Abstract
The pharmacokinetics of once‐daily extended‐release tacrolimus tablets (LCPT) in de novo liver transplantation have not been previously reported. In this phase II, randomized, open‐label study, de novo liver transplant recipients were randomized to LCPT 0.07–0.13 mg/kg/day (taken once daily; n = 29) or twice‐daily immediate‐release tacrolimus capsules (IR‐Tac) at 0.10–0.15 mg/kg/day (divided twice daily; n = 29). Subsequent doses of both drugs were adjusted to maintain tacrolimus trough concentrations of 5 to 20 ng/mL through day 90, and 5–15 ng/mL thereafter. Twenty‐four‐hour pharmacokinetic profiles were obtained on days 1, 7, and 14, with trough concentration and efficacy/safety monitoring through year 1. Similar proportions of patients in both groups achieved therapeutic trough concentrations on days 7 and 14 (day 7: LCPT = 78%, IR‐Tac = 75%; day 14: LCPT = 86%, IR‐Tac = 91%) as well as similar systemic and peak exposure. There was a robust correlation between drug concentration at time 0 and area under the concentration‐time curve for both LCPT and IR‐Tac (respectively, day 7: r = 0.86 and 0.79; day 14: r = 0.93 and 0.86; P < .0001 for all). Dose adjustments during days 1 to 14 were frequent. Thirty‐five patients completed the extended‐use period. No significant differences in adverse events were seen between groups. Incidence of biopsy‐proven acute rejection (LCPT = 6 and IR‐Tac = 4) was similar on day 360. Between formulations, overall exposure was similar at 1 week after transplant with the characteristic delayed‐release pharmacokinetic profile of LCPT demonstrated in this novel population. These data support further investigation of the safety and efficacy of LCPT in de novo liver transplantation.
Keywords: acute rejection, calcineurin inhibitor, immunosuppression, phase II, safety
Immediate‐release, twice‐daily tacrolimus (Prograf; Astellas Pharma US, Inc., Northbrook, Illinois) was approved by the US Food and Drug Administration in 1994 for prophylaxis of organ rejection in patients receiving liver transplants; since then, tacrolimus has become the cornerstone of immunosuppressive therapy in liver transplantation. Currently, approximately 90% of de novo and maintenance liver transplant recipients are on an immunosuppression regimen that contains tacrolimus.1
While tacrolimus is effective in preventing rejection,2, 3 the traditional formulation of immediate‐release twice‐daily capsules (IR‐Tac) exhibits some challenging pharmacokinetic (PK) properties (eg, significant inter‐ and intraindividual variability in absorption and metabolism of tacrolimus).4, 5 Tacrolimus has poor bioavailability, poor water solubility, undergoes extensive presystemic metabolism by cytochrome P450 3A isoenzymes in the gut, and is a substrate for P‐glycoprotein. The biliary route is the primary elimination method for tacrolimus metabolites. As a substrate for cytochrome P450 3A4 and cytochrome P450 3A5, tacrolimus is subject to many drug‐drug interactions with other substrates, inhibitors, and inducers.4 In addition, 2 doses per day are required; more frequent daily dosing is associated with an increased risk for poor adherence.6, 7, 8, 9 Such noncompliance is discussed by Morrissey et al10 as a factor that contributes to acute rejection and graft failure in renal transplantation.
A novel formulation of extended‐release tacrolimus marketed as Envarsus XR (Veloxis Pharmaceuticals, Inc., Cary, North Carolina) was approved by the US Food and Drug Administration in December 2015. This extended‐release once‐daily tablet (LCPT) increases the bioavailability of tacrolimus by the creation of a solid dispersion, or a solid solution, of the drug substance through a physical process called “controlled agglomeration.” Prior randomized trials in renal transplant recipients comparing LCPT with IR‐Tac have shown that LCPT has greater bioavailability, a steadier and more consistent concentration time profile over 24 hours, and reduced fluctuation (the peak‐to‐trough change in drug concentrations around the average concentration)11 and swing (the peak‐to‐trough change in drug concentrations relative to the minimum concentration)11 compared to IR‐Tac.12 In addition, LCPT has demonstrated comparable efficacy,13 improved tolerability in regard to hand tremors,14 and robust correlations between area under the concentration‐time curve (AUC) and minimum whole blood concentration (Cmin) in trials evaluating the product in renal transplantation.12, 15, 16, 17
A single study has been published evaluating LCPT in liver transplant recipients. In that phase II trial, stable liver transplant recipients who were converted from IR‐Tac to LCPT demonstrated consistent exposure, as defined by AUC, at a lower total daily dose compared to IR‐Tac at steady state.18 Maximum whole blood concentration (Cmax), the Cmax/minimum concentration (Cmin) ratio, and the percent of fluctuation and swing were significantly lower with LCPT (P < .001), while time to maximum concentration (tmax) was significantly longer for LCPT vs IR‐Tac (P < .001). AUC0–24 and Cmin correlation coefficients were ≥0.93 after 7 and 14 days of therapy following conversion. During the 52‐week trial extension period, none of the 49 patients in the intent‐to‐treat population experienced graft loss or death.18
The current phase II trial is the first to report the PK of LCPT in a de novo liver transplant population randomized to LCPT tablets once daily or IR‐Tac capsules twice daily.
Methods
Study Design
This phase II, open label, multicenter, randomized trial (NCT00772148; Pharmacokinetics of LCP‐Tacro™ Once Daily and Prograf® Twice a Day in Adult De Novo Liver Transplant Patients) was approved by the following institutional review boards (IRBs): California Pacific Medical Center IRB; Western IRB; Mayo Clinic IRB; Biomedical Research Alliance of New York, LLC; Piedmont Healthcare IRB; Stanford University Medical Center Administrative Panels Office; University of California, San Francisco Committee on Human Research, Office of Research; University of Cincinnati Medical Center IRB; University of Miami Human Subjects Research Office; University of Michigan IRBMED; and the Washington University St. Louis Human Research Protection Office. All subjects provided written informed consent before participating in the study. The study was conducted according to the International Conference on Harmonisation Guideline for Good Clinical Practice and the United States Code of Federal Regulations. The study was carried out at the following sites: California Pacific Medical Center, LifeLink Healthcare Institute, Mayo Clinic Arizona, Mayo Clinic Rochester, New York University, Piedmont Hospital, Stanford University Medical Center, University of Alabama Birmingham, University of California San Francisco, University of Cincinnati, University of Miami, University of Michigan, and Washington University in St. Louis.
Patient enrollment occurred between October 2008 and May 2010. Adult de novo liver transplant recipients were randomized to receive LCPT tablets once daily or IR‐Tac capsules twice daily, beginning within 72 hours of reperfusion. Patients swallowed all LCPT tablets whole and without manipulation. Study centers were permitted to follow their respective induction protocols; however, alemtuzumab use was not permitted. Concomitant immunosuppressive therapy with mycophenolate mofetil, mycophenolic acid sodium, prednisone, or azathioprine was allowed; use of everolimus or sirolimus was not permitted.
LCPT tablets were administered at a starting dose of 0.07 to 0.11 mg/kg/day for all patients, except those who self‐identified as black (2 patients in each arm), who received a starting dose of 0.09 to 0.13 mg/kg/day. The differences in starting dose in this population was a response to higher rates of tacrolimus metabolism observed in blacks in previous clinical studies. Dose selection within the dosing range was left to the clinician's discretion. The initial total daily dose of IR‐Tac twice‐daily capsules was 0.10 to 0.15 mg/kg/day for all patients.19 All subsequent doses of both formulations were investigator adjusted to achieve target whole blood trough concentrations of 5 to 20 ng/mL until day 90, and between 5 and 15 ng/mL thereafter, according to standards of local practice. Dose adjustments to maintain tacrolimus whole blood trough levels within the predefined therapeutic ranges were to be based on local laboratory determinations.
Pharmacokinetic Assessment
PK assessments following overnight fasts were performed on days 1 (24 hours after drug initiation), 7, and 14. Blood samples for LCPT or IR‐Tac were collected 14 or 18 times per day, respectively, per the following schedules: patients on LCPT were tested before their morning LCPT dose (0.0) and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 14, 16, 20, and 24 hours after the single morning dose; patients on IR‐Tac were monitored at the same time points with the addition of tests at 12.5, 13, 13.5, and 15 hours after the morning dose to better capture the second, midday peak that occurs with this formulation.
All PK samples were analyzed in a central laboratory using high‐performance liquid chromatography–tandem mass spectrometry with an XTerra MS C8, 2.1′100 mm, 5‐µm (Waters) column. Tacrolimus and the internal standard, rapamycin, were extracted from the whole blood sample using potassium ethylenediaminetetraacetic acid as an anticoagulant, by solid phase extraction, into an organic medium, evaporated under nitrogen, and reconstituted in 200.0 µL of mobile phase. The mobile phase was 90:10 v/v methanol:0.002M ammonium acetate in 0.1% formic acid. An aliquot of this extract was injected into a high‐performance liquid chromatography system, detected using a TSQ Quantum tandem mass spectrometer, and quantitated using the peak area ratio method. Method sensitivity and selectivity were achieved by detecting distinct precursor‐to‐product ion mass transitions for tacrolimus (821.5 → 768.5) and the internal standard, rapamycin, (931.6 → 864.7) at a defined retention time under reverse phase chromatographic conditions. The procedure used to analyze tacrolimus is validated over the range of 0.200 to 25.600 ± 10%. The within‐day accuracy range was –2.9% to 0.5% and the between‐day accuracy range was –4.9 to 0.4% (percent coefficient of variation).
Patients
Inclusion and Exclusion Criteria
Patients eligible for the study were adult men and women ≥18 years of age who were recipients of a liver transplant from a deceased donor and had a Model for End‐Stage Liver Disease score of ≤30 at the time of transplantation.
The main exclusion criteria included receipt of a liver from a non–heart‐beating donor (ie, a donor who was pronounced dead after cardiac death), receipt of any transplanted organ other than a liver, or ABO incompatibility. Additionally, patients with gastrointestinal disorders or documented frank manipulation of the gastrointestinal tract that could have affected the absorption of tacrolimus were excluded. The protocol did not specifically exclude patients with a history of Roux‐en‐Y hepaticojejunostomy.
Objectives
The primary objectives were to demonstrate the PK of LCPT (AUC0‐24h and Cmax) and 24‐hour trough concentrations (C24) within the first 14 days after transplantation, and to compare the proportion of patients in each treatment group who achieved sufficient tacrolimus whole blood trough concentrations within the first 14 days after transplantation. Secondary objectives were to compare the PK of LCPT with the PK of IR‐Tac on study days 1, 7, and 14, and to evaluate the efficacy and safety of LCPT compared to IR‐Tac in the first 12 months after liver transplantation.
Statistical Analysis
Analysis Populations
The analysis population included the modified intent‐to‐treat population (all randomized patients who received ≥1 dose of study medication).
Statistical Methods
All demographic and baseline characteristics data, immunosuppression dosing data, laboratory data, and adverse events (AEs) were summarized using descriptive statistics.
Pharmacokinetic Parameters
The tacrolimus concentration vs time profiles and PK parameters were calculated based on data from a central laboratory. PK parameters were calculated using noncompartmental analyses for tacrolimus as follows: AUC0‐24h, Cmax, average plasma concentration (Cavg), plasma concentration 24 hours after dosing (C24), tmax, percent fluctuation, percent swing, and accumulation ratio. PK parameters and C24 were summarized using descriptive statistics on days 1, 7, and 14. Comparisons for the PK parameters between treatments were performed using a 1‐way analysis of variance model. The proportion of patients in each study arm who achieved tacrolimus whole blood trough concentrations within the recommended therapeutic range (5–20 ng/mL) was summarized on days 1, 7, and 14 based on the data from local laboratories; the difference between the groups was compared using Fisher's exact test. Differences between the groups in total number of dose adjustments during days 1 to 14 or during the course of study were evaluated using a generalized linear model for Poisson distribution based on likelihood ratio statistics and normalized to the extent of exposure (AUC0–24h) during each period of each subject.
Efficacy and Safety
Patient and graft survival, and the cumulative incidence of biopsy‐proven acute rejection (BPAR) (Banff grade ≥1) at day 360 after transplantation were estimated using the product‐limit (Kaplan‐Meier) method and compared between treatment groups using the z‐statistic. The severity of BPAR episodes was compared between treatment groups using a proportional odds model. Liver function tests were conducted and data collected throughout the study period. A measure of renal function was based on the change from baseline for the estimated glomerular filtration rate (derived using Equation 7 of the Modification of Diet in Renal Disease study). Baseline is defined as renal function at day 42 after transplantation; this time point allows for postoperative recovery and stabilization of renal function. Renal function was then compared between groups using a mixed‐effects linear regression (random intercept) model.
The proportion of LCPT and IR‐Tac patients experiencing AEs was compared using Fisher's exact test.
The primary goal of this study was to assess PK and intended to be descriptive; based on LCPT phase I and phase II study results, a sample size of 25 to 30 evaluable patients was determined to be sufficient to characterize the PK parameters of LCPT. A power analysis was not conducted to determine the sample size needed to assess efficacy and safety outcomes; as such, the efficacy and safety results should be interpreted considering this limitation.
Results
Patients
A total of 58 de novo liver patients were randomized and received at least 1 dose of a study drug (LCPT, n = 29; IR‐Tac, n = 29). The PK portion of the study was completed by 76% of patients (LCPT, n = 21; IR‐Tac, n = 23); patients completing the study through day 360 numbered 17 (59% of the original 29) in the LCPT group and 18 (62% of the original 29) in the IR‐Tac group (Figure 1).
Figure 1.
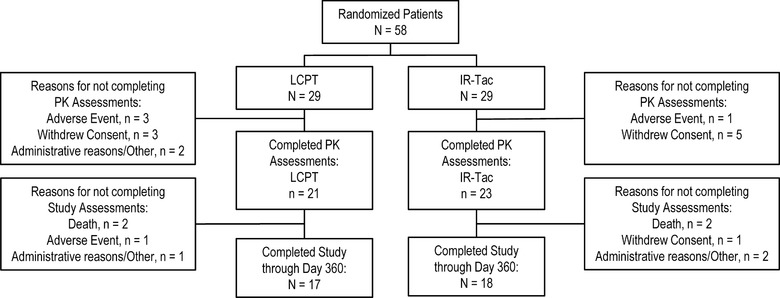
Subject disposition.
In the LCPT group, a total of 12 patients discontinued the study early; reasons documented were AEs (4 patients), death (2 patients), and “other” (n = 6); in the IR‐Tac group, of the 11 early discontinuations, 2 were due to deaths, 1 to an AE, and 8 were attributed to “other.”
Of the 58 patients enrolled in the study, 84.5% were white. Overall, there were 40 (69%) men and 18 (31%) women; the mean (standard deviation) age was 54.4 (8.55) years. The proportion of males in the LCPT group (24; 82.8%) was higher than in the IR‐Tac group (16; 55.2%). For full demographic details, see Table 1.
Table 1.
Demographic Characteristics—Modified Intent‐to‐Treat Population
| Characteristics | LCPT (N = 29)a | IR‐Tac (N = 29)a | Overall (N = 58)a |
|---|---|---|---|
| Sex, nb (%) | |||
| Male | 24 (82.8) | 16 (55.2) | 40 (69.0) |
| Female | 5 (17.2) | 13 (44.8) | 18 (31.0) |
| Age (y) | |||
| Mean (SD) | 54.1 (7.27) | 54.6 (9.78) | 54.4 (8.55) |
| Median | 55.0 | 55.0 | 55.0 |
| Min‐max | 27–63 | 21–72 | 21–72 |
| Race, nb (%) | |||
| American Indian/Alaska native | 0 (0.0) | 1 (3.4) | 1 (1.7) |
| Asian | 1 (3.4) | 2 (6.9) | 3 (5.2) |
| Asian, native Hawaiian/Pacific Islander | 1 (3.4) | 0 (0.0) | 1 (1.7) |
| Black/African American | 2 (6.9) | 2 (6.9) | 4 (6.9) |
| White | 25 (86.2) | 24 (82.8) | 49 (84.5) |
| Ethnicity, nb (%) | |||
| Hispanic or Latino | 5 (17.2) | 2 (6.9) | 7 (12.1) |
| Not Hispanic or Latino | 24 (82.8) | 27 (93.1) | 51 (87.9) |
IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; SD, standard deviation.
Percentages based on the total number of patients in the modified intent‐to‐treat analysis data set (N).
n represents number of patients contributing to summary.
Source: Table 14.1.2.1, Listing 16.2.4.1.
Immunosuppression
Tacrolimus was initiated between 1 and 3 days after reperfusion; the median time to initiation was 2 days. Mean total daily doses are presented in Table 2. A higher percentage of patients achieved therapeutic tacrolimus trough concentrations (5–20 ng/mL) in the IR‐Tac group (32.1%) on day 1 compared to LCPT (13.8%). Mean locally analyzed trough concentrations over the first week are presented in Table 3. On days 7 and 14, the proportions of patients achieving whole blood therapeutic tacrolimus trough concentrations were similar in both the LCPT and IR‐Tac groups (day 7, 78.3% vs 75.0%; day 14, 85.7% vs 91.3%, respectively).
Table 2.
Summary of Tacrolimus PK Parameters—Modified Intent‐to‐Treat Analysis Set
| Day 1a | Day 7 | Day 14 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean (%CV) Arithmetic Mean ± SD | Geometric Mean (%CV) Arithmetic Mean ± SD | Geometric Mean (%CV) Arithmetic Mean ± SD | |||||||
| PK Parameter | LCPT (n = 29) | IR‐Tac (n = 28) | P‐Valueb | LCPT (n = 23) | IR‐Tac (n = 26) | P‐Valueb | LCPT (n = 21) | IR‐Tac (n = 23) | P‐Valueb |
| Total daily dose (mg) | 6.48 ± 1.27 | 8.29 ± 2.55 | 9.78 ± 4.17 | 9.33 ± 3.77 | 9.43 ± 4.17 | 9.65 ± 4.14 | |||
| (mg/kg) | 0.076 ± 0.014 | 0.096 ± 0.017 | 0.122 ± 0.059 | 0.118 ± 0.054 | 0.121 ± 0.064 | 0.129 ± 0.070 | |||
| AUC0–24h (ng · h/mL) |
|
116.17 (54.51) 135.62 ± 73.92 |
<.0001 |
|
|
.85 |
|
229.55 (33.12) 241.22 ± 79.90 |
.38 |
| Cmax (ng/mL) |
|
|
<.0001 |
|
|
.10 |
|
|
.83 |
| Cmin (ng/mL) |
|
|
.057 |
|
|
.85 |
|
|
.55 |
| Cavg (ng/mL) | 2.84 ± 1.55 | 5.65 ± 3.08 | <.0001 | 10.45 ± 4.25 | 10.22 ± 4.24 | .86 | 11.66 ± 5.82 | 10.06 ± 3.33 | .37 |
| Fluctuation (%) | 95.25 ± 80.45 | 159.89 ± 86.33 | .005 | 92.70 ± 48.81 | 134.22 ± 51.77 | .007 | 121.82 ± 62.34 | 140.49 ± 81.52 | .40 |
| Swing (%) | 137.92 ± 164.76 | 248.59 ± 183.80 | .02 | 147.75 ± 96.90 | 201.52 ± 105.38 | .08 | 204.95 ± 127.82 | 196.42 ± 131.51 | .83 |
| Tmax (hr) Median (min‐max) | 12.00 (1.48–24.20) | 2.67 (1.00–20.00) | .009 | 4.00 (0.00–12.00) | 1.51 (0.67–16.50) | .03 | 4.00 (1.00–16.00) | 2.00 (1.00–14.00) | .02 |
AUC0–24, area under the concentration‐time curve, 0 to 24 hours; Cavg, average concentration; Cmax, maximum concentration; Cmin, minimum concentration; CV, coefficient of variation; IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; PK, pharmacokinetics; SD, standard deviation; tmax, maximum time.
Day 1 refers to level drawn 24 hours after drug initiation.
P‐value determined by analysis of variance.
Table 3.
Locally Analyzed Tacrolimus Whole Blood Trough (ng/mL) at Each Visit—PK mITT Population
| LCPT Arithmetic Mean (SD) | IR‐Tac Arithmetic Mean (SD) | |
|---|---|---|
| Day 2a | 5.02 (2.88) | 5.86 (3.58) |
| (n) | 22 | 23 |
| Day 3a | 6.83 (4.13) | 7.42 (3.23) |
| (n) | 26 | 25 |
| Day 4a | 6.57 (3.19) | 7.80 (2.80) |
| (n) | 28 | 26 |
| Day 7a | 8.90 (4.30) | 9.59 (3.82) |
| (n) | 25 | 25 |
IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; mITT, modified intent‐to‐treat; PK, pharmacokinetics.
Day refers to time after initiation of tacrolimus.
Dose adjustments were frequent; there was an average of 3.9 adjustments per LCPT patient and 4.8 per IR‐Tac patient in the first 14 days of treatment.
Concomitant immunosuppressive medications were used by the modified intent‐to‐treat population; during days 1 to 14, a total of 48 patients received prednisone, 47 received CellCept (mycophenolate mofetil), and 57 received methylprednisolone. Concomitant antifungals were more frequent in the LCPT vs IR‐Tac group (fluconazole, LCPT 12 [41%] vs IR‐Tac 8 [28%]; voriconazole, LCPT 2 [7%] vs IR‐Tac 0).
Pharmacokinetics
Table 2 provides a detailed description of PK findings of this study. Figure 2 shows the mean whole blood tacrolimus concentrations on days 1, 7, and 14 for LCPT and IR‐Tac. Table 4 and Figure 3 detail the exposure‐normalized PK parameters of LCPT vs IR‐Tac and the exposure‐normalized mean whole blood concentration profiles, respectively. Exposure normalization is an adjustment by which the AUC of LCPT and IR‐Tac are made equal, and all other PK parameters mathematically adjusted, to allow direct comparisons of PK parameters under assumption of equivalent exposure.
Figure 2.
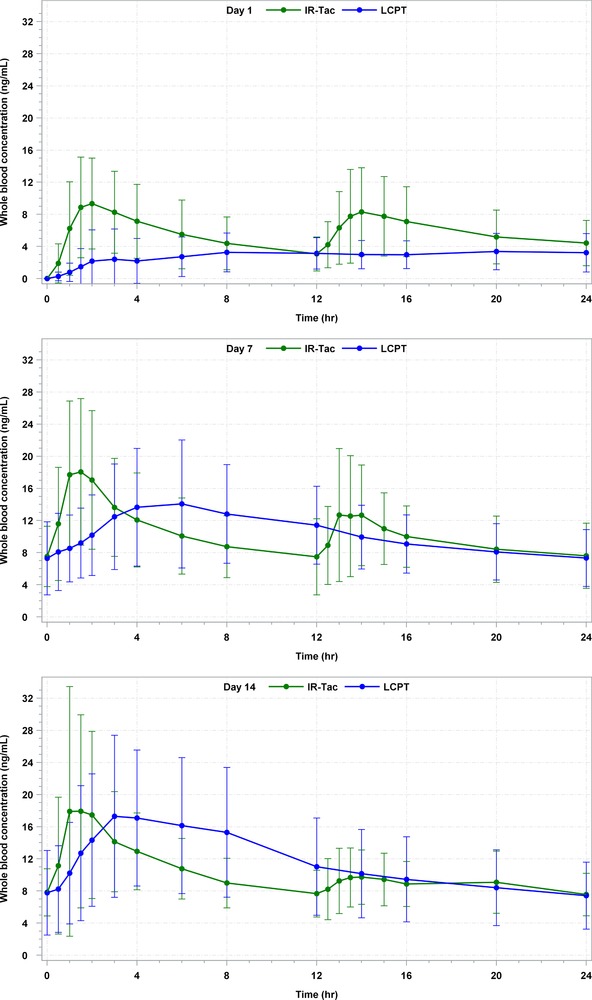
24‐hour arithmetic mean whole blood tacrolimus concentrations (ng/mL) and SD on days 1, 7, and 14 for LCPT and IR‐Tac. All BLQ values entered as zero and included as such in the calculation of means. BLQ, below limit of quantification; IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; SD, standard deviation.
Table 4.
Summary of Exposure Normalizeda Tacrolimus PK Parameters—mITT Analysis Set
| Day 1ab (Adjustment Factor 1 + 0.95) | Day 7 (Adjustment Factor 1 – 0.015) | Day 14 (Adjustment Factor 1 – 0.105) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean (%CV), Arithmetic Mean ± SD | Geometric Mean (%CV), Arithmetic Mean ± SD | Geometric Mean (%CV), Arithmetic Mean ± SD | |||||||
| PK Parameter | LCPT (n = 29) | IR‐Tac (n = 28) | P‐Valuec | LCPT (n = 23) | IR‐Tac (n = 26) | P‐Valuec | LCPT (n = 21) | IR‐Tac (n = 23) | P‐Valuec |
| Total daily dose (mg) | 12.64 ± 2.48 | 8.29 ± 2.55 | 9.64 ± 4.1 | 9.33 ± 3.77 | 8.44 ± 3.73 | 9.65 ± 4.14 | |||
| AUC0–24h (ng · h/mL) |
|
|
.999 |
|
|
.95 |
|
|
.94 |
| Cmax (ng/mL) |
|
|
.44 |
|
|
.083 |
|
|
.38 |
| Cmin (ng/mL) |
|
|
.082 |
|
|
.77 |
|
|
.13 |
AUC0–24h, area under the concentration‐time curve, 0 to 24 hours; Cmax, maximum concentration; Cmin, minimum concentration; CV, coefficient of variation; IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; mITT, modified intent‐to‐treat; PK, pharmacokinetics; SD, standard deviation.
Exposure normalization is an adjustment by which the AUC of LCPT and IR‐Tac are made equal, and all other PK parameters mathematically adjusted, to allow direct comparisons of PK parameters under assumption of equivalent exposure.
Day 1 refers to level drawn 24 hours after drug initiation.
P‐value determined by analysis of variance.
Figure 3.
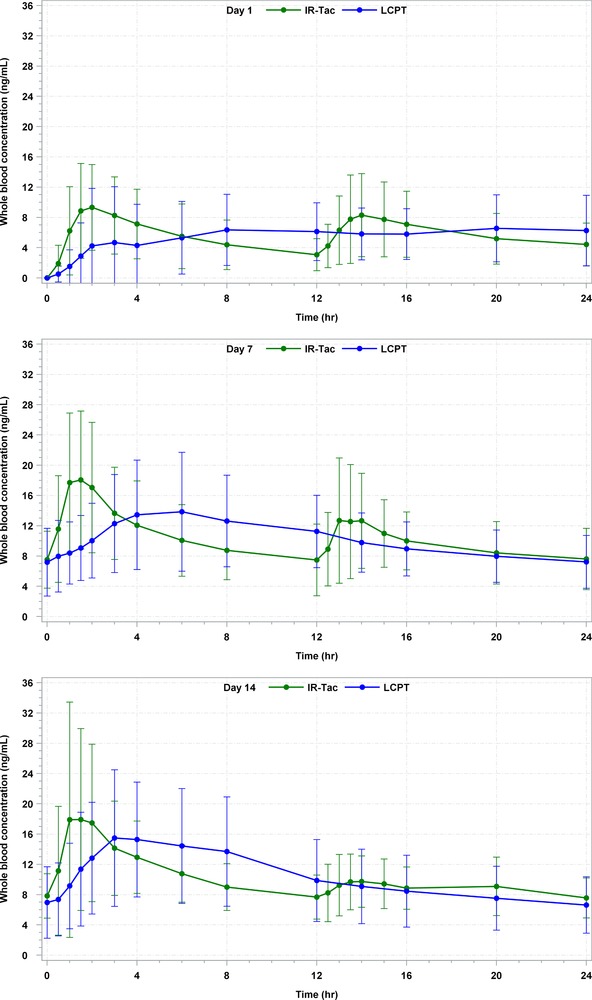
24‐hour arithmetic mean whole blood tacrolimus concentrations (ng/mL) and SD on days 1, 7, and 14 for LCPT and IR‐Tac, exposure normalized.* All BLQ values entered as zero and included as such in the calculation of means. BLQ, below limit of quantification; IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; SD, standard deviation.
*Exposure normalization is an adjustment by which the AUC of LCPT and IR‐Tac are made equal, and all other pharmacokinetic parameters mathematically adjusted, to allow direct comparisons of pharmacokinetic parameters under assumption of equivalent exposure.
Following the administration of LCPT on Day 1, the overall systemic exposure (AUC0–24h) of tacrolimus was approximately 49% lower (P < .0001), the peak systemic exposure (Cmax) was approximately 55% lower (P < .0001), and the concentration at the end of the dosing interval (Cmin) was approximately 30% lower for LCPT (P = .057). However, a higher degree of fluctuation (P = .005) and swing (P = .020) followed the administration of IR‐Tac vs LCPT. The median tmax of LCPT was 12 hours, substantially longer compared to 2.67 hours for IR‐Tac (P = .009).
On day 7, the overall systemic exposure of tacrolimus was comparable following administration of LCPT vs IR‐Tac. The Cmax was approximately 20% lower for LCPT vs IR‐Tac, though this did not reach statistical significance (P = .10). The Cmin was comparable for both groups (P = .85). While the degree of fluctuation was significantly higher for the IR‐Tac group compared to LCPT (P = .007), there were no statistically significant differences in the degree of swing (P = .08). The tmax of LCPT was significantly longer (4 hours) compared to IR‐Tac (1.51 hours) (P = .03).
On day 14, following administration of LCPT vs IR‐Tac, the overall systemic exposure of LCPT was approximately 11% higher (P = .38). The Cmax was comparable for the 2 groups (P = .83), and the Cmin was also comparable (P = .55). There were no statistically significant differences in the degree of fluctuation (P = .40) or swing (P = .83). Again, the median tmax of LCPT was longer (4 hours) compared to IR‐Tac (2 hours) (P = .017). Normalization of the curves for AUC did not alter the statistical significance of any PK parameters.
The correlation between dose‐normalized AUC0–24h and Cmin on day 1 was r = 0.53 for LCPT (P = .001) and r = 0.74 for IR‐Tac (P < .0001). By day 7, the correlation between AUC0–24h and Cmin was high for both LCPT (r = 0.86; P < .0001) and IR‐Tac (r = 0.79; P < .0001) (Figure 4). They remained highly correlated at day 14 (LCPT: r = 0.93, P < .0001; IR‐Tac: r = 0.86, P < .0001) (Figure 5).
Figure 4.
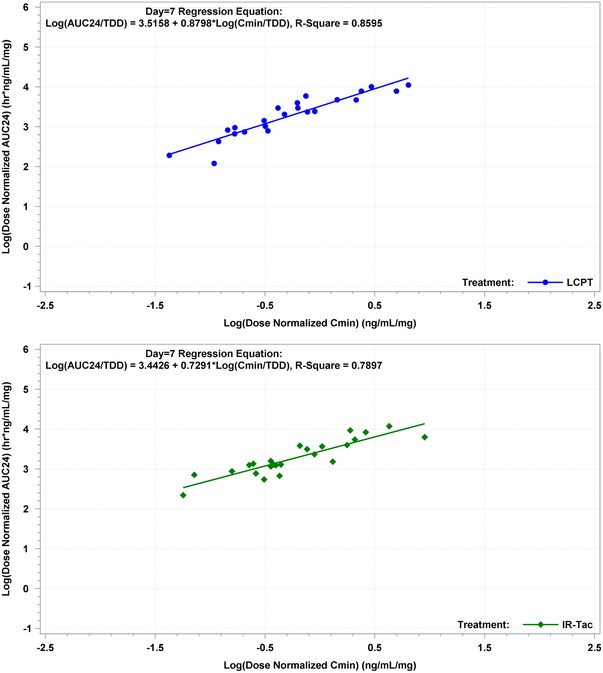
Correlation between dose‐normalized AUC0–24h and Cmin on day 7 for LCPT and IR‐Tac. IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus.
Figure 5.
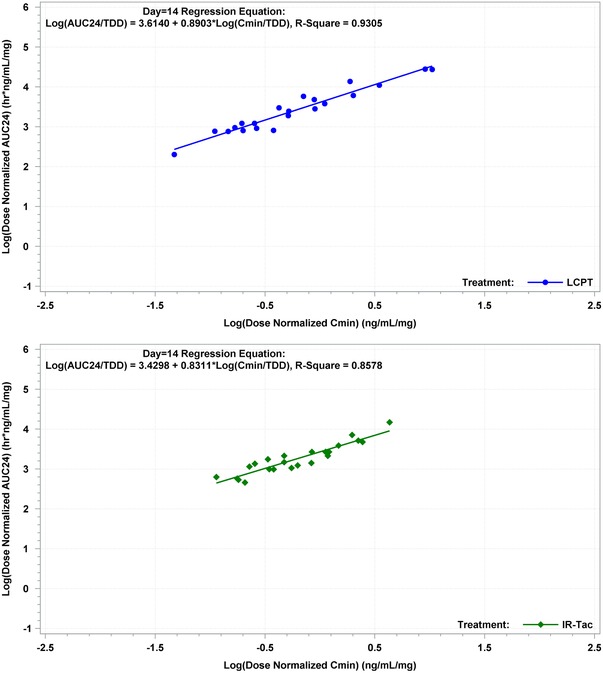
Correlation between dose‐normalized AUC0–24h and Cmin on day 14 for LCPT and IR‐Tac. IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus.
Efficacy
The incidence of BPAR was similar in both groups; at day 360, 6 (21%) patients on LCPT and 4 (14%) on IR‐Tac had experienced BPAR. The severity of the rejection events among patients treated with LCPT included: no Banff grade 3 events, 3 grade 2 events, and 3 grade 1 events. In comparison, of the 4 rejection events in IR‐Tac patients, 2 were grade 3 BPAR, and 2 were grade 2 BPAR events. One additional patient in each group was reported as having rejection without confirmatory biopsy. The Kaplan‐Meier estimate of freedom from BPAR at day 180 was 79% in the LCPT group and 87% (P = .61) in the IR‐Tac group, and on day 360 was 74% in the LCPT group and 82% in the IR‐Tac group (P = .65).
Safety
All patients experienced at least 1 AE during this open‐label study; serious AEs were reported in 58.6% of LCPT patients (72% of which were suspected of being related to the study drug by the investigator) and 34.5% of IR‐Tac patients (45% of which were suspected of being related to the study drug by the investigator). Although a larger number of patients experienced serious AEs in the LCPT group, when comparing AE percentages for both groups, the difference was not statistically significant (Table 5). The 6 most common AEs reported in the entire study population were diarrhea (41.4%), nausea (37.9%), headache (36.2%), peripheral edema (36.2%), anemia (31.0%), and tremor (31.0%).
Table 5.
Adverse Events Experienced by ≥20% of Patients, n (%)
| Preferred Term | LCPT (N = 29) | IR‐Tac (N = 29) | Overall (N = 58) |
|---|---|---|---|
| Any adverse events | 29 (100.0) | 29 (100.0) | 58 (100.0) |
| Diarrhea | 13 (44.8) | 11 (37.9) | 24 (41.4) |
| Edema, peripheral | 11 (37.9) | 10 (34.5) | 21 (36.2) |
| Constipation | 10 (34.5) | 7 (24.1) | 17 (29.3) |
| Headache | 10 (34.5) | 11 (37.9) | 21 (36.2) |
| Anemia | 9 (31.0) | 9 (31.0) | 18 (31.0) |
| Hepatitis C | 9 (31.0) | 8 (27.6) | 17 (29.3) |
| Nausea | 9 (31.0) | 13 (44.8) | 22 (37.9) |
| Hyperkalemia | 8 (27.6) | 4 (13.8) | 12 (20.7) |
| Hypokalemia | 8 (27.6) | 8 (27.6) | 16 (27.6) |
| Insomnia | 8 (27.6) | 6 (20.7) | 14 (24.1) |
| Tremor | 8 (27.6) | 10 (34.5) | 18 (31.0) |
| Fluid overload | 7 (24.1) | 5 (17.2) | 12 (20.7) |
| Liver transplant rejection | 7 (24.1) | 5 (17.2) | 12 (20.7) |
| Back pain | 6 (20.7) | 7 (24.1) | 13 (22.4) |
| Hypomagnesemia | 5 (17.2) | 10 (34.5) | 15 (25.9) |
IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus.
There were 2 deaths in each group during the study, none of which were suspected to be study drug related. The composite patient and graft survival rates out to 360 days after transplantation were 90.3% for LCPT and 91.1% for IR‐Tac (P = .952).
Liver function, as measured by aspartate transaminase, alanine transaminase, albumin, and total bilirubin were similar between the 2 treatment groups throughout the course of the study with the exception of total bilirubin on day 7, which was higher in the LCPT group (Table 6).
Table 6.
Liver Function Tests—mITT Population
| Day 1a | Day 7 | Day 14 | Day 360 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic Mean ± SD | Arithmetic Mean ± SD | Arithmetic Mean ± SD | Arithmetic Mean ± SD | |||||||||
| Liver Function Parameter | LCPT (n = 29) | IR‐Tac (n = 29) | P‐Valueb | LCPT (n = 26) | IR‐Tac (n = 24) | P‐Valueb | LCPT (n = 26) | IR‐Tac (n = 22) | P‐Valueb | LCPT (n = 12) | IR‐Tac (n = 15) | P‐Valueb |
| AST (U/L) | 542.1 ± 586.89 | 386.2 ± 416.13 | .401 | 54.6 ± 30.34 | 47.3 ± 24.37 | .373 | 33.6 ± 22.36 | 44.4 ± 78.54 | .584 | 73.8 ± 109.58 | 45.8 ± 44.92 | .399 |
| ALT (U/L) | 694.2 ± 873.08 | 434.1 ± 333.8 | .127 | 161.5 ± 128.95 | 135.2 ± 91.14 | .588 | 74.4 ± 56.72 | 78.2 ± 133.08 | .916 | 79.1 ± 106.91 | 39.3 ± 30.17 | .189 |
| Tbili (mg/dL) | 3.46 ± 4.08 | 2.52 ± 1.86 | .089 | 3.66 ± 4.87 | 1.70 ± 1.04 | .019 | 1.50 ± 1.31 | 1.31 ± 0.70 | .21 | 0.92 ± 0.72 | 0.79 ± 0.46 | .26 |
ALT, alanine transaminase; AST, aspartate transaminase; IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tacrolimus tablets; mITT, modified intent‐to‐treat; SD, standard deviation; Tbili, total bilirubin.
Day 1 refers to level drawn 24 hours after drug initiation.
P‐value determined by analysis of variance.
The mean change from baseline in the estimated glomerular filtration rate was not significantly different between the 2 groups (P = .78). There were no statistically significant differences between the treatment groups for any of the predefined clinically significant laboratory parameters, including abnormalities in fasting plasma glucose or hematologic parameters (platelets, white blood cell counts).
Discussion
The characteristic peak exposure and larger degree of fluctuation in exposure associated with IR‐Tac were evident during the PK portion of this study, while LCPT showed a smaller degree of fluctuation and the characteristically longer tmax. As a narrow therapeutic index drug, a lower Cmax/Cmin ratio and less fluctuation may represent more time within the target range; however, studies have not yet established the clinical significance of these PK parameters with tacrolimus. At days 7 and 14, peak concentration and exposure were comparable between LCPT and IR‐Tac. Although a lower proportion of patients on LCPT achieved therapeutic troughs on day 1, a similar proportion of patients in both groups met the therapeutic tacrolimus trough targets at days 7 and 14. While bioavailability was not specifically assessed in this PK study, dose‐adjusted AUC can be used as a surrogate for bioavailability. As demonstrated in other studies, the increased bioavailability associated with LCPT allows for utilization of a lower dose to achieve similar systemic exposure.12, 13, 15, 16
On days 1, 7, and 14, tmax was significantly prolonged with LCPT, which is consistent with a controlled‐release formulation. Additionally, there was a robust correlation between AUC0–24h and Cmin with LCPT, demonstrating that the current practice of therapeutic drug monitoring of Cmin as a measure of tacrolimus exposure can be applied to LCPT in liver transplant patients. The day 1 PK of LCPT did not align with day 7 and day 14 PK or with previously published steady‐state PK data in stable liver transplants.18 However, this is the first study to evaluate LCPT PK in the immediate posttransplant period. In this study, day 1 (ie, 24 hours after the initiation of LCPT; median time to drug initiation was 2 days) PK of LCPT exhibited lower exposure, lower peak concentration, and lower troughs compared with IR‐Tac. Despite the improved bioavailability of LCPT, it is possible that the recommended starting dose was too low. Furthermore, early posttransplant PK are highly variable, and a patient's pretransplant health, transplanted liver, concomitant medications, and postoperative care can all affect the PK of administered drugs.20 Azoles were more frequently used in the LCPT group than in the IR‐Tac group, although the concentration of tacrolimus would be increased due to azoles’ inhibition of the hepatic metabolism of tacrolimus.21 These variables may have contributed to the low day 1 exposure. Considering that LCPT has demonstrated higher bioavailability, it is unclear why the variables would have disproportionately affected day 1 exposure of LCPT compared with IR‐Tac. Interestingly, lower day 1 exposure has also been reported with once‐daily extended‐release tacrolimus capsules (Tac‐XL, Astagraf XL; Astellas Pharma US, Inc., Northbrook, Illinois) compared with IR‐Tac, suggesting a possible challenge related to absorption of extended‐release formulations or morning medication administration immediately after transplantation.22
Dose adjustments were frequent in both groups. As a result, the PK profiles observed do not reflect steady‐state kinetics, nor should they be assumed to reflect a gradual approach to steady state. Based on a tacrolimus half‐life of approximately 36 hours and assuming that 5 half‐lives are required to approximate steady‐state conditions, a steady‐state profile would be expected following approximately 7 days of stable LCPT or IR‐Tac dosing. Those numbers would suggest that steady state could be achieved with only 1 dose adjustment during the initial 14 days of PK monitoring. But, in fact, all 58 patients randomized in the present study had >1 dose adjustment, with an average of 4 and 5 for LCPT and IR‐Tac, respectively, in the first 14 days of treatment. Future studies of LCPT in the de novo setting are warranted to evaluate first‐dose kinetics and true steady‐state kinetics far enough into the treatment period to reflect consistent dosing. PK of LCPT vs IR‐Tac in stable liver transplant recipients have been previously published.18 Conversion from IR‐Tac to LCPT in stable liver transplant recipients showed that patients who were switched to LCPT at a dose that was 30% less than their IR‐Tac dose had similar preconversion tacrolimus exposure (similar AUC and troughs) 7 and 14 days after conversion.18
The primary outcome in the present study was PK; however, efficacy and safety data were also captured. The data suggest that LCPT and IR‐Tac were associated with similar efficacy and safety in de novo liver transplant use. Please note that the relatively small sample size for efficacy and safety outcomes and lack of a power analysis preclude making definitive statements regarding efficacy and safety. There were few BPAR episodes in either group. Despite a higher number of rejections in patients treated with LCPT (6 vs 4), patients treated with IR‐Tac tended to experience more severe rejection than those treated with LCPT. The incidence and types of AEs observed in this study were representative of those typically seen in a de novo liver transplant population being treated with a tacrolimus‐based immunosuppressive regimen, and results did not differ between the LCPT and IR‐Tac groups.
This phase II PK study provides insight into early posttransplant LCPT and IR‐Tac PK; however, interpretation of the results is complicated by variable posttransplant PK conditions, lack of exclusion of interacting medications, and frequent dose adjustments. Another limitation of the study is the small sample size and lack of power analysis that limit the ability to draw safety and efficacy conclusions.
Conclusions
This first analysis of LCPT PK in the de novo liver transplant setting supports a conclusion that the novel formulation allows for once‐daily administration of tacrolimus in de novo liver transplantation. Further phase III study is warranted to confirm the similarity between the drugs in efficacy and safety suggested here. The PK results indicate that LCPT regimens may benefit from utilizing slightly higher starting doses than were used in this de novo study.
Results of this 1‐year study, in addition to those of previous kidney de novo and conversion trials, a liver conversion trial, and PK comparisons among LCPT, IR‐Tac, and Tac‐XL support further investigation of LCPT for de novo liver transplant patients.12, 13, 15, 16, 17, 23 Future studies are warranted to examine whether once‐daily dosing and the unique PK parameters provided by LCPT are associated with safety or efficacy differences in liver transplant recipients.
Declaration of Conflicting Interest
K.U. receives honoraria from Veloxis Pharmaceuticals, Inc. and Astellas Pharma US, Inc. for serving on speakers' bureaus. A.S. receives honoraria from Veloxis Pharmaceuticals, Inc. for service on their speakers' bureau. A.E.A. receives honoraria from Eisai and Bayer for consulting on advisory boards, and from Bayer and Novartis for serving on speakers' bureaus. C.T. and D.R.S. received their standard salary from Veloxis Pharmaceuticals, Inc. during their work on the manuscript. D.A.D., W.C., and L. T. declare that they have no conflicts of interest to disclose.
Acknowledgments
The authors thank medical writer Kristin Kistler and editor Janet Dooley for their assistance in the preparation and review of this manuscript. They are both employees of Evidera, a research and consulting company with which Veloxis contracted for these services. The authors also acknowledge Wei Du for her statistical support in the preparation and review of this manuscript.
Funding
This manuscript and the study on which it is based were funded by Veloxis Pharmaceuticals A/S.
Data Sharing
Reasonable requests for access to data may be submitted to the corresponding author.
ClinicalTrials.gov Identifier: NCT00772148
References
- 1. Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 Annual data report: kidney. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). Am J Transplant. 2013;13(suppl 1):11–46. [Google Scholar]
- 2. Webster AC, Taylor RRS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005;(4):CD003961. [DOI] [PubMed] [Google Scholar]
- 3. Kramer BK, Del Castillo D, Margreiter R, et al. Efficacy and safety of tacrolimus compared with ciclosporin A in renal transplantation: three‐year observational results. Nephrol Dial Transplant. 2008;23(7):2386–2392. [DOI] [PubMed] [Google Scholar]
- 4. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–653. [DOI] [PubMed] [Google Scholar]
- 5. Venkataramanan R, Jain A, Cadoff E, et al. Pharmacokinetics of FK 506: preclinical and clinical studies. Transplant Proc. 1990;22(1):52–56. [PMC free article] [PubMed] [Google Scholar]
- 6. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. [DOI] [PubMed] [Google Scholar]
- 7. Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150(9):1881–1884. [PubMed] [Google Scholar]
- 8. Feldman HI, Hackett M, Bilker W, Strom BL. Potential utility of electronic drug compliance monitoring in measures of adverse outcomes associated with immunosuppressive agents. Pharmacoepidemiol Drug Saf. 1999;8(1):1–14. [DOI] [PubMed] [Google Scholar]
- 9. Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22–e33. [PubMed] [Google Scholar]
- 10. Morrissey PE, Reinert S, Yango A, Gautam A, Monaco A, Gohh R. Factors contributing to acute rejection in renal transplantation: the role of noncompliance. Transplant Proc. 2005;37(5):2044–2047. [DOI] [PubMed] [Google Scholar]
- 11. Endrenyi L, Tothfalusi L. Metrics for the evaluation of bioequivalence of modified‐release formulations. AAPS J. 2012;14(4):813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunnapradist S, Rostaing L, Alloway RR, et al. LCPT once‐daily extended‐release tacrolimus tablets versus twice‐daily capsules: a pooled analysis of two phase 3 trials in important de novo and stable kidney transplant recipient subgroups. Transpl Int. 2016;29(5):603–611. [DOI] [PubMed] [Google Scholar]
- 14. Langone A, Steinberg SM, Gedaly R, et al. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP‐TacrO (STRATO): an open‐label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29(9):796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budde K, Bunnapradist S, Grinyo JM, et al. Novel once‐daily extended‐release tacrolimus (LCPT) versus twice‐daily tacrolimus in de novo kidney transplants: one‐year results of phase III, double‐blind, randomized trial. Am J Transplant. 2014;14(12):2796–2806. [DOI] [PubMed] [Google Scholar]
- 16. Bunnapradist S, Ciechanowski K, West‐Thielke P, et al. Conversion from twice‐daily tacrolimus to once‐daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant. 2013;13(3):760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rostaing L, Bunnapradist S, Grinyo JM, et al. Novel once‐daily extended‐release tacrolimus versus twice‐daily tacrolimus in de novo kidney transplant recipients: two‐year results of phase 3, double‐blind, randomized trial. Am J Kidney Dis. 2016;67(4):648–659. [DOI] [PubMed] [Google Scholar]
- 18. Alloway RR, Eckhoff DE, Washburn WK, Teperman LW. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): phase II trial of stable liver transplant recipients. Liver Transpl. 2014;20(5):564–575. [DOI] [PubMed] [Google Scholar]
- 19. Wiesner RH. A long‐term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66(4):493–499. [DOI] [PubMed] [Google Scholar]
- 20. Gerard C, Stocco J, Hulin A, et al. Determination of the most influential sources of variability in tacrolimus trough blood concentrations in adult liver transplant recipients: a bottom‐up approach. AAPS J. 2014;16(3):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang S, Pillai VC, Mada SR, Strom S, Venkataramanan R. Effect of voriconazole and other azole antifungal agents on CYP3A activity and metabolism of tacrolimus in human liver microsomes. Xenobiotica. 2012;42(5):409–416. [DOI] [PubMed] [Google Scholar]
- 22. Fischer L, Trunecka P, Gridelli B, et al. Pharmacokinetics for once‐daily versus twice‐daily tacrolimus formulations in de novo liver transplantation: a randomized, open‐label trial. Liver Transpl. 2011;17(2):167–177. [DOI] [PubMed] [Google Scholar]
- 23. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady‐state head‐to‐head pharmacokinetic comparison of all FK‐506 (tacrolimus) formulations (ASTCOFF): an open‐label, prospective, randomized, two‐arm, three‐period crossover study. Am J Transplant. 2017;17(2):432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]


