Abstract
Background
Surgeons have traditionally been reluctant to perform total pancreatectomy because of concerns for brittle diabetes and poor quality of life (QoL). Several recent studies have suggested that outcomes following total pancreatectomy have improved, but a systematic review is lacking.
Methods
A systematic review was undertaken of studies reporting on outcomes after total pancreatectomy for all indications, except chronic pancreatitis. PubMed, EMBASE (Ovid), and Cochrane Library were searched (2005–2018). Endpoints included functional outcome and QoL.
Results
A total of 21 studies, including 1536 patients, fulfilled the eligibility criteria. During a median follow‐up of 20·8 (range 1·5–96·0) months, 18·6 per cent (45 of 242 patients) were readmitted for endocrine‐related morbidity, with associated mortality in 1·6 per cent (6 of 365 patients). No diabetes‐related mortality was reported in studies including only patients treated after 2005. Symptoms related to exocrine insufficiency were reported by 43·5 per cent (143 of 329 patients) during a median follow‐up of 15·9 (1·5–96·0) months. Overall QoL, reported by 102 patients with a median follow‐up of 28·6 (6·0–66·0) months, using the EORTC QLQ‐C30 questionnaire, showed a moderately reduced summary score of 76 per cent, compared with a general population score of 86 per cent (P = 0·004).
Conclusion
Overall QoL after total pancreatectomy is affected adversely, in particular by the considerable impact of diarrhoea that requires better treatment. There is also room for improvement in the management of diabetes after total pancreatectomy, particularly with regards to prevention of diabetes‐related morbidity.
Report of a systematic review of outcomes following total pancreatectomy.
Diabetes and diarrhea should be improved
Antecedentes
Los cirujanos tradicionalmente se han mostrado reacios a realizar una pancreatectomía total (total pancreatectomy, TP) debido a la preocupación por la aparición de diabetes lábil y la mala calidad de vida (quality of life, QoL). Varios estudios recientes han sugerido que el resultado de la TP ha mejorado, sin embargo no existe una revisión sistemática.
Métodos
Se ha realizado una revisión sistemática de los estudios que incluían los resultados después de una TP efectuada para todas las indicaciones, excepto para una pancreatitis crónica. Se realizaron búsquedas en PubMed, Embase (Ovid) y Cochrane Library (2005‐2018). Los resultados principales eran el resultado funcional y la calidad de vida.
Resultados
En total, 21 estudios cumplieron los criterios de elegibilidad, incluyendo 1.536 pacientes que se habían sometido a una TP. Durante una mediana de seguimiento de 20,8 meses (rango 1,5‐96,0), el 18,6% de los pacientes (45 de 242) fueron reingresados por morbilidad relacionada con la función endocrina, con una mortalidad asociada del 1,6% (6 de 365 pacientes). No hubo mortalidad relacionada con la diabetes en los estudios que incluyeron solo aquellos pacientes tratados después de 2005. Se observaron síntomas relacionados con insuficiencia exocrina en un 43,5% de los pacientes (143 de 329) durante una mediana de seguimiento de 15,9 meses (rango 1,5‐96,0). La calidad de vida global, informada para 102 pacientes con una mediana de seguimiento de 28,6 meses (rango 6,0‐66,0) utilizando el cuestionario EORTC QLQ‐C30, mostró una puntuación sumatoria moderadamente reducida del 76% en comparación con la puntuación del 86% para la población general (P = 0,004).
Conclusión
Es necesario seguir mejorando el manejo de la diabetes después de la TP, sobre todo en lo que respecta a la prevención de la morbilidad relacionada con la diabetes. La calidad de vida global se ve afectada después de la TP y, especialmente, el impacto de la diarrea es considerable y requiere mejorar este aspecto en el futuro.
Introduction
The most common indication for total pancreatectomy is pancreatic cancer, but total pancreatectomy is increasingly performed for main‐duct/mixed‐type intraductal papillary mucinous neoplasm (IPMN)1, 2, 3. Total pancreatectomy for IPMN requires extensive counselling, as this is prophylactic surgery. Other indications for total pancreatectomy could be multifocal pancreatic disease (such as neuroendocrine tumours and renal cell cancer metastases) and technical difficulties, or patients with a very high risk of having a pancreatic anastomosis (for instance in arterial reconstruction)4, 5, 6.
Most surgeons have traditionally been reluctant to perform total pancreatectomy because of the associated postoperative morbidity and resulting lifelong insulin‐dependent brittle diabetes, with risk of severe hypoglycaemia and substantial impact on quality of life (QoL).
In recent years, several investigators have suggested that the role of total pancreatectomy in IPMN should be expanded as the postoperative management has improved7. Of note, a recent international survey8 showed no consensus on the use of total pancreatectomy in patients with main‐duct/mixed‐type IPMN. This lack of consensus is likely to be related to concerns regarding total pancreatectomy‐related diabetes and exocrine insufficiency. Others1, 4 have questioned whether any indication remains for an upfront total pancreatectomy in the current era of improved imaging.
Knowledge of long‐term functional (endocrine and exocrine insufficiency) outcomes and QoL after total pancreatectomy is therefore essential during preoperative counselling and shared decision‐making. This systematic review focuses on short‐ and long‐term functional outcomes after total pancreatectomy, and its impact on QoL.
Methods
This systematic review was registered with PROSPERO (registration number CRD420016051093)9 and performed in accordance with the PRISMA guidelines10.
Search strategy
A systematic literature search was conducted in PubMed, EMBASE (Ovid), and the Cochrane Library for studies published from 1 January 2005 to 31 January 2018. This inclusion period was chosen because management of endocrine and exocrine insufficiency seems to have improved in recent years11. The main search term was ‘total pancreatectomy’. The full search strategy is shown in Table S1 (supporting information). Restrictions on English language and studies older than 13 years (arbitrary cutoff) were applied. After excluding duplicates, studies were screened by two authors independently, according to the eligibility criteria by title, abstract and full text. The final decision on eligibility was made by consensus.
Eligibility criteria
Studies of at least ten patients reporting on functional outcomes and QoL after total pancreatectomy were considered eligible. Studies or subgroups where more than 10 per cent of patients had undergone total pancreatectomy for chronic pancreatitis were excluded unless results were given separately. Benign indications with no further description were considered as chronic pancreatitis. Conference abstracts and review articles were also excluded. When there were overlapping cohorts, only unique results were used. If there were overlapping outcomes between these cohorts, the largest study was chosen for analysis.
Definitions
Total pancreatectomy was defined as removal of the entire pancreas, with or without splenectomy, performed via either an open or minimally invasive technique. Primary total pancreatectomy was defined as elective total pancreatectomy (planned before surgery as well as decided during the operation), and emergency total pancreatectomy as surgery performed as a result of trauma. Urgent completion total pancreatectomy was defined as emergency total pancreatectomy for postoperative complications after pancreatoduodenectomy, whereas elective completion total pancreatectomy was performed mostly for recurrence in the remnant pancreas.
Endocrine insufficiency was defined as diabetes after total pancreatectomy, classified by the WHO12 and the American Diabetes Association13 as one of the causes of pancreatogenic (type 3c) diabetes. Exocrine insufficiency was defined as inadequate pancreatic enzyme function, with or without related symptoms, that required enzyme substitution.
Where only readmissions were reported, these were not defined as complications to avoid underestimation of diabetes‐related morbidity. Invasive or malignant IPMNs were grouped under carcinomas as ‘invasive IPMN’. When studies did not report the grade of dysplasia, the IPMNs were grouped as ‘potentially malignant’. When studies presented only the minimum duration of follow‐up, that value was chosen to calculate the pooled weighted median. When studies presented the maximum follow‐up or the range, the half of this value was used to calculate the pooled weighted median. The postoperative period was defined as a maximum of 90 days after surgery.
Extracted data
The following data were extracted per study by two authors: first author, country, year of publication, study design, age, sex, diagnosis/indication for surgery, duration of follow‐up, number of patients undergoing total pancreatectomy, and outcomes of exocrine and endocrine insufficiency and QoL. Two authors cross‐checked all data independently. Corresponding authors of the included studies were contacted where there was missing or insufficient data. The European Organization for Research and Treatment in Cancer Quality of Life Questionnaire Cancer (EORTC QLQ‐C30) global health status, functioning and symptom scores were determined, and differences with the general population calculated. Clinically important differences were categorized and defined according to Osoba et al. 14: less than 5 per cent, no change; 5–10 per cent, a little change; 10–20 per cent, moderate change; more than 20 per cent, very much change.
Quality assessment
The methodological quality of the included studies was assessed by two authors independently, using the Newcastle–Ottawa Scale (NOS)15. Methodological quality was scored for ‘selection of patients’, ‘comparability’ and ‘outcome of study participants’, and was ranked with a maximum of 5 points. In this systematic review, the parts of the NOS score (‘selection of the non‐exposed’, ‘outcome of interest’ and ‘comparability’) were not applicable, because of its descriptive nature. In this study, cohort studies with a NOS score of 5 or higher were considered of high quality.
Statistical analysis
Age, length of follow‐up and QoL parameters from the different questionnaires are presented as mean(s.d.) and median (range) values. Categorical data are presented as frequencies and percentages. Overall follow‐up values are presented as pooled weighted median. When included studies reported median (range) values, means(s.d.) values were calculated using statistical algorithms according to the method of Wan and colleagues16 and Hozo and co‐workers17. P < 0·050 was considered statistically significant. Statistical analysis was performed using SPSS version 24 (IBM, Armonk, New York, USA).
Results
The systematic search (Table S1 , supporting information) identified 21 eligible studies, all cohort studies, comprising 1536 patients undergoing total pancreatectomy2, 6, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36. The PRISMA diagram of studies included in this systematic review is presented in Fig. 1. Most patients were men (52·6 per cent), and the median age was 65 years; the methodological quality was scored as high in 20 of the 21 studies (Table S2 , supporting information).
Figure 1.
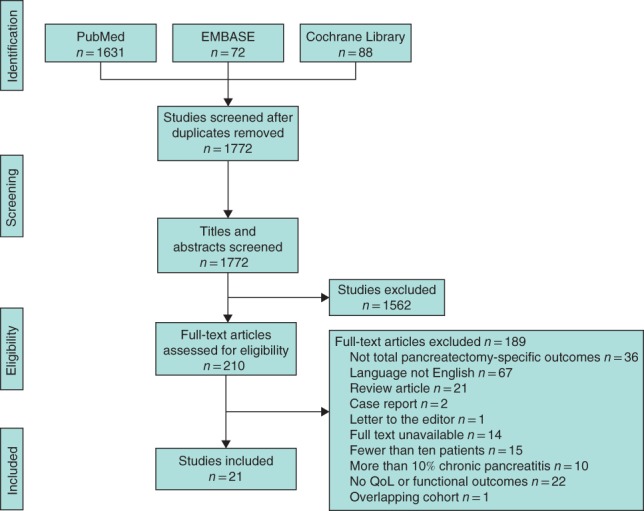
PRISMA diagram for the systematic review QoL, quality of life.
Surgical details
Of 1536 patients, indication for total pancreatectomy was pancreatic ductal adenocarcinoma (PDAC) in 806 patients (52·5 per cent) and IPMN in 454 patients (29·6 per cent); IPMN consisted of 227 patients (14·8 per cent) with non‐invasive IPMN and 227 (14·8 per cent) with invasive IPMN (Table 1).
Table 1.
Diagnosis in patients who had a total pancreatectomy
| Non‐carcinomas | ||||||
|---|---|---|---|---|---|---|
| Reference | n | Carcinomas | Potentially malignant | Benign | CP* | Other |
| Barbier et al.18 | 56 | PDAC (4) | IPMN (20) | – | 1 (2) | Renal mets (2) |
| Invasive IPMN (22) | NET (6) | |||||
| Acinar cell carcinoma (1) | ||||||
| Casadei et al.19, † | 20 | PDAC (7) | IPMN (2) | – | 1 (5) | Renal mets (2) |
| Invasive IPMN (6) | NET (2) | |||||
| Casadei et al.20, † | 73 | PDAC (38) | IPMN (15) | – | 2 (3)§ | Mets (3) |
| Periampullary cancer (3) | NET (6) |
Other (5) |
||||
| Serous cystic tumours (1) | ||||||
| Crippa et al.21, † | 65 | PDAC (19) | IPMN (6) | SCA (1) | 1 (2) | Renal mets (6) |
| Invasive IPMN (25) | NET (6) | |||||
| Periampullary cancer (1) | ||||||
| Crippa et al.22, †, ‡ | 29¶ | Invasive IPMN (13) | IPMN (16) | – | 0 (0) | – |
| Epelboym et al.23 | 77 | PDAC (50) | IPMN (15) | SCA (1) | 4 (5)§ | Renal mets (1) |
| Invasive IPMN (2) | NET (4) | |||||
| Fujino et al.24 | 36 | PDAC (17) | NET (2) | Acute pancreatitis (1) | 1 (3) | Mets (1) |
| Invasive IPMN (14) | ||||||
| Hartwig et al.6 | 434 | PDAC (289) | IPMN (44) | SCN (4) | 4 (0·9)§ | Other (4) |
| Invasive IPMN (31) | NET (28) | |||||
| Adenosquamous carcinoma (8) | ||||||
| Acinar cell carcinoma (4) | ||||||
| Other (18) | ||||||
| Hata et al.25 | 43 | PDAC (21) | IPMN (11) | Solid pseudopapillary neoplasm (1) | 1 (2) | Mets (3) |
| NET (2) | SCN (1) AVM (3) | |||||
| Jamil et al.26 | 14 | IPMN (9) | IPMN (5) | – | 0 (0) | – |
| Kitagawa et al.27 | 10 | Carcinoma (4) | IPMN (5) | Gastrinoma (1) | 0 (0) | – |
| Müller et al.2, ‡ | 147 | PDAC (78) | IPMN (12) | Cystic tumours (5) | 13 (8·8) | Renal mets (9) |
| Invasive IPMN (10) | NET (11) | |||||
| Periampullary cancer (7) | ||||||
| Cystic tumour (2) | ||||||
| Nikfarjam et al.28 | 15 | Adenocarcinoma (13) | IPMN (1) | Cystadenoma (1) | 0 (0) | – |
| Parsaik et al.29, ‡ | 97 | PDAC (49) | Cystic neoplasm (7) | – | 0 (0) | Other (14) |
| Invasive IPMN (24) | ||||||
| Periampullary adenocarcinoma (3) | ||||||
| Reddy et al.30 | 100 | PDAC (100) | – | – | 0 (0) | – |
| Shi et al.31 | 52 | PDAC (43) | – | – | 0 (0) | Renal mets (1) |
| Invasive IPMN (4) | ||||||
| Adenosquamous carcinoma (1) | ||||||
| Acinar adenocarcinoma (1) | ||||||
| Cystadenocarcinoma (2) | ||||||
| Stauffer et al.32 | 47 | PDAC (10) | IPMN (21) | – | 2 (4) | Renal mets (1) |
| Invasive IPMN (10) | NET (1) | Trauma (1) | ||||
| Cholangiocarcinoma (1) | ||||||
| Suzuki et al.33 | 41 | PDAC (13) | IPMN (5) | – | 0 (0) | Renal mets (2) |
| Invasive IPMN (20) | Intraductal tubulopapillary neoplasm (1) | |||||
| Takami et al.34, ‡ | 33 | PDAC (25) | IPMN (7) | – | 1 (3)§ | – |
| Watanabe et al.35 | 44 | PDAC (20) | IPMN (2) | – | 1 (2)§ | Renal mets (1) |
| Invasive IPMN (18) | NET (1) | |||||
| Distal cholangiocarcinoma (1) | ||||||
| Zakaria et al.36 | 103 | PDAC (23) | IPMN (40) | Ampullary adenoma with IPMN (1) | 8 (7·8) | Renal mets (1) |
| Invasive IPMN (19) | NET (7) | |||||
| Ampullary adenocarcinoma (1) | Trauma (1) | |||||
| Cholangiocarcinoma (1) | ||||||
| Sarcoma (1) | ||||||
| Overall* | 1536 | 1106 (72·0) | 312 (20·3) | 20 (1·3) | 40 (2·6) | 58 (3·8) |
Values in parentheses are percentages.
No overlapping cohorts; used unique results and the biggest group in case of non‐unique results;
subgroup used; §benign tumours (possible chronic pancreatitis (CP));
after contacting author. PDAC, pancreatic ductal adenocarcinoma; IPMN, intraductal papillary mucinous neoplasm; NET, neuroendocrine tumour; mets, metastases; SCA, serous cystadenoma; SCN, serous cystic neoplasm; AVM, arteriovenous malformation.
In 1254 patients (81·6 per cent), the type of total pancreatectomy (primary versus completion pancreatectomy) was described. Primary total pancreatectomy was performed in 1120 patients (89·3 per cent), of whom 1117 (99·7 per cent) had elective surgery and three (0·3 per cent) had emergency surgery. Completion total pancreatectomy was performed in 134 patients (10·7 per cent), electively in 110 (82·1 per cent) and as an emergency in 24 (17·9 per cent).
Endocrine insufficiency
Outcomes related to endocrine insufficiency after total pancreatectomy were reported in 19 studies2, 6, 18, 19, 20, 21, 22, 23, 24, 26, 27, 28, 29, 30, 31, 32, 33, 35, 36, including 809 patients, after a median follow‐up of 20·8 (range 1·5–96·0) months. The endocrine‐related morbidity rate was 25·9 per cent (112 of 432 patients). Ten studies18, 19, 20, 21, 22, 23, 24, 32, 33, 35, including 299 patients, reported the number of prescribed insulin units per day (mean 27 units/day). Mean(s.d.) HbA1c levels at 6 and 12 months after surgery were 7·5(0·5) and 7·2(0·7) per cent respectively24, 26, 31, 32, 33. Two studies31, 33 found an increase in HbA1c concentration during the first 3 months after surgery, although stabilization was seen afterwards. During the first year after surgery, HbA1c levels were increased in both benign and malignant diseases, after which the levels remained stable at acceptable levels6. For endocrine‐related reasons, 45 of 242 patients (18·6 per cent) were admitted to hospital2, 18, 19, 21, 23, 26, 27, 32, 35.
Two studies21, 29 reported on long‐term diabetes‐related complications. Crippa and colleagues21 reported long‐term diabetes‐related complications in six of 45 patients after total pancreatectomy (follow‐up at least 60 months), including peripheral vascular disease (4 patients), stroke (1) and retinopathy (1). During a median follow‐up of 2 years, Parsaik et al 29. found development of target organ complications in seven of 26 patients.
Nine studies2, 18, 19, 21, 22, 24, 31, 32, 35 described diabetes‐related mortality, which occurred in six of 365 patients (1·6 per cent). Three patients died from hypoglycaemia, two after 8 and 22 months, and one patient at an unknown time during a median follow‐up of 35 (range 4–168) months. Two patients died from ketoacidosis, one patient after 5 months and the other at an unknown time during a median follow‐up of 21 (2–222) months. Hyperglycaemia was the cause of death in one patient 17 days after surgery. In addition, diabetes‐related mortality was 0 per cent (0 of 94 patients) in the most recent series, comprising the studies that included patients treated after 200519, 22, 31 (Table 2).
Table 2.
Endocrine insufficiency in patients who had a total pancreatectomy
| Reference | n | Follow‐up (months)* | Insulin (units/day)* | Glycaemic events/week* | Complications | Hospitalization | Mortality |
|---|---|---|---|---|---|---|---|
| Barbier et al. 18 | 52 | 35 (4–168) | R: 21 (7–70) | n.r. | n.r. | Hypoglycaemic coma (6) | Ketoacidosis (1) |
| L: 16 (7–48) | Hypoglycaemia (1) | ||||||
| Insulin pump (n = 1) | |||||||
| Subgroup | 25 | 66 (7–168) | n.r. | 10 (1–36)/month | Loss of consciousness due to hypoglycaemia (10) | Diabetes equilibration (14) | n.r. |
| Casadei et al. 19 | 13 | 23 (6–60) | R: 18 (0–18) | 4 (1–10) hypoglycaemia and hyperglycaemia | n.r. | Poor glycaemic control (3) | Severe hypoglycaemia (0) |
| L: 7 (4–20) | |||||||
| Casadei et al. 20 | 35 | > 1 year | R: 7 (0–18) | n.r. | n.r. | n.r. | n.r. |
| I: 3 (0–19) | |||||||
| L: 19 (4–40) | |||||||
| Crippa et al. 21 | 45 | 2–14 years | 32 (18–52) | 2 (0–5) | PVD (4) | Hyperglycaemia (3) | Hypoglycaemia (0) |
| Stroke (1) | Hypoglycaemia (7) | ||||||
| Retinopathy (1) | |||||||
| Crippa et al. 22 | 29 | 62 (6–91) | n.r. | n.r. | n.r. | n.r. | Insulin‐related coma (0) |
| Subgroup | 15 | 63 (30–91) | R: 25 (19–38) | n.r. | Severe hypoglycaemia (7) | n.r. | n.r. |
| L: 4 (0–14) | |||||||
| Epelboym et al. 23 | 17 | 40·3 (i.q.r. 32·8) | 29·8(18·6)† | 2 (i.q.r. 2) | Hypoglycaemic episodes (16) | Hypoglycaemic episodes (4) | n.r. |
| Fujino et al. 24 | 36 | n.r. | 22(6)† | n.r. | n.r. | n.r. | Severe hypoglycaemia (2) |
| Hartwig et al. 6 | 83 | ≥ 1 year | n.r. | n.r. | n.r. | n.r. | n.r. |
| Jamil et al. 26 | 14 | Up to 24 | Insulin pump (n = 3) | n.r. | Hypoglycaemic episodes (7) | Hypoglycaemia (1) | n.r. |
| Hyperglycaemia (0) | |||||||
| Kitagawa et al. 27 | 10 | 7·9 (2·1–28·6) | 0·45(0·13) units/kg/day‡ at discharge† | n.r. | n.r. | Hypoglycaemia (1) | n.r. |
| Müller et al. 2 | 47 | > 6 after surgery | n.r. | n.r. | n.r. | Hyperglycaemia (4) | Mortality (0) |
| Hypoglycaemia (3) | |||||||
| Nikfarjam et al. 28 | 15 | Postoperative period | n.r. | n.r. | Hypoglycaemia (4) | n.r. | n.r. |
| Parsaik et al. 29 | 26 | 2 (i.q.r. 0·6–5·3) years | n.r. | n.r. | Mild hypoglycaemic episodes (19) | n.r. | n.r. |
| Severe hypoglycaemic episodes (7) | |||||||
| Target organ complications (7) | |||||||
| Reddy et al. 30 | 100 | Postoperative period | n.r. | n.r. | Glycaemic control: CD I (20), CD IV (1) | n.r. | n.r. |
| Shi et al. 31 | 52 | To 12 months after surgery | 0·56(0·12) units/kg/day‡ at 12 months† | n.r. | n.r. | n.r. | Hypoglycaemia or diabetic complications (0) |
| Stauffer et al. 32 | 47 | Postoperative period | L: 10 (0–80) at discharge | n.r. | Major glycaemic events (2) | n.r. | Hyperglycaemia (1) |
| Subgroup | 46 | To 12 months after surgery | n.r. | n.r. | n.r. | Glycaemic events (5) | n.r. |
| Suzuki et al. 33 | 41 | To 12 months after surgery | 30 (12–50) | n.r. | n.r. | n.r. | n.r. |
| 0·56(0·16) units/kg/day at 12 months† | |||||||
| Watanabe et al. 35 | 44 | 21 (2–222) | n.r. | n.r. | n.r. | n.r. | Ketoacidosis (1) |
| Subgroup | 25 | 22 (2–73) | R: 17 (10–28) | 2 (1–4) (12 patients) | Loss of consciousness (0) | Hyperglycaemia or hypoglycaemia (0) | n.r. |
| L: 6 (0–16) | |||||||
| Zakaria et al. 36 | 103 | Postoperative period | n.r. | n.r. | Major glycaemic event (6) | n.r. | n.r. |
| Overall ¶ | 809 | 20·8 months§ | 26†, § | 2·3 (2–4) (125 patients)§ | 112 of 432 (25·9) | 45 of 242 (18·6) | 6 of 365 (1·6) |
| R: 16 | |||||||
| L: 12 |
Values are
median (range) and
mean(s.d.).
Not used in the analysis.
Weighted average of medians.
Values in parentheses are percentages unless indicated otherwise. R, rapid‐acting insulin; n.r., not reported; L, long‐acting insulin; I, intermediate‐acting insulin; PVD, peripheral vascular disease; CD, Clavien–Dindo co‐morbidity grade.
Exocrine insufficiency
Outcomes related to exocrine insufficiency were reported in 15 studies2, 6, 18, 19, 21, 24, 25, 26, 27, 29, 31, 32, 33, 34, 35, including 495 patients, during a median follow‐up of 19·6 (range 1·5–96·0) months (Table 3). Exocrine insufficiency‐related symptoms were reported by 43·5 per cent (143 of 329 patients) during a median follow‐up of 15·9 (1·5–96·0) months. Among 136 patients receiving pancreatic enzyme substitution, 32 (23·5 per cent) still reported symptoms18, 21, 33, 35. In general, the most common symptom was diarrhoea, which occurred in 0–64 per cent of the patients2, 18, 21, 33, 34, 35. Steatorrhoea was described by two studies21, 26, varying between 14 and 27 per cent of patients. Ten studies18, 19, 21, 24, 27, 29, 32, 33, 34, 35 reported on loss of bodyweight after surgery, with an occurrence of 44–85 per cent of the patients and a median loss of 6·7 (range 3·1–15) kg. Shi and co‐workers31 observed stabilization of postoperative weight loss after 6–12 months of follow‐up. One study33 reported the dosages of pancreatic enzymes during the first year after operation and revealed that patients needed higher dosages as time progressed. In that study, pancreatic enzyme dosage was based on patients' stool consistency, BMI, serum albumin and signs of liver steatosis on CT, achieving a stabile BMI and no diarrhoea.
Table 3.
Exocrine insufficiency in patients who had a total pancreatectomy
| Weight loss | ||||||
|---|---|---|---|---|---|---|
| Reference | n | Follow‐up (months)* | Pancreatic lipase (units/day)* | Symptoms and related readmissions | No. of patients | kg* |
| Barbier et al.18 | 25 | 66 (7–168) | 150 000 (75 000–450 000) | Diarrhoea (6) | 15 | 9 (2–14) |
| 6 (3–18) capsules | ||||||
| Casadei et al.19 | 13 | 23 (6–60) | 8 (6–11) capsules | n.r. | 11 | 15 (1–32) |
| Crippa et al.21 | 45 | 2–14 years | 80 000 (30 000–160 000) | Steatorrhoea (12) | 20 | 5 (1–18) |
| Diarrhoea (6) | ||||||
| Fujino et al.24 | n.r. | > 12 | n.r. | n.r. | n.r. | 8·8(10·4)% |
| Hartwig et al.6 | 75 | ≥ 1 year | n.r. | n.r. | n.r. | n.r. |
| Hata et al.25 | 43 | > 6 | 18 patients: high dose (1800 mg/day) | Hepatic steatosis (16) | n.r. | n.r. |
| Pancrelipase within 2 weeks of TP | ||||||
| Jamil et al.26 | 14 | ≥ 24 | n.r. | Steatorrhoea because of intolerance to medication (1) | n.r. | n.r. |
| Inadequate dosing (1) | ||||||
| Kitagawa et al.27 | 10 | 7·9 (2·1–28·6) | n.r. | Readmission because of diarrhoea (1) | n.r. | 4·1† |
| Use of antidiarrhoeal drugs (4) | ||||||
| Müller et al.2 | 47 | > 6 | n.r. | Flatulence (28) | n.r. | n.r. |
| Diarrhoea (30) | ||||||
| Parsaik et al.29 | 26 | 2 (i.q.r. 0·6–5·3) years | n.r. | n.r. | n.r. | 3·1(0·54)† |
| Shi et al.31 | 52 | To 12 months after surgery | n.r. | n.r. | n.r. | n.r. |
| Stauffer et al.32 | 46 | To 12 months after surgery | n.r. | Readmission because of malnutrition, failure to thrive, nausea, vomiting, diarrhoea or weakness (20) | n.r. | 8·8 (1·2–39·5) |
| Suzuki et al.33 | 41 | To 12 months after surgery | 125 145(97 487) at 12 months† | Diarrhoea (0) | 18 | 4·23 |
| Takami et al.34 | 33 | Early postoperative period | n.r. | Severe diarrhoea (10) | n.r. | > 10% at 6 months |
| Dumping syndrome (0) | ||||||
| Watanabe et al.35 | 25 | 22 (2–73) | 150 000 (0–225 000) | Diarrhoea (8) | 17 | 4·5 (1–15) |
| Overall‡ | 495 | 19·6‡ | 143 of 329 (43·5) | 81 of 149 (54·4) | 6·7 (3·1–15)‡ | |
Values are
median (range) and
mean(s.d.);
‡values in parentheses are percentages. ‡Weighted average of medians. n.r., Not reported; TP, total pancreatectomy.
Several studies6, 18, 24, 25, 31, 33, 34 investigated laboratory tests to assess patients' nutritional status. Serum nutritional markers decreased in the first 1–6 months after total pancreatectomy, but had stabilized or normalized by 12 months after surgery6, 24, 31, 33, 34. Barbier and colleagues18 showed that, also in the long term, prealbumin and albumin levels remained stabile within normal ranges.
Hata et al.25 investigated long‐term consequences by describing development of hepatic steatosis in 16 of 43 patients, which was associated with poor nutritional status early after surgery. None of these patients developed liver cirrhosis or liver failure during long‐term follow‐up. Suzuki and co‐workers33 suggested that all patients tended to develop hepatic steatosis at 1 year after surgery, but signs of steatosis decreased by enhancing the dose of pancreatic enzymes. Liver function also remained normal in the long term (median follow‐up 66 months), according to Barbier et al.18.
Quality of life
QoL after total pancreatectomy for all indications was reported in seven studies2, 6, 18, 19, 20, 23, 35, including 243 patients, using various validated questionnaires. The median weighted follow‐up in these studies was 28·6 (range 6·0–66·0) months. The following questionnaires were used: EORTC QLQ‐C30 (5 studies2, 6, 18, 19, 23), EORTC QLQ after pancreatic resection (EORTC QLQ‐PAN26) (3 studies)6, 18, 23, Audit of Diabetes Dependent Quality of Life (ADD‐QoL) (1 study23), Short Form 36 (SF‐36) (1 study35), Problem Areas in Diabetes scale (PAID20) (1 study20) and EQ‐5D‐5L (1 study20).
The EORTC QLQ‐C30 questionnaire, designed to measure overall health status in patients with cancer37, was used in four studies2, 18, 19, 23, including 102 patients. One study6 with 81 patients was excluded for this analysis, because EORTC QLQ‐C30 scores were shown only in figures and no exact data were available. The mean global health status score was 64 per cent, a small clinically important difference (58–69 per cent) compared with the general population score of 71 per cent (Table 4). Mean scores in the functioning scales varied from 67 to 78 per cent and, compared with the general population scores, they varied from 9 per cent (little change) to 14 per cent (moderate change). Global health status and all functioning domains differed significantly in comparison with those for the general population. Mean scores in the symptom scales varied from 6 to 38 per cent, with fatigue, dyspnoea and especially diarrhoea as the main symptoms.
Table 4.
Quality of life assessed with the EORTC QLQ‐C30 questionnaire
| Barbier et al.18 (n = 25)¶ | Casadei et al.19 (n = 13) | Epelboym et al.23 (n = 17) | Müller et al.2 (n = 47) | Total (n = 102) | General population38 (n = 7802) | P # | Clinically relevant difference (%)14 | |
|---|---|---|---|---|---|---|---|---|
| Global health status * | 64(4) | 58(21) | 69(n.r.) | n.r. | 64(13) | 71(22) | 0·019 | 7 (low) |
| Functioning scales † | n.r. | n.r. | 79(n.r.) | n.r. | 79 | n.r. | ||
| Physical | 84(3) | 65(25) | n.r. | n.r. | 78(17) | 90(16) | < 0·001 | 12 (moderate) |
| Role | 75(2) | 67(25) | n.r. | n.r. | 72(15) | 85(25) | 0·001 | 13 (moderate) |
| Emotional | 76(3) | 67(21) | n.r. | 63(24) | 67(11) | 76(23) | < 0·001 | 9 (low) |
| Cognitive | 76(2) | 75(25) | n.r. | n.r. | 76(15) | 86(20) | 0·002 | 10 (moderate) |
| Social | 73(2) | 75(25) | n.r. | n.r. | 74(15) | 88(23) | < 0·001 | 14 (moderate) |
| Symptom scales ‡ | n.r. | n.r. | 18(n.r.) | n.r. | 18 | n.r. | ||
| Fatigue | 38(3) | 39(22) | n.r. | n.r. | 38(13) | 24(24) | < 0·001 | 14 (moderate) |
| Nausea and vomiting | 15(2) | 17(17) | n.r. | n.r. | 16(10) | 4(12) | < 0·001 | 12 (moderate) |
| Pain | 26(2) | 21(21) | n.r. | n.r. | 24(13) | 21(28) | 0·509 | 3 (none) |
| Dyspnoea | 19(1) | 42(25) | n.r. | n.r. | 27(18) | 12(23) | < 0·001 | 15 (moderate) |
| Insomnia | 20(1) | 42(25) | n.r. | n.r. | 28(18) | 22(30) | 0·218 | 6 (low) |
| Appetite loss | 13(1) | 25(25) | n.r. | n.r. | 17(16) | 7(18) | < 0·001 | 10 (moderate) |
| Constipation | 9(1) | 8(8) | n.r. | 4·2(17) | 6(13) | 7(18) | 0·646 | 1 (none) |
| Diarrhoea | 25(1) | 33(17) | n.r. | n.r. | 28(11) | 7(18) | < 0·001 | 21 (high) |
| Financial difficulties | 16(1) | 0(0) | n.r. | n.r. | 11(8) | 10(23) | 0·789 | 1 (none) |
| EORTC QLQ‐C30 summary score § | 78 | 71 | n.a. | n.a. | 76 | 86 | 0·004 | 11 (moderate) |
Values are mean(s.d.). n.r., Not reported; n.a., not applicable.
The higher the score, the better the global health status;
the higher the score, the better the functioning;
the higher the score, the greater the symptoms.
(Physical functioning + Role functioning + Social functioning + Emotional functioning + Cognitive functioning + (100 − Fatigue) + (100 − Pain) + (100 – Nausea and Vomiting) + (100 – Dyspnoea) + (100 – Insomnia) + (100 – Appetite loss) + (100 – Constipation) + (100 – Diarrhoea))/13.
Data received from corresponding author.
Student's t test.
Long‐term QoL was evaluated in 81 patients by Hartwig and colleagues6, using the EORTC QLQ‐C30. After matching data with an age‐ and sex‐matched healthy control population, no differences in global health status were found. During the first year after surgery, global health status was significantly lower in patients with benign disease, but no differences between benign and malignant disease were seen after this time. Patients with malignant disease suffered significantly more from diarrhoea than those with benign disease, compared with the control population.
The EORTC QLQ‐PAN26 questionnaire, designed to evaluate disease symptoms, treatment side‐effects and emotional issues specific to pancreatic cancer, was used in three studies6, 18, 23 (67 patients). The most frequently described symptom was altered bowel habit.
One study35 with 25 patients used the SF‐36, a questionnaire that evaluates eight domains of physical and mental well‐being. The physical aspects of QoL were significantly lower than those in the national population (which included many young people) (P < 0·050). In addition, scores of vitality and mental well‐being were significantly worse in patients with diarrhoea‐related complaints. However, QoL was comparable with that in age‐matched controls. These authors concluded that QoL after total pancreatectomy is acceptable if the patient is capable of self‐management.
Diabetes‐specific quality of life
Epelboym and colleagues23 assessed the impact of diabetes on the QoL in 17 patients after total pancreatectomy and in eight patients following pancreatoduodenectomy. Insulin was used in all patients in the total pancreatectomy group versus half of those in the pancreatoduodenectomy group. All average weighted scores, measured by the ADD‐QoL questionnaire, did not differ significantly between the groups. However, leisure time and physical activity were lower in patients after total pancreatectomy. These authors concluded that total pancreatectomy‐induced diabetes has a negative impact on functioning and activities, and that overall QoL is comparable with that in patients who have had a pancreatoduodenectomy.
One study20 used the PAID20 questionnaire in 35 patients after total pancreatectomy and in 43 after pancreatoduodenectomy, and did not find any significant differences, except for the question about feelings of guilt or anxiety about their diabetes management when glucose values were off‐track. These authors concluded that diabetes after total pancreatectomy is manageable, with acceptable QoL.
Quality of life after pancreatoduodenectomy versus total pancreatectomy
Two studies20, 23 compared QoL after total pancreatectomy with that following pancreatoduodenectomy. Epelboym et al.23 performed a matched‐paired analysis of age, postoperative pathology, and preoperative and postoperative presence of diabetes. After a median follow‐up of 45 months, patients in both groups had similar functional and symptom scales and global health status. Overall QoL after total pancreatectomy was good, and showed no significant difference from that in patients who had a pancreatoduodenectomy, according to Casadei and colleagues20. Diabetes impacted poorly on QoL in both groups, but the overall PAID20 score tended to favour pancreatoduodenectomy (P = 0·081). Nevertheless, Casadei et al.20 concluded that overall QoL and the impact of diabetes after total pancreatectomy were acceptable, and comparable with those following pancreatoduodenectomy.
Discussion
This systematic review of 1536 patients after total pancreatectomy has shown that, over a follow‐up period of 20·8 months, endocrine‐related readmission was fairly common (18·6 per cent) and should be improved. Diabetes‐related mortality (1·6 per cent) did occur, but only in patients treated before 2005. The burden of diarrhoea seems to be significant, with a negative impact on QoL, requiring accurate personalized management. Although overall QoL appears acceptably reduced after total pancreatectomy, data are sparse as few studies have reported on long‐term endocrine and exocrine insufficiency and QoL.
Most surgeons favour partial over total pancreatectomy in order to prevent the consequences of insulin‐dependent diabetes and its effects on QoL. Based on this information, new‐onset diabetes after total pancreatectomy seems similar to type 1 diabetes mellitus26, 39, 40. Multiple studies2, 20, 26, 35, 39, 41 concluded that diabetes after total pancreatectomy could be managed well, whereas others18, 19 still emphasized its complexity. The present review revealed a substantial rate of diabetes‐related morbidity, but nevertheless acceptable and stabilized levels of HbA1c in the first year after surgery, indicating reasonable management. All diabetes‐related mortality occurred in studies that included patients between 1990 and 2013. More recent studies, which included patients treated only after 2005, did not report any diabetes‐related mortality at all. Hence, brittle diabetes may no longer be a correct term in current practice of total pancreatectomy, owing to improved diabetes management.
Diarrhoea was the most frequently reported symptom after total pancreatectomy; 23·5 per cent of patients still had symptoms despite pancreatic enzyme substitution. The wide range of incidence of diarrhoea (0–64 per cent) suggests that management is difficult, but achievable. In the literature6, 18, 25, the extent of resection is mentioned as a contributing cause of diarrhoea. Extended resection could lead to autonomic denervation and therefore impaired bowel control, explaining the difference in severity of diarrhoea between benign and malignant indications2, 6. Nevertheless, the potential for sufficient stool management and improvement of nutritional status over time suggests a crucial role for extensive patient education and follow‐up to optimize pancreatic enzyme replacement therapy18, 33, 42. Moreover, optimal nutritional status seems to be important to reduce the risk of hepatic steatosis25, 33. In 1991, Dresler et al.43 found that three of 49 patients died as consequence of liver cirrhosis, but cirrhosis was no longer seen in recent literature.
In this review, QoL was reduced significantly in 11 of 15 domains in comparison with that in the general population. Decreases in global health status and functioning were classified as small and moderate, indicating a reasonable reduction. Specifically, two studies6, 35 compared QoL with age‐ and/or sex‐matched healthy individuals and found no significant differences during a median follow‐up of 22 (range 2–73) months and up to 5 years respectively. Other studies, including some not in the present systematic review, described impaired but still acceptable QoL, and even comparability with other types of diabetes and partial pancreatectomy2, 18, 20, 23, 40, 41, 44, 45. Whereas the impact of diabetes on QoL is suggested to be comparable with that of partial pancreatoduodenectomy20, 23, several articles2, 6, 18, 35 mentioned the major impact of diarrhoea.
The current state of knowledge about functional outcomes after total pancreatectomy and its impact on QoL seems to be limited, and a variety of opinions exist concerning the acceptability of outcomes. Nevertheless, recent studies1, 18, 36 have shown an increase in total pancreatectomy performed over time, especially in patients with IPMN. In the present review, however, the majority of patients (52·5 per cent) underwent total pancreatectomy for PDAC and 29·6 per cent for IPMN. Despite the potential benefits of total pancreatectomy as radical treatment for IPMN, international consensus regarding the role of total pancreatectomy is lacking, possibly as result of limited data on functional outcomes, QoL and long‐term survival rates compared with those for partial pancreatectomy8.
This review should be interpreted in the light of some shortcomings. First, the heterogeneity between studies was substantial, mainly because of different indications for total pancreatectomy. Second, follow‐up periods varied (from 1 month to several years). Long‐term follow‐up was limited, and therefore data on long‐term consequences of total pancreatectomy are sparse. In addition, one‐third of all included studies made no distinction between elective and emergency total pancreatectomy. Potentially, outcomes of (completion) total pancreatectomy in emergency settings may have influenced outcomes negatively. A strength of this review is that studies with relative small cohorts and dated series were excluded.
Several further studies are needed in this field. First, few studies of long‐term outcomes on endocrine and exocrine insufficiency and QoL have been published, and this needs to be investigated in large prospective series, evaluating clinicophysiological parameters and their courses over time. Second, such studies should compare outcomes with those following partial pancreatectomy, especially for IPMN. Third, evidence‐based guidelines are needed for optimal management of endocrine and exocrine insufficiency.
Supporting information
Table S1. Search syntax
Table S2. Baseline characteristics of included studies
Acknowledgements
L.S. and T.F.S. contributed equally to this publication.
The authors acknowledge the work of F. S. van Etten (clinical librarian) for her help with the literature search.
L.S. has received a grant from ZEALAND for studies on management of postpancreatectomy diabetes. M.G.B. has received a grant (number UVA2013‐5842) from the Dutch Cancer Society for studies on pancreatic cancer.
Disclosure: The authors declare no conflict of interest.
References
- 1. Almond M, Rob KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J et al Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB (Oxford) 2015; 17: 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Müller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U et al Is there still a role for total pancreatectomy? Ann Surg 2007; 246: 966–975. [DOI] [PubMed] [Google Scholar]
- 3. Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M et al Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002; 123: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 4. Andrén‐Sandberg A, Ansorge C, Yadav TD. Are there indications for total pancreatectomy in 2016? Dig Surg 2016; 33: 329–334. [DOI] [PubMed] [Google Scholar]
- 5. Del Chiaro M, Rangelova E, Segersvärd R, Arnelo U. Are there still indications for total pancreatectomy? Updates Surg 2016; 68: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartwig W, Gluth A, Hinz U, Bergmann F, Spronk PE, Hackert T et al Total pancreatectomy for primary pancreatic neoplasms: renaissance of an unpopular operation. Ann Surg 2015; 261: 537–546. [DOI] [PubMed] [Google Scholar]
- 7. Griffin JF, Poruk KE, Wolfgang CL. Is it time to expand the role of total pancreatectomy for IPMN? Dig Surg 2016; 33: 335–342. [DOI] [PubMed] [Google Scholar]
- 8. Scholten L, van Huijgevoort NCM, Bruno MJ, Fernandez‐Del Castillo C, Satoi S, Sauvanet A et al; European Study Group on Cystic Tumours of the Pancreas and the International Association of Pancreatology . Surgical management of intraductal papillary mucinous neoplasm with main duct involvement: an international expert survey and case‐vignette study. Surgery 2018; doi:10.1016/j.surg.2018.01.025 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Scholten L. PROSPERO – International Prospective Register of Systematic Reviews http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD420016051093 [accessed November 2018].
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki S, Kajiyama H, Takemura A, Shimazaki J, Nishida K, Shimoda M. The clinical outcomes after total pancreatectomy. Dig Surg 2017; 34: 142–150. [DOI] [PubMed] [Google Scholar]
- 12. WHO . Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus; 1999. http://apps.who.int/iris/bitstream/10665/66040/1/WHO_NCD_NCS_99.2.pdf [accessed 2 February 2018].
- 13. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2016; 39(Suppl 1): S13–S22. [DOI] [PubMed] [Google Scholar]
- 14. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M et al; The Ottawa Hospital Research Institute. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of NonRandomised Studies in Meta‐Analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 15 February 2018].
- 16. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P et al Impact of total pancreatectomy: short‐ and long‐term assessment. HPB (Oxford) 2013; 15: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casadei R, Monari F, Buscemi S, Laterza M, Ricci C, Rega D et al Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg 2010; 62: 41–46. [DOI] [PubMed] [Google Scholar]
- 20. Casadei R, Ricci C, Taffurelli G, Guariniello A, Di Gioia A, Di Marco M et al Is total pancreatectomy as feasible, safe, efficacious, and cost‐effective as pancreaticoduodenectomy? A single center, prospective, observational study. J Gastrointest Surg 2016; 20: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 21. Crippa S, Tamburrino D, Partelli S, Salvia R, Germenia S, Bassi C et al Total pancreatectomy: indications, different timing, and perioperative and long‐term outcomes. Surgery 2011; 149: 79–86. [DOI] [PubMed] [Google Scholar]
- 22. Crippa S, Pergolini I, Rubini C, Castelli P, Partelli S, Zardini C et al Risk of misdiagnosis and overtreatment in patients with main pancreatic duct dilatation and suspected combined/main‐duct intraductal papillary mucinous neoplasms. Surgery 2016; 159: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 23. Epelboym I, Winner M, DiNorcia J, Lee MK, Lee JA, Schrope B et al Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J Surg Res 2014; 187: 189–196. [DOI] [PubMed] [Google Scholar]
- 24. Fujino Y, Matsumoto I, Ajiki T, Kuroda Y. Clinical reappraisal of total pancreatectomy for pancreatic disease. Hepatogastroenterology 2009; 56: 1525–1528. [PubMed] [Google Scholar]
- 25. Hata T, Ishida M, Motoi F, Sakata N, Yoshimatsu G, Naitoh T et al Clinical characteristics and risk factors for the development of postoperative hepatic steatosis after total pancreatectomy. Pancreas 2016; 45: 362–369. [DOI] [PubMed] [Google Scholar]
- 26. Jamil LH, Chindris AM, Gill KR, Scimeca D, Stauffer JA, Heckman MG et al Glycemic control after total pancreatectomy for intraductal papillary mucinous neoplasm: an exploratory study. HPB Surg 2012; 2012: 381328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitagawa M, Ikoma H, Ochiai T, Ishii H, Shiozaki A, Kuriu Y et al Total pancreatectomy for pancreatic carcinoma: evaluation of safety and efficacy. Hepatogastroenterology 2012; 59: 907–910. [DOI] [PubMed] [Google Scholar]
- 28. Nikfarjam M, Low N, Weinberg L, Chia PH, He H, Christophi C. Total pancreatectomy for the treatment of pancreatic neoplasms. ANZ J Surg 2014; 84: 823–826. [DOI] [PubMed] [Google Scholar]
- 29. Parsaik AK, Murad MH, Sathananthan A, Moorthy V, Erwin PJ, Chari S et al Metabolic and target organ outcomes after total pancreatectomy: Mayo Clinic experience and meta‐analysis of the literature. Clin Endocrinol (Oxf) 2010; 73: 723–731. [DOI] [PubMed] [Google Scholar]
- 30. Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD et al Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long‐term survival. Ann Surg 2009; 250: 282–287. [DOI] [PubMed] [Google Scholar]
- 31. Shi HJ, Jin C, Fu DL. Impact of postoperative glycemic control and nutritional status on clinical outcomes after total pancreatectomy. World J Gastroenterol 2017; 23: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stauffer JA, Nguyen JH, Heckman MG, Grewal MS, Dougherty M, Gill KR et al Patient outcomes after total pancreatectomy: a single centre contemporary experience. HPB (Oxford) 2009; 11: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki S, Miura J, Shimizu K, Tokushige K, Uchigata Y, Yamamoto M. Clinicophysiological outcomes after total pancreatectomy. Scand J Gastroenterol 2016; 51: 1526–1531. [DOI] [PubMed] [Google Scholar]
- 34. Takami H, Fujii T, Kanda M, Suenaga M, Yamamura K, Kodera Y. Preservation of the pyloric ring confers little benefit in patients undergoing total pancreatectomy. World J Surg 2014; 38: 1807–1813. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe Y, Ohtsuka T, Matsunaga T, Kimura H, Tamura K, Ideno N et al Long‐term outcomes after total pancreatectomy: special reference to survivors' living conditions and quality of life. World J Surg 2015; 39: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 36. Zakaria HM, Stauffer JA, Raimondo M, Woodward TA, Wallace MB, Asbun HJ. Total pancreatectomy: short‐ and long‐term outcomes at a high‐volume pancreas center. World J Gastrointest Surg 2016; 8: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al The European Organisation for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 38. Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M et al; EORTC Quality of Life Group . EORTC QLQ‐C30 Reference Values. EORTC: Brussels, 2008. [Google Scholar]
- 39. Jethwa P, Sodergren M, Lala A, Webber J, Buckels JA, Bramhall SR et al Diabetic control after total pancreatectomy. Dig Liver Dis 2006; 38: 415–419. [DOI] [PubMed] [Google Scholar]
- 40. Roberts KJ, Blanco G, Webber J, Marudanayagam R, Sutcliffe RP, Muiesan P et al How severe is diabetes after total pancreatectomy? A case‐matched analysis. HPB (Oxford) 2014; 16: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu W, Dodson R, Makary MA, Weiss MJ, Hirose K, Cameron JL et al A contemporary evaluation of the cause of death and long‐term quality of life after total pancreatectomy. World J Surg 2016; 40: 2513–2518. [DOI] [PubMed] [Google Scholar]
- 42. Struyvenberg MR, Fong ZV, Martin CR, Tseng JF, Clancy TE, Fernández‐Del Castillo C et al Impact of treatments on diabetic control and gastrointestinal symptoms after total pancreatectomy. Pancreas 2017; 46: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 43. Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belyaev O, Herzog T, Chromik AM, Meurer K, Uhl W. Early and late postoperative changes in the quality of life after pancreatic surgery. Langenbecks Arch Surg 2013; 398: 547–555. [DOI] [PubMed] [Google Scholar]
- 45. Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG et al Quality‐of‐life after total pancreatectomy: is it really that bad on long‐term follow‐up? J Gastrointest Surg 2005; 9: 1059–1067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search syntax
Table S2. Baseline characteristics of included studies


