ABSTRACT
Aim
Haemoglobin (Hb) variability has been reported to be associated with mortality in dialysis patients in some but not all studies. We aimed to establish the prognostic significance of Hb variability with all‐cause mortality in haemodialysis patients through this meta‐analysis.
Methods
The Medline, Embase, Cochrane Library and Web of Science databases were searched for studies assessing the association between Hb variability and all‐cause mortality in haemodialysis patients after adjustment for other covariates.
Results
We included three studies of five cohorts with a total of 262 641 patients. Forest plots showed that the combined hazard ratio for all‐cause mortality was 1.09 (95% CI = 1.01–1.08; P = 0.03) per 1 g/dL increase in Hb variability.
Conclusion
Based on the current evidence, our meta‐analysis found an association between Hb variability and all‐cause mortality in patients receiving haemodialysis therapy.
Keywords: all‐cause mortality, haemodialysis patients, Hb variability, meta‐analysis, systematic review
SUMMARY AT A GLANCE
Three studies of five cohorts with a total of 262 641 patients showed a combined hazard ratio for all‐cause mortality in HD patients to be 1.09 (95% CI = 1.01–1.08; P = 0.03) per 1 g/dL increase in the haemoglobin level. A significant heterogeneity exists between the studies.
Anaemia is a common complication that is associated with adverse cardiovascular complications and poor outcomes in patients with chronic kidney disease (CKD), especially those on dialysis.1 Since initiation of the use of erythropoiesis‐stimulating agents (ESA) in dialysis patients 20 years ago, the average haemoglobin (Hb) levels have risen steadily and transfusion needs have declined, but the optimal concentration is still a matter of debate. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend target Hb levels in the range of 11–12 g/dL, whereas a Hb level of 13 g/dL should be avoided.2 Maintaining patients’ Hb levels in such a narrow range is difficult due to the loss of physiological regulation of red cell formation and many other factors, such as iron deficiency, chronic inflammation, secondary hyperparathyroidism, malnutrition and inadequate‐dose dialysis. The data show that only 30% of patients will fall within this range at any point in time because fluctuations in the Hb level result in frequent under‐ and overshooting of the target level.3
Over the last decade, much attention has been paid to variability in Hb levels for dialysis patients. Evidence from various clinical trials and the US Renal Data System suggests that Hb variability is very common in maintenance dialysis populations,3, 4, 5, 6, 7 and the standard deviation (SD) of Hb in these populations in the United States is 1.1–1.3 g/dL.8, 9 However, is Hb variability independently associated with mortality? Many large population‐based studies investigating Hb variability have been performed to date, but the results are controversial.3, 4, 6, 9 Therefore, we conducted this meta‐analysis to assess the predictive value of Hb variability in haemodialysis patients for all‐cause mortality.
METHODS
Study selection
We searched the Medline, Embase, Cochrane Library and Web of Science databases to 25 November 2018. Combinations of the search terms ‘Hb variability’ and ‘haemodialysis’ were used. Studies were included if they met the following criteria: (i) adult haemodialysis participants aged ≥18 years with at least 6 months of dialysis, which is regarded as the enrollment phase for the description of Hb variability; (ii) use of SD or residual SD to describe Hb variability; and (iii) assessed the association between Hb variability and all‐cause mortality after adjustment for other covariates.
Two investigators (LFZ and CXH) independently reviewed all identified articles for eligibility using the above criteria. The titles and abstracts of the identified articles were reviewed, and those deemed ineligible were excluded. The full text of the remainder of the articles was retrieved and reviewed. Discrepancies on whether to include a study were resolved by discussion.
Data extraction
Data were extracted from all articles by two separate investigators (LFZ and CXH) independently. The data extracted from each study included the authors, year of publication, design of the trial, patient characteristics, sample size, follow‐up, Hb variability parameters, adjusted covariates and hazard ratios (HR). Discrepancies in data abstraction were resolved by discussion. When the required data were not available, we emailed the study authors.
Quality assessment
We assessed the quality of cohort studies using the Newcastle‐Ottawa scale,10 including selection (0–4 points), comparability (0–2 points) and outcome (0–3 points). The maximum score is 8 points, which represents the highest methodological quality.
Data analysis and statistical methods
All statistical analyses were performed using the review manager 5.3 statistical software (Cochrane Collaboration, Oxford, UK) for the meta‐analysis. The pooled HR and 95% confidence intervals (CI) were calculated per 1 g/dL increase in the SD or residual SD of Hb using a random‐effects model. We also assessed the heterogeneity of the outcomes with the χ2 test and evaluated the extent of inconsistency using the I2 measure. P < 0.05 was considered statistically significant.
RESULTS
Eligible studies and quality assessment
The electronic and manual search retrieved 189 citations. A total of 18 studies did not use residual SD or SD to describe Hb variability, whereas 1 study reported the facility‐level Hb variability. Another study by Brunelli et al. was a reanalysis of Yang's study and thus was excluded. Finally, three studies with five cohorts were included in this analysis3, 11, 12 (Fig. 1).
Figure 1.
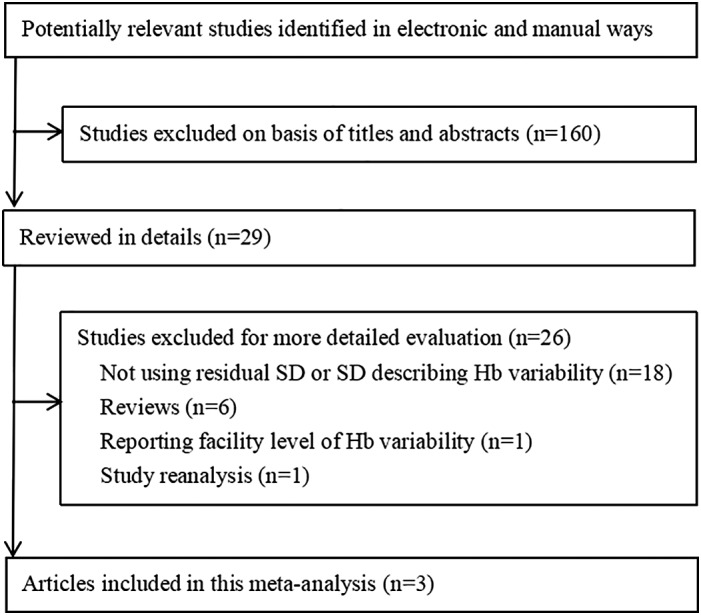
Flowchart of meta‐analysis.
The characteristics of the included studies are summarized in Table 1. The total sample size of the five cohorts was 262 641. Weinhandl et al. included two cohorts of prevalent haemodialysis patients and one cohort of incident haemodialysis patients, whereas the study by Brunelli et al. only contained incident haemodialysis patients.3, 11 Yang et al. analyzed data originating from both incident and prevalent haemodialysis patients.12 The definition of incident or prevalent haemodialysis patients varied in each study. For example, Brunelli et al. characterized incident haemodialysis patients as those on haemodialysis for a period of less than 1 month before enrollment.3 The incident haemodialysis patients included in the study by Weinhandl et al. were those who initiated dialysis therapy between 1 January 2005 and 30 June 2006.11 Yang et al. did not describe the population characteristics in their study; we obtained this information from the study by Brunelli et al., which was a reanalysis of the data from Yang's study.12, 13 All of the studies used residual SD as a measurement of Hb variability and assessed the association between Hb variability and all‐cause mortality after adjustment for other covariates. The details of the adjusted covariates in each study are listed in Table 2. Generally, these adjusted covariates could be classified into six categories (demographic data, comorbid conditions, laboratory findings, medication, hospitalization and other Hb parameters). The study by Weinhandl et al. did not adjust for laboratory and medication as covariates, whereas hospitalization was not mentioned by Brunelli et al. Because of their strict inclusion criteria, Yang et al. were able to adjust for the hospitalization covariate; this study only included subjects with no missing Hb values based on the hypothesis that such patients probably were stable haemodialysis patients whose Hb variability would not be the result of variations in their health status. Regarding the other Hb parameters, instead of using the mean Hb and Hb slope as adjusted covariates as reported by Brunelli et al. and Yang et al., Weinhandl et al. introduced the concept of a low Hb duration, which was defined as the number of months with a Hb level < 10 g/dL.
Table 1.
Characteristics of included studies
| Author | Year of the cohort | Design of the study | Population | Sample size (n) | Follow‐up | Hb variability parameters | HR |
|---|---|---|---|---|---|---|---|
| Weinhandl (2011) | 2006 | Retrospective cohort study | Prevalent haemodialysis patients | 133 246 | 1 year | Residual SD (per 1 g/dL increase) | 1.02 (95% CI = 0.99–1.05) |
| Weinhandl (2011 p) | 1996 | Retrospective cohort study | Prevalent haemodialysis patients | 78 602 | 1 year | Residual SD (per 1 g/dL increase) | 1.07 (95% CI = 1.03–1.12) |
| Weinhandl (2011 i) | 2006 | Retrospective cohort study | Incident haemodialysis patients | 24 999 | 1 year | Residual SD (per 1 g/dL increase) | 1.01 (95% CI = 0.95–1.06) |
| Brunelli (2008) | 2004 | Retrospective cohort study | Incident haemodialysis patients | 6644 | 3082 patient‐years | Residual SD (per 1 g/dL increase) | 1.11 (95% CI = 0.92–1.33) |
| Yang (12) | 1996 | Retrospective cohort study | Incident and prevalent haemodialysis patients | 19 150 | 527 967 patient‐months | Residual SD (per 1 g/dL increase) | 1.33 (95% CI = 1.22–1.45) |
Hb, haemoglobin; HR, hazard ratio; SD, standard deviation.
Table 2.
Adjusted covariates of included studies
| Author | Adjusted covariates | |||||
|---|---|---|---|---|---|---|
| Demographic data | Comorbid conditions | Laboratory | Medication | Hospitalization | Other Hb parameters | |
| Weinhandl (2011) | Age, sex, race | Primary cause of ESRD, ESRD duration, AHD, CHF, arrhythmia, other cardiac disease, DM, GIB, cerebrovascular accident, TIA, PVD, cancer, COPD, hepatic disease | NM | NM | Cumulative hospitalization days | Number of months with Hb level < 10 g/dL |
| Weinhandl (2011 p) | Age, sex, race | Primary cause of ESRD, ESRD duration, AHD, CHF, arrhythmia, other cardiac disease, DM, GIB, cerebrovascular accident, TIA, PVD, cancer, COPD, hepatic disease | NM | NM | Cumulative hospitalization days | Number of months with Hb level < 10 g/dL |
| Weinhandl (2011 i) | Age, sex, race | Primary cause of ESRD, ESRD duration, AHD, CHF, arrhythmia, other cardiac disease, DM, GIB, cerebrovascular accident, TIA, PVD, cancer, COPD, hepatic disease bleeding, hepatic disease | NM | NM | Cumulative hospitalization days | Number of months with Hb level < 10 g/dL |
| Brunelli (2008) | BMI, sex, race | DM, hypertension, arterial disease, CHF, cancer | URR, Kt/V, albumin, bicarbonate, calcium, phosphate, creatine, iPTH, ferritin, TS | Intravenous iron dose, EPO dose | NM | Mean Hb, Hb slope |
| Yang (12) | Age, sex, race | DM, duration of ESRD | URR, Kt/V, albumin, AST, bicarbonate, calcium, phosphate, iPTH, ferritin, TS | Intravenous iron dose, EPO dose | All included subjects with no missing Hb values | Mean Hb, Hb slope |
AHD, atherosclerotic heart disease; AST, aspartate aminotransferase; BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; EPO, erythropoietin; ESRD, end‐stage renal disease; GIB, gastrointestinal bleeding; Hb, haemoglobin; iPTH, intact parathyroid hormone; NM, not mentioned; PVD, peripheral vascular disease; TIA, transient ischaemic attack; TS, transferrin saturation; URR, urea reduction ratio.
The quality assessment analysis results are presented in Table 3. All three studies were assessed as high quality.
Table 3.
Quality assessment of included studies
| Author | Representativeness | Selection of the non exposed cohort | Ascertainment | Outcome was not present at start of study | Comparability | Assessment of outcome | Follow‐up time | Adequacy of follow‐up | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Yang (12) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | High | |
| Weinhandl (2011) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | High | |
| Brunelli (2008) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | High |
The star symbol means the corresponding assessment has been met.
Prognostic value of Hb variability
The relationship between Hb variability and all‐cause mortality is plotted in Figure 2. The forest plots showed that the combined HR for all‐cause mortality among the five cohorts was 1.09 (95% CI = 1.01–1.18; P = 0.03) per 1 g/dL increase in Hb variability.
Figure 2.
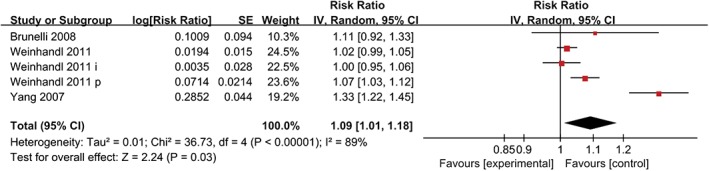
The relationship between Hb variability and all‐cause mortality.
Subgroup analysis
To reduce the heterogeneity of this meta‐analysis, we conducted a subgroup analysis. First, according to the different populations included in these five cohorts, we subsequently divided the patients into three subgroups (the incident haemodialysis patient group, prevalent haemodialysis patient group and combined group); the combined group contained both incident and prevalent haemodialysis patients. The HR for all‐cause mortality in each subgroup were 1.01 (95% CI = 0.96–1.07; P = 0.67), 1.04 (95% CI = 0.99–1.10; P = 0.10) and 1.33 (95% CI = 1.22–1.45; P < 0.00001) per 1 g/dL increase in Hb variability, respectively (Fig. 3). The subgroup differences between the incident haemodialysis and prevalent haemodialysis patient groups were not significant (P = 0.4). In contrast, significant subgroup differences were found between the combined group and both the incident haemodialysis patient group (P < 0.00001) and the prevalent haemodialysis patient group (P < 0.00001) (see Figures [Link], [Link], [Link]).
Figure 3.
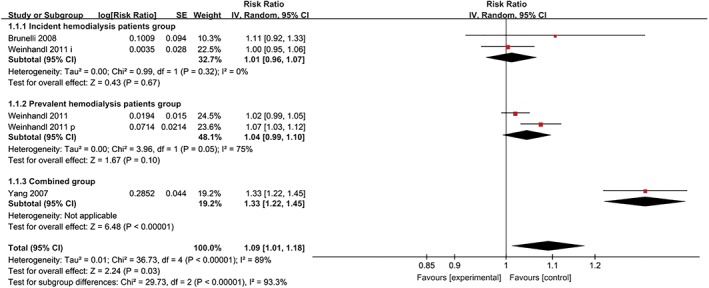
Subgroup analysis based on the different populations included in these five cohorts.
Different adjusted covariates in these five cohorts were another primary source of heterogeneity in the results of this meta‐analysis. The results did not change after excluding the study by Brunelli et al., which did not contain hospitalization as an adjusted covariate (P < 0.00001) (Fig. 4). Medication and laboratory findings were also key covariates. An analysis of the effect of Hb variability on all‐cause mortality adjusted for medication and laboratory findings is plotted in Figure 5. The combined HR among the two cohorts was 1.24 (95% CI = 1.04–1.47; P = 0.02) per 1 g/dL increase in Hb variability, which was consistent with the previous result.
Figure 4.
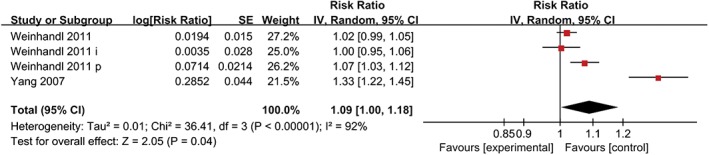
Analysis result after excluding a study which did not contain hospitalization as an adjusted covariate.
Figure 5.

Analysis result after excluding studies which did not contain medication and laboratory as adjusted covariates.
DISCUSSION
Hb variability in haemodialysis patients was first described by Lacson and Berns in 2003,14, 15 but for decades whether Hb variability was independently associated with mortality remained controversial. To the best of our knowledge, this report is the first meta‐analysis of an association between Hb variability and all‐cause mortality in haemodialysis patients. Our study demonstrated a 9% increase in the adjusted rate of death for each 1 g/dL increase in Hb variability.
Maintenance of steady Hb levels is mandatory to ensure consistent and adequate oxygen delivery to tissues.16 In normal subjects, individual variation in the Hb level occurs within the range of normal values and generally does not exceed 1 g/dL.17, 18 However, for haemodialysis patients, variability in the Hb level over time is very common, with almost 90% of patients experiencing Hb level changes over a 6‐month period.6 Fluctuations in the Hb levels induce repeated episodes of relative ischaemia and tissue hypoxia, which may result in organ dysfunction or injury, such as left ventricular hypertrophy19, 20 and autonomic nervous system dysfunction.21
Is the association between Hb variability and mortality different between incident and prevalent haemodialysis patients? The health status among the incident haemodialysis patients was worse than that of the prevalent haemodialysis patients, which might diminish the influence of Hb variability on mortality.1 Brunelli et al. demonstrated in their article that the effect of Hb variability on mortality among incident haemodialysis patients might differ from that among prevalent patients. After reanalysing the data from Yang's study, which were limited to incident haemodialysis patients, the authors found that the detrimental effect of Hb variability on mortality disappeared.3 To answer this question, we conducted a subgroup analysis. Although we found a tendency for a statistically significant difference in the prevalent haemodialysis patient group (HR = 1.04; 95% CI = 0.99–1.10; P = 0.10), current evidence did not support the hypothesis that a difference might exist in the association between Hb variability and mortality in incident and prevalent haemodialysis patients.
The included studies adjusted for different covariates, which might lead to different results and subsequently an unreliable conclusion from our meta‐analysis. For example, when the data in the study by Brunelli et al. were adjusted for the covariates mentioned in Yang's study, the HR increased (1.22 vs 1.11).3 Some strong covariates, such as comorbid conditions, hospitalization, absolute Hb levels, medication and laboratory findings, may diminish the prognostic effect of Hb variability on the associated mortality risk. The relationship between Hb variability and intercurrent illness is intertwined. Various intercurrent events, including infections, vascular access complications and malnutrition, have been shown to be associated with an increased mortality risk.22, 23 Fluctuations in the Hb level during hospitalization are very common.24, 25 Some experts have considered that the HR of mortality reflected by Hb variability may be merely a surrogate for the health status. In our meta‐analysis, the results did not change after excluding the study by Brunelli et al., which did not adjust for hospitalization, suggesting that Hb variability was associated with the mortality risk independent of hospitalization (Fig. 4). Similar results were obtained after excluding cohorts not adjusted for medication and laboratory findings (Fig. 5). The results of Brunelli et al., who adjusted for medication and laboratory findings as time‐dependent confounding factors in a history‐adjusted marginal structural model, further confirmed the result that Hb variability was an independent risk factor for all‐cause mortality in haemodialysis patients regardless of the medication and laboratory covariates.13 Comorbid conditions and the absolute Hb levels are also important covariates that should be adjusted. Comorbid conditions, including cardiac disease, hypertension, cerebrovascular disease, diabetes mellitus, gastrointestinal bleeding and so on, have been verified to present a higher risk of death.26 The mortality of patients with intractable anemia or who have Hb levels less than 11 g/dL 80–100% of the time was increased by several fold.27, 28 In our meta‐analysis, all five included cohorts adjusted for these two confounding factors, which improved the reliability of this research.
In addition to SD or residual SD, many other methodologies have been used as tools to describe Hb variability in other studies. These parameters include average real variability, coefficient of variation, Hb cycling and fluctuations across thresholds. Variation may exist when different Hb variability parameters are used to assess its impact on mortality. For example, Gilbertson et al. divided Hb values into the following three categories: L (Hb < 10 g/dL), M (Hb: 11–12.5 g/dL) and H (Hb > 12.5 g/dL). After adjusting for covariates, the HR for mortality events were significantly increased in the L‐H group (HR = 2.38; 95% CI = 1.20–4.71; P: 0.013), indicating that Hb variability could be a primary driver of an increased risk of death.29 However, when Hb cycling (Hb variability with an amplitude >1.5 g/dL and duration >8 weeks) was used to describe Hb variability in 150 haemodialysis patients, Thanakitcharu et al. could not demonstrate a significant influence on mortality and hospitalization.30 Because no single method could completely characterize Hb variability in haemodialysis patients, Arneson summarized the strengths and limitations of these parameters in their review; they concluded that no method could be judged better than another but that SD and residual SD could produce a single and scaled value representing variability that was more feasible for comparisons between different groups.31 Use of residual SD to analyse Hb variability was first applied by Yang et al..12 This model first drew a regression line based on a series of Hb values and then calculated variations from the regressive average Hb. This method was easy to understand and was useful when trends were generally linear,32 but it could not provide insights into different types of fluctuations and was inaccurate when patients experienced U‐shaped patterns. For example, most incident haemodialysis patients do not receive ESA therapy until the initiation of dialysis. Therefore, they may experience a robust increase in Hb at the beginning due to ESA supplementation. Subsequently, the Hb level would be maintained at a relatively steady state, leading to a curvilinear trajectory. Application of a linear model in these cases would overestimate the Hb variability and create bias.3 To solve this problem, Heinzl et al. demonstrated in their article that the use of cubic spline functions allowed investigation of this type of exposure.33 Flythe et al. also mentioned that metrics based on residuals from mixed models might be the best choice for blood pressure variability metrics.34 Because we could not obtain metrics based on residuals from mixed models from the original studies, we had no choice but to select SD and residual SD as parameters to describe Hb variability.
How can we translate this result into clinical experience? The potential factors that might influence patients’ Hb variability were summarized in a review by Aronoff.35 These authors concluded that drug‐related factors, patient demographics, iron deficiency, infections, inflammation, malignancies and reimbursement‐related factors all had an impact on Hb variability. Of these many factors, the ESA dose was the most actionable factor in the management of anaemia for patients on dialysis therapy, as mentioned by Jay B. Wish,36 and Fishbane demonstrated that ESA dose patterns might contribute to Hb level cycling.5 In consideration of the different pharmacokinetic and pharmacodynamic characteristics of ESA, some authors hypothesized that longer acting ESA might have the potential to reduce Hb variability. However, according to a recent meta‐analysis by Perez‐Ruixo, subject variability in Hb and the ESA dose administered to dialysis patients were associated neither with the type of ESA nor with the dose interval or administration route.37 To be more precise, personalized dose adjustments of ESA have great importance for anaemia management and the patient's well‐being, which was also supported by the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD.38 As artificial intelligence has progressed in recent years, a variety of predictive algorithms based on sophisticated modeling approaches have been proposed to offer personalized treatment in line with the predicted Hb trend and have shown promising outcomes.39, 40, 41, 42 Thus, individual use of ESA has been made possible for haemodialysis patients and may reduce Hb variability.
Some limitations in our study should be noted. First, this study was a meta‐analysis of observational studies and thus may have difficulty determining cause‐and‐effect relationships. Second, we included both incident and prevalent patients in our study. Although the subgroup analysis indicated that no differences existed in the association between Hb variability and mortality in the incident and prevalent haemodialysis patients, the heterogeneity analysis presented that the main source of heterogeneity in this meta‐analysis came from the combined group that included both incident and prevalent haemodialysis patients from the study of Yang et al. However, this study was a high‐quality study based on the Newcastle‐Ottawa scale or the adjusted covariates in this study, and therefore excluding it was impractical. Moreover, considering the small number of studies available for pooling, we could not perform meta‐regression to reduce the heterogeneity mentioned above. Third, many interference factors may have an impact on Hb variability, such as the comorbid diseases, laboratory results and medication variables and the absolute Hb levels, which may diminish the prognostic effect of Hb variability associated with the mortality risk. Although we have conducted a subgroup analysis to minimize the heterogeneity and improve the reliability of this meta‐analysis, some differences related to different adjusted covariates in different included studies may still exist and contribute to the heterogeneity of the results. However, to date, no study had completely adjusted for all covariates. Fourth, multiple observation studies were excluded from this meta‐analysis for not using SD or residual SD to describe Hb variability, which might cause bias in this meta‐analysis. However, most of these studies applied methodologies such as fluctuations across thresholds or Hb cycling to describe Hb variability, which were difficult to pool. The limitation of using SD or residual SD to describe Hb variability was mentioned above. Exploring a better Hb variability parameter or metric combination is an urgent need. Finally, the studies included in this meta‐analysis did not adjust for time‐varying covariates. Time‐varying covariates are variables that can act as both confounders and intermediates between the exposure and outcome. During the search phase, we found a study by Brunelli et al. that adjusted for time‐dependent confounding in a history‐adjusted marginal structural model.13 However, this study was a reanalysis of Yang's study and did not adjust for covariates such as comorbid conditions and hospitalization, which might have caused heterogeneity in our meta‐analysis.
Based on the current evidence, our meta‐analysis found an association between Hb variability and all‐cause mortality in patients receiving haemodialysis therapy.
DISCLOSURE
We have no conflict of interest to report.
Supporting information
Fig. S1 Subgroup analysis of the relationship between Hb variability and all‐cause mortality in incident haemodialysis patients group and prevalent haemodialysis patients group.
Fig. S2 Subgroup analysis of the relationship between Hb variability and all‐cause mortality in incident haemodialysis patients group and combined group.
Fig. S3 Subgroup analysis of the relationship between Hb variability and all‐cause mortality in prevalent haemodialysis patients group and combined group.
REFERENCES
- 1. U.S. Renal Data System . USRDS 2007 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disorders, 2007. [Google Scholar]
- 2. KDOQI . Clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am. J. Kidney Dis. 2007; 50: 471–530. [DOI] [PubMed] [Google Scholar]
- 3. Brunelli SM, Lynch KE, Ankers ED et al Association of hemoglobin variability and mortality among contemporary incident hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008; 3: 1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foley RN, Zhang R, Gilbertson DT, Dunning S, Ishani A, Collins AJ. Exceeding hemoglobin target levels in US hemodialysis patients receiving epoetin, 1999 to 2002. Hemodial. Int. 2007; 11: 333–9. [DOI] [PubMed] [Google Scholar]
- 5. Fishbane S, Berns JS. Evidence and implications of haemoglobin cycling in anaemia management. Nephrol. Dial. Transplant. 2007; 22: 2129–32. [DOI] [PubMed] [Google Scholar]
- 6. Ebben JP, Gilbertson DT, Foley RN, Collins AJ. Hemoglobin level variability: Associations with comorbidity, intercurrent events, and hospitalizations. Clin. J. Am. Soc. Nephrol. 2006; 1: 1205–10. [DOI] [PubMed] [Google Scholar]
- 7. Collins AJ, Kasiske B, Herzog C et al Excerpts from the United States renal data system 2006 annual data report. Am. J. Kidney Dis. 2007; 49: A6–7 S1‐296. [DOI] [PubMed] [Google Scholar]
- 8. Regidor DL, Kopple JD, Kovesdy CP et al Associations between changes in hemoglobin and administered erythropoiesis‐stimulating agent and survival in hemodialysis patients. J. Am. Soc. Nephrol. 2006; 17: 1181–91. [DOI] [PubMed] [Google Scholar]
- 9. Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ. Hemoglobin level variability: Associations with mortality. Clin. J. Am. Soc. Nephrol. 2008; 3: 133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. Ontario, Canada: Department of Epidemiology and Community Medicine, University of Ottawa. Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 11. Weinhandl ED, Peng Y, Gilbertson DT, Bradbury BD, Collins AJ. Hemoglobin variability and mortality: Confounding by disease severity. Am. J. Kidney Dis. 2011; 57: 255–65. [DOI] [PubMed] [Google Scholar]
- 12. Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI. Hemoglobin variability and mortality in ESRD. J. Am. Soc. Nephrol. 2007; 18: 3164–70. [DOI] [PubMed] [Google Scholar]
- 13. Brunelli SM, Joffe MM, Israni RK et al History‐adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008; 3: 777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lacson E, Ofsthun N, Lazarus JM. Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am. J. Kidney Dis. 2003; 41: 111–24. [DOI] [PubMed] [Google Scholar]
- 15. Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, Deoreo PB. Hemoglobin variability in epoetin‐treated hemodialysis patients. Kidney Int. 2003; 64: 1514–21. [DOI] [PubMed] [Google Scholar]
- 16. De Nicola L, Minutolo R, Conte G. Anaemia management in non‐dialysis chronic kidney disease: Flexibility of target to target stability. Nephron Clin. Pract. 2010; 114: c236–41. [DOI] [PubMed] [Google Scholar]
- 17. Ross DW, Ayscue LH, Watson J, Bentley SA. Stability of hematologic parameters in healthy subjects. Intraindividual versus interindividual variation. Am. J. Clin. Pathol. 1988; 90: 262–7. [DOI] [PubMed] [Google Scholar]
- 18. Dot D, Miró J, Fuentes‐Arderiu X. Within‐subject biological variation of hematological quantities and analytical goals. Arch. Pathol. Lab. Med. 1992; 116: 825–6. [PubMed] [Google Scholar]
- 19. Georgieva Z, Georgieva M. Compensatory and adaptive changes in microcirculation and left ventricular function of patients with chronic iron‐deficiency anaemia. Clin. Hemorheol. Microcirc. 1997; 17: 21–30. [PubMed] [Google Scholar]
- 20. Meerson FZ, Evsevieva ME. Disturbances of the heart structure and function in chronic hemolytic anemia, their compensation with increased coronary flow, and their prevention with ionol, an inhibitor of lipid peroxidation. Adv. Myocardiol. 1985; 5: 201–11. [DOI] [PubMed] [Google Scholar]
- 21. Romero MJC, Hernández A, Agramonte O, Hernández P. Cardiovascular autonomic dysfunction in sickle cell anemia: A possible risk factor for sudden death. Clin. Auton. Res. 1997; 7: 121–5. [DOI] [PubMed] [Google Scholar]
- 22. den Elzen WP, van Manen JG, Boeschoten EW, Krediet RT, Dekker FW. The effect of single and repeatedly high concentrations of C‐reactive protein on cardiovascular and non‐cardiovascular mortality in patients starting with dialysis. Nephrol. Dial. Transplant. 2006; 21: 1588–95. [DOI] [PubMed] [Google Scholar]
- 23. Goldwasser P, Mittman N, Antignani A et al Predictors of mortality in hemodialysis patients. J. Am. Soc. Nephrol. 1993; 3: 1613–22. [DOI] [PubMed] [Google Scholar]
- 24. Fishbane S, Berns JS. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005; 68: 1337–43. [DOI] [PubMed] [Google Scholar]
- 25. Solid CA, Foley RN, Gilbertson DT, Collins AJ. Perihospitalization hemoglobin‐epoetin associations in U.S. hemodialysis patients, 1998 to 2003. Hemodial. Int. 2007; 11: 442–7. [DOI] [PubMed] [Google Scholar]
- 26. Goodkin DA, Bragg‐Gresham JL, Koenig KG et al Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J. Am. Soc. Nephrol. 2003; 14: 3270–7. [DOI] [PubMed] [Google Scholar]
- 27. Ishani A, Solid CA, Weinhandl ED, Gilbertson DT, Foley RN, Collins AJ. Association between number of months below K/DOQI haemoglobin target and risk of hospitalization and death. Nephrol. Dial. Transplant. 2008; 23: 1682–9. [DOI] [PubMed] [Google Scholar]
- 28. Kausz AT, Solid C, Pereira BJ, Collins AJ, St PW. Intractable anemia among hemodialysis patients: A sign of suboptimal management or a marker of disease. Am. J. Kidney Dis. 2005; 45: 136–47. [DOI] [PubMed] [Google Scholar]
- 29. Kainz A, Mayer B, Kramar R, Oberbauer R. Association of ESA hypo‐responsiveness and haemoglobin variability with mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2010; 25: 3701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thanakitcharu P, Jirajan B. Prevalence of hemoglobin cycling and its clinical impact on outcomes in Thai end‐stage renal disease patients treated with hemodialysis and erythropoiesis‐stimulating agent. J. Med. Assoc. Thai. 2016; 99: S28–37. [PubMed] [Google Scholar]
- 31. Arneson TJ, Zaun D, Peng Y, Solid CA, Dunning S, Gilbertson DT. Comparison of methodologies to characterize haemoglobin variability in the US Medicare haemodialysis population. Nephrol. Dial. Transplant. 2009; 24: 1378–83. [DOI] [PubMed] [Google Scholar]
- 32. Shafi T, Sozio SM, Bandeen‐Roche KJ et al Predialysis systolic BP variability and outcomes in hemodialysis patients. J. Am. Soc. Nephrol. 2014; 25: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput. Methods Programs Biomed. 1997; 54: 201–8. [DOI] [PubMed] [Google Scholar]
- 34. Flythe JE, Brunelli SM. Blood pressure variability among chronic dialysis patients: Recent advances in knowledge. Curr. Opin. Nephrol. Hypertens. 2015; 24: 163–9. [DOI] [PubMed] [Google Scholar]
- 35. Kalantar‐Zadeh K, Aronoff GR. Hemoglobin variability in anemia of chronic kidney disease. J. Am. Soc. Nephrol. 2009; 20: 479–87. [DOI] [PubMed] [Google Scholar]
- 36. Wish JB. Hemoglobin variability as a predictor of mortality: What's a practitioner to do. Am. J. Kidney Dis. 2011; 57: 190–3. [DOI] [PubMed] [Google Scholar]
- 37. Pérez‐Ruixo JJ, Cucala‐Ramos M, García‐Gonzalo E, del Val Romero B, Valveny N. Between subjects variability in haemoglobin and dose are not associated with the erythropoiesis‐stimulating agent used to treat anaemia in dialysis: A meta‐analysis. Br. J. Clin. Pharmacol. 2013; 75: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int. 2012; 82: 952–60. [DOI] [PubMed] [Google Scholar]
- 39. Brier ME, Gaweda AE. Predictive modeling for improved anemia management in dialysis patients. Curr. Opin. Nephrol. Hypertens. 2011; 20: 573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nichols B, Shrestha RP, Horowitz J et al Simplification of an erythropoiesis model for design of anemia management protocols in end stage renal disease. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011; 2011: 83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martínez‐Martínez JM, Escandell‐Montero P, Barbieri C et al Prediction of the hemoglobin level in hemodialysis patients using machine learning techniques. Comput. Methods Programs Biomed. 2014; 117: 208–17. [DOI] [PubMed] [Google Scholar]
- 42. Escandell‐Montero P, Chermisi M, Martínez‐Martínez JM et al Optimization of anemia treatment in hemodialysis patients via reinforcement learning. Artif. Intell. Med. 2014; 62: 47–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Subgroup analysis of the relationship between Hb variability and all‐cause mortality in incident haemodialysis patients group and prevalent haemodialysis patients group.
Fig. S2 Subgroup analysis of the relationship between Hb variability and all‐cause mortality in incident haemodialysis patients group and combined group.
Fig. S3 Subgroup analysis of the relationship between Hb variability and all‐cause mortality in prevalent haemodialysis patients group and combined group.


