Abstract
Aim
To estimate gingivitis effects of a bioavailable gluconate chelated 0.454% stannous fluoride (SnF2) family of dentifrices in adult subjects versus positive (triclosan) and negative (NaF or MFP) controls when used ≤3 months.
Materials and methods
A meta‐analysis evaluated bioavailable gluconate chelated SnF2 dentifrices versus a negative or positive control for gingival bleeding.
Results
In 18 randomized controlled trials (RCTs) with 2,890 subjects assessing SnF2 paste versus a negative or positive control, the average number of bleeding sites was reduced by 51% and 31%, respectively. The average change (95% CI) in number of bleeding sites was −16.3 (−27.8, −4.9) versus the negative control and −3.6 (−5.4, −1.8) versus the positive control. Subjects with localized or generalized gingivitis had 3.7 times better odds (95% CI [2.8, 5.0]) of shifting to generally healthy using SnF2 versus a negative control and 2.8 times better odds (95% CI [2.1, 3.9]) of shifting to generally healthy using SnF2 versus a positive control. The individual study risk of bias was deemed to be low in all categories of bias.
Conclusion
This meta‐analysis demonstrates significant gingivitis benefits of bioavailable SnF2 dentifrices when used ≤3 months versus positive (triclosan) and negative (NaF or MFP) controls.
Keywords: bioavailability, dentifrices, gingivitis, meta‐analysis, stannous fluoride
Clinical Relevance.
Scientific rationale for the study: Published meta‐analyses on SnF2 dentifrices typically exclude shorter‐term studies and include a variety of different SnF2 formulations, which can vary in bioavailable levels of SnF2 and performance. This meta‐analysis examined shorter‐term gingival health effects of a bioavailable gluconate chelated 0.454% SnF2 family of dentifrices.
Principal findings: In gingivitis studies assessing a bioavailable gluconate chelated SnF2 paste versus a negative or positive control, the SnF2 paste significantly reduced the number of bleeding sites.
Practical implications: Bioavailable gluconate chelated SnF2 dentifrices consistently reduced gingival bleeding across the spectrum of gingivitis levels, regardless of baseline level of disease.
1. INTRODUCTION
Periodontal diseases are a group of chronic inflammatory diseases of the gingiva and periodontium that have a high global prevalence and are a primary aetiology of tooth loss (Kassebaum et al., 2014; Marcenes et al., 2013). Periodontal disease initially presents as gingivitis, a plaque‐induced inflammation of the marginal and attached gingiva (Page, 1986). The clinical symptoms of gingivitis include redness, oedema and bleeding at the gingival margin. The high prevalence of gingivitis in adult populations has been established, with multiple global studies estimating the prevalence to be 75% or higher (Beaglehole et al., 2009; Hugoson & Jordon, 1982). Treatment and management of gingivitis is of clinical importance, because left untreated it can progress to periodontitis which includes loss of clinical attachment, recession, increased tooth mobility and in some events tooth loss. The absence of bleeding on probing over repetitive dental examinations has been shown to be a reliable predictor for the maintenance of periodontal health, as measured by lack of attachment loss, in patients following active periodontal therapy (Lang, Adler, Joss, & Nyman, 1990).
The microbial aetiology of gingivitis was clearly established by Löe, Theliade, and Jensen, through his seminal research on induced gingivitis through the cessation of daily oral hygiene (Löe et al., 1965). This experiment established that gingivitis could be initiated through plaque biofilm development and maturation, and then reversed by removal of the plaque biofilm. In the ensuing 50 years, researchers have elucidated the underlying microbial and host pathways that are operative in the initiation, propagation and reversal of gingivitis. Loesch and Syed (1978) reported on the microbial succession that occurred in the plaque biofilm during the experimental gingivitis phase, with a shift from a Streptococcus dominated biofilm to an Actinomyces biofilm as the biofilm aged over the 3‐week period of no oral hygiene (Loesch & Syed, 1978; Syed & Loesche, 1978). Importantly, the bacterial species Actinomyces viscosus and Bacteroides melaninogenicus were increased significantly at gingival sites that bled, establishing an association (Loesch & Syed, 1978). More recently, Actinomyces viscosus and Prevotella intermedia were found in overabundance (p < .05) in twins at sites that were not flossed relative to the companion twin at sites that were flossed over a two‐week treatment period (Corby et al., 2008). The flossing treatment group had ~40% reductions in gingival bleeding versus the non‐flossing treatment group, supporting the association of specific bacteria to gingival bleeding in chronic gingivitis (Biesbrock et al., 2006). Inflammation is induced by specific activity of the microbial biofilm that includes the production of a number of bacterial virulence factors (“toxins”) that are capable of triggering an immune inflammatory response, including lipopolysaccharide, lipoteichoic acid, short chain fatty acids, collagenase and elastase enzymes (gingipain) (Ginsburg, 2002; Hamada, Takada, Ogawa, Fujiwara, & Mihara, 1990; Holt & Bramanti, 1991; Madianos, Bobetis, & Kinane, 2005). These microbial virulence factors can interact with host epithelial and immune cells through specialized cell receptors, such as toll receptors, that mediate the initiation of the host inflammatory cascade (Beutler et al., 2006; Hans & Hans, 2011; Takeda & Akira, 2005).
Treatment and management of gingivitis is predicated on direct removal of plaque or suppression of plaque biofilm and its metabolites. Historically, the primary treatment modality to physically disrupt and remove plaque biofilm has been daily oral hygiene, primarily toothbrushing and flossing. Lang et al. established a minimal brushing frequency of once every 48 hr as a necessity to suppress the initiation and progression of gingivitis (Lang, Cumming, & Löe, 1973). Routine dental prophylaxis is also used as a treatment in the management of gingivitis. Two separate cohorts report median reductions in gingival bleeding of 40% and 66%, respectively (McClanahan, Bartizek, & Biesbrock, 2001), one week following dental prophylaxis. Several antimicrobial chemotherapeutic dentifrices and mouthrinses have been clinically proven for the treatment and management of gingivitis, including stannous fluoride (SnF2), triclosan, essential oils, cetylpyridinium chloride and chlorhexidine. These actives have all been shown to have direct bactericidal mechanisms through which they reduce gingival inflammation.
A number of meta‐analyses examining the effects of SnF2 dentifrices on plaque and gingivitis have been reported in the past fifteen years (Gunsolley, 2006; Paraskevas & van der Weijden, 2006; Sälzer, Slot, Dörfer, & van der Weijden, 2015). All of these meta‐analyses confirm the ability of SnF2 dentifrices to reduce gingivitis and gingival bleeding. Importantly, these meta‐analyses were limited to studies of at least 6 months in duration, which led to the exclusion of a number of shorter‐term gingivitis studies. The SnF2 dentifrices included in these meta‐analyses varied in formulation composition, which may introduce differences in the amount of bioavailable SnF2 across formulations. Formulation composition is critically important to maintaining bioavailable SnF2 concentrations in the dentifrice over time, which in turn affects antimicrobial and antigingivitis efficacy (Tinannoff, 1995; White, 1995). The meta‐analysis reported in this paper was explicitly designed to include earlier timepoints (2 to 12 weeks) in randomized clinical trials (RCTs), as a preponderance of the clinical evidence exists in the form of shorter duration gingivitis studies. We used the PICO model: Patient: adult gingivitis sufferer; Intervention: family of 0.454% bioavailable gluconate chelated SnF2 dentifrices from a single manufacturer (The Procter & Gamble Company); Comparator: positive or negative control; Outcome: number of bleeding sites derived from the Löe–Silness Gingival Index (LSGI) or Gingival Bleeding Index (GBI). The objective was to estimate gingivitis effects of a bioavailable gluconate chelated 0.454% SnF2 family of dentifrices in adult subjects versus positive (triclosan) and negative (NaF or MFP) controls when used for 3 months or less (PICO question). The primary rationale for examining bioavailable SnF2 dentifrice from a single manufacturer was to ensure products had bioavailable levels of SnF2 through access to both analytical and antimicrobial performance data. Secondarily, it was important to have access to subject‐level data from each RCT to facilitate transition analyses.
2. MATERIALS AND METHODS
The meta‐analysis was conducted in accordance with the general principles of the PRISMA statement (Moher, Liberati, Tetzlaff, Altman, & The PRISMA Group, 2009).
2.1. Search
A search limited to the Procter & Gamble Oral Care Clinical archive was undertaken to identify relevant studies with results available as of March 2018 for inclusion in this meta‐analysis.
2.2. Eligibility criteria
We included data from evaluations at 3 months or less from parallel randomized, blinded, controlled clinical trials that had intervention and control groups in human subjects and reported the effect of a family of 0.454% bioavailable SnF2 dentifrices on gingivitis outcomes.
2.3. Study selection and data collection
Two authors (TH and YZ) independently assessed the eligibility of all studies retrieved from the archives. Disagreements between the evaluators concerning the selected studies were resolved by discussion. From the studies included in the final analysis, we extracted the following data: study name and year; country; study design; participants; age and gender; intervention; follow‐up period; oral health condition; and values of outcome measurements (subject‐level data, sample size, means and standard deviations) in both intervention and control groups. If the study had more than one follow‐up visit, we used the assessment up to and including the 3‐month visit for data extraction.
2.4. Risk of bias assessment
We assessed the quality of the included individual studies based on the revised Cochrane collaboration tool for assessing the risk of bias (Sterne et al., 2019).
2.5. Statistical analysis
For the gingivitis (bleeding sites derived from LSGI or GBI) efficacy variable, the generic inverse variance method with both fixed and random effects models was used to calculate the summary differences between the SnF2 dentifrice and the control. If there were four or more studies to be analysed, the “random effects” model was chosen to calculate the weighted average of the treatment effects across the studies (Higgins & Green, 2009). If there were fewer than four studies, the “fixed effects” model was used (Poklepovic et al., 2012). Results included gingivitis assessments up to 12 weeks. For studies with multiple interventions or controls, the weighted average of the scores and pooled standard deviation was calculated to obtain a single pairwise comparison and to mitigate the unit‐of‐analysis error.
The estimated mean differences and the 95% confidence intervals (CIs) are presented in forest plots. Tests for overall effects were based on z‐statistics and associated p values. Percent change from control was calculated by the weighted percent change from the control from different studies where the weights were calculated from the random or fixed effects model. Additionally, a subgroup analysis was performed using mean bleeding site data. The study subgroups were defined using the study‐level baseline mean bleeding scores as follows: mild (<17 bleeding sites), moderate (17–50 bleeding sites) or severe (>50 bleeding sites).
The current Gingivitis Case Definition from the 2017 World Workshop in Periodontology (Trombelli, Farina, Silva, & Tatakis, 2018) was used to classify subject‐level gingival bleeding at baseline and the final visit used in the analysis as: generally healthy (<10% bleeding sites), localized gingivitis (10%–30% bleeding sites) or generalized gingivitis (>30% bleeding sites). Transition rates by treatment were then calculated and used to generate odds ratios with 95% confidence intervals.
All summary‐level meta‐analyses were conducted using the “metafor” package in R version 3.2.3 (R Development Core Team, 2015; Viechtbauer, 2010). All subject‐level analyses were conducted using SAS 9.4 (SAS Institute).
3. RESULTS
Eighteen RCTs with 2,890 subjects across three countries assessed gingivitis health benefits. In 12 gingivitis studies assessing a SnF2 dentifrice versus a negative control (NaF and MFP), a change in the average number of bleeding sites of −16.3 (95% CI: −27.8, −4.9) was observed equating to a 51% benefit versus the negative control (p < .001). (Figure 1a) In 7 gingivitis studies assessing a SnF2‐containing dentifrice versus a positive control (triclosan), a change in the average number of bleeding sites of −3.6 (95% CI: −5.4, −1.8) was observed equating to a 31% benefit versus the positive control (p < .001). (Figure 1b).
Figure 1.
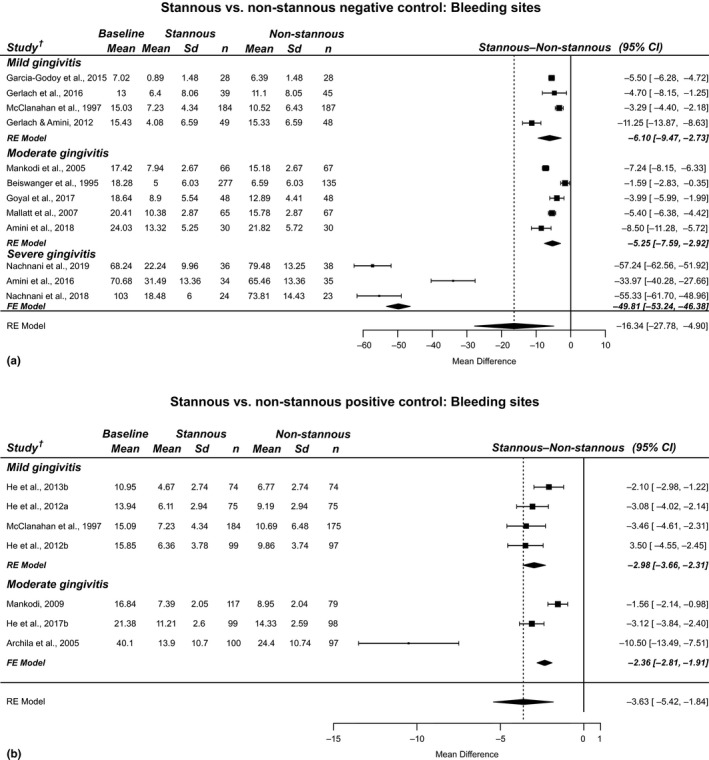
Results from RCTs included in the bleeding site meta‐analyses: SnF2 dentifrice versus a negative control (a) and positive control (b)
Further analysis of the effect of baseline gingivitis status was assessed at both the mean and subject levels. The changes (95% CI) in the average number of bleeding sites were −6.1 (−9.5, −2.7), −5.3 (−7.6, −2.9) and −49.8 (−53.2, −46.4) for studies in the mild, moderate and severe groups compared to the negative control. (Figure 1a). These reductions equate to a 59%, 35% and 67% benefit versus the negative control, respectively (p < .001). Study subgroup analysis showed a change (95% CI) in the average number of bleeding sites of −3.0 (−3.7, −2.3) and −2.4 (−2.8, −1.9) for mild and moderate groups compared to the positive control. (Figure 1b) These reductions equate to a 33% and 20% benefit versus the positive control, respectively (p < .001).
The subjects receiving the SnF2 dentifrice had a greater response than positive and negative controls at every percentile of the percent change from baseline bleeding response distribution (Figure 2a,b).
Figure 2.
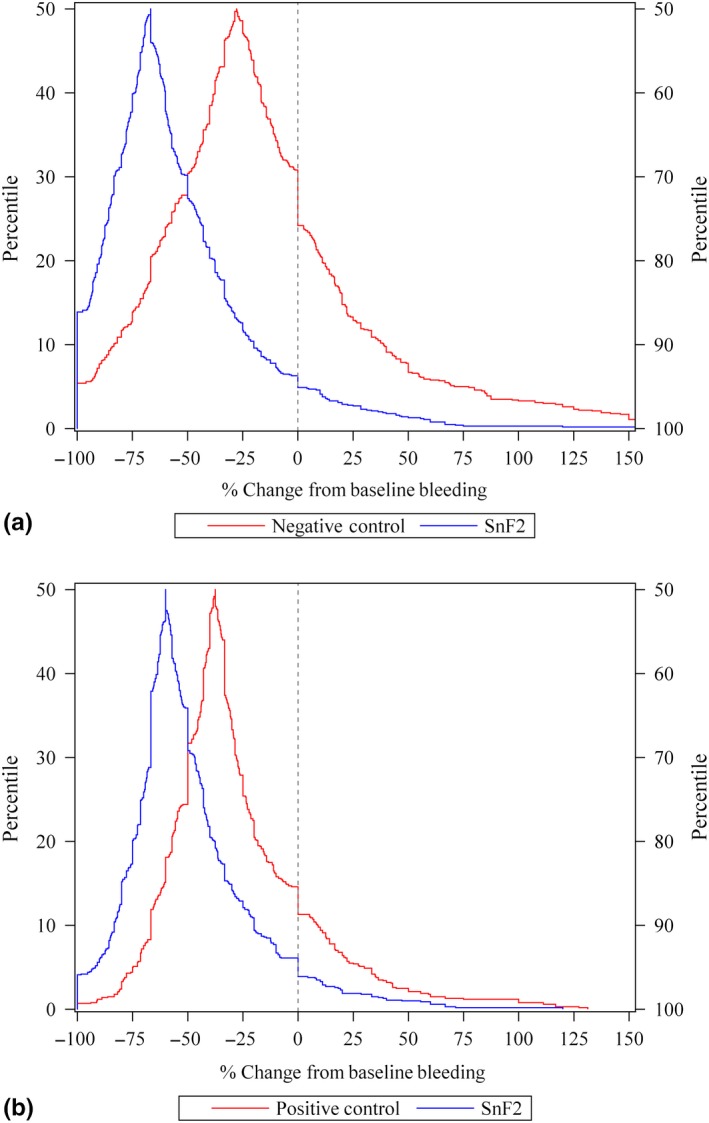
Gingival bleeding response versus baseline bleeding levels: SnF2 dentifrice versus a negative control (a) and positive control (b)
Additionally, at the subject level, the SnF2 dentifrice benefit on gingival bleeding response relative to the negative and positive control was observed across the entire range of baseline bleeding. (Figure 3a,b).
Figure 3.
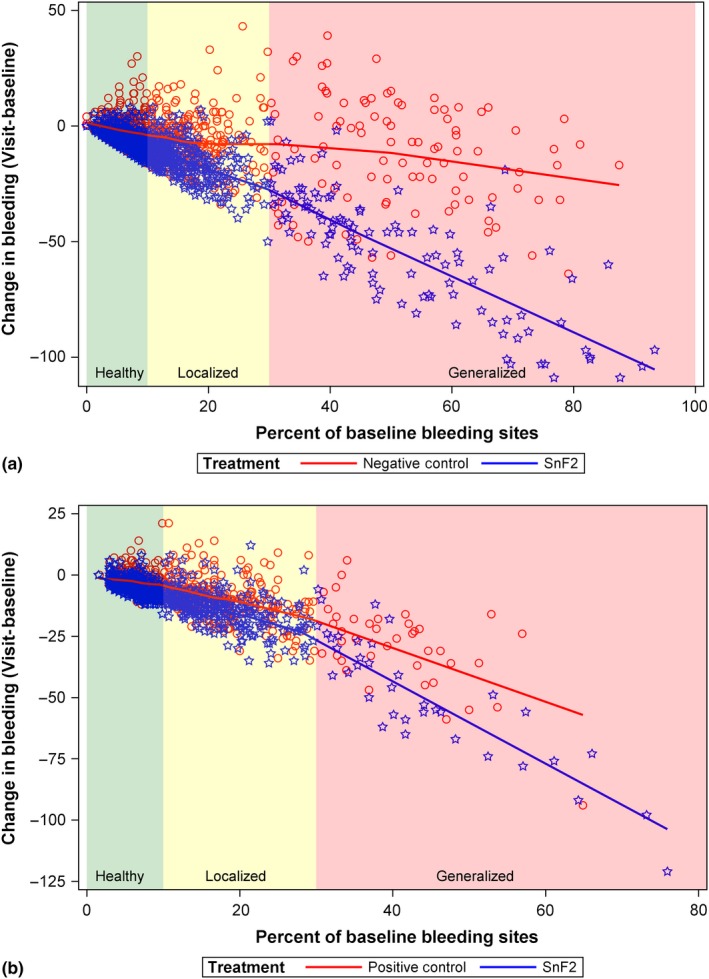
Distribution of percent change from baseline in the number of bleeding sites: SnF2 dentifrice versus a negative control (a) and positive control (b)
In negative‐controlled studies, of the 444 subjects in the SnF2 treatment group with localized or generalized gingivitis at baseline, 301 (68%) transitioned to generally healthy while only 139 (36%) of the 386 subjects in the negative control transitioned to generally healthy. (Table 1a) That is, subjects with localized or generalized gingivitis had 3.7 times better odds (95% CI [2.8, 5.0]) of shifting to generally healthy using SnF2 versus a negative control. In positive‐controlled studies, of the 348 subjects in the SnF2 treatment group with localized or generalized gingivitis at baseline, 246 (71%) transitioned to generally healthy while only 152 (46%) of the 331 subjects in the positive control transitioned to generally healthy. (Table 1b) That is, subjects with localized or generalized gingivitis had 2.8 times better odds (95% CI [2.1, 3.9]) of shifting to generally healthy using SnF2 versus a positive control.
Table 1.
Baseline and post‐baseline % bleeding sites in (a) negative‐controlled studies; (b) positive‐controlled studies
| Treatment | Baseline | Total | Post‐baseline | |
|---|---|---|---|---|
| Generally healthy (<10%) | Gingivitis (≥10%) | |||
| (a) | ||||
| SnF2 | <10% | 436 | 430 (99%) | 6 (1%) |
| ≥10% | 444 | 301 (68%) | 143 (32%) | |
| Neg Control | <10% | 365 | 332 (91%) | 33 (9%) |
| ≥10% | 386 | 139 (36%) | 247 (64%) | |
| (b) | ||||
| SnF2 | <10% | 400 | 397 (99%) | 3 (1%) |
| ≥10% | 348 | 246 (71%) | 102 (29%) | |
| Pos Control | <10% | 363 | 354 (98%) | 9 (2%) |
| ≥10% | 331 | 152 (46%) | 179 (54%) | |
(a) Subjects with localized or generalized gingivitis had 3.7 times better odds (95% CI [2.8, 5.0]) of shifting to generally healthy (<10% bleeding) using SnF2 versus a negative control.
(b) Subjects with localized or generalized gingivitis had 2.8 times better odds (95% CI [2.1, 3.9]) of shifting to generally healthy (<10% bleeding) using SnF2 versus a positive control.
3.1. Risk of bias
The risk of bias in individual studies was deemed to be low in all categories of bias (Figure S1, File S1). All studies included in the meta‐analysis were randomized and blinded with the allocation sequence concealed until all participants were enrolled and assigned to treatment (bias arising from the randomization process). The individual study analyses were based on the per‐protocol population to assess the effect of adhering to intervention, and examiners were not aware of a participants’ assigned intervention during the trial (bias due to deviations from the intended interventions). Outcome data were available for all or nearly all subjects (bias due to missing outcome data). Valid and reliable outcome measures were used in all studies, and in the case of single‐blind studies, the examiners were not aware of the participants’ assigned intervention during the trial (bias in measurement of the outcome). All published and unpublished studies had a pre‐specified analysis plan, and results were included regardless of outcome (bias in the selection of the reported result).
All of the studies included in this meta‐analysis were sponsored by The Procter & Gamble Company, which is a potential source of systematic bias. However, this across‐study risk of bias is mitigated to a large degree in that all studies were randomized, blinded and controlled and by the robust nature and scope of the research involving 18 studies across 7 sites in 3 different countries, which support the results are valid and reproducible.
4. DISCUSSION
The results of this meta‐analysis provide further evidence that bioavailable gluconate chelated 0.454% SnF2 dentifrices deliver significantly greater reductions in gingivitis as measured by gingival bleeding than negative control (NaF or MFP) dentifrices. The Paraskevas and van der Weijden (2006) meta‐analysis confirmed that SnF2 dentifrices provide statistically significant reductions in gingivitis versus sodium fluoride negative control dentifrices. The Gunsolley (2006) meta‐analysis similarly found that SnF2 provides statistically significant gingivitis reductions versus sodium fluoride negative control dentifrices. More recently, the Sälzer et al. (2015) meta‐analysis compared the gingivitis effects of SnF2 and triclosan dentifrices (Sälzer et al., 2015). SnF2 dentifrices statistically significantly reduced gingival bleeding scores relative to triclosan‐containing dentifrices, with no statistically significant difference observed in gingivitis scores between the two treatments. In this study, the meta‐analysis results from 18 studies in 2,890 subjects demonstrated a 51% reduction and a 31% reduction in gingival bleeding for SnF2 dentifrices versus negative control and positive control dentifrices, respectively. The results were observed over a 2‐ to 12‐week treatment period and are generally consistent with results observed at 6 months in individual RCTs (Archila et al., 2005; Mallatt et al., 2007; Mankodi et al., 2005). Furthermore, the observed results are generally consistent with results reported in previous meta‐analyses of 6‐month studies (Gunsolley, 2006; Paraskevas & van der Weijden, 2006; Sälzer et al., 2015). The majority of the dentifrices in these previously published meta‐analyses contained 0.454% SnF2 products with gluconate chelant systems, although they also included SnF2 products without gluconate chelant systems.
There are four publications in the peer‐reviewed published literature evaluating gingivitis effects of gluconate chelated SnF2 dentifrices that were not included in this meta‐analysis because they did not meet a key criterion of access to subject‐level data (Ayad, Stewart, Zhang, & Proskin, 2010; Boneta et al., 2010; Seriwatanachai et al., 2019; Singh et al., 2010). All four studies were sponsored by a single manufacturer (Colgate‐Palmolive). Three RCTs compared the SnF2 dentifrice to triclosan dentifrice (Colgate Total) over periods of 6 weeks to 6 months. Two of the studies also included a negative control. These studies reported that the triclosan dentifrice produced statistically significantly greater improvements in gingival health compared to the SnF2 dentifrice and the SnF2 dentifrice produced statistically significantly greater improvements in gingival health compared to the negative control. Importantly, the only two global manufacturers to market triclosan dentifrices (Procter & Gamble and Colgate‐Palmolive) chose to discontinue triclosan dentifrice in favour of stannous fluoride formulations. The most recent study by Seriwatanachai et al. evaluated two SnF2 dentifrices, including gluconate chelated dentifrice and a novel formulation with zinc phosphate. Both SnF2 dentifrices improved gingival health statistically significantly better than the negative control with no statistically significant differences between the SnF2 dentifrices.
Two other recent studies assessing SnF2 with zinc phosphate and SnF2 with tripolyphosphate dentifrices, not gluconate chelant systems, demonstrated that they deliver reductions in gingivitis and gingival bleeding relative to negative control dentifrice at both 3 and 6 months (Hu et al., 2019; Parkinson, Amini, Wu, & Gallob, 2018). Taken collectively, numerous 0.454% SnF2 dentifrices have been reported to provide reductions in gingival inflammation and bleeding. One limitation of this current meta‐analysis is that it cannot address potential differences in the clinical efficacy of varying 0.454% SnF2 dentifrice formulations, which will require more comparative testing in randomized clinical trials.
At the recent 2017 World Workshop of Periodontology, new guidelines were proposed that define generalized gingivitis cases and localized gingivitis cases (Trombelli et al., 2018). Furthermore, patients with < 10% bleeding sites, without attachment loss and radiographic bone loss, are considered clinically periodontally healthy, albeit with localized sites of gingival inflammation. The relative baseline gingivitis level of the patients in the studies included in this meta‐analysis spanned a large distribution of the disease ranging from generally periodontally healthy with localized gingival inflammation sites to generalized gingivitis cases, with baseline bleeding sites per subject ranging from 0 to 147 bleeding sites. Importantly, SnF2 dentifrices provided reductions in gingivitis across the entire baseline disease spectrum, including generalized gingivitis case types, localized gingivitis case types and generally periodontally healthy case types with isolated sites of gingival inflammation (Figure 3a,b). The mean percent reduction in bleeding sites in studies classified as mild, moderate and severe was 59%, 35% and 67%, respectively. These represent large meaningful reductions in gingival bleeding, which are similar in magnitude to the 40%–66% delivered one week following a dental prophylaxis (McClanahan et al., 2001). The magnitude of reduction in bleeding provided by SnF2 dentifrices is also similar to that observed with flossing treatment, which delivers ~40% reductions in gingival bleeding versus non‐flossing treatment (Biesbrock et al., 2006).
At every percentile of the percent change from baseline bleeding response distribution, SnF2 had a greater response than positive and negative controls (Figure 2a,b). For negative control studies, half of the SnF2 subjects had a 67% reduction from baseline compared to half of the negative control subjects who had a 28% reduction. For positive control studies, half of the SnF2 subjects had a 60% reduction from baseline compared to half of the positive control subjects had a 38% reduction. Importantly, results from the subject‐level analyses support that subjects using a SnF2 dentifrice had 3.7 times better odds of transitioning from localized or generalized gingivitis to generally healthy relative to those using the negative control and 2.8 times better odds of transitioning to generally healthy relative to those using the positive control (Table 1).
Gingival bleeding is an objective measure of inflammation that has been positively correlated to histologic changes in the gingiva, which include a greater percentage of cell‐rich collagen‐poor connective tissue consistent with an inflammatory infiltrate, as compared to non‐bleeding sites (Greenstein, Caton, & Polson, 1981). The clinical significance of gingival bleeding should not be underestimated, as chronic inflammation of the gingiva and periodontium has been shown to be a significant risk factor for both periodontal attachment loss and recession. Sites with persistent gingival bleeding over multiple periodic examinations have been shown to have higher odds for progressive attachment loss compared to non‐bleeding sites (Schatzle et al., 2003). Over a 26‐year observation period in a population of well‐maintained, well‐educated men who practiced regular oral hygiene, sites that bled consistently throughout the course of the study had approximately 70% more attachment loss than sites that were consistently non‐inflamed, yielding an odds ratio of 3.22 for inflamed sites (bleeding) converting to attachment loss (Schatzle et al., 2003). These persistent bleeding sites can exist even if patients are considered generally healthy. In addition, the absence of persistent gingival bleeding on probing has also been shown to have a high negative predictive value of 98.1% for disease progression, as measured by ≥2 mm attachment loss, in a periodontal maintenance population over a 2.5‐year observation period (Lang et al., 1990). It should be noted that bleeding on probing and gingival bleeding upon sweeping may not represent the same inflammatory lesion in deeper pockets (≥5 mm); however in shallower pocket depths (≤3 mm), it is likely that these clinical measures significantly overlap. The established relationship between persistent gingival bleeding and attachment loss is the mechanistic basis for gingival inflammation as a risk factor for tooth mortality. Importantly, teeth surrounded by persistent inflamed gingival tissue (presence of bleeding) had a 46‐fold higher risk of being lost over a 26‐year observation period, compared to teeth surrounded by inflammation‐free gingival tissues (absence of bleeding) (Schatzle et al., 2004).
Results of this meta‐analysis quantify the gingivitis effects of a bioavailable gluconate chelated 0.454% SnF2 family of dentifrices in adult subjects versus positive (triclosan) and negative (NaF or MFP) controls when used for 3 months or less and add further supporting evidence of clinically meaningful benefits. The generalizability of these studies is limited to the bioavailable gluconate chelated SnF2 formulas examined in this report.
CONFLICT OF INTEREST
The roles of the study sponsor (P&G) and clinical sites were consistent across all studies. The study sponsor was responsible for investigator and site identification and monitoring and statistical analyses. The independent clinical sites were responsible for IRB submission, recruitment and study conduct. Protocol development and study interpretation were jointly performed by the investigator and sponsor.
Supporting information
ACKNOWLEDGEMENT
The protocol for this meta‐analysis has been registered in clinicaltrials.gov (NCT03970759).
APPENDIX 1.
STUDIES IN FIGURE 1A.
| Study reference | Formula | Year Study initiated | Location | Measures & inclusion criteria | Control | Length of measurement | N | Female | Mean age in years (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nachnani et al., 2019, Summary S2 |
0.45% SnF2 0.51% SnCl2 0.53% Zn citrate |
2018 | California, USA | Bleeding sites | BS: ≥20 | Negative, SMFP | 12 weeks | 84 | 69% | 38.7 (15.62) |
| Nachnani, Lee, Gurich, Zou, & Anastasia, 2018, Summary S3 |
0.45% SnF2 2.5% Zn lactate |
2017 | California, USA | Bleeding sites LSGI | BS: ≥20 | Negative, SMFP | 4 weeks | 49 | 59% | 42.2 (15.26) |
| Amini, Miner, & Gerlach, 2016, Summary S4 |
0.45% SnF2 2.5% Zn lactate |
2015 | Nevada, USA | Bleeding sites | BS: n/a | Negative, NaF | 2 weeks | 70 | 57% | 36.1 (11.53) |
| Amini, Amini, & Gerlach, 2018 |
0.45% SnF2 2.5% Zn lactate |
2013 | Nevada, USA | Bleeding sites MGI | BS: ≥15 | Negative, SMFP | 3 weeks | 61 | 57% | 33.4 (11.95) |
| Goyal, Qaqish, He, Anastasia, & Winston, 2017, Summary S5 |
0.45% SnF2 0.51% SnCl2 0.53% Zn citrate |
2013 | Nevada, USA | Bleeding sites MGI |
BS: ≥5 MGI: 1.75 to 2.3 |
Negative, SMFP | 12 weeks | 100 | 65% | 41.6 (14.12) |
| Garcia‐Godoy, Duque, Rothrock, Anastasia, & Gerlach, 2015, Summary S6 |
0.45% SnF2 2.5% Zn lactate |
2012 | Florida, USA | Bleeding sites LSGI | Mild‐to‐moderate gingivitis | Negative, SMFP | 4 weeks | 57 | 65% | 29.1 (8.68) |
| Gerlach & Amini, 2012 |
0.45% SnF2 0.46% SnCl2 1.9% Zn lactate |
2009 | Nevada, USA | Bleeding sites MGI |
BS: n/a MGI: 1.75 to 2.3 |
Negative, SMFP | 12 weeks | 100 | 60% | 33.6 (11.14) |
| Gerlach, Sagel, Winston, Garcia‐Godoy, & Garcia‐Godoy, 2016, Summary S7 |
0.45% SnF2 2.5% Zn lactate |
2007 | Florida, USA | TMQHI | Adults with plaque and gingivitis | SMFP | 11 weeks | 91 | 76% | 33.4 (11.19) |
| Mallatt et al., 2007 |
0.45% SnF2 2.5% Zn lactate |
2003 | Florida, USA | Bleeding sites MGI | MGI: 1.75 to 2.3 | Negative, SMFP | 12 weeks | 140 | 62% | 37.7 (11.66) |
| Mankodi et al., 2005 |
0.45% SnF2 2.5% Zn lactate |
2002 | Florida, USA | Bleeding sites MGI | MGI: 1.75 to 2.3 | Negative, SMFP | 12 weeks | 143 | 67% | 37.2 (10.87) |
| McClanahan et al., 1997 |
0.45% SnF2 1.5% SnCl2 |
1992 | Indiana, USA | Bleeding sites LSGI | BS: >5 | Negative, NaF | 12 weeks | 383 | 67% | 35.9 (11.35) |
| Beiswanger et al., 1995 |
0.45% SnF2 1.5% SnCl2 |
1988 | Indiana, USA | Bleeding sites LSGI | BS: >5 | Negative, SMFP | 12 weeks | 463 | 69% | 33.2 (9.76) |
Abbreviations: BS, bleeding sites; LSGI, Löe–Silness Gingival Index; MGI, Modified Gingival Index; N, number of randomized subjects; NaF, sodium fluoride; SMFP, sodium monofluorophosphate; TMQHI, Turesky's modification of Quigley–Hein index.
STUDIES IN FIGURE 1B.
| Study reference or Investigator, Year | Formula | Year Study initiated | Location | Measures & inclusion criteria | Control | Length of measurement | N | Female | Mean age in years (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|
| He, Eusebio, Goyal, & Qaqish, 2017 |
0.45% SnF2 0.51% SnCl2 0.53% Zn citrate |
2016 | Ontario, Canada | Bleeding sites MGI |
BS: 10 to 50 MGI: 1.75 to 2.3 |
Positive, NaF/triclosan | 8 weeks | 200 | 73% | 47.9 (10.63) |
| He, Barker, Biesbrock, Miner, et al., 2013 |
0.45% SnF2 0.46% SnCl2 1.9% Zn lactate |
2011 | Nevada, USA | Bleeding sites MGI |
BS: ≥5 MGI: 1.75 to 2.3 |
Positive, NaF/triclosan | 8 weeks | 150 | 63% | 37.4 (11.48) |
| He, Barker, Biesbrock, et al., 2012 |
0.45% SnF2 0.46% SnCl2 1.9% Zn lactate |
2011 | Ontario, Canada | Bleeding sites MGI |
BS: ≥5 MGI: 1.75 to 2.3 |
Positive, NaF/triclosan | 8 weeks | 150 | 60% | 42.4 (10.61) |
| He, Barker, Goyal, & Biesbrock, 2012 |
0.45% SnF2 0.46% SnCl2 1.9% Zn lactate |
2010 | Nevada, USA | Bleeding sites MGI |
BS: ≥5 MGI: 1.75 to 2.3 |
Positive, NaF/triclosan | 8 weeks | 200 | 61% | 38.1 (13.28) |
| Mankodi, 2009 Unpublished, Summary S8 |
0.45% SnF2 0.46% SnCl2 1.9% Zn lactate |
2009 | Florida, USA | Bleeding sites MGI |
BS: n/a MGI: 1.75 to 2.3 |
Positive, NaF/triclosan | 12 weeks | 205 | 70% | 42.1 (12.91) |
| Archila et al., 2005 |
0.45% SnF2 2.5% Zn lactate |
2002 | Guatemala | Bleeding sites LSGI | Mild‐to‐moderate gingivitis | Positive, NaF/triclosan | 12 weeks | 197 | 76% | 29.8 (9.42) |
| McClanahan et al., 1997 |
0.45% SnF2 1.5% SnCl2 |
1992 | Indiana, USA | Bleeding sites LSGI |
BS: >5 LSGI: n/a |
Positive, NaF/triclosan | 12 weeks | 359 | 67% | 36.1 (11.11) |
Abbreviations: BS, bleeding sites; LSGI, Löe–Silness Gingival Index; MGI, Modified Gingival Index; N, number of randomized subjects; NaF, sodium fluoride; SMFP, sodium monofluorophosphate.
Biesbrock A, He T, DiGennaro J, Zou Y, Ramsey D, Garcia‐Godoy F. The effects of bioavailable gluconate chelated stannous fluoride dentifrice on gingival bleeding: Meta‐analysis of eighteen randomized controlled trials. J Clin Periodontol. 2019;46:1205–1216. 10.1111/jcpe.13203
Funding information
The studies in this meta‐analysis were sponsored by The Procter and Gamble Company. Aaron Biesbrock, Tao He, Joe DiGennaro, Yuanshu Zou, and Dave Ramsey are employees of The Procter & Gamble Company. Franklin Garcia‐Godoy has done consulting work for The Procter & Gamble Company.
REFERENCES
- Amini, P. , Amini, A. , & Gerlach, R. W. (2018). Randomized controlled trial evaluating concurrent gingivitis and stain effects of a two‐step dentifrice/gel sequence. American Journal of Dentistry, 31(Sp Is A), 13A–17A. [PubMed] [Google Scholar]
- Amini, P. , Gerlach, R. W. , Walanski, A. , Cheng, R. , & Anastasia, M. K. (2014). A randomized clinical trial to evaluate a daily two‐step dentifrice and gel system in the prevention of stain, plaque and calculus following a dental prophylaxis. Retrieved from https://www.dentalcare.com/-/media/dentalcareus/research/pdf/paste/cphhd041final.pdf?la=en&v=1-201604260558 [Google Scholar]
- Amini, P. , Miner, M. , & Gerlach, R. W. (2016). Gingivitis response comparing SnF2/H2O2 and KNO3 daily use whitening products. Journal of Dental Research, 95(Spec Iss B), Abstract 0320. Summary S4. [Google Scholar]
- Archila, L. , He, T. , Winston, J. L. , Biesbrock, A. R. , McClanahan, S. F. , & Bartizek, R. D. (2005). Antigingivitis efficacy of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice in subjects previously nonresponsive to a triclosan/copolymer dentifrice. Compendium of Continuing Education in Dentistry, 26(Supp 1), 12–18. [PubMed] [Google Scholar]
- Ayad, F. , Stewart, B. , Zhang, Y. P. , & Proskin, H. M. (2010). A comparison of the efficacy of a triclosan/copolymer/sodium fluoride dentifrice, a stannous fluoride/sodium hexametaphosphate/zinc lactate dentifrice, and a sodium fluoride dentifrice for the control of established supragingival plaque and gingivitis: A six‐week clinical study. Journal of Clinical Dentistry, 21, 111–116. [PubMed] [Google Scholar]
- Barker, M. L. , Gerlach, R. W. , Sagel, P. , & Magnusson, I. (2015). Plaque imaging comparison of two‐step SnF2/H2O2 dentifrices versus triclosan dentifrice. Journal of Dental Research, 94(Spec Iss A), Abstract 294. [Google Scholar]
- Beaglehole, R. , Benzian, H. , Crail, J. , Mackay, J. , King, J. , Lacey, C. , & Wyse, E. (2009). The oral health atlas: Mapping a neglected global health issue (pp. 1–124). Brighton, UK: Dental Education Ltd & Myriad Editions. [Google Scholar]
- Beiswanger, B. B. , Doyle, P. M. , Jackson, R. D. , Mallatt, M. E. , Mau, M. S. , Bollmer, B. W. , … McClanahan, S. F. (1995). The clinical effect of dentifrices containing stabilized stannous fluoride on plaque formation and gingivitis–a six‐month study with ad libitum brushing. Journal of Clinical Dentistry, 6, 46–53. [PubMed] [Google Scholar]
- Beutler, B. , Jiang, Z. , Georgel, P. , Crozat, K. , Croker, B. , Rutschman, S. , … Hoebe, K. (2006). Genetic analysis of host resistance: Toll‐like receptor signaling and immunity at large. Annual Review of Immunology, 24, 353–389. 10.1146/annurev.immunol.24.021605.090552 [DOI] [PubMed] [Google Scholar]
- Biesbrock, A. , Corby, P. M. A. , Bartizek, R. , Corby, A. L. , Coelho, M. , Costa, S. , … Bretz, W. A. (2006). Assessment of treatment responses to dental flossing in twins. Journal of Periodontology, 77, 1386–1391. 10.1902/jop.2006.050399 [DOI] [PubMed] [Google Scholar]
- Boneta, A. E. , Aguilar, M. M. , Romeu, F. L. , Stewart, B. , DeVizio, W. , & Proskin, H. M. (2010). Comparative investigation of the efficacy of triclosan/copolymer/sodium fluoride and stannous fluoride/sodium hexametaphosphate/zinc lactate dentifrices for the control of established supragingival plaque and gingivitis in a six‐month clinical study. The Journal of Clinical Dentistry, 21, 117–123. [PubMed] [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, P. T. , & Rothstein, H. R. (2009). Introduction to meta‐analysis 2009. Chichester, UK: John Wiley & Sons Ltd. [Google Scholar]
- Corby, P. M. A. , Biesbrock, A. , Bartizek, R. , Corby, A. L. , Montverde, R. , Ceschin, R. , & Bretz, W. A. (2008). Treatment outcomes of dental flossing in twins: Molecular analysis of the interproximal microflora. Journal of Periodontology, 79, 1426–1433. 10.1902/jop.2008.070585 [DOI] [PubMed] [Google Scholar]
- Friesen, L. R. , Goyal, C. R. , Qaqish, J. G. , He, T. , Eusebio, R. , Zsiska, M. , … Schneiderman, E. (2017). Comparative antiplaque effect of two antimicrobial dentifrices: Laboratory and clinical evaluations. Journal of Clinical Dentistry, 28(4 Spec No B), B6–B11. [PubMed] [Google Scholar]
- Garcia‐Godoy, C. , Duque, N. , Rothrock, J. , Anastasia, M. K. , & Gerlach, R. W. (2015). Comparative effectiveness of two‐step paste/gel plus CrissCross brush on gingivitis. Journal of Dental Research, 94(Spec Iss A), Abstract 1645. Summary S6. [Google Scholar]
- Gerlach, R. W. , & Amini, P. (2012). Randomized controlled trial of 0.454% stannous fluoride dentifrice to treat gingival bleeding. Compendium of Continuing Education in Dentistry, 33(2), 134–136, 138. [PubMed] [Google Scholar]
- Gerlach, R. , Sagel, P. , Winston, J. L. , Garcia‐Godoy, C. , & Garcia‐Godoy, F. (2016). Randomized Controlled Trial Evaluating Oral Health Effects of Two‐Step Hygiene. Journal of Dental Research, 95(Spec Issue A), Abstract 91. Summary S7. [Google Scholar]
- Ginsburg, J. (2002). Role of lipoteichoic acid in infection and inflammation. The Lancet Infectious Diseases, 2, 171–179. 10.1016/S1473-3099(02)00226-8 [DOI] [PubMed] [Google Scholar]
- Goyal, C. R. , Qaqish, J. , He, T. , Anastasia, M. K. , & Winston, J. L. (2017). Clinical anti‐gingivitis efficacy of a 0.454% SnF2 dentifrice. Journal of Dental Research, 96(Spec Iss A), Abstract 889. Summary S5. [Google Scholar]
- Greenstein, G. , Caton, J. , & Polson, A. M. (1981). Histologic characteristics associated with bleeding after probing and visual signs of inflammation. Journal of Periodontology, 52, 420–425. 10.1902/jop.1981.52.8.420 [DOI] [PubMed] [Google Scholar]
- Gunsolley, J. C. (2006). A meta‐analysis of six‐month studies of antiplaque and antigingivitis agents. Journal of the American Dental Association, 137, 1649–1657. 10.14219/jada.archive.2006.0110 [DOI] [PubMed] [Google Scholar]
- Hamada, S. , Takada, H. , Ogawa, T. , Fujiwara, T. , & Mihara, J. (1990). Lipopolysaccharides of oral anaerobes associated with chronic inflammation: Chemical and immunomodulating properties. International Reviews of Immunology, 6, 247–261. 10.3109/08830189009056635 [DOI] [PubMed] [Google Scholar]
- Hans, M. , & Hans, V. M. (2011). Toll‐like receptors and their dual role in periodontitis: A review. Journal of Oral Science, 53, 263–271. 10.2334/josnusd.53.263 [DOI] [PubMed] [Google Scholar]
- He, T. , Anastasia, M. K. , Wilberg, A. , & Eusebio, R. (2017). Anti‐plaque effects of a SnF2 dentifrice relative to marketed controls. Journal of Dental Research, 96(Spec Iss A), Abstract 2815. [Google Scholar]
- He, T. , Barker, M. L. , Biesbrock, A. R. , Eynon, H. , Milleman, J. L. , Milleman, K. R. , … Wintergerst, A. M. (2013). Digital plaque imaging evaluation of a stabilized stannous fluoride dentifrice compared with a triclosan/copolymer dentifrice. American Journal of Dentistry, 26, 303–306. [PubMed] [Google Scholar]
- He, T. , Barker, M. L. , Biesbrock, A. , Miner, M. , Amini, P. , Goyal, C. R. , & Qaqish, J. (2013). Evaluation of anti‐gingivitis benefits of stannous fluoride dentifrice among triclosan dentifrice users. American Journal of Dentistry, 26, 175–179. [PubMed] [Google Scholar]
- He, T. , Barker, M. L. , Biesbrock, A. R. , Sharma, N. C. , Qaqish, J. , & Goyal, C. R. (2012). Assessment of the effects of a stannous fluoride dentifrice on gingivitis in a two‐month positive‐controlled clinical study. Journal of Clinical Dentistry, 23, 80–85. [PubMed] [Google Scholar]
- He, T. , Barker, M. L. , Goyal, C. R. , & Biesbrock, A. R. (2012). Anti‐gingivitis effects of a novel 0.454% stabilized stannous fluoride dentifrice relative to a positive control. American Journal of Dentistry, 25, 136–140. [PubMed] [Google Scholar]
- He, T. , Eusebio, R. , Goyal, C. R. , & Qaqish, J. G. (2017). Assessment of the effects of a novel stabilized stannous fluoride dentifrice on gingivitis in a two‐month positive‐controlled clinical study. Journal of Clinical Dentistry, 28(4 Spec no B), B12–B16. [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Green, S. (2009). Handbook for systematic reviews of interventions; Version 5.1.0. Retrieved from http://cochrane-handbook.org [Google Scholar]
- Holt, S. C. , & Bramanti, T. E. (1991). Factors in virulence expression and their role in periodontal pathogenesis. Critical Reviews in Oral Biology & Medicine, 2, 177–281. [DOI] [PubMed] [Google Scholar]
- Hu, D. , Li, X. , Liu, H. , Mateo, L. R. , Sabharwal, A. , Xu, G. , … Zhang, Y.‐P. (2019). Evaluation of a stabilized stannous fluoride dentifrice on dental plaque and gingivitis in a randomized controlled trial with 6 month follow‐up. Journal of the American Dental Association, 150(4 suppl), S32–S37. 10.1016/j.adaj.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Hugoson, A. , & Jordon, T. (1982). Frequency distribution of individuals aged 20–70 according to severity of periodontal disease. Community Dentistry and Oral Epidemiology, 10, 187–192. [DOI] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Bernabe, E. , Dahiya, M. , Bhandari, B. , Murray, C. J. , & Marcenes, W. (2014). Global burden of severe periodontitis in 1990–2010. A systematic review and meta‐regression. Journal of Dental Research, 93, 1045–1053. 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, N. P. , Adler, R. , Joss, A. , & Nyman, S. (1990). Absence of bleeding on probing: An indicator of periodontal stability. Journal of Clinical Periodontology, 17, 714–721. 10.1111/j.1600-051X.1990.tb01059.x [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Cumming, B. R. , & Löe, H. (1973). Toothbrushing frequency as it relates to plaque development and gingival health. Journal of Periodontology, 44, 396–405. 10.1902/jop.1973.44.7.396 [DOI] [PubMed] [Google Scholar]
- Löe, H. , Theliade, E. , & Jensen, S. B. (1965). Experimental gingivitis in man. Journal of Periodontology, 36, 177–187. 10.1902/jop.1965.36.3.177 [DOI] [PubMed] [Google Scholar]
- Loesch, W. J. , & Syed, S. A. (1978). Bacteriology of human experimental gingivitis: Effect of plaque and gingivitis score. Infection and Immunity, 21, 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madianos, P. N. , Bobetis, Y. A. , & Kinane, D. F. (2005). Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. Journal of Clinical Periodontology, 32(Suppl 6), 57–71. 10.1111/j.1600-051X.2005.00821.x [DOI] [PubMed] [Google Scholar]
- Mallatt, M. , Mankodi, S. , Bauroth, K. , Bsoul, S. A. , Bartizek, R. D. , & He, T. (2007). A controlled 6‐month clinical trial to study the effects of a stannous fluoride dentifrice on gingivitis. Journal of Clinical Periodontology, 34(9), 762–767. [DOI] [PubMed] [Google Scholar]
- Mankodi, S. , Bartizek, R. D. , Winston, J. L. , Biesbrock, A. R. , McClanahan, S. F. , & He, T. (2005). Anti‐gingivitis efficacy of a stabilized 0.454% stannous fluoride/sodium hexametaphosphate dentifrice. Journal of Clinical Periodontology, 32(1), 75–80. [DOI] [PubMed] [Google Scholar]
- Marcenes, W. , Kassebaum, N. J. , Bernabe, E. , Flaxman, A. , Naghavi, M. , Lopez, A. , & Murray, C. J. (2013). Global burden of oral conditions in 1990–2010. A systematic analysis. Journal of Dental Research, 92, 592–597. 10.1177/0022034513490168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClanahan, S. F. , Beiswanger, B. B. , Bartizek, R. D. , Lanzalaco, A. C. , Bacca, L. , & White, D. J. (1997). A comparison of stabilized stannous fluoride dentifrice and triclosan/copolymer dentifrice for efficacy in the reduction of gingivitis and gingival bleeding: six‐month clinical results. Journal of Clinical Dentistry, 8(2 Spec No), 39–45. [PubMed] [Google Scholar]
- McClanahan, S. F. , Bartizek, R. D. , & Biesbrock, A. R. (2001). Identification and consequences of distinct Löe‐Silness Gingival Index examiner styles for the clinical assessment of gingivitis. Journal of Periodontology, 72, 383–392. 10.1902/jop.2001.72.3.383 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & The PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachnani, S. , He, T. , Peters, J. , Eusebio, R. , Lee, S. , & Farrell, S. (2019). Clinical effects of bioavailable SnF2 dentifrice on plaque and gingivitis. Journal of Dental Research, 98(Spec Iss A), Abstract 2326. Summary S2. [Google Scholar]
- Nachnani, S. , Lee, S. , Gurich, N. , Zou, Y. , & Anastasia, M. (2018). Gingivitis and malodor improvement with the use of 2‐step system. Journal of Dental Research, 97(Spec Iss B), Abstract 2596. Summary S3. [Google Scholar]
- Page, R. C. (1986). Gingivitis*. Journal of Clinical Periodontology, 13, 345–355. 10.1111/j.1600-051X.1986.tb01471.x [DOI] [PubMed] [Google Scholar]
- Paraskevas, S. , & Van der Weijden, G. A. (2006). A review of the effects of stannous fluoride on gingivitis. Journal of Clinical Periodontology, 33, 1–13. 10.1111/j.1600-051X.2005.00860.x [DOI] [PubMed] [Google Scholar]
- Parkinson, C. , Amini, P. , Wu, J. , & Gallob, J. (2018). A 24‐week randomized clinical study investigating the anti‐gingivitis efficacy of a 0.454% w/w stannous fluoride dentifrice. American Journal of Dentistry, 31, 17–23. [PubMed] [Google Scholar]
- Poklepovic, T. , Sambunjak, D. , Johnson, T. , Imai, P. , Tugwell, P. , Nickerson, J. , … Worthington, H. (2012). Interdental brushing for the management of periodontal diseases and dental caries in adults. Cochrane Database of Systematic Reviews, 10.1002/14651858.CD009857 [DOI] [PubMed] [Google Scholar]
- R Development Core Team . (2015). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3‐900051‐07‐0. Retrieved from https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing [Google Scholar]
- Sälzer, S. , Slot, D. E. , Dörfer, C. E. , & Van der Weijden, G. A. (2015). Comparison of triclosan and stannous fluoride dentifrices on parameters of gingival inflammation and plaque scores: A systemic review and meta‐analyses. International Journal of Dental Hygiene, 13, 1–17. [DOI] [PubMed] [Google Scholar]
- Schatzle, M. , Löe, H. , Burgin, W. , Anerud, A. , Boysen, H. , & Lang, N. P. (2003). Clinical course of periodontics. I. Role of gingivitis. Journal of Clinical Periodontology, 30, 887–901. [DOI] [PubMed] [Google Scholar]
- Schatzle, M. , Löe, H. , Lang, N. P. , Burgin, W. , Anerud, A. , & Boysen, H. (2004). The clinical course of chronic periodontitis. IV. Gingival inflammation as a risk factor in tooth mortality. Journal of Clinical Periodontology, 31, 1122–1127. 10.1111/j.1600-051X.2004.00634.x [DOI] [PubMed] [Google Scholar]
- Seriwatanachai, D. , Triratana, T. , Kraivaphan, P. , Amaornchat, C. , Mateo, L. , Sabharwal, A. , … Zhang, Y. P. (2019). Effect of stannous fluoride and zinc phosphate dentifrice on dental plaque and gingivitis: A randomized clinical trial with 6 month follow‐up. Journal of the American Dental Association, 150(4 suppl), S25–S31. [DOI] [PubMed] [Google Scholar]
- Sharma, N. C. , He, T. , Barker, M. L. , & Biesbrock, A. R. (2013). Plaque control evaluation of a stabilized stannous fluoride dentifrice compared to a triclosan dentifrice in a six‐week trial. Journal of Clinical Dentistry, 24, 31–36. [PubMed] [Google Scholar]
- Singh, M. , Papas, A. , & Gerlach, R. W. (2018). Safety and effectiveness of a two‐step dentifrice/gel sequence with medication‐associated hyposalivation: A randomized controlled trial in a vulnerable population. American Journal of Dentistry, 31(Sp Is A), 24A–28A. [PubMed] [Google Scholar]
- Singh, S. , Chaknis, P. , DeVizio, W. , Petrone, M. , Panagakos, F. S. , & Proskin, H. M. (2010). A clinical investigation of the efficacy of three commercially available dentifrices for controlling established gingivitis and supragingival plaque. The Journal of Clinical Dentistry, 21, 105–110. [PubMed] [Google Scholar]
- Sterne, J. A. C. , Savović, J. , Page, M. J. , Elbers, R. G. , Blencowe, N. S. , Boutron, I. , … Higgins, J. P. T. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ, 28(366), l4898 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Syed, S. A. , & Loesche, W. J. (1978). Bacteriology of human experimental gingivitis: Effect of plaque age. Infection and Immunity, 21, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, K. , & Akira, S. (2005). Toll‐like receptors in innate immunity. International Immunology, 2005(17), 1–14. [DOI] [PubMed] [Google Scholar]
- Tinannoff, N. (1995). Progress regarding the use of stannous fluoride in clinical dentistry. Journal of Clinical Dentistry, 6(special issue), 37–40. [PubMed] [Google Scholar]
- Trombelli, L. , Farina, R. , Silva, C. O. , & Tatakis, D. N. (2018). Plaque‐induced gingivitis: Case definition and diagnostic considerations. Journal of Periodontology, 89(Suppl 1), S46–S73. 10.1002/JPER.17-0576 [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metaphor package. Journal of Statistical Software, 36, 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- White, D. J. (1995). A “return” to stannous fluoride dentifrices. Journal of Clinical Dentistry, 6(special issue), 29–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


