Abstract
Introduction
The Myasthenia Gravis Patient Registry (MGR) is a voluntary, patient‐submitted database dedicated to improve understanding of care/burden of myasthenia gravis (MG).
Methods
In this study we present analyses of baseline records through July 2017 (n = 1140) containing data on the MG—Activities of Daily Living (MG‐ADL) and the MG 15‐item Quality of Life (MG‐QOL15) instruments, two validated scales assessing quality of life in MG patients at sign‐up into the MGR.
Results
Most registrants reported moderate to severe impairment of health‐related quality of life, with a median MG‐ADL score of 6 and a median MG‐QOL15 score of 21. Seventy‐one percent of the patients had received pyridostigmine. Corticosteroids, mycophenolate mofetil, and azathioprine were the most common immunomodulators/immunosuppressants, with 85% of participants having ever using one of these agents. Forty‐seven registrants reported receiving intravenous immunoglobulin, and 30% received plasma exchange. Twelve percent reported other treatments, and 40% were unsure whether they received less common therapies. Forty percent had undergone thymectomy.
Discussion
The MGR data correlate well with other MG cohorts. Many MG patients remain negatively impacted despite treatment.
Keywords: ADL, MG, MGFA, myasthenia, QOL
Abbreviations
- AChR
acetylcholine receptor
- IVIg
intravenous immunoglobulin
- MG
myasthenia gravis
- MG‐ADL
Myasthenia Gravis—Activities of Daily Living
- MGFA
Myasthenia Gravis Foundation of America
- MG‐QOL15
15‐item Myasthenia Gravis Quality of Life
- MGR
Myasthenia Gravis Patient Registry
- MuSK
muscle‐specific kinase
- NARCOMS
North American Research Committee on Multiple Sclerosis
- PLEx
plasmapheresis
1. INTRODUCTION
Myasthenia gravis (MG) is an autoimmune disease caused by antibodies directed toward proteins localized to the neuromuscular junction that result in failure of neuromuscular transmission.1, 2 Although advances have been made in understanding of disease pathogenesis and treatment, many patients have MG exacerbations, which often require hospitalization, and disease‐ and treatment‐related morbidity remains high.2 Common treatment regimens for MG include relatively benign, but often inadequate, cholinesterase inhibitors, surgical removal of the thymus, and immunomodulatory or immunosuppressive treatments (high‐dose corticosteroids, cyclosporine, azathioprine, rituximab, mycophenolate mofetil, intravenous immunoglobulin [IVIg], plasmapheresis [PLEx], and eculizumab), each with their unique adverse‐effect profiles.3, 4 Despite the many treatment options, an estimated 15% to 20% of MG patients are considered treatment refractory. Although national and international consensus guidelines have been established, there is limited understanding of how patients are being treated in everyday clinical practice.3, 4, 5, 6
To better understand the status of patient care, the Myasthenia Gravis Foundation of America (MGFA) established a patient registry in collaboration with the University of Alabama at Birmingham, starting in 2013. The MG Patient Registry (MGR) is modeled after the North American Research Committee on Multiple Sclerosis Registry (NARCOMS) and includes voluntarily provided information from patients obtained through questionnaires addressing basic demographics, diagnosis, treatment, and insurance status.7 The data obtained also included validated MG‐specific disease severity scales: the MG—Activities of Daily Living (MG‐ADL) and the 15‐item MG Quality of Life‐15 (MG‐QOL15) instruments.8, 9, 10, 11, 12 The MGR has previously identified differential gender effects of MG and its treatment in quality of life and assessment of adverse effects to prednisone treatment.13, 14 Our investigation of the MGR provides an assessment of registrant demographics, treatments, and disability in a broad patient sample.
2. METHODS
2.1. Myasthenia Gravis Patient Registry
The MGR is an active database of persons with MG. It is a voluntary, confidential, and patient‐submitted research project, funded and managed by the MGFA and the Coordinating Center at the University of Alabama at Birmingham (UAB) (http://www.mgregistry.org/). An extensive description of the MGR can be found in earlier publications.14 The MGR was developed for several purposes, including research to assess disease course and management. Initiated on July 1, 2013, the MGR involves subject completion of voluntarily submitted disease and health‐related information through a web‐based portal with the option to submit a paper questionnaire. The MGR is advertised through the MGFA website, support groups, the MGFA national meeting, and brochures for patient distribution at the offices of physicians belonging to the MGFA Medical and Scientific Advisory Board.
In addition to the general approval of the MGR at UAB, the present study was approved by the institutional review board of UAB. Consent is obtained electronically by acknowledging completion of the survey. Data have been de‐identified.
The data, including completion of MG‐ADL and MG‐QOL15, are directly entered by registrants without confirmation by a physician. The MG‐ADL thus differs from the original instructions that require that a health‐care professional ask the questions and note the answers.
2.2. Inclusion and exclusion criteria
Registrants were required to be at least 18 years of age and answered “Yes” to “Has your doctor diagnosed you with MG”? They were also required to be living in the United States and completed survey enrollment between July 1, 2013 and June 30, 2017. Each patient record is unique with no known duplications. Only data collected at baseline (sign‐up into the registry) were used in these analyses.
2.3. Main measures
The MG‐QOL15 is a 15‐item (range 0–60, higher scores indicate more severe disease) disease‐specific quality‐of‐life scale that was derived from a 60‐item MG‐specific health‐related quality‐of‐life scale.15, 16 The MG‐ADL is an eight‐question survey (range 0–24, higher scores indicate more severe disease) of MG symptoms.8, 12 Both are Likert‐type scales. A commonly accepted clinically meaningful difference in MG‐ADL is 2 points, whereas the clinically meaningful difference in MG‐QOL15 is not clearly established.8, 17
2.4. Statistical methods
All data presented is collected at sign‐up into the MGR. Demographics included age at entry into the MGR, age at symptom onset, race, and gender. Disease characteristics include disease duration, self‐reported acetylcholine receptor (AChR) antibody status, and muscle‐specific kinase (MuSK) antibody status. Quality of life was assessed by MG‐QOL15 and MG‐ADL. Subjects reported either being told they are antibody positive, not told they are antibody positive, or they were unsure. We also describe reported use of MG‐specific treatments including PLEx, IVIg, rituximab, cyclophosphamide, methotrexate, tacrolimus, cyclosporine, mycophenolate mofetil, azathioprine, and steroids. The form used for collecting immunotherapy data is provided online in Figure S1 A relatively recent treatment for MG, eculizimab, was not assessed here because its US Food and Drug Administration approval was obtained after the cutoff time of our study sample. Quality‐of‐life measures are described within the various demographic and disease characteristic subgroups. We characterized the association between the MG‐ADL and the aforementioned immunotherapies by comparing means (95% CI) within each immunotherapy subgroup with the overall means (95% CI) for the full sample, and similarly for the MG‐QOL15. Details on subgroup definitions are given in Table S1 online. Mean, SD, median, and range statistics were used for continuous variables, and frequency and proportion were used for categorical variables to describe the data. Correlations between MG‐QOL15 and MG‐ADL were assessed using the Pearson correlation. ADL and MG‐QOL15 group means were compared between those told they were AChR antibody positive vs those not told, as well as those told they were MuSK antibody positive vs those not told. t Tests were used and P values were reported for group mean comparisons. Ninety‐five percent CIs were reported for group means and correlations. Statistical significance was achieved at the .05 alpha level. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
3. RESULTS
3.1. Demographics
Data from 1140 registrants out of a total of 2084 records met the inclusion criteria and were included in the present analysis. Figure S2 online shows details on subjects included or excluded from the present analyses. The mean age at sign‐up in the MGR was 54.6 years (Table 1), with a plurality of registrants being between 45 and 65 (45%), 26% below 45, and 29% above 65 years of age. Sixty‐six percent of registrants were women. Eighty percent of registrants were white. The most common states of residence for the sample were Florida (10%), California (7%), Texas (7%), North Carolina (6%), Pennsylvania (6%), New York (5%), Virginia (4%), Michigan (4%), Georgia (4%), and Illinois (3%).
Table 1.
Demographics and disease characteristics at MGR sign‐up
| Characteristic | Statistic |
|---|---|
| Age at MGR (years) | |
| Mean ± SD | 54.6 ± 14.8 |
| Median (range) | 57.0 (18.0–93.0) |
| Gender female (%) | 66.2% |
| Race (%) | |
| White | 80.4 |
| Black | 6.2 |
| Hispanic | 0.2 |
| Disease duration (years) | |
| Mean ± SD | 9.9 ± 10.1 |
| Median (range) | 6.0 (2.0–58.0) |
| Age at first MG symptoms (years) | |
| Mean ± SD | 40.3 ± 18.9 |
| Median (range) | 40.0 (1.0–81.0) |
| AChR antibody status | |
| Told positive | 23.2% |
| Not told positive | 10.4% |
| Unsure or missing | 66.5% |
| MuSK antibody status | |
| Told positive | 5.8% |
| Not told positive | 8.0% |
| Unsure or missing | 86.2% |
| MG‐ADL total score (N = 1140) | |
| Mean ± SD | 6.2 ± 4.0 |
| Median (range) | 6 (0–21) |
| MG‐QOL total score (N = 1138) | |
| Mean ± SD | 22.2 ± 15.0 |
| Median (range) | 21 (0–60) |
Abbreviations: AChR, acetylcholine receptor; MG‐QOL15, Myasthenia Gravis 15‐item Quality of Life; MG‐ADL, Myasthenia Gravis—Activities of Daily Living; MGR, Myasthenia Gravis Patient Registry.
3.2. Disease characteristics
The mean (SD) disease duration was 9.9 (10.1) years, with approximately half the registrants having MG for 0 to 5 years (Table 1). The mean age at first symptoms was 40.3 years with a wide range from 1 to 81 years. Most registrants (72%) had their first symptoms between the age of 18 and 64 years. Analyses by gender and age were consistent with the known disease onset at a younger age for women compared with men (Figure 1). The mean (SD) onset age for women was 34.4 (17.0) years. The mean (SD) onset age for men was 51.7 (17.3) years.
Figure 1.
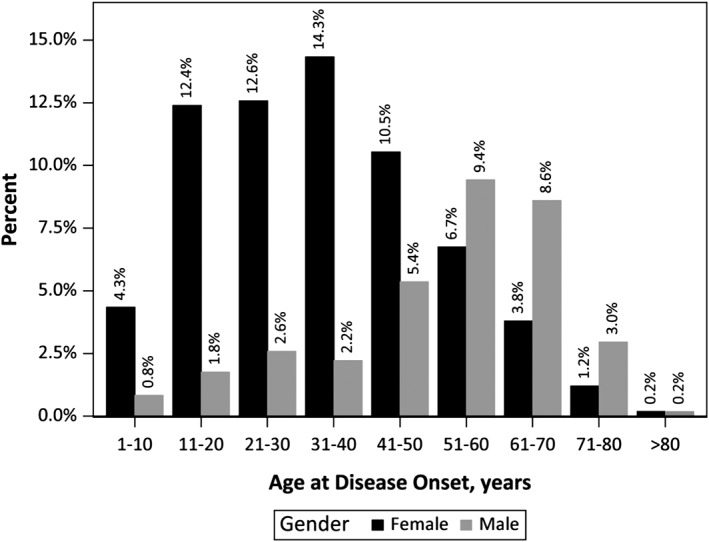
Age at disease onset. Although incidence was similar from the second to seventh decade overall, the peak age at disease onset was in the fourth decade in female patients and in the sixth decade in male patients
Twenty‐three percent of registrants indicated they were told they were positive for AChR antibodies, and 6% indicated they were told they were positive for MuSK antibodies (Table 1). High percentages reported unknown or gave no response for AChR or MuSK antibody. One hundred eighteen (10%) subjects reported that they were not told they were positive for the AChR antibody, whereas 91 (8%) reported that they were not told they were positive for the MuSK antibody.
The mean (SD) MG‐ADL score was 6.2 (4.0). On average, half the registrants reported moderate to severe symptoms or disability that limited their activities of daily living at MGR sign‐up (Figure 2A). The mean (SD) MG‐QOL15 score was 22.2 (15.0), indicating that most registrants had substantial disease burden. A wide range of MG‐QOL15 scores were recorded (0–60) (Figure 2B).
Figure 2.
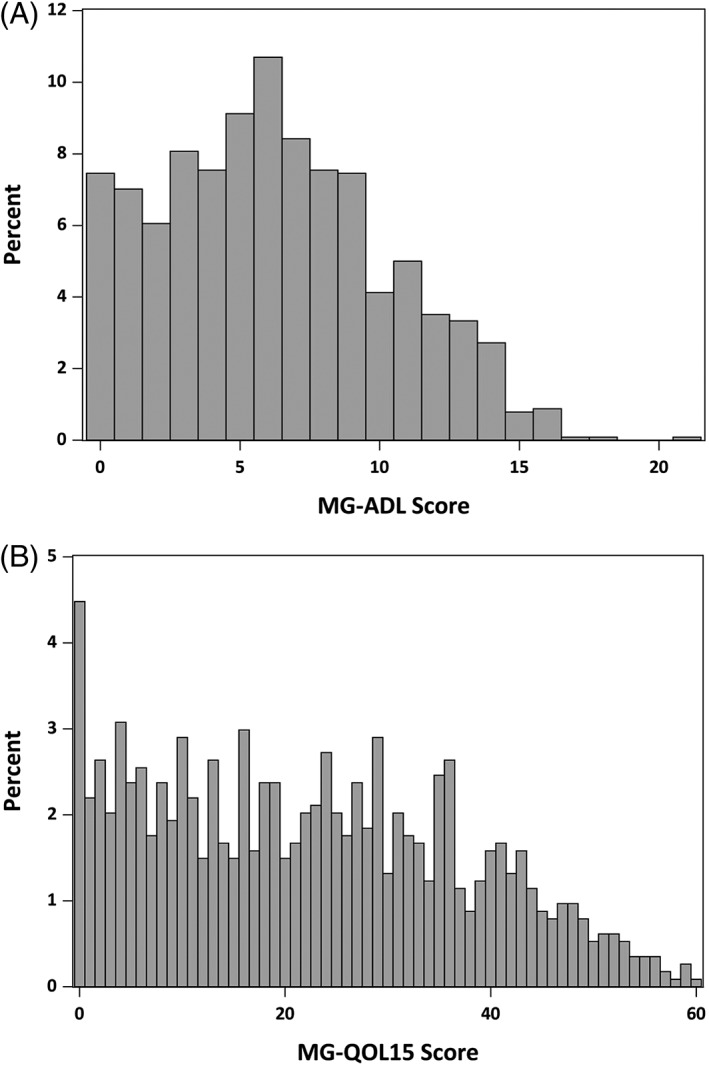
Distribution of (A) MG‐ADL and (B) MG‐QOL15 at sign‐up. The majority of patients reported moderate to severe impairment in their activities of daily living, as measured by MG‐ADL (N = 1140) and MG‐QOL15 (N = 1138) scale scores. MG‐ADL, Myasthenia Gravis—Activities of Daily Living; MG‐QOL15, 15‐item Myasthenia Gravis Quality of Life
3.3. MG‐specific therapy at sign‐up and treatment history
At sign‐up to the MGR, 71% of registrants reported receiving pyridostigmine, 42% were receiving corticosteroids, 24% mycophenolate mofetil, 19% azathioprine, 19% IVIg, and 4% PLEx. Forty percent had undergone a thymectomy, 36% reported previous steroid use, 28% had received IVIg, and 26% reported PLEx (Figure 3). Only a small proportion of registrants (12%) reported ever receiving other immunosuppressive therapies, although approximately 40% were unsure whether they had received those treatments or did not provide an answer.
Figure 3.
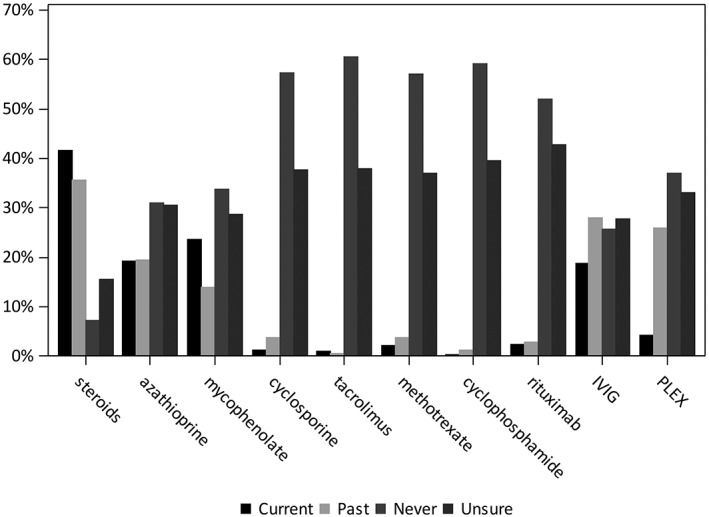
Immunotherapies in MG Patient Registry at sign‐up. Current and past medication use as reported by patients is generally consistent with current treatment guidelines for MG standard of care. MG, myathenia gravis; PLEX, plasmapheresis
The proportion of registrants receiving the most frequently prescribed disease‐modifying medications was further investigated (Figure 4). We identified 723 (63%) registrants who were on at least one of three medications at sign‐up. Of these, a similar number were receiving either: (1) prednisone alone (21%); (2) azathioprine or mycophenolate mofetil alone (21%); or (3) a combination of prednisone and either mycophenolate mofetil or azathioprine (20%). Nine hundred sixty‐six (85%) reported receiving at least one of these medications at any time. Of these, 53% reported to have used prednisone plus either azathioprine or mycophenolate mofetil (n = 604); 29% had received prednisone only (n = 277) and 18% (n = 170) had received all 3 medications. Only 10 registrants reported that they had received azathioprine or mycophenolate mofetil, but not prednisone.
Figure 4.
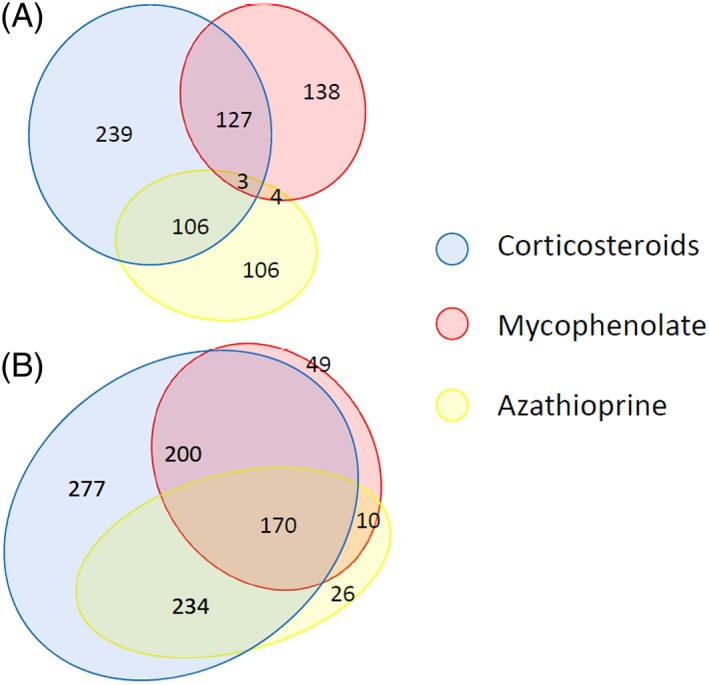
Analysis of most frequently used immunomodulatory therapies at sign‐up. Patients using corticosteroids, azathioprine, and/or mycophenolate mofetil (A), at present (n = 723), and (B) at any time (n = 966), past or present, for treatment of myasthenia of the 1140 MGR patients assessed. The Venn diagrams with areas proportional to the number of patients in each group have been generated using EulerAPE (http://www.eulerdiagrams.org/eulerAPE/). MGR, Myasthenia Gravis Patient Registry
3.4. Correlation between MG‐ADL and MG‐QOL15
Based on 1138 subjects who completed both questionnaires at sign‐up to the MGR, the estimated correlation (95% CI) between MG‐ADL and MG‐QOL15 was r = 0.78 (0.75–0.80) (P < .0001). This estimated correlation (95% CI) in the subgroups above an MG‐ADL score of 6 (n = 624) was lower at r = 0.58 (0.53–0.63) (P < .0001), with this cutoff often considered the lower boundary of substantial clinical impairment.
3.5. MG‐ADL and MG‐QOL15 in subgroups
We found that younger registrants tended to be more severely impaired in their health‐related quality of life than those over 65 years of age, and women were more affected (Table 2). Those with shorter disease duration generally reported higher impairment, and age over 65 years at first symptoms was correlated with lower disease burden. We also found that registrants who reported being told they were MuSK antibody positive, irrespective of AChR antibody, had a mean MG‐ADL score of 6.3 and a mean MG‐QOL15 score of 25.5. Those who reported being told they were AChR antibody positive, irrespective of MuSK antibody, had a mean MG‐ADL score of 5.7 and a mean MG‐QOL15 score of 22.2.
Table 2.
Functionality by subgroup
| MG‐QOL | MG‐ADL | |||
|---|---|---|---|---|
| N (%) | Mean (95% CI) | N (%) | Mean (95% CI) | |
| Age at MGR sign‐up (years) | ||||
| 18–44 | 297 (26%) | 24.1 (22.5–25.8) | 297 (26%) | 6.7 (6.3–7.2) |
| 45–64 | 517 (45%) | 24.6 (23.3–25.9) | 518 (45%) | 6.9 (6.6–7.3) |
| 65+ | 324 (28%) | 16.5 (15.0–18.1) | 325 (29%) | 4.6 (4.2–5.0) |
| Gender | ||||
| Female | 755 (66%) | 24.4 (23.4–25.5) | 755 (66%) | 6.9 (6.6–7.2) |
| Male | 383 (34%) | 17.8 (16.3–19.2) | 385 (34%) | 4.8 (4.5–5.2) |
| MG duration (years) | ||||
| 0–5 | 546 (48%) | 23.7 (22.4–24.9) | 547 (48%) | 6.3 (5.9–6.6) |
| 6–10 | 241 (21%) | 21.1 (19.2–23.0) | 241 (21%) | 6.2 (5.7–6.7) |
| 11–15 | 136 (12%) | 22.8 (20.3–25.2) | 136 (12%) | 6.5 (5.8–7.1) |
| 16–20 | 67 (6%) | 19.2 (15.7–22.7) | 67 (6%) | 6.0 (5.1–6.9) |
| 21–40 | 120 (11%) | 19.7 (16.8–22.6) | 120 (11%) | 6.0 (5.2–6.7) |
| 41+ | 28 (2%) | 16.9 (12.3–21.6) | 29 (3%) | 5.0 (3.7–6.3) |
| Age at first symptoms (years) | ||||
| Unknown | 58 (5%) | 24.6 (20.8–28.5) | 58 (5%) | 7.1 (6.1–8.1) |
| 0–17 | 145 (13%) | 25.1 (22.5–27.8) | 146 (13%) | 7.1 (6.4–7.8) |
| 18–44 | 454 (40%) | 23.8 (22.5–25.2) | 454 (40%) | 6.9 (6.6–7.3) |
| 45–64 | 363 (32%) | 20.6 (19.1–22.1) | 364 (32%) | 5.5 (5.1–5.9) |
| 65+ | 118 (10%) | 15.9 (13.4–18.4) | 118 (10%) | 4.1 (3.5–4.8) |
| AChR antibody status* | ||||
| Told positive | 262 (23%) | 22.2 (20.3–24.0) | 264 (23%) | 5.7 (5.2–6.2) |
| Not told positive | 118 (10%) | 25.3 (23.0–27.7) | 118 (10%) | 7.5 (6.9–8.2) |
| Unsure or missing | 758 (67%) | 21.7 (20.6–22.8) | 758 (66%) | 6.2 (5.9–6.5) |
| MuSK antibody status* | ||||
| Told positive | 66 (6%) | 25.5 (21.5–29.5) | 66 (6%) | 6.3 (5.4–7.2) |
| Not told positive | 91 (8%) | 27.0 (24.2–29.7) | 91 (8%) | 8.0 (7.2–8.9) |
| Unsure or missing | 981 (86%) | 21.5 (20.6–22.5) | 981 (86%) | 6.0 (5.8–6.3) |
| Overall | 1138 (100%) | 22.2 (21.3–23.1) | 1140 (100%) | 6.2 (6.0–6.4) |
Abbreviations: AChR, acetylcholine receptor; MG‐ADL, Myasthenia Gravis—Activities of Daily Living; MG‐QOL15, Myasthenia Gravis 15‐item Quality of Life; MGR, Myasthenia Gravis Patient Registry; MuSK, muscle‐specific kinase.
In the MGR questionnaires, subjects report being told if the results of antibody tests were positive (yes, no, unsure). Thus, it is possible for a subject to be positive for the antibody but not told so by their clinician.
Figure 5 displays the mean (95% CI) MG‐ADL and MG‐QOL15 acores for past and current users of the different immunotherapies. The overall means were 6.2 for MG‐ADL and 22.2 for MG‐QOL15, irrespective of therapy. We found that those currently using rituximab, PLEx, or IVIg had worse quality‐of‐life scores, on average, when compared with overall means on the MG‐ADL or MG‐QOL15. Meanwhile, those reporting past use of mycophenolate mofetil, methotrexate, rituximab, or cyclosporine had worse quality‐of‐life scores when compared with the overall means. Clinically significant differences in mean ADL, compared with the overall mean ADL, were observed for those currently using rituximab (+2.4) and PLEx (+2.7), as well as for those with past use of methotrexate (+3.1), rituximab (+2.1), and cyclosporine (+2.2).
Figure 5.
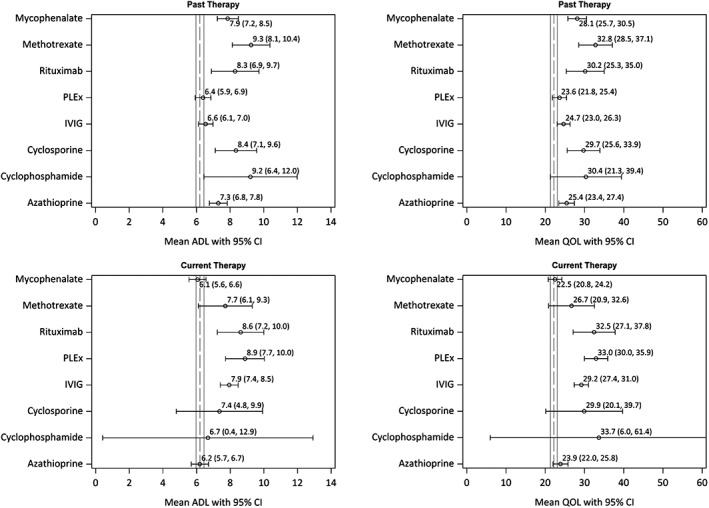
MG‐ADL and MG‐QOL15 in the immunotherapy subgroups at sign‐up. Mean and 95% confidence intervals for MG‐ADL and MG‐QOL15 measured at entry into the MGR for immunotherapy subgroups based on their self‐reported current or past medication use. Black vertical lines indicate overall means and 95% confidence intervals for the entire study sample, irrespective of therapy. MG‐ADL, Myasthenia Gravis—Activities of Daily Living; MG‐QOL15, 15‐item Myasthenia Gravis Quality of Life; MGR, Myasthenia Gravis Patient Registry
Registrants reporting prior thymectomy had similar quality‐of‐life scores when compared to those without. Those with prior thymectomy reported a mean MG‐ADL score of 6.2, whereas those without reported a mean MG‐ADL score of 6.2 (P = .72). Those with prior thymectomy reported a mean MG‐QOL score of 22.0 and those without reported a mean score of 22.3 (P = .81).
4. DISCUSSION
4.1. Validity and representativeness of the MGR
The MGR is the largest patient‐reported database of MG patients, comprised of data from over 1000 patients in the United States. Within the limits of patient‐reported data, the MGR provides unique insights into the MG patient experience. The MGR was modeled after the NARCOMS registry, which has existed since the mid‐1990s and has been used in over 100 investigations. Multiple sclerosis and MG have similarities in being chronic neurological disorders, which may at times be difficult to diagnose clinically or may be misreported by registry participants. Considering this, NARCOMS underwent a validation of diagnosis by a formal review process and showed a 99% confirmation of diagnosis in subjects sampled.18
A critical issue for any registry is the extent to which the data may be biased, especially due to the likelihood that more symptomatic patients may participate more readily in a patient organization (MGFA) and its patient registry. Although such registry participation bias cannot be eliminated, comparison of the MGR data to published clinical data from several other studies indicates that participation bias is likely modest.9, 19, 20 The age and gender distribution of the MGR is consistent with the known distribution of MG.21 However, there was an underrepresentation of nonwhite races in the MGR, despite there being no racial bias of MG incidence. Thus, the MGR data may be fairly generalizable to other patients with MG in the United States. For example, our results are comparable to those of a 2008–2009 11‐center North American study designed to validate the MG Composite and MG‐QOL15.9 We found a mean difference in MG‐ADL of 1.3 points, and a mean difference in MG‐QOL15 of 3.5 in the MGR compared with the 11‐center study.9 Mean age at baseline was 3.4 years lower, and mean disease duration was 2.9 years higher in the MGR compared with the 11‐center study (7 years). Women were also more common in the MGR compared with the 11‐center study.9 For registrants who reported requiring IVIg or PLEx, mean MG‐QOL15 score was 29, similar to published scores from a randomized clinical trial in Toronto, Canada.19 Reported MG medication use in MGR participants was somewhat similar compared with a 2008–2010 study of 1288 MG patients, with the exception of markedly higher use of mycophenolate mofetil and IVIg in the MGR.20 The discrepancy in mycophenolate mofetil use is expected, however, considering that use has increased for the population of MG patients over the past decade in the United States. Increased IVIg in the MGR may suggest a higher disease severity, but could be affected by aspects of patient‐reported data in the MGR.
We found that the MG‐ADL and MG‐QOL15 scores correlated well (Pearson r = 0.78), similar to what was observed in the 11‐center North American study (r = 0.76), further validating the MGR data and use of these two patient‐reported, disease‐specific measures.9 Overall, comparisons with the 11‐center study suggest the MGR participants reported slightly worse disease statuses as measured by MG‐ADL and MG‐QOL15, were slightly younger, had slightly longer disease durations, and included a moderately larger proportion of women. Medication usage was also slightly different in the MGR compared with the 2008–2010 study, but these differences may be consistent with known trends over time. Despite this, the MGR data appear to be similar enough to generalize our observations to a larger population of MG patients in the United States. We also found a lower correlation between MG‐ADL and MG‐QOL15 scores in the subgroup with an MG‐ADL score above 6, which is in line with the narrower dynamic range for both scales in this subgroup vs the entire population.
4.2. MG disease burden and immunotherapies
Our data suggest that a large proportion of patients have a significant disease burden, as measured by two well‐established, validated, disease‐specific, and patient‐reported measures—the MG‐ADL and the MG‐QOL15. These data highlight that MG remains a disease that may significantly affect the disease‐specific quality of life of a large number of patients, despite current treatments.
Our analyses also indicate that younger patients and women generally report more symptoms and limitations, and poorer disease‐specific QOL. Patients may suffer disproportionately during their younger active years. The findings also suggest that there may be clinician reluctance to provide more aggressive treatment to such patients, especially given the significant acute side effects and long‐term risks related to immunosuppressive therapies. These observations may in part also reflect that, in women, onset of MG predominantly occurs at a younger age compared with men.
In subgroup analyses, around 80% of registrants reported current or past use of pyridostigmine or corticosteroids, which is consistent with standard‐of‐care recommendations.3 Approximately 40% reported having been on azathioprine and/or mycophenolate mofetil, whereas 30% reported never receiving either one or the other, and 30% were not sure. This observation is unexpected given the overall level of impairment identified. Based on consensus guidelines, patients not responding well to cholinesterase inhibition and corticosteroids should be moved to immunosuppressive treatments. This point is magnified by the observation that impairment was similar among patients receiving vs not receiving immunosuppression. The reasons for this finding cannot be addressed by the data at hand, but are critical to better understand any potential gaps in current treatment practices. Do underlying practice patterns of physicians vary to the point that, despite significant disease burden, some patients are not prescribed standard of care treatment? Do physicians and patients vary in the level of acceptable disability prior to treatment escalation? An alternative explanation may be that a substantial population of patients may have limited knowledge of their treatments or may have forgotten about participation in an oral immunosuppressant trial, such as with azathioprine in prior years.
Only a small proportion of patients knowingly received third‐line therapeutics such as methotrexate, tacrolimus, cyclophosphamide, or rituximab, which are typically used after failure of azathioprine and mycophenolate mofetil. Not surprisingly, those who did receive these treatments tended to have the highest disease burden. Patients with or without thymectomy had similar levels of disease burden, which is not consistent with a clinical trial demonstrating the effectiveness of thymus removal for patients with AChR antibodies and age younger than 65 years.22 This may reflect a bias of MGR participants with greater severity of disease regardless of treatment.
4.3. Limitations
Limitations of our study include the voluntary nature of the MGR, which leads to the potential for selection bias with an overrepresentation of patients with more severe disease. This may decrease the generalizability of our results to all MG subjects, but it also indicates a need for future studies to continue characterization of their unmet needs. Another limitation stems from the self‐reporting of all data from patients. With self‐reporting comes the chance of recall bias and the potential for error. This likely contributes to the high number of registrants who do not recall autoantibody status, specific treatments, or specific clinical measures. The chance of inaccuracies and bias in patient‐reported data is present in most observational studies and registries. Thus, conclusions drawn from them must be made cautiously, especially for data pertaining to antibody statuses or seronegativity. Another limitation is the lack of diagnostic certainty for MGR participants, so there is the possibility that MGR participants do not have MG. The majority of subjects were not aware of their antibody status, suggesting a lack of discussion with their physicians regarding antibodies. However, we believe it is more likely that typical participants are not fully informed regarding their antibody status or diagnostic methodology, and that this is not an immediate invalidation of their MG status. Given that therapies such as eculizumab and thymectomy are focused on patients with AChR antibodies, greater effort should be made by physicians to educate patients on their diagnosis. Similar issues are present in the reporting of MG immunotherapy, in which 15% to 30% of subjects were unsure of the three common drugs. Despite these limitations, reasonably valid results and insights may still be derived. The MGR was designed to be largely similar to the NARCOMS registry, in which a validation assessment showed concurrence with self‐reported diagnosis and independent record evaluations. This supports that the accuracy of our patient‐reported sample should be in line with other similar neurological disease registries.
In conclusion, our analyses document in a large sample that many patients with MG still suffer from a significant disease burden despite current standard‐of‐care treatment.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
CONFLICT OF INTEREST
R.F.F. is an employee of Ra Pharmaceuticals, Inc, which funded the study. P.W.D. is an employee of Ra Pharmaceuticals. G.C. is employed by the University of Alabama at Birmingham and president of Pythagoras, Inc, a private consulting company in Birmingham, Alabama. G.C. is also a member of the data safety and monitoring boards for AMO Pharmaceuticals, Biolinerx, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Merck, Merck/Pfizer, Opko Biologics, Neurim, Novartis, Ophazyme, Sanofi‐Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva Pharmaceuticals, the NHLBI (protocol review committee), and the NICHD (OPRU oversight committee), and is on the consulting or advisory boards of Atara Biotherapeutics, Axon, Biogen, Argenix, Brainstorm Cell Therapeutics, Charleston Labs, Click Therapeutics, Genzyme, Genentech, GW Pharma, Klein‐Buendel, Medimmune, Medday, Novartis, Roche, Scifluor, Somahlution, Teva Pharmaceuticals, TG Therapeutics, and University of Texas at Houston. H.J.K. is a member of the data safety and monitoring boards for Novartis and the National Institutes of Health; receives consulting fees from Alnylam Pharmaceuticals, UCB, Biocatalyst, RA Pharmaceuticals, and Momenta Pharmaceuticals; receives grant support from Akari Pharmaceuticals and the Muscular Dystrophy Association; and holds a patent related to technology to focus a complement inhibitor on the neuromuscular junction for the treatment of myasthenia gravis (US Patent No. 8,961,981). The remaining authors have no potential conflicts of interest to disclose related to this report.
AUTHOR CONTRIBUTIONS
Statistical analyses were performed jointly by H.X., I.A., and G.C. G.C. is responsible for curating the data of the MG Patient Registry (MGR), crafted the statistical analysis plan, supported the statistical analyses, and critically reviewed the manuscript. H.X. extracted the data, completed the statistical analyses, and critically reviewed the manuscript. I.A. is responsible for curating the data of the MGR, completed statistical analyses, and critically reviewed the manuscript. H.J.K. provided input for statistical analyses and interpretation of the data and critically reviewed and edited the manuscript. T.M.B. provided input for the statistical analyses and interpretation of the data and critically reviewed and edited the manuscript. P.A. critically reviewed the analysis and edited the manuscript. R.F.F. and P.W.D. provided input for the statistical analysis plan, cowrote the first draft of the manuscript, and critically reviewed and edited the manuscript.
Supporting information
FIGURE S1 Questionnaire for collection of MG immunotherapy data.
FIGURE S2 STROBE diagram.
TABLE S1 Definitions of subgroups
Cutter G, Xin H, Aban I, et al. Cross‐sectional analysis of the Myasthenia Gravis Patient Registry: Disability and treatment. Muscle Nerve. 2019;60:707–715. 10.1002/mus.26695
G.C. and H.X. contributed equally to this study.
Funding information Myasthenia Gravis Foundation of America, Grant/Award Number: Core Grant; Ra Pharmaceuticals, Inc.
REFERENCES
- 1. Berrih‐Aknin S, Le Panse R. Myasthenia gravis and autoantibodies: pathophysiology of the different subtypes. Rev Med Interne. 2014;35:413‐420. [DOI] [PubMed] [Google Scholar]
- 2. Gilhus NE. Myasthenia gravis. N Engl J Med. 2017;376:e25. [DOI] [PubMed] [Google Scholar]
- 3. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87:419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Breskovska I, Gandhy S, Punga AR, Guptill HJ. Advances in autoimmune myasthenia gravis management. Expert Rev Neurother. 2018;18:573‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sussman J, Farrugia ME, Maddison P, Hill M, Leite MI, Hilton‐Jones D. Myasthenia gravis: Association of British Neurologists' management guidelines. Pract Neurol. 2015;15:199‐206. [DOI] [PubMed] [Google Scholar]
- 6. Murai H. Japanese clinical guidelines for myasthenia gravis: putting into practice. Clin Exp Neuroimmunol. 2015;2015:21‐31. [Google Scholar]
- 7. Nickerson M, Cofield SS, Tyry T, Salter AR, Cutter GR, Marrie RA. Impact of multiple sclerosis relapse: the NARCOMS participant perspective. Mult Scler Relat Disord. 2015;4:234‐240. [DOI] [PubMed] [Google Scholar]
- 8. Muppidi S, Wolfe GI, Conaway M, et al. MG‐ADL: still a relevant outcome measure. Muscle Nerve. 2011;44:727‐731. [DOI] [PubMed] [Google Scholar]
- 9. Burns TM, Conaway M, Sanders DB. The MG Composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74:1434‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burns TM, Conaway MR, Cutter GR, Sanders DB. Construction of an efficient evaluative instrument for myasthenia gravis: the MG composite. Muscle Nerve. 2008;38:1553‐1562. [DOI] [PubMed] [Google Scholar]
- 11. Mullins LL, Carpentier MY, Paul RH, Sanders DB. Disease‐specific measure of quality of life for myasthenia gravis. Muscle Nerve. 2008;38:947‐956. [DOI] [PubMed] [Google Scholar]
- 12. Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487‐1489. [DOI] [PubMed] [Google Scholar]
- 13. Lee I, Kaminski HJ, McPherson T, Feese M, Cutter GC. gender differences in prednisone adverse effects: survey result from the MG Registry. Neurol Neuroimmunol Neuroinflamm. 2018;5:e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee I, Kaminski HJ, Xin H, Cutter GC. Gender and Quality of Life in Myasthenia Gravis Patients from the Myasthenia Gravis Foundation of America Registry. Muscle Nerve. 2018;58:90‐98. [DOI] [PubMed] [Google Scholar]
- 15. Burns TM, Conaway MR, Cutter GR, Sanders DB. Less is more, or almost as much: a 15‐item quality‐of‐life instrument for myasthenia gravis. Muscle Nerve. 2008;38:957‐963. [DOI] [PubMed] [Google Scholar]
- 16. Burns TM, Grouse CK, Wolfe GI, Conaway MR, Sanders DB. The MG‐QOL15 for following the health‐related quality of life of patients with myasthenia gravis. Muscle Nerve. 2011;43:14‐18. [DOI] [PubMed] [Google Scholar]
- 17. Wolfe GI, Herbelin R, Nations SP, Foster B, Bryan WW. Myasthenia Gravis Activities of Daily Living profile. Neurology. 1999;52:1487. [DOI] [PubMed] [Google Scholar]
- 18. Marrie RA, Cutter G, Tyry D, Campagmolo D, Vollmer T. Validation of the NARCOMS registry: diagnosis. Mult Scler. 2007;13:770‐775. [DOI] [PubMed] [Google Scholar]
- 19. Barnett TC, Bril V, Davis AM. Performance of individual items of the quantitative myasthenia gravis score. Neuromuscul Disord. 2013;23:413‐417. [DOI] [PubMed] [Google Scholar]
- 20. Guptill JT, Marano A, Krueger A, Sanders DB. Cost analysis of myasthenia gravis from a large U.S. insurance database. Muscle Nerve. 2011;44:907‐911. [DOI] [PubMed] [Google Scholar]
- 21. Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurol Clin. 2018;36:253‐260. [DOI] [PubMed] [Google Scholar]
- 22. Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375:511‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Questionnaire for collection of MG immunotherapy data.
FIGURE S2 STROBE diagram.
TABLE S1 Definitions of subgroups


