Abstract
Background
Secondary resection of initially unresectable colorectal cancer liver metastases (CRLM) can prolong survival. The added value of selective internal radiotherapy (SIRT) to downsize lesions for resection is not known. This study evaluated the change in technical resectability of CRLM with the addition of SIRT to FOLFOX‐based chemotherapy.
Methods
Baseline and follow‐up hepatic imaging of patients who received modified FOLFOX (mFOLFOX6: fluorouracil, leucovorin, oxaliplatin) chemotherapy with or without bevacizumab (control arm) versus mFOLFOX6 (with or without bevacizumab) plus SIRT using yttrium‐90 resin microspheres (SIRT arm) in the phase III SIRFLOX trial were reviewed by three or five (of 14) expert hepatopancreatobiliary surgeons for resectability. Reviewers were blinded to one another, treatment assignment, extrahepatic disease status, and information on clinical and scanning time points. Technical resectability was defined as at least 60 per cent of reviewers (3 of 5, or 2 of 3) assessing a patient's liver metastases as surgically removable.
Results
Some 472 patients were evaluable (SIRT, 244; control, 228). There was no significant baseline difference in the proportion of technically resectable liver metastases between SIRT (29, 11·9 per cent) and control (25, 11·0 per cent) arms (P = 0·775). At follow‐up, significantly more patients in both arms were deemed technically resectable compared with baseline: 159 of 472 (33·7 per cent) versus 54 of 472 (11·4 per cent) respectively (P = 0·001). More patients were resectable in the SIRT than in the control arm: 93 of 244 (38·1 per cent) versus 66 of 228 (28·9 per cent) respectively (P < 0·001).
Conclusion
Adding SIRT to chemotherapy may improve the resectability of unresectable CRLM.
Secondary resection of initially unresectable colorectal cancer liver metastases (CRLM) can prolong survival. This study of 472 patients suggests that chemotherapy with FOLFOX with or without bevacizumab may improve the technical resectability rate of CRLM, especially in patients with an initial hepatic tumour burden of 25 per cent or less, and that this effect is enhanced substantially by adding selective internal radiotherapy to systemic chemotherapy.
Might improve resectability
Antecedentes
La resección secundaria de metástasis hepáticas de cáncer colorrectal (colorectal cancer liver metastases, CRLM) inicialmente irresecables puede prolongar la supervivencia. Se desconoce el valor añadido de la radioterapia interna selectiva (selective internal radiation therapy, SIRT). Este estudio evaluó el cambio en la resecabilidad técnica de las CRLM secundario a la adición de SIRT a una quimioterapia tipo FOLFOX.
Métodos
Las pruebas de radioimagen basales y durante el seguimiento de pacientes tratados con un régimen FOLFOX modificado (mFOLFOX6: fluorouracilo, leucovorina, oxaliplatino) ± bevacizumab (grupo control) versus mFOLFOX6 (± bevacizumab) más SIRT usando microesferas de resina de yttrium‐90, en el ensayo de fase III SIRFLOX, fueron revisadas por 3‐5 (de 14) cirujanos expertos hepatobiliares para determinar la resecabilidad. Los expertos efectuaron la revisión de forma ciega unos respecto a otros en relación con la asignación al tratamiento, estado de la enfermedad extra‐hepática y situación clínica en el momento del estudio radiológico. La resecabilidad técnica se definió como ≥ 60% de revisores evaluando las metástasis del paciente como quirúrgicamente resecables.
Resultados
Fueron evaluables un total de 472 pacientes (control, n = 228; SIRT, n = 244). No hubo diferencias significativas basales en la proporción de metástasis hepáticas técnicamente resecables entre SIRT (29/244; 11,9%) y el grupo control (25/228; 11,0%: P = 0,775). Durante el seguimiento y en ambos brazos de tratamiento, un número significativamente mayor de pacientes se consideraron técnicamente resecables en comparación con la situación basal (54/472 (11,4%) basal y 159/472 (33,7%) al seguimiento). Hubo más pacientes resecables en el grupo SIRT que en el control (93/244 (38,1%) y 66/228 (28,9%); P < 0,001, respectivamente).
Conclusión
La adición de SIRT a la quimioterapia puede mejorar la resecabilidad de las CRLM irresecables.
Introduction
An increasingly aggressive surgical approach in patients with colorectal cancer liver metastases (CRLM) has led to higher numbers of long‐term survivors. However, 80–90 per cent of patients with CRLM are not amenable to surgery at diagnosis1, 2, 3, 4 and liver metastases remain the principal site of fatal colorectal cancer5, 6, 7, 8. Secondary resection and/or thermal ablation of primarily unresectable CRLM following systemic treatment may prolong survival or be curative in some patients4, 9, 10, 11, 12, 13, 14, 15, 16. However, there is uncertainty surrounding the best treatment combination to achieve downsizing of the metastatic lesions for resection, as few trials have specifically addressed this17. In the absence of a clear definition of unresectability, effective treatments for downsizing of metastatic liver tumours are difficult to evaluate objectively. Many factors influence the decision to perform these procedures, and studies focusing on secondary resection/ablation after downsizing treatment have used very heterogeneous inclusion criteria15, 18, 19. Few studies on the effects of liver‐directed therapy on the resectability of CRLM have been undertaken20. Selective internal radiotherapy (SIRT) with yttrium‐90 (90Y) labelled microspheres is a liver‐directed therapy that could downsize tumours and render them amenable to surgery20.
In the randomized SIRFLOX trial that compared chemotherapy with chemotherapy plus a single SIRT procedure in patients with CRLM deemed unresectable, the secondary resection rate was low in both arms21. It is possible that this reflects the presence of poor prognostic features unrelated to the extent of liver tumour involvement, such as extrahepatic disease or patient co‐morbidity, or the reluctance of surgeons to operate on the liver following SIRT because of safety concerns22. Hence, the secondary resection rate in SIRFLOX does not provide information on how resectability would have been affected by chemotherapy or chemotherapy plus SIRT in patients otherwise suitable for surgery.
SIRT has yielded encouraging tumour response rates in uncontrolled or small‐scale studies23. In SIRFLOX, the tumour response rate in the liver was approximately 10 per cent higher in the SIRT than in the control arm. Response to chemotherapy had good correlation with secondary resection rates in previous trials13, 14, but some patients may remain unresectable owing to the distribution of the CRLM. These aspects are poorly covered by defined resectability criteria used in studies, making image assessment by experienced hepatopancreatobiliary (HPB) surgeons the most reliable tool with which to determine resectability. This approach was first used in the CELIM (Cetuximab in Neoadjuvant Treatment of Non‐Resectable Colorectal Liver Metastases) trial24, where a blinded surgical image review revealed that almost one‐third of patients who were enrolled because their metastases had been deemed unresectable by local assessment were in fact considered candidates for resection on expert review. The proportion of potentially resectable patients increased to 60 per cent after cetuximab‐containing chemotherapy, making CELIM the first study to prove objectively the principle of ‘conversion’ chemotherapy. To date, no such data exist for interventional treatments such as SIRT that may play a role in conversion of unresectable liver metastases to surgery, and, therefore, this post hoc analysis of SIRFLOX data was performed.
Methods
The REsect study, a retrospective analysis of the SIRFLOX study patient cohort, was designed to determine the quasi‐objective change in resectability of CRLM from baseline to follow‐up after first‐line treatment with mFOLFOX6 chemotherapy (oxaliplatin, folinic acid and fluorouracil regimen) with or without bevacizumab (control arm) or mFOLFOX6 with or without bevacizumab plus SIRT (SIRT arm) with 90Y‐labelled resin microspheres (SIR‐Spheres®; Sirtex Medical, Sydney, New South Wales, Australia).
As REsect relied on existing CT hepatic imaging data from SIRFLOX and no new data were acquired for the analysis, no formal ethics committee approval was needed. The SIRFLOX study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the relevant institutional review boards for each participating centre. All patients had to provide written informed consent. SIRFLOX had the http://clinicaltrials.gov identifier NCT00724503.
Patients
All patients who had received first‐line treatment for CRLM in the SIRFLOX study were eligible for inclusion in the REsect study. Patients had to have evaluable CT data at baseline and follow‐up, and have received the allocated treatment. For enrolment in SIRFLOX, patients had to have histologically confirmed adenocarcinoma of the colon or rectum, with or without the primary tumour in situ, with proven liver‐dominant metastatic disease. Patients had to be chemotherapy‐naive, have a WHO performance status of 0–1, and a life expectancy of more than 3 months. Predefined stratification factors included the presence of extrahepatic metastases (liver‐only versus liver‐dominant metastatic colorectal cancer), hepatic tumour burden (25 per cent or less versus more than 25 per cent), intent to use bevacizumab, and treatment centre. The protocol for SIRFLOX, including full details of inclusion and exclusion criteria, has been published previously25.
Treatment and assessment in the SIRFLOX study
Patients were randomized in a 1 : 1 ratio to treatment with an mFOLFOX6 regimen alone or to mFOLFOX6 plus SIRT with 90Y‐labelled resin microspheres25. Chemotherapy was continued until disease progression. Patients could receive bevacizumab from cycle 1 in the control arm and from cycle 4 onwards in the SIRT arm.
Patients in the SIRT arm had baseline hepatic angiography and a liver‐to‐lung breakthrough nuclear medicine scan to assess suitability to receive SIRT25. The patient's body surface area, percentage tumour involvement, and magnitude of liver‐to‐lung shunting were used to determine the activity (GBq) administered. SIRT was given in a single procedure on day 3–4 of cycle 1 or cycle 2 of mFOLFOX6 treatment.
Patients were assessed by CT every 8–12 weeks until hepatic progression. Other follow‐up assessments included clinical assessment25 and suitability for liver resection, at every CT time point. All patients were followed up until death, or to the end of the study.
Blinded review of CT scans in REsect
A pool of 14 HPB surgeons were recruited to perform the blinded review. All reviewers viewed a training video (Video S1 , supporting information) to ensure consistency in reading the scans. The criterion for resectability was that resection of all visible lesions, with negative margins and a sufficient future liver remnant (FLR) size, was feasible. Baseline and best response follow‐up imaging of 100 randomly selected patients were presented independently to five HPB surgeons to determine the pooled interobserver agreement and validate the analysis. This initial series of assessments was designed to demonstrate the feasibility of the study methodology. The assessments showed good pooled interobserver agreement and provided the rationale for proceeding with three reviewers per case for the remainder of cases.
All reviewers were blinded to clinical data, extent of extrahepatic disease, study arm assignment, and one another's assessments. To ensure that assessment was not biased by a reviewer's expectations on changes in resectability after treatment, reviewers were also blinded to the timing of CT (baseline versus follow‐up). As the objective was to assess the change in technical resectability of liver metastases, reviewers were explicitly instructed to ignore any patient features other than the extent of liver lesions that may have been visible on the CT scan (such as extrahepatic metastases), even if these might affect the decision for, or against, surgery in real life. A patient was deemed to be resectable or unresectable by majority agreement (3 of 5, or 2 of 3 surgeons: at least 60 per cent).
Objectives of REsect
The primary objective was to determine the change in technical resectability of all liver lesions between baseline and time of best treatment response in the liver (defined as the deepest response (nadir) when the sum of the diameters of all hepatic target lesions was lowest).
Subanalyses stratified by the presence or absence of miliary disease in the liver (defined as at least 15 metastases, with reason to assume the patient would remain unresectable after treatment) and by hepatic tumour burden (25 per cent or less or more than 25 per cent) were performed.
Statistical analysis
All statistical analysis presented here was preplanned. The analysis was performed on an evaluable population that was a subset of the SIRFLOX per protocol population, defined as all patients with evaluable baseline and follow‐up CT results who had received allocated treatment. Analyses from the statistical analysis plan are not presented in their entirety owing to space constraints. Descriptive statistics are provided for patient and disease characteristics at baseline. Change in resectability before and after treatment was tested with the McNemar test, with matched pairs. Agreement based on votes each at baseline and at the time of best response was reported using percentage agreement. Fisher's exact test was used for all comparisons except age, body surface area and tumour burden (t test). A significance level of P < 0·050 was used in all comparisons.
Results
SIRFLOX recruited 530 patients (263 in the control and 267 in the SIRT arm)21. Of these, 472 patients had evaluable baseline and follow‐up imaging, and received the allocated treatment (control, 228; SIRT, 244), forming the population for this analysis (Fig. 1). The median length of follow‐up was 23·5 months. Baseline characteristics were similar for patients in the two treatment arms (Tables 1 and 2).
Figure 1.
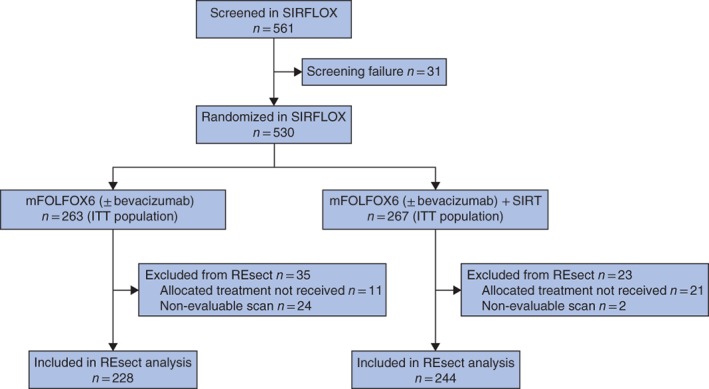
CONSORT diagram for the study
Table 1.
Baseline characteristics of patients eligible for the REsect study
| Control arm (n = 228) | SIRT arm (n = 244) | P ¶ | |
|---|---|---|---|
| Age (years) * | 62·6(10·9) | 61·8(10·5) | 0·484# |
| Sex ratio (M : F) | 154 : 74 | 163 : 81 | 0·922 |
| Body surface area (m 2 ) * | 1·87(0·21) | 1·87(0·22) | 0·884# |
| Estimated tumour burden (%) * | 17·6 (15·3)† | 18·3 (16·6)† | 0·637# |
| Extrahepatic metastasis | 91 (39·9) | 99 (40·6) | 0·925 |
| Hepatic tumour burden (%) | 0·416 | ||
| ≤ 25 | 166 (72·8)† | 169 (69·3)† | |
| > 25 | 61 (26·8)† | 74 (30·3)† | |
| Primary tumour in situ | 103 (45·2) | 108 (44·3) | 0·854 |
| Hepatic miliary disease ‡ | 89 (39·0) | 94 (38·5) | 0·925 |
| Side of primary tumour | 0·100 | ||
| Left | 177 (77·6) | 169 (69·3) | |
| Right | 46 (20·2) | 68 (27·9) | |
| Both or unknown | 5 (2·2) | 7 (2·9) | |
| WHO performance status | 0·923 | ||
| 0 | 149 (65·4) | 160 (65·8) | |
| 1 | 79 (34·6) | 83 (34·2) | |
| Missing | 0 | 1 | |
| Bevacizumab § | 133 (58·3) | 122 (50·0) | 0·079 |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.).
Data unknown for one patient in each arm.
Defined as at least 15 metastases, with reason to assume the patient would remain unresectable after treatment.
Patients who received bevacizumab before disease progression.
SIRT, selective internal radiotherapy; n.d., not done.
Fisher's exact test, except
t test.
Table 2.
Baseline characteristics of patients eligible for the REsect study, stratified by absence or presence of miliary disease
| No miliary disease (n = 289) | Miliary disease (n = 183) | |||||
|---|---|---|---|---|---|---|
| Control arm (n = 139) | SIRT arm (n = 150) | P ‡ | Control arm (n = 89) | SIRT arm (n = 94) | P ‡ | |
| Age (years) * | 63·2(11·0) | 63·0(10·7) | 0·885§ | 61·6(10·8) | 60·0(10·1) | 0·330§ |
| Sex ratio (M : F) | 90 : 49 | 96 : 54 | 0·903 | 64 : 25 | 67 : 27 | 1·000 |
| Body surface area (m 2 ) * | 1·87(0·22) | 1·86(0·21) | 0·905§ | 1·89(0·19) | 1·89(0·22) | 0·941§ |
| Estimated tumour burden (%) * | 13·0(14·1)† | 12·9(14·9)† | 0·952§ | 24·8(14·5) | 27·0(15·6) | 0·941§ |
| Extrahepatic metastasis | 54 (38·8) | 56 (37·3) | 0·875 | 37 (42) | 43 (46) | 0·340 |
| Hepatic tumour burden (%) | ||||||
| ≤ 25 | 116 (84·1)† | 124 (83·2)† | 0·875 | 50 (56) | 45 (48) | 0·655 |
| > 25 | 22 (15·9)† | 25 (16·8)† | 39 (44) | 49 (52) | ||
| 1 | 1 | |||||
| Primary tumour in situ | 53 (38·1) | 57 (38·0) | 1·000 | 50 (56) | 51 (54) | 0·882 |
| Side of primary tumour | ||||||
| Left | 110 (79·1) | 100 (66·7) | 67 (75) | 69 (73) | 0·560 | |
| Right | 27 (19·4) | 45 (30·0) | 19 (21) | 23 (24) | ||
| Both or unknown | 2 (1·4) | 5 (3·3) | 3 (3) | 2 (2) | ||
| WHO performance status | ||||||
| 0 | 95 (68·3) | 108 (72·5) | 0·073 | 54 (61) | 52 (55) | 0·549 |
| 1 | 44 (31·7) | 41 (27·5) | 35 (39) | 42 (45) | ||
| Missing | 0 | 1 | 0 | 0 | ||
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.).
Data unknown for one patient in each arm. SIRT, selective internal radiotherapy.
Fisher's exact test, except
t test.
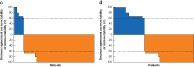
Among the 100 patients reviewed by five surgeons, agreement on resectability/unresectability was 92·1 per cent at baseline and 86·1 per cent at follow‐up. In the study population as a whole, the agreement between reviewers was 100 per cent for 80·4 per cent of patients deemed to have unresectable disease at baseline and around 64·8 per cent at follow‐up. In each treatment arm at baseline and follow‐up, the agreement between the surgeons was 100 per cent for 41·5 per cent of the patients deemed resectable and 80 per cent (4 of 5 reviewers), 60 per cent (3 of 5 reviewers) or 67 per cent (2 of 3 reviewers) for the remainder (Fig. 2).
Figure 2.

Blinded surgical review panel agreement on resectability at baseline and follow‐up for each patient Percentage agreement on resectability or unresectability in a,b control and c,d selective internal radiotherapy (SIRT) arms at a,c baseline and b,d follow‐up. The dotted lines denote the 60 per cent threshold of agreement by the surgical review panel on resectability or unresectability.
At baseline there was no statistically significant difference in the technical resectability rate of liver metastases between the SIRT and control arms (11·9 versus 11·0 per cent respectively; P = 0·775) (Fig. 3 a). At follow‐up, the technical resectability rate increased significantly in both arms (SIRT: 38·1 (95 per cent c.i. 32·0 to 45·0) per cent, P < 0·001; control: 28·9 (23·0 to 35·0) per cent, P < 0·001). The difference in resectability between SIRT and control arms at follow‐up was also statistically significant (relative risk 1·25, 95 per cent c.i. 1·10 to 1·54; P < 0·001) (Fig. 3 a). Of patients in the control and SIRT arms deemed unresectable at baseline, 46 of 203 (22·7 per cent) and 67 of 215 (31·2 per cent) respectively, were converted to technical resectability at follow‐up (P < 0·001) (Fig. 3 b).
Figure 3.

Resectability of colorectal liver metastases Baseline and follow‐up resectability rates in a the whole cohort; b patients deemed unresectable at baseline; c patients with a hepatic tumour burden of 25 per cent or less; d patients with a hepatic tumour burden greater than 25 per cent; e patients without hepatic miliary disease; f patients with hepatic miliary disease.
At baseline, in patients with an initial hepatic tumour burden of 25 per cent or less, there was no significant difference in the technical resectability rate of liver metastases between the two treatment arms (P = 0·551) (Fig. 3 c). In both arms the increase in technical resectability from baseline to follow‐up tended to be more pronounced in this population compared with the full data set (SIRT: 49·4 versus 17·1 per cent, P < 0·001; control: 34·7 versus 14·4 per cent, P < 0·001), and significantly more patients in the SIRT arm with a tumour burden of 25 per cent or less had technically resectable liver metastases at follow‐up than those in the control arm (P = 0·008) (Fig. 3 c). At follow‐up, few patients in either arm with an initial tumour burden greater than 25 per cent were deemed to have technically resectable liver metastases, with no significant difference between the control and SIRT arms (13 versus 12 per cent respectively; P = 1·000) (Fig. 3 d).
In patients without miliary disease, the increase in technical resectability rate from baseline to follow‐up tended to be more pronounced in both treatment arms compared with the full data set (SIRT: 54·0 versus 19·3 per cent, P < 0·001; control: 45·3 versus 18·0 per cent, P < 0·001). At follow‐up, a greater proportion of patients without miliary disease in the SIRT arm (81 of 150, 54·0 per cent) had resectable liver metastases compared with that in the control arm (63 of 139, 45·3 per cent), but the difference was not statistically significant (P = 0·158) (Fig. 3 e). Surprisingly, three of 89 patients (3 per cent) with miliary disease in the control arm and 12 of 94 (13 per cent) in the SIRT arm were considered to have resectable lesions at follow‐up (P = 0·029) (Fig. 3 f).
In the SIRFLOX study, 70 patients had a resection, some in combination with other local procedures or preoperative hepatic hypertrophy induction (SIRT, 36; control, 34). Forty of these patients were also classified in the REsect study as having technically resectable disease (25 SIRT, 15 control), and 30 patients (11 SIRT, 19 control) as having unresectable disease (Table 3). All 30 of these patients were deemed to be candidates for complete hepatic tumour clearance through a combination of metastasectomy and one or more additional procedures (such as thermal ablation or preoperative induction of parenchymal hypertrophy).
Table 3.
Observed resections in SIRFLOX study versus assessment of technical resectability in REsect
| Technical resectability (REsect) | |||
|---|---|---|---|
| Resectable | Unresectable | Total | |
| Observed resections (SIRFLOX) | |||
| Resected | 40 | 30 | 70 |
| Unresected | 119 | 283 | 402 |
| Total | 159 | 313 | 472 |
Discussion
REsect showed that in both treatment arms of the SIRFLOX study higher numbers were deemed to be technically resectable after treatment than at baseline. Moreover, the addition of SIRT to the FOLFOX‐based chemotherapy regimen increased the technical resectability rate of metastatic liver lesions at follow‐up.
However, in the SIRFLOX study, as in studies investigating different chemotherapy regimens in patients not selected for potential secondary resectability26, 27, there was no difference between treatment arms in the rate of liver resection21. In addition, SIRFLOX did not demonstrate any difference in progression‐free survival at any site between treatment arms. It has been suggested that inadequate patient selection has been partly responsible for these negative results. Patients whose tumours have spread far beyond oligometastatic liver disease may not derive any survival benefit from liver‐directed treatment, whereas benefit may be obtained in patients with earlier disease stages28.
Several reasons may explain the difference between the actual resection rates in SIRFLOX and the technical resectability rate determined in this blinded analysis. Rates of resection are influenced by a range of different factors, such as a patient's overall fitness to undergo surgery, access to a specialized hepatobiliary surgical centre, presence and extent of concomitant extrahepatic disease, and the surgeon's general attitude and experience. This has also been reported in other studies29, and was seen here. With these factors in mind, many patients in the SIRFLOX study may not have been considered for surgical resection owing to the presence of extrahepatic disease (40 per cent of enrolled patients had extrahepatic metastases and 45 per cent had a primary tumour in situ). Moreover, the reluctance of surgeons to operate on livers treated with 90Y‐labelled resin microspheres, owing to uncertainties over the safety of such an approach, may also have reduced the resection rate in the SIRT arm of SIRFLOX. Recently published data30 have demonstrated that mortality and complication rates in patients undergoing liver surgery after SIRT appeared acceptable given the risk profile of those particular patients; although further data are needed, these results may reassure physicians regarding the feasibility of this approach.
The criterion for resectability in REsect was that the expert HPB surgeons deemed the resection of all visible lesions to be feasible, with negative margins and sufficient FLR size. It is well established, and recommended in treatment guidelines, that the resectability of liver metastases should be assessed by surgeons with extensive experience in HPB surgery. HPB surgeons generally assess more patients as resectable than general surgeons or surgeons from other specialties31, 32. This is also why, in studies focusing on chemotherapy for unresectable CRLM, the reported rates of conversion to resectability are extremely heterogeneous. The addition of SIRT to chemotherapy improved the technical resectability rate in the subgroup of patients with a hepatic tumour burden of 25 per cent or less, but not in those with a greater tumour burden. The cutoff of 25 per cent for tumour burden may therefore be useful for selecting patients likely to derive benefit from the addition of SIRT in terms of potential secondary resectability of their liver disease.
A blinded retrospective review of the CELIM trial scans gave similar results to the present study. In the CELIM trial, patients with metastatic colorectal cancer deemed unresectable at baseline by the treating institution received cetuximab with either FOLFOX or FOLFIRI (folinic acid, fluorouracil and irinotecan); the resectability rate, by blinded surgical assessment, increased from 32 per cent at baseline to 60 per cent after treatment24. Despite higher objective tumour response rates in the SIRFLOX study (68·8–78·7 per cent) than in CELIM (57–68 per cent), resectability rates were higher in CELIM. Population differences between these trials probably account for the differences: CELIM focused specifically on conversion to secondary resectability in a potentially curative treatment approach, whereas patients in SIRFLOX were not selected for potential for secondary resectability21.
The limitations of this analysis are those inherent to a post hoc analysis. For example, SIRFLOX may not have been powered adequately for such an analysis. Although the blinded approach is useful to remove potential bias, it also obscures clinically important reasons why resection was not performed in specific patients. No attempt was made to correlate the theoretical technical resectability, in the judgement of the panel of HPB surgeons, with any resections performed in patients who participated in the study. However, REsect was designed bearing this in mind, and factors precluding resection other than technical unresectability were disregarded intentionally, as the aim was to evaluate how SIRT might have performed as a conversion treatment in patients who were potential candidates for resection. Given that no formal hypothesis testing was undertaken due to the post hoc nature of the analysis, demonstration of clinical significance requires additional validation in an adequately powered sample population.
Nevertheless, the present results provide a rationale for considering the addition of SIRT to FOLFOX‐based chemotherapy (with or without bevacizumab) in such patients, and this approach should be tested in a prospective trial focusing on this population of patients with non‐diffuse, technically unresectable, liver‐only metastatic colorectal cancer who are fit for surgery. The protocol for this trial, with secondary resection rate and disease‐free survival following resection as co‐primary endpoints, is currently in development.
Supporting information
Video S1 Training video for assessment of CT scans
Acknowledgements
The authors thank all investigators in the SIRFLOX Study Group, and in particular the principal investigators. They acknowledge the editorial assistance provided by M. Gilmour of ESP Bioscience (Crowthorne, UK), funded by Sirtex, during preparation of this article.
This study was supported by Sirtex Medical (Sydney, Australia). Where patient data can be anonymized, the authors will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted at http://www.sirtexgrants.com, and will be assessed by a scientific review board. Data are available from 6 months to 5 years after publication of this study; after this time, only raw data may be available.
B.G. has received research funding as well as honoraria from Sirtex for participation in advisory boards and for giving presentations. P.G. has received honoraria from Sirtex, Roche, Amgen and Merck for participation in advisory boards and for giving presentations. R.J. has received honoraria from Sirtex. G.A.V.H. has received honoraria from Amgen, Boehringer, Lilly, Merck, Roche, Sanofi and Sirtex for consultancy, participation in advisory boards and presentations, and his institution has received study grants from Amgen, Boehringer, Lilly, Merck, Roche, Sanofi and Sirtex. C.J.B. has received research funding and advisory board honoraria from Sirtex, and participated in presentations for Sirtex, Novartis, Celgene, Excellence in Oncology. D.M.M. has received support for travel to meetings, and honoraria for lecturing and attendance at advisory boards from Sirtex. F.P. has received lecture and consulting fees from Sirtex. J.R. has received honoraria from Sirtex. A.M.d.l.C. has received honoraria from Sirtex as clinical consultant, proctor and blind reader for the SIRFLOX study. D.G. has received support for travel to meetings from Merck Serono. T.M.v.G. has received lecture and consulting fees from Sirtex. S.A. was an employee of Sirtex Medical at the time of the study. M.S. has received honoraria from Sirtex for giving presentations, and his institution has received study grants from Sirtex.
Disclosure: The authors declare no other conflicts of interest.
Presented to the 12th Biennial European–African Hepato‐Pancreato‐Biliary Association Congress, Mainz, Germany, May 2017, and the Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, USA, June 2017; published in abstract form as J Clin Oncol 2017; 5(Suppl): 3532
References
- 1. Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 2005; 23: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 2. Navarra G, Ayav A, Weber JC, Jensen SL, Smadga C, Nicholls JP et al Short‐ and long‐term results of intraoperative radiofrequency ablation of liver metastases. Int J Colorectal Dis 2005; 20: 521–528. [DOI] [PubMed] [Google Scholar]
- 3. Rothbarth J, van de Velde CJ. Treatment of liver metastases of colorectal cancer. Ann Oncol 2005; 16(Suppl 2): ii144–ii149. [DOI] [PubMed] [Google Scholar]
- 4. Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G et al Towards a pan‐European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006; 42: 2212–2221. [DOI] [PubMed] [Google Scholar]
- 5. Abbas S, Lam V, Hollands M. Ten‐year survival after liver resection for colorectal metastases: systematic review and meta‐analysis. ISRN Oncol 2011; 2011: 763245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D et al Five‐year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol 2001; 8: 347–353. [DOI] [PubMed] [Google Scholar]
- 7. Welch JP, Donaldson GA. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg 1979; 189: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol 2014; 21: 501–506. [DOI] [PubMed] [Google Scholar]
- 9. Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D et al Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long‐term survival. Ann Surg 2004; 240: 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A et al; European Colorectal Metastases Treatment Group . Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer 2007; 43: 2037–2045. [DOI] [PubMed] [Google Scholar]
- 11. Adam R, Bhangui P, Poston G, Mirza D, Nuzzo G, Barroso E et al Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg 2010; 252: 774–787. [DOI] [PubMed] [Google Scholar]
- 12. Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P et al; European Colorectal Metastases Treatment Group; Sixth International Colorectal Liver Metastases Workshop . Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 2009; 20: 985–992. [DOI] [PubMed] [Google Scholar]
- 13. Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005; 16: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 14. Jones RP, Hamann S, Malik HZ, Fenwick SW, Poston GJ, Folprecht G. Defined criteria for resectability improves rates of secondary resection after systemic therapy for liver limited metastatic colorectal cancer. Eur J Cancer 2014; 50: 1590–1601. [DOI] [PubMed] [Google Scholar]
- 15. Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B et al Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009; 27: 1829–1835. [DOI] [PubMed] [Google Scholar]
- 16. Basso M, Dadduzio V, Ardito F, Lombardi P, Strippoli A, Vellone M et al Conversion chemotherapy for technically unresectable colorectal liver metastases: a retrospective, STROBE‐compliant, single‐center study comparing chemotherapy alone and combination chemotherapy with cetuximab or bevacizumab. Medicine (Baltimore) 2016; 95: e3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D et al ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 18. Malik H, Khan AZ, Berry DP, Cameron IC, Pope I, Sherlock D et al Liver resection rate following downsizing chemotherapy with cetuximab in metastatic colorectal cancer: UK retrospective observational study. Eur J Surg Oncol 2015; 41: 499–505. [DOI] [PubMed] [Google Scholar]
- 19. Devaud N, Kanji ZS, Dhani N, Grant RC, Shoushtari H, Serrano PE et al Liver resection after chemotherapy and tumour downsizing in patients with initially unresectable colorectal cancer liver metastases. HPB (Oxford) 2014; 16: 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henry LR, Hostetter RB, Ressler B, Bowser I, Yan M, Vaghefi H et al Liver resection for metastatic disease after Y90 radioembolization: a case series with long‐term follow‐up. Ann Surg Oncol 2015; 22: 467–474. [DOI] [PubMed] [Google Scholar]
- 21. van Hazel GA, Heinemann V, Sharma NK, Findlay MP, Ricke J, Peeters M et al SIRFLOX: Randomized phase III trial comparing first‐line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016; 34: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 22. Sangro B, Gil‐Alzugaray B, Rodriguez J, Sola I, Martinez‐Cuesta A, Viudez A et al Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 2008; 112: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 23. Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G et al Randomised phase 2 trial of SIR‐Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004; 88: 78–85. [DOI] [PubMed] [Google Scholar]
- 24. Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT et al Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010; 11: 38–47. [DOI] [PubMed] [Google Scholar]
- 25. Gibbs P, Gebski V, Van Buskirk M, Thurston K, Cade DN, Van Hazel GA; SIRFLOX Study Group . Selective internal radiation therapy (SIRT) with yttrium‐90 resin microspheres plus standard systemic chemotherapy regimen of FOLFOX versus FOLFOX alone as first‐line treatment of non‐resectable liver metastases from colorectal cancer: the SIRFLOX study. BMC Cancer 2014; 14: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A et al Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–1417. [DOI] [PubMed] [Google Scholar]
- 27. Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L et al Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 28. Tabernero J, Salazar R. Gastrointestinal cancer: light and shade of intrahepatic arterial radiotherapy in mCRC. Nat Rev Clin Oncol 2016; 13: 467–468. [DOI] [PubMed] [Google Scholar]
- 29. Modest DP, Denecke T, Pratschke J, Ricard I, Lang H, Bemelmans M et al Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer – central evaluation of FIRE‐3. Eur J Cancer 2018; 88: 77–86. [DOI] [PubMed] [Google Scholar]
- 30. Pardo F, Sangro B, Lee RC, Manas D, Jeyarajah R, Donckier V et al The Post‐SIR‐Spheres Surgery Study (P4S): retrospective analysis of safety following hepatic resection or transplantation in patients previously treated with selective internal radiation therapy with yttrium‐90 resin microspheres. Ann Surg Oncol 2017; 24: 2465–2473. [DOI] [PubMed] [Google Scholar]
- 31. Aubin JM, Bressan AK, Grondin SC, Dixon E, MacLean AR, Gregg S et al Assessing resectability of colorectal liver metastases: how do different subspecialties interpret the same data? Can J Surg 2018; 61: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Homayounfar K, Bleckmann A, Helms HJ, Lordick F, Ruschoff J, Conradi LC et al Discrepancies between medical oncologists and surgeons in assessment of resectability and indication for chemotherapy in patients with colorectal liver metastases. Br J Surg 2014; 101: 550–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Training video for assessment of CT scans


