adult seroprevalence. Bars indicate 95% confidence intervals, calculated by us when not stated in the study, if age class size was available (not available for the population in Mali). *indicates that calves <1-year-old were not included. **indicates that the study was conducted in dromedary populations in response to an epidemiologically linked human MERS-CoV infection.
3.2.2. Geographic location
Although MERS-CoV seroprevalence is consistently high across West, North, East Africa and the Middle East at the country level, some studies measured considerable within-country variation in seroprevalence, particularly in Africa (ranges given in Table 1). In Kenya all three studies that presented sub-nationally disaggregated results showed seroprevalence to vary greatly by province. Tunisia, Morocco and Mali also showed considerable regional seroprevalence variation. Less regional variation in seroprevalence was observed within Middle Eastern countries.
3.2.3. Sample population characteristics
Several studies found imported animals to have significantly higher seroprevalence than their locally bred counterparts (Gutierrez et al., 2015; Ali et al., 2017a, b; Islam et al., 2018). Dromedaries sampled at markets, abattoirs and quarantine sites had higher seroprevalence than those in farms, villages and research facilities (Ali et al., 2017a, b; van Doremalen et al., 2017; Islam et al., 2018). In some cases, dromedary origin varied with the type of site sampled suggesting confounding.
In a study conducted across Burkina Faso, Morocco and Ethiopia, dromedaries used for milk and meat had higher seroprevalence than those used for transport (Miguel et al., 2017). In most of the studies that stratified by sex, little difference was seen, but in Kenya females had statistically significantly higher seroprevalence than males (93% vs. 81% in one study (Munyua et al., 2017), and 74% vs. 54% in another (Ommeh et al. 2018)) whereas males had significantly higher seroprevalence in Egypt and in KSA (72% and 84% in males vs. 66% in females) (Ali et al., 2017b; Kasem et al., 2018b).
Large and medium herd-size was a significant risk factor for seropositivity across Burkina Faso, Morocco and Ethiopia, as well as nomadic and sedentary husbandry systems as opposed to a mixed lifestyle (Miguel et al., 2017). Conversely, in Kenya there was a non-statistically significant trend for smaller herds to have higher seroprevalence (Deem et al., 2015). Two other studies in Kenya showed higher seroprevalence in nomadic herds compared with those kept on ranches or those with agro-pastoralist management. However, ranches were in a different region from nomadic herds and the sample size for agro-pastoralist management was very small (Corman et al., 2014; Munyua et al., 2017).
3.3. Prevalence of active MERS-CoV infection – cross-sectional studies
Our search found that dromedary populations in 16 countries have been tested for MERS-CoV RNA, 13 of which report positive results indicating active infection. These include KSA (0.12–56%) (Alagaili et al., 2014; Khalafalla et al., 2015; Sabir et al., 2016; Alfuwaires et al., 2017; Kasem et al., 2018a, b), UAE (0–29% (Wernery et al., 2015a, b; Yusof et al., 2015; Li et al., 2017; Yusof et al., 2017) or 0–100% if dromedaries epidemiologically linked to human MERS-CoV cases are included(Al Hammadi et al., 2015; Muhairi et al., 2016; Paden et al., 2018)), Qatar (22–79%) (Haagmans et al., 2014; Reusken et al., 2014a; Farag et al., 2015; Mohran et al., 2016), Oman (7%) (Nowotny and Kolodziejek, 2014), Iraq (15%) (Al Salihi and Alrodhan, 2017), and Jordan (62%) (van Doremalen et al., 2017), as well as Egypt (4–15%) (Chu et al. 2014, Ali et al., 2017a, b), Ethiopia (10%) (Miguel et al., 2017), Kenya (0.35-0.95%) (Kiambi et al., 2018; Ommeh et al., 2018), Nigeria (3–11%) (Chu et al., 2015; So et al., 2018), Burkina Faso (5%) (Miguel et al., 2017), Morocco (2%) (Miguel et al., 2017), and Pakistan (3%) (Zohaib et al., 2018). See Fig. 4 for a map of RNA prevalence, and Table 1). Despite moderate seropositivity, surveys have not detected active MERS-CoV infection in the dromedary populations of Bangladesh or Israel (David et al., 2018; Islam et al., 2018). RNA prevalence in dromedary populations linked to human cases was similar to more randomly sampled populations in the same country, with the exception of two small outbreak studies in the UAE which found 100% of epidemiologically linked dromedaries to be infected (Al Hammadi et al., 2015; Paden et al., 2018).
Fig. 4.
Map of prevalence of active MERS-CoV infection in dromedaries. Measures of MERS-CoV RNA prevalence in dromedaries, aggregated at the country level. Total sample size tested is given in parenthesis. Camel density is calculated using FAOSTAT country-level camel population data (FAO, 2016) and World Bank data (World Bank, 2016) on country surface area. *value calculated by us from disaggregated sub-national measures of RNA prevalence. Red text highlights studies conducted in dromedary populations in response to an epidemiologically linked human MERS-CoV infection. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Factors affecting prevalence of infection
3.4.1. Age
Age stratified studies in KSA and Jordan found that juveniles had a higher RNA-positivity than adults (Alagaili et al., 2014; van Doremalen et al., 2017; Kasem et al., 2018b). RNA-positivity also had an inverse association with age in dromedaries across Morocco, Burkina Faso and Ethiopia (Miguel et al., 2017). Two studies in Egypt measured similar positivity rates between juveniles and adults (Ali et al., 2017a, b).
3.4.2. Sample population characteristics
Higher prevalence of RNA shedding was found in imported animals by three studies in Egypt, however site-type is a potential confounder, with local camels being sampled from farms and villages, whilst imported animals were sampled in markets, quarantine centres and abattoirs (Chu et al., 2014; Ali et al., 2017a, b). A study in KSA sampled both local and imported dromedaries within live animal markets found that locally-reared animals had significantly higher prevalence of viral shedding (Sabir et al., 2016). Overall, three studies reported abattoirs and one reported wholesale markets to be associated with an increase in measured prevalence of shedding compared to villages, farms and quarantines (Yusof et al., 2015; Sabir et al., 2016; Alfuwaires et al., 2017; Miguel et al., 2017). Much like seroprevalence, RNA positivity was significantly higher in dromedaries bred for meat or milk compared with those used as transport in Burkina Faso, Morocco and Ethiopia, and shedding was higher amongst females, albeit sex and function were highly correlated (Miguel et al., 2017).
3.4.3. Potential temporal trends
Five studies measured RNA prevalence at a defined site at multiple points in time. Animals were not themselves sampled longitudinally. Three studies in Egypt and KSA showed a clear peak in prevalence of viral RNA shedding from December to May (Khalafalla et al., 2015; Ali et al., 2017b; Kasem et al., 2018a). The fourth, conducted in wholesale markets in KSA, saw lower rates infection during July and August, and higher positivity in December (Sabir et al., 2016). At an abattoir in Nigeria, no infection was seen from October to mid-January, with prevalence of infection peaking in February after which no more samples were taken (So et al., 2018).
3.5. Evidence of infection and immunity from longitudinal studies
We found 10 longitudinal studies describing 9 incidences of natural infection on farms and in quarantine facilities – 1 in Egypt (Ali et al., 2017b), 4 in KSA (Azhar et al., 2014; Hemida et al., 2014a; Memish et al., 2014; Hemida et al., 2017a), 5 in UAE (Al Hammadi et al., 2015; Wernery et al., 2015a; Meyer et al., 2016; Muhairi et al., 2016; Yusof et al., 2017) and 1 study taking monthly samples of 430 dromedaries in Kenya (Kiambi et al., 2018).
3.5.1. Duration of viral shedding
Four studies of natural infection measured viral shedding in dromedaries at approximately weekly intervals. The maximum time window in which all consecutive nasal samples taken were positive for MERS-CoV RNA ranged from 7 to 45 days across published studies, with most positive animals becoming negative within 2 weeks (Al Hammadi et al., 2015; Wernery et al., 2015a; Muhairi et al., 2016; Yusof et al., 2017). All available studies followed animals that were found to be MERS-CoV RNA positive at the first instance of sampling and the duration of shedding prior to sampling is unknown. Further to this, intermittent RNA shedding, and evidence of potential rapid reinfection/coinfection has been observed (Muhairi et al., 2016; Yusof et al., 2017).
3.5.2. Evidence of reinfection
Three studies have found dromedaries to be shedding MERS-CoV RNA despite having high antibody titres months or weeks prior to detectable infection. Both older animals whose antibodies reflect past exposure (Meyer et al., 2016; Ali et al., 2017b; Hemida et al., 2017a), and young calves whose high antibody titres were maternally-acquired immediately post-partum, became infected (Meyer et al., 2016).
One study directly observed recurring infection amongst a herd of dromedaries in Egypt. Four animals were shedding 1–3 months prior to a herd-wide epidemic in which they were RNA-positive once more (Ali et al., 2017b). Sequenced isolates from a market in UAE showed lineage switching from week to week which is also supportive of rapid reinfection or coinfection in calves (Yusof et al., 2017).
Longitudinal studies also indicated that maternally acquired immunity may offer some protection. In both studies of calf-mother-pairs conducted in UAE, MERS-CoV infection became highly prevalent in calves between 4–6 months of age when maternally-acquired antibody titres had waned (Wernery et al., 2015a; Meyer et al., 2016). Samples from reinfected animals have been found to have lower viral loads, suggesting that past infection may ameliorate future infections (Hemida et al., 2017a). Viral load and probability of isolating infectious virus were greater when sampling calves, than adults (Meyer et al., 2016; Ommeh et al., 2018).
4. Discussion
The results of our systematic review show that MERS-CoV circulates widely in dromedaries across the Middle East and Africa, but transmission varies spatially, and temporally. The sub-national range of MERS-CoV seroprevalence appears to be larger in countries outside of the Arabian Peninsula. Within-country variation in seroprevalence is potentially indicative of differences in transmission dynamics, meaning vaccine strategy evaluation and mathematical modelling will need to be conducted at a sub-national resolution.
The rise of MERS-CoV seroprevalence from 40 to 90% in juveniles, to 80–100% in adult dromedaries across much of West, North and East Africa and the Middle East, is signature of an endemic disease where the probability of infection increases with time. High seroprevalence in calves <1-year-old in some populations in UAE and KSA suggests high transmission intensity in the Arabian Peninsula, with most dromedaries becoming infected during the first year of life, though maternally acquired antibodies may contribute to seropositivity in young calves (Hemida et al., 2013; Wernery et al., 2015a, b). The age-dependent seroprevalence values synthesised here should be used to fit models of MERS-CoV transmission in dromedaries and elucidate the likely transmission intensity of the virus in the Middle East and in Africa – a key parameter for estimating vaccination impact. Reporting finer age stratification of young dromedaries would allow a better comparison of transmission intensity in different regions through fitting models of seroconversion.
Despite locally-acquired human cases predominantly being reported within the Arabian Peninsula, the major unilateral trade of camels from the Horn of Africa to the Arabian Peninsula (Younan et al., 2016) means that the endemicity of MERS-CoV in African dromedary populations has implications for the scope of control programs. Thus far, MERS-CoV isolates have been grouped into 3 clades based on genetic similarity. In the most recent phylogeny, viruses isolated in Africa (Egypt, Ethiopia, Morocco, Nigeria, Burkina Faso and most recently Kenya) have all been classified into Clade C (with West African isolates comprising sub-clade C1(Chu et al., 2018)), while isolates from the Arabian Peninsula have only ever been classed as clades A or B (Chu et al., 2014, 2015; Chu et al., 2018; Kiambi et al., 2018). Spike region sequences from Pakistan are similar to those from the Arabian Peninsula (Zohaib et al., 2018). Virus neutralisation tests have shown that isolates from different clades are antigenically similar and therefore that it is likely that a single clade vaccine would be cross-protective against other clades too (Chu et al., 2018). More extensive testing to confirm cross-protection would be useful for vaccine development. Phenotypically, sub-clade C1, so far restricted to West Africa, does differ from other isolates tested in that it is less able to reproduce in humanised mice and ex-vivo human lung tissue (Chu et al., 2018). This difference did not apply to other clade C isolates so cannot explain the lack of reported cases in Africa. Further investigation of the geographical restrictions of MERS-CoV clades would help clarify the extent to which MERS-CoV circulates intercontinentally.
Age-dependent seroprevalence patterns suggest that the higher prevalence of viral shedding in juveniles compared with adults is likely due to immunological naivety. The age-distribution of reported infections synthesised here, suggests that contact with juveniles may pose greater risks of human transmission than adults, making them potential targets for vaccination. However, frequency of human contact with dromedaries may also be animal-age-dependent (Wernery et al., 2015a). Calf-focused vaccination may reduce the overall number of dromedary infections but, the reduced risk of exposure would mean that any remaining infections would likely occur at an older age than in the absence of vaccination. It will therefore be important to further investigate the age-dependency of human-dromedary contact patterns and how these vary in different countries and husbandry systems. Vaccination strategies should be evaluated, not only on their likely impact on prevalence of active infection in dromedaries, but also on the age-distribution of infections.
Mapping the movement of dromedaries is necessary to understand the underlying spatial transmission dynamics of MERS-CoV. The mixing of dromedaries underpins interaction between infectious and susceptible individuals and therefore the dynamics of MERS-CoV transmission. Live markets and abattoirs which both had higher prevalence of RNA shedding compared to other site-types in multiple studies, are key locations for animal mixing (Perera et al., 2013; Yusof et al., 2015; Sabir et al., 2016; Miguel et al., 2017). Quantitative data describing the movement and trading patterns of dromedary populations will be essential for informing models and considering where potential vaccination should take place. A role for markets as drivers of disease dissemination is characteristic of other zoonotic diseases such as avian influenza (Gilbert et al., 2014; Fournié et al., 2016).
Move evidence is required to establish whether MERS-CoV infection in dromedaries is seasonal. The temporal studies in this review observed higher prevalence of active infection between December and June (Khalafalla et al., 2015; Sabir et al., 2016; Ali et al., 2017b; Kasem et al., 2018a). These were conducted in Egypt and KSA where dromedary calving occurs between October and February (Almutairi et al., 2010; Hemida et al., 2017b; Ali et al., 2018), and Nigeria which has a similar calving season (Abdussamad et al., 2011). Assuming seasonal calving was driving the trend, and calves become susceptible between 4–6 months (Wernery et al., 2015a; Meyer et al., 2016), we might expect the number of susceptible dromedaries be higher between January and May – which overlaps with the peaks observed. If infection is driven by seasonal calving, vaccination would need to occur annually prior to the infection of newly susceptible calves. Based on phylogenetic analysis of MERS-CoV genomes isolated from humans and dromedaries, a seasonal period of elevated risk of zoonotic transmission has been estimated to exist from April through to July (Dudas et al., 2018), however, this is not consistent with the epidemiology of primary human MERS-CoV cases reported to the World Health Organisation (WHO) since 2012 (Al-Tawfiq and Memish, 2018). As well as seasonal calving, environmental factors have also been hypothesised to be associated with zoonotic transmission (measured as the incidence of primary human cases). Lower temperatures and humidity were found to be associated with higher numbers of reported primary human MERS cases in KSA (Gardner et al., 2019). Further investigation of potential seasonality has been highlighted as a priority by the FAO-OIE-WHO MERS-CoV Technical Working Group (FAO-OIE-WHO MERS-CoV Technical Working Group, 2018).
The results of longitudinal studies included in this review demonstrate re-infection of dromedaries despite high titres of MERS-CoV specific antibodies being present in their sera. Unfortunately, the degree and duration of protection afforded by maternally-acquired antibodies and those acquired from infection is unclear. Informative surveys of a better proxy for protective immunity in dromedaries would improve the accuracy of models of reinfection and the likely effects of vaccination.
Although we found maximum duration of RNA shedding to range from 7 to 45 days across studies, intermittent shedding or rapid reinfection has been seen to occur for 6 weeks which complicates interpretation of RT-PCR derived RNA shedding results for infectious period. A controlled challenge study in 4 dromedaries performed daily sampling and saw the maximum duration of shedding to be 35 days post inoculation, with infectious virus (as determined by plaque assay) isolatable for the first 7 days (Adney et al., 2014). However, the biological relevance of the challenge dose is not known. More frequent sampling that includes genotyping and captures of the onset of shedding is needed to more accurately estimate the duration of infectiousness following a single natural infection.
Limitations of our study include that a single author completed the systematic search and data extraction, and that we only included published literature thereby introducing potential publication bias. The available studies exhibit differences in study design, criteria for seropositivity, sample-site type and sample population characteristics. Some studies report that the latter two variables are associated with statistically significant differences in seroprevalence or prevalence of infection within individual studies (Corman et al., 2014; Yusof et al., 2015; Sabir et al., 2016; Ali et al., 2017a, b; Miguel et al., 2017; van Doremalen et al., 2017). These heterogeneities made quantitative pooling inappropriate.
Available published studies do not include results from camel dense regions of northern Africa or Rajasthan, India, and Yemen. In addition to the countries included in this systematic review of the published literature, OIE has received reports of RNA positive camels in Iran and Kuwait (OIE - World Organisation for Animal Health, 2014a; OIE, World Organisation for Animal Health, 2014b). Members at WHO and the Food and Agriculture Organisation of the United Nations (FAO), together with country counterparts are planning and/or implementing further RNA testing studies in several countries in Africa (e.g. Ethiopia, Kenya, Egypt, Somalia, Sudan, Algeria and Morocco), the Middle East (e.g. Jordan), and South Asia (e.g. Pakistan) (personal communication, Maria D. Van Kerkhove).
Implementing MERS-CoV vaccination in dromedaries will be challenging. First, since MERS-CoV infection does not cause significant disease in camels, a dromedary vaccine will be implemented solely for the purpose of preventing human infections. While there are several examples of vaccinating animals to protect humans from infection (Monath, 2013) (e.g., low pathogenic avian influenza (Busani et al., 2009; Halvorson, 2009), Hendra virus (Broder et al., 2013), and rabies (Lavan et al., 2017)), overcoming cultural and economic concerns of camel owners and the camel industry will require strong advocacy with owners, industry and political leaders in countries with high dromedary density. It is critical that any animal vaccine will not harm the livelihoods of animal owners and those involved in the selling, trading or recreational use of the animals. Second, despite ample scientific evidence that MERS-CoV is a zoonotic virus and can transmit between camels and humans (FAO-OIE-WHO MERS Technical Working Group, 2018), in some contexts there is still reluctance to accept that dromedaries play a role MERS transmission. While member states are working with Ministries of Health, Ministries of Agriculture, and communities to address this, a dromedary vaccine that can be implemented with existing accepted dromedary vaccines would be preferential. Third, at this stage in their development, it is not clear whether a dromedary vaccine will provide adequate protection against re-infection. Available studies (Haagmans et al., 2016; Alharbi et al., 2017) suggest that a dromedary vaccine may greatly reduce viral shedding, rather than providing sterilizing immunity. The infectiousness of dromedaries shedding low levels of the virus is currently uncertain. Genetic diversity is unlikely to demand a vaccine based on more than one clade due to antigenic similarity and cross-protection. Further discussion of these wider challenges of implementing a MERS-CoV vaccine in dromedaries, would benefit from being informed by a clearer picture of what an epidemiologically optimal vaccination strategy would involve.
5. Conclusions
Our findings provide strong evidence that MERS-CoV is endemic in dromedary populations across much of West, North, East Africa and the Middle East, in agreement with the similar systematic review conducted in parallel with our own (Sikkema et al., 2019).
In addition, our findings highlight several epidemiological characteristics of MERS-CoV that must be considered in the design of MERS-CoV animal vaccination strategies. Calves are likely to play a central role in sustaining circulation of MERS-CoV and should be a target of potential dromedary vaccination. However, the potential for mass vaccination of calves to change the age distribution of infected individuals should be investigated through mathematical modelling of transmission dynamics in dromedary populations and considered in the context of age-dependent human-camel contact frequency patterns. Sites where dromedaries mix may also play a role in driving transmission. A better understanding of dromedary husbandry and trade patterns, as well as quarantine facilities, is needed to identify where dromedaries become infected with MERS-CoV – critical for focussing potential vaccination strategies both geographically and within the dromedary camel value chain. Although in a few studies, prevalence of infection appears to peak in the first half of the year, which may be facilitated by the increase in susceptible animals after the calving season, further studies are needed to confirm this. More longitudinal studies are required to investigate the temporal dynamics of viral shedding and immunity in the animal host and should ideally be capable of distinguishing co-circulating MERS-CoV lineages.
The remaining gaps in our understanding of MERS-CoV transmission dynamics in dromedary populations are in agreement with the prioritized research outlined in the FAO-OIE-WHO Technical Working group report (FAO-OIE-WHO MERS Technical Working Group, 2018) and must be addressed to obtain a clearer picture of what an optimal epidemiological vaccination strategy would involve, as well as its likely impact, before wider challenges of implementation can be considered further.
Declarations of interest
None.
Author contributions
The search was designed and conducted by A. Dighe with supervision and advice from N. Ferguson, T. Jombart and M.D. Van Kerkhove. Data extraction and analysis was conducted by A Dighe. A. Dighe wrote the manuscript with editing from N. Ferguson, T. Jombart and M.D. Van Kerkhove.
Funding statement
This work was supported by a Wellcome Trust Studentship awarded to Amy Dighe (203871/Z/16/Z).
Other authors declare the following additional funding sources.
Thibaut Jombart: Global Challenges Research Fund (GCRF) for the project ‘RECAP – research capacity building and knowledge generation to support preparedness and response to humanitarian crises and epidemics’ (ES/P010873/1), UK Public Health Rapid Support Team, National Institute for Health Research - Health Protection Research Unit for Modelling Methodology.
Neil Ferguson: Programme funding from Bill and Melinda Gates Foundation (OPP1092240), Centre funding from the MRC and DfID (MR/R015600/1), Health Protection Research Unit funding from the National Institute of Health Research (HPRU-2012-10080), MIDAS programme funding from the National Institute of General Medical Sciences (1U01GM110721-01).
Acknowledgements
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.epidem.2019.100350.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Abdussamad A., Holtz W., Gauly M., Suleiman M., Bello M. Reproduction and breeding in dromedary camels: insights from pastoralists in some selected villages of the Nigeria-Niger corridor. Livest. Res. Rural Dev. 2011;23(8) http://www.lrrd.org/lrrd23/8/abdu23178.htm 2011. [Google Scholar]
- Adney D.R., van Doremalen N., Brown V.R., Bushmaker T., Scott D., de Wit E., Bowen R.A., Munster V.J. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg. Infect. Dis. 2014;20(12):1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Memish Z.A. Lack of seasonal variation of middle east respiratory syndrome coronavirus (MERS-CoV) Travel Med. Infect. Dis. 2018 doi: 10.1016/j.tmaid.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hammadi Z.M., Chu D.K., Eltahir Y.M., Al Hosani F., Al Mulla M., Tarnini W., Hall A.J., Perera R.A., Abdelkhalek M.M., Peiris J.S., Al Muhairi S.S., Poon L.L. Asymptomatic MERS-CoV infection in humans possibly linked to infected dromedaries imported from Oman to United Arab Emirates, may 2015. Emerg. Infect. Dis. 2015;21(12):2197–2200. doi: 10.3201/eid2112.151132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Salihi S.F., Alrodhan M.A. Phylogenetic analysis of MERSCoV in human and camels in Iraq. Int. J. Pharm. Res. Allied Sci. 2017;6(1):120–129. [Google Scholar]
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., Kapoor A., Epstein J.H., Karesh W.B., Daszak P., Mohammed O.B., Lipkin W.I. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. Erratum appears in MBio. 2014;2014(2):5. doi: 10.1128/mBio.00884-14. e01002-14 Note: Burbelo, Peter D [added]]." mBio 5(2): e00884-00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Kobinger G.P., Soule G., Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound. Emerg. Dis. 2014;61(2):105–108. doi: 10.1111/tbed.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfuwaires M., Altaher A., Alhafufi A., Kandeel M. Middle east respiratory syndrome coronavirus in healthy and diseased dromedaries. J. Camel Pract. Res. 2017;24(3):217–220. doi: 10.5958/2277-8934.2017.00036.4. [DOI] [Google Scholar]
- Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A., Grehan K., Temperton N., Lambe T., Warimwe G., Becker S., Hill A.V.S., Gilbert S.C. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35(30):3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Derar D., Alsharari A., Alsharari A., Khalil R., Almundarij T.I., Alboti Y., Al-Sobayil F. Factors affecting reproductive performance in dromedary camel herds in Saudi Arabia. Trop. Anim. Health Prod. 2018;50(5):1155–1160. doi: 10.1007/s11250-018-1545-3. [DOI] [PubMed] [Google Scholar]
- Ali M., El-Shesheny R., Kandeil A., Shehata M., Elsokary B., Gomaa M., Hassan N., El Sayed A., El-Taweel A., Sobhy H., Fasina F.O., Dauphin G., El Masry I., Wolde A.W., Daszak P., Miller M., VonDobschuetz S., Morzaria S., Lubroth J., Makonnen Y.J. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro Surveill. 2017;2017(May 19):22. doi: 10.2807/1560-7917.ES.2017.22.11.30487. PMID: 28537549." Euro Surveillance: Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin 22(11): 16(Erratum appears in) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.A., Shehata M.M., Gomaa M.R., Kandeil A., El-Shesheny R., Kayed A.S., El-Taweel A.N., Atea M., Hassan N., Bagato O., Moatasim Y., Mahmoud S.H., Kutkat O., Maatouq A.M., Osman A., McKenzie P.P., Webby R.J., Kayali G. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microbes Infect. 2017;6(1):e1. doi: 10.1038/emi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi S.E., Boujenane I., Musaad A., Awad-Acharari F. Non-genetic factors influencing reproductive traits and calving weight in Saudi camels. Trop. Anim. Health Prod. 2010;42(6):1087–1092. doi: 10.1007/s11250-010-9529-y. [DOI] [PubMed] [Google Scholar]
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., Madani T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Breban R., Riou J., Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382(9893):694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder C.C., Xu K., Nikolov D.B., Zhu Z., Dimitrov D.S., Middleton D., Pallister J., Geisbert T.W., Bossart K.N., Wang L.-F. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral Res. 2013;100(1):8–13. doi: 10.1016/j.antiviral.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busani L., Toson M., Stegeman A., Dalla Pozza M., Comin A., Mulatti P., Cecchinato M., Marangon S. Vaccination reduced the incidence of outbreaks of low pathogenicity avian influenza in northern Italy. Vaccine. 2009;27(27):3655–3661. doi: 10.1016/j.vaccine.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Cauchemez S., Van Kerkhove M.D., Riley S., Donnelly C.A., Fraser C., Ferguson N.M. Transmission scenarios for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and how to tell them apart. Euro Surveill. 2013;18(24) doi: 10.2807/ese.18.24.20503-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.M., Damdinjav B., Perera R.A., Chu D.K., Khishgee B., Enkhbold B., Poon L.L., Peiris M. Absence of MERS-Coronavirus in Bactrian camels, Southern Mongolia, November 2014. Emerg. Infect. Dis. 2015;21(7):1269–1271. doi: 10.3201/eid2107.150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Oladipo J.O., Perera R.A., Kuranga S.A., Chan S.M., Poon L.L., Peiris M. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Nigeria, 2015. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2015;20(49) doi: 10.2807/1560-7917.ES.2015.20.49.30086. [DOI] [PubMed] [Google Scholar]
- Chu D.K.W., Hui K.P.Y., Perera R., Miguel E., Niemeyer D., Zhao J.C., Channappanavar R., Dudas G., Oladipo J.O., Traore A., Fassi-Fihri O., Ali A., Demissie G.F., Muth D., Chan M.C.W., Nicholls J.M., Meyerholz D.K., Kuranga S.A., Mamo G., Zhou Z.Q., So R.T.Y., Hemida M.G., Webby R.J., Roger F., Rambaut A., Poon L.L.M., Perlman S., Drosten C., Chevalier V., Peiris M. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl. Acad. Sci. U. S. A. 2018;115(12):3144–3149. doi: 10.1073/pnas.1718769115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K.W., Poon L.L.M., Gomaa M.M., Shehata M.M., Perera R., Abu Zeid D., El Rifay A.S., Siu L.Y., Guan Y., Webby R.J., Ali M.A., Peiris M., Kayali G. MERS coronaviruses in dromedary camels, Egypt. Emerg. Infect. Dis. 2014;20(6):1049–1053. doi: 10.3201/eid2006.140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarivate Analytics . 2018. EndnoteX8.2. (Version X8.2) [Software] Clarivate Analytics. 2018. [Google Scholar]
- Clarivate Analytics . 2018. Web of Science. [Database] Clarivate Analytics. Available from: http://apps.webofknowledge.com/WOS_GeneralSearch_input.do?product=WOS&search_mode=GeneralSearch&SID=D12CW4GmjGuwicdeqUC&preferencesSaved= [Accessed 31st December 2018] [Google Scholar]
- Corman V., Eckerle I., Bleicker T., Zaki A., Landt O., Eschbach-Bludau M., van Boheemen S., Gopal R., Ballhause M., Bestebroer T. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Eurosurveillance. 2012;17(39) doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Said M.Y., Gluecks I., Lattwein E., Bosch B.J., Drexler J.F., Bornstein S., Drosten C., Muller M.A. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992-2013. Emerg. Infect. Dis. 2014;20(8):1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri G., Durr P.A., Barr J., Yu M., Graham K., Williams O.J., Kayali G., Smith D., Peiris M., Mackenzie J.S., Wang L.F. Absence of MERS-CoV antibodies in feral camels in Australia: implications for the pathogen’s origin and spread. One Health. 2015;1:76–82. doi: 10.1016/j.onehlt.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D., Rotenberg D., Khinich E., Erster O., Bardenstein S., van Straten M., Okba N.M.A., Raj S.V., Haagmans B.L., Miculitzki M., Davidson I. Middle East respiratory syndrome coronavirus specific antibodies in naturally exposed Israeli llamas, alpacas and camels. One Health. 2018;5:65–68. doi: 10.1016/j.onehlt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem S.L., Fevre E.M., Kinnaird M., Browne A.S., Muloi D., Godeke G.J., Koopmans M., Reusken C.B. Serological evidence of MERS-CoV antibodies in dromedary camels (Camelus dromedaries) in Laikipia County, Kenya. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140125. [Erratum appears in PLoS One. 2017 May 18;12 (5):e0178310; PMID: 28542448] [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas G., Carvalho L.M., Rambaut A., Bedford T. MERS-CoV spillover at the camel-human interface.[Erratum appears in Elife. 2018 Apr 19;7: PMID: 29669683] eLife. 2018;7(01):16. doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsevier B.V. 2018. Embase Classic+Embase 1947 to 2018 December 31. [Database] Ovid Wolters Kluwer. Available from: http://ovidsp.tx.ovid.com/sp-3.32.0a/ovidweb.cgi?&S=BCCGFPDLLKDDGPKGNCDKCCLBGDPNAA00&New+Database=Single%7c4 [Accessed 31st December 2018] [Google Scholar]
- Falzarano D., Kamissoko B., de Wit E., Maiga O., Cronin J., Samake K., Traore A., Milne-Price S., Munster V.J., Sogoba N., Niang M., Safronetz D., Feldmann H. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health. 2017;3:41–43. doi: 10.1016/j.onehlt.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . 2016. FAOSTAT [Internet]. 2016. Available from: http://www.fao.org/faostat/en/. [Accessed September 2018] [Google Scholar]
- Farag E.A., Reusken C.B., Haagmans B.L., Mohran K.A., Stalin Raj V., Pas S.D., Voermans J., Smits S.L., Godeke G.J., Al-Hajri M.M., Alhajri F.H., Al-Romaihi H.E., Ghobashy H., El-Maghraby M.M., El-Sayed A.M., Al Thani M.H., Al-Marri S., Koopmans M.P. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect. Ecol. Epidemiol. 2015;5:28305. doi: 10.3402/iee.v5.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournié G., Tripodi A., Nguyen T.T.T., Tran T.T., Bisson A., Pfeiffer D.U., Newman S.H. Investigating poultry trade patterns to guide avian influenza surveillance and control: a case study in Vietnam. Sci. Rep. 2016;6:29463. doi: 10.1038/srep29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi S., Fukuma A., Kurosu T., Watanabe S., Shimojima M., Shirato K., Iwata-Yoshikawa N., Nagata N., Ohnishi K., Ato M., Melaku S.K., Sentsui H., Saijo M. Characterization of novel monoclonal antibodies against the MERS-coronavirus spike protein and their application in species-independent antibody detection by competitive ELISA. J. Virol. Methods. 2018;251:22–29. doi: 10.1016/j.jviromet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner E.G., Kelton D., Poljak Z., Van Kerkhove M., von Dobschuetz S., Greer A.L. A case-crossover analysis of the impact of weather on primary cases of Middle East respiratory syndrome. BMC Infect. Dis. 2019;19(1):113. doi: 10.1186/s12879-019-3729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M., Golding N., Zhou H., Wint G.W., Robinson T.P., Tatem A.J., Lai S., Zhou S., Jiang H., Guo D. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat. Commun. 2014;5:4116. doi: 10.1038/ncomms5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO-OIE-WHO MERS Technical Working Group MERS: progress on the global response, remaining challenges and the way forward. Antiviral Res. 2018;159:35–44. doi: 10.1016/j.antiviral.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Tejedor-Junco M.T., Gonzalez M., Lattwein E., Renneker S. Presence of antibodies but no evidence for circulation of MERS-CoV in dromedaries on the Canary Islands, 2015. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2015;20(37) doi: 10.2807/1560-7917.ES.2015.20.37.30019. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R., Godeke G.J., Jonges M., Farag E., Diab A., Ghobashy H., Alhajri F., Al-Thani M., Al-Marri S.A., Al Romaihi H.E., Al Khal A., Bermingham A., Osterhaus A.D., AlHajri M.M., Koopmans M.P. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., van den Brand J.M., Raj V.S., Volz A., Wohlsein P., Smits S.L., Schipper D., Bestebroer T.M., Okba N., Fux R., Bensaid A., Solanes Foz D., Kuiken T., Baumgartner W., Segales J., Sutter G., Osterhaus A.D. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351(6268):77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- Halvorson D.A. Prevention and management of avian influenza outbreaks: experiences from the United States of America. Revue scientifique et technique. 2009;28(1):359. doi: 10.20506/rst.28.1.1866. [DOI] [PubMed] [Google Scholar]
- Harcourt J.L., Rudoler N., Tamin A., Leshem E., Rasis M., Giladi M., Haynes L.M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health. 2018;31:31. doi: 10.1111/zph.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrath R., Abu Duhier F.M. Sero-prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) specific antibodies in dromedary camels in Tabuk, Saudi Arabia. J. Med. Virol. 2018;90(8):1285–1289. doi: 10.1002/jmv.25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Alnaeem A., Chu D.K., Perera R.A., Chan S.M., Almathen F., Yau E., Ng B.C., Webby R.J., Poon L.L., Peiris M. Longitudinal study of Middle East Respiratory Syndrome coronavirus infection in dromedary camel herds in Saudi Arabia, 2014-2015. Emerg. Microbes Infect. 2017;6(6):e56. doi: 10.1038/emi.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Chu D.K.W., Poon L.L.M., Perera R., Alhammadi M.A., Ng H.Y., Siu L.Y., Guan Y., Alnaeem A., Peiris M. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg. Infect. Dis. 2014;20(7):1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Elmoslemany A., Al-Hizab F., Alnaeem A., Almathen F., Faye B., Chu D.K., Perera R.A., Peiris M. Dromedary camels and the transmission of middle east respiratory syndrome coronavirus (MERS-CoV) Transbound. Emerg. Dis. 2017;64(2):344–353. doi: 10.1111/tbed.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Perera R.A., Al Jassim R.A., Kayali G., Siu L.Y., Wang P., Chu K.W., Perlman S., Ali M.A., Alnaeem A., Guan Y., Poon L.L., Saif L., Peiris M. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2014;19(23):12. doi: 10.2807/1560-7917.ES2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Perera R.A., Wang P., Alhammadi M.A., Siu L.Y., Li M., Poon L.L., Saif L., Alnaeem A., Peiris M. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2013;18(50):20659. doi: 10.2807/1560-7917.ES2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- Islam A., Epstein J.H., Rostal M.K., Islam S., Rahman M.Z., Hossain M.E., Uzzaman M.S., Munster V.J., Peiris M., Flora M.S., Rahman M., Daszak P. Middle east respiratory syndrome coronavirus antibodies in dromedary camels, Bangladesh, 2015. Emerg. Infect. Dis. 2018;24(5):926–928. doi: 10.3201/eid2405.171192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasem S., Qasim I., Al-Doweriej A., Hashim O., Alkarar A., Abu-Obeida A., Saleh M., Al-Hofufi A., Al-Ghadier H., Hussien R., Al-Sahaf A., Bayoumi F., Magouz A. The prevalence of Middle East respiratory Syndrome coronavirus (MERS-CoV) infection in livestock and temporal relation to locations and seasons. J. Infect. Public Health. 2018;29:29. doi: 10.1016/j.jiph.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasem S., Qasim I., Al-Hufofi A., Hashim O., Alkarar A., Abu-Obeida A., Gaafer A., Elfadil A., Zaki A., Al-Romaihi A., Babekr N., El-Harby N., Hussien R., Al-Sahaf A., Al-Doweriej A., Bayoumi F., Poon L.L.M., Chu D.K.W., Peiris M., Perera R. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J. Infect. Public Health. 2018;11(3):331–338. doi: 10.1016/j.jiph.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafalla A.I., Lu X., Al-Mubarak A.I., Dalab A.H., Al-Busadah K.A., Erdman D.D. MERS-CoV in upper respiratory tract and lungs of dromedary camels, Saudi Arabia, 2013-2014. Emerg. Infect. Dis. 2015;21(7):1153–1158. doi: 10.3201/eid2107.150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiambi S., Corman V.M., Sitawa R., Githinji J., Ngoci J., Ozomata A.S., Gardner E., von Dobschuetz S., Morzaria S., Kimutai J. Detection of distinct MERS-Coronavirus strains in dromedary camels from Kenya, 2017. Emerg. Microbes Infect. 2018;7(1):195. doi: 10.1038/s41426-018-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavan R.P., King A.I.M., Sutton D.J., Tunceli K. Rationale and support for a one Health program for canine vaccination as the most cost-effective means of controlling zoonotic rabies in endemic settings. Vaccine. 2017;35(13):1668–1674. doi: 10.1016/j.vaccine.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Li Y., Khalafalla A.I., Paden C.R., Yusof M.F., Eltahir Y.M., Al Hammadi Z.M., Tao Y., Queen K., Hosani F.A., Gerber S.I., Hall A.J., Al Muhairi S., Tong S. Identification of diverse viruses in upper respiratory samples in dromedary camels from United Arab Emirates. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184718. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wen Z., Wang J., Ge J., Chen H., Bu Z. Absence of Middle East respiratory syndrome coronavirus in Bactrian camels in the West Inner Mongolia autonomous Region of China: surveillance study results from July 2015. Emerg. Microbes Infect. 2015;4(no pagination):e73. doi: 10.1038/emi.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Cotten M., Meyer B., Watson S.J., Alsahafi A.J., Al Rabeeah A.A., Corman V.M., Sieberg A., Makhdoom H.Q., Assiri A., Al Masri M., Aldabbagh S., Bosch B.J., Beer M., Muller M.A., Kellam P., Drosten C. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. 2014;20(6):1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Juhasz J., Barua R., Das Gupta A., Hakimuddin F., Corman V.M., Muller M.A., Wernery U., Drosten C., Nagy P. Time course of MERS-CoV infection and immunity in dromedary camels. Emerg. Infect. Dis. 2016;22(12):2171–2173. doi: 10.3201/eid2212.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Muller M.A., Corman V.M., Reusken C.B., Ritz D., Godeke G.J., Lattwein E., Kallies S., Siemens A., van Beek J., Drexler J.F., Muth D., Bosch B.J., Wernery U., Koopmans M.P., Wernery R., Drosten C. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg. Infect. Dis. 2014;20(4):552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E., Chevalier V., Ayelet G., Ben Bencheikh M.N., Boussini H., Chu D.K., El Berbri I., Fassi-Fihri O., Faye B., Fekadu G., Grosbois V., Ng B.C., Perera R.A., So T.Y., Traore A., Roger F., Peiris M. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2017;22(13):30. doi: 10.2807/1560-7917.ES.2017.22.13.30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E., Perera R.A., Baubekova A., Chevalier V., Faye B., Akhmetsadykov N., Ng C.Y., Roger F., Peiris M. Absence of middle east respiratory syndrome coronavirus in camelids, Kazakhstan, 2015. Emerg. Infect. Dis. 2016;22(3):555–557. doi: 10.3201/eid2203.151284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohran K.A., Farag E., Reusken C., Raj V.S., Lamers M.M., Pas S.D., Voermans J., Smits S.L., AlHajri M.M., Alhajri F., Al-Romaihi H.E., Ghobashy H., El-Maghraby M.M., Al Dhahiry S.H.S., Al-Mawlawi N., El-Sayed A.M., Al-Thani M., Al-Marri S.A., Haagmans B.L., Koopmans M.P.G. The sample of choice for detecting Middle East respiratory syndrome coronavirus in asymptomatic dromedary camels using real-time reverse-transcription polymerase chain reaction. Revue Scientifique Et Technique-Office International Des Epizooties. 2016;35(3):905–911. doi: 10.20506/rst.35.3.2578. [DOI] [PubMed] [Google Scholar]
- Monath T.P. Vaccines against diseases transmitted from animals to humans: a one health paradigm. Vaccine. 2013;31(46):5321–5338. doi: 10.1016/j.vaccine.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhairi S.A., Hosani F.A., Eltahir Y.M., Mulla M.A., Yusof M.F., Serhan W.S., Hashem F.M., Elsayed E.A., Marzoug B.A., Abdelazim A.S. Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2016;52(6):848–854. doi: 10.1007/s11262-016-1367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Bosch B.J., Lattwein E., Hilali M., Musa B.E., Bornstein S., Drosten C. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg. Infect. Dis. 2014;20(12):2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyua P., Corman V.M., Bitek A., Osoro E., Meyer B., Muller M.A., Lattwein E., Thumbi S.M., Murithi R., Widdowson M.A., Drosten C., Njenga M.K. No serologic evidence of middle east respiratory syndrome coronavirus infection among camel farmers exposed to highly seropositive camel herds: a household linked study, Kenya, 2013. Am. J. Trop. Med. Hyg. 2017;96(6):1318–1324. doi: 10.4269/ajtmh.16-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Library of Medicine . 2018. National Library of Medicine Ovid MEDLINE(R) ALL 1946 to December 31. [Database] Ovid Wolters Kluwer. Available from: http://ovidsp.tx.ovid.com/sp-3.32.0a/ovidweb.cgi?&S=BCCGFPDLLKDDGPKGNCDKCCLBGDPNAA00&New+Database=Single%7c7 [Accessed 31st December 2018] [Google Scholar]
- Nowotny N., Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2014;19(16):20781. doi: 10.2807/1560-7917.ES2014.19.16.20781. [DOI] [PubMed] [Google Scholar]
- OIE - World Organisation for Animal Health . 2014. Infection With Coronavirus in Camels, Iran. [Internet]. Available from: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=16411 [Accessed December 2018] [Google Scholar]
- OIE, World Organisation for Animal Health . 2014. Immediate Notification of MERS-CoV in Kuwait [Internet]. 2014. Available from: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=15407 [Accessed December 2018] [Google Scholar]
- OIE, World Organisation for Animal Health . 2017. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Case Definition for Reporting to OIE. [Internet] Available from http://www.oie.int/scientific-expertise/specific-information-and-recommendations/mers-cov/. [Accessed December 2018] [Google Scholar]
- Ommeh S., Zhang W., Zohaib A., Chen J., Zhang H., Hu B., Ge X.-Y., Yang X.-L., Masika M., Obanda V. Genetic evidence of middle east respiratory syndrome coronavirus (MERS-Cov) and widespread seroprevalence among camels in Kenya. Virol. Sin. 2018:1–9. doi: 10.1007/s12250-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden C.R., Yusof M., Al Hammadi Z.M., Queen K., Tao Y., Eltahir Y.M., Elsayed E.A., Marzoug B.A., Bensalah O.K.A., Khalafalla A.I., Al Mulla M., Khudhair A., Elkheir K.A., Issa Z.B., Pradeep K., Elsaleh F.N., Imambaccus H., Sasse J., Weber S., Shi M., Zhang J., Li Y., Pham H., Kim L., Hall A.J., Gerber S.I., Al Hosani F.I., Tong S., Al Muhairi S.S.M. Zoonotic origin and transmission of Middle East respiratory syndrome coronavirus in the UAE. Zoonoses Public Health. 2018;65(3):322–333. doi: 10.1111/zph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.A., Wang P., Gomaa M.R., El-Shesheny R., Kandeil A., Bagato O., Siu L.Y., Shehata M.M., Kayed A.S., Moatasim Y., Li M., Poon L.L., Guan Y., Webby R.J., Ali M.A., Peiris J.S., Kayali G. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2013;18(36):pii=20574. doi: 10.2807/1560-7917.ES2013.18.36.20574. [DOI] [PubMed] [Google Scholar]
- Raj V.S., Farag E.A., Reusken C.B., Lamers M.M., Pas S.D., Voermans J., Smits S.L., Osterhaus A.D., Al-Mawlawi N., Al-Romaihi H.E., AlHajri M.M., El-Sayed A.M., Mohran K.A., Ghobashy H., Alhajri F., Al-Thani M., Al-Marri S.A., El-Maghraby M.M., Koopmans M.P., Haagmans B.L. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg. Infect. Dis. 2014;20(8):1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C., Haagmans B.L., Muller M.A., Gutierrez C., Godeke G.J., Meyer B., Muth D., Raj V.S., Smits-De Vries L., Corman V.M., Drexler J.F., Smits S.L., El Tahir Y.E., De Sousa R., van Beek J., Nowotny N., van Maanen K., Hidalgo-Hermoso E., Bosch B.J., Rottier P., Osterhaus A., Gortazar C., Drosten C., Koopmans M.P.G. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Ababneh M., Raj V.S., Meyer B., Eljarah A., Abutarbush S., Godeke G.J., Bestebroer T.M., Zutt I., Muller M.A., Bosch B.J., Rottier P.J., Osterhaus A.D., Drosten C., Haagmans B.L., Koopmans M.P. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2013;18(50):20662. doi: 10.2807/1560-7917.ES2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- Reusken C.B., Farag E.A., Jonges M., Godeke G.J., El-Sayed A.M., Pas S.D., Raj V.S., Mohran K.A., Moussa H.A., Ghobashy H., Alhajri F., Ibrahim A.K., Bosch B.J., Pasha S.K., Al-Romaihi H.E., Al-Thani M., Al-Marri S.A., AlHajri M.M., Haagmans B.L., Koopmans M.P. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2014;19(23):12. doi: 10.2807/1560-7917.ES2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- Reusken C.B.E.M., Messadi L., Feyisa A., Ularamu H., Godeke G.J., Danmarwa A., Dawo F., Jemli M., Melaku S., Shamaki D., Woma Y., Wungak Y., Gebremedhin E.Z., Zutt I., Bosch B.J., Haagmans B.L., Koopmans M.P.G. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg. Infect. Dis. 2014;20(8):1370–1374. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir J.S., Lam T.T., Ahmed M.M., Li L., Shen Y., Abo-Aba S.E., Qureshi M.I., Abu-Zeid M., Zhang Y., Khiyami M.A., Alharbi N.S., Hajrah N.H., Sabir M.J., Mutwakil M.H., Kabli S.A., Alsulaimany F.A., Obaid A.Y., Zhou B., Smith D.K., Holmes E.C., Zhu H., Guan Y. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351(6268):81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Saqib M., Sieberg A., Hussain M.H., Mansoor M.K., Zohaib A., Lattwein E., Muller M.A., Drosten C., Corman V.M. Serologic Evidence for MERS-CoV Infection in Dromedary Camels, Punjab, Pakistan, 2012-2015. Emerg. Infect. Dis. 2017;23(3):550–551. doi: 10.3201/eid2303.161285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Azumano A., Nakao T., Hagihara D., Ishida M., Tamai K., Yamazaki K., Kawase M., Okamoto Y., Kawakami S., Okada N., Fukushima K., Nakajima K., Matsuyama S. Middle East respiratory syndrome coronavirus infection not found in camels in Japan. Jpn. J. Infect. Dis. 2015;68(3):256–258. doi: 10.7883/yoken.JJID.2015.094. [DOI] [PubMed] [Google Scholar]
- Sikkema R., Farag E., Islam M., Atta M., Reusken C., Al-Hajri M.M., Koopmans M. Global status of Middle East respiratory syndrome coronavirus in dromedary camels: a systematic review. Epidemiol. Infect. 2019;147 doi: 10.1017/S095026881800345X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So R.T., Perera R.A., Oladipo J.O., Chu D.K., Kuranga S.A., Chan K.-h., Lau E.H., Cheng S.M., Poon L.L., Webby R.J. Lack of serological evidence of Middle East respiratory syndrome coronavirus infection in virus exposed camel abattoir workers in Nigeria, 2016. Euro Surveill. 2018;23(32) doi: 10.2807/1560-7917.ES.2018.23.32.1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwiny H.T., Al Hamed T.A., Nazzal A.R. Seroepidemiological study of Middle East respiratory syndrome (MERS) virus infection in Iraqi dromedary camels. Vet. Arch. 2018;88(2):191–200. doi: 10.24099/vet.arhiv.161224. [DOI] [Google Scholar]
- van Doremalen N., Hijazeen Z.S., Holloway P., Al Omari B., McDowell C., Adney D., Talafha H.A., Guitian J., Steel J., Amarin N., Tibbo M., Abu-Basha E., Al-Majali A.M., Munster V.J., Richt J.A. High prevalence of middle east respiratory coronavirus in young dromedary camels in Jordan. Vector Borne Zoonotic Dis. 2017;17(2):155–159. doi: 10.1089/vbz.2016.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernery U., Corman V.M., Wong E.Y., Tsang A.K., Muth D., Lau S.K., Khazanehdari K., Zirkel F., Ali M., Nagy P., Juhasz J., Wernery R., Joseph S., Syriac G., Elizabeth S.K., Patteril N.A., Woo P.C., Drosten C. Acute middle East respiratory syndrome coronavirus infection in livestock Dromedaries, Dubai, 2014. Emerg. Infect. Dis. 2015;21(6):1019–1022. doi: 10.3201/eid2106.150038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernery U., El Rasoul I.H., Wong E.Y., Joseph M., Chen Y., Jose S., Tsang A.K., Patteril N.A., Chen H., Elizabeth S.K., Yuen K.Y., Joseph S., Xia N., Wernery R., Lau S.K., Woo P.C. A phylogenetically distinct Middle East respiratory syndrome coronavirus detected in a dromedary calf from a closed dairy herd in Dubai with rising seroprevalence with age. Emerg. Microbes Infect. 2015;4(12):e74. doi: 10.1038/emi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank . 2016. The World Bank: Land Area [Internet] Available from: https://data.worldbank.org/indicator/AG.LND.TOTL.K2. [Accessed September 2018] [Google Scholar]
- World Health Organisation . 2018. WHO MERS-CoV Global Summary and Assessment of Risk [Internet]. Geneva: WHO. Available from: https://www.who.int/csr/disease/coronavirus_infections/risk-assessment-august-2018.pdf?ua=1 [Accessed September 2018] [Google Scholar]
- World Health Organisation . World Health Organization (WHO/MERS/LAB/15.1/Rev1/2018). Licence: CC BY-NC-SA 3.0 IGO; Geneva, Switzerland: 2018. Laboratory Testing for Middle East Respiratory Syndrome Coronavirus, Interim Guidance [Internet] Available from: https://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en/ [Accessed September 2018] [Google Scholar]
- Younan M., Bornstein S., Gluecks I.V. MERS and the dromedary camel trade between Africa and the Middle East. Trop. Anim. Health Prod. 2016;48(6):1277–1282. doi: 10.1007/s11250-016-1089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof M.F., Eltahir Y.M., Serhan W.S., Hashem F.M., Elsayed E.A., Marzoug B.A., Abdelazim A.S., Bensalah O.K., Al Muhairi S.S. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2015;50(3):509–513. doi: 10.1007/s11262-015-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof M.F., Queen K., Eltahir Y.M., Paden C.R., Al Hammadi Z., Tao Y., Li Y., Khalafalla A.I., Shi M., Zhang J., Mohamed M., Abd Elaal Ahmed M.H., Azeez I.A., Bensalah O.K., Eldahab Z.S., Al Hosani F.I., Gerber S.I., Hall A.J., Tong S., Al Muhairi S.S. Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg. Microbes Infect. 2017;6(11):e101. doi: 10.1038/emi.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zohaib A., Saqib M., Athar M.A., Chen J., Khan S., Taj Z., Sadia H., Tahir U., Tayyab M.H., Qureshi M.A. Countrywide survey for MERS-Coronavirus antibodies in dromedaries and humans in Pakistan. Virol. Sin. 2018;33(5):410–417. doi: 10.1007/s12250-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.