Abstract
Aim
To evaluate the use of recombinant human fibroblast growth factor (rhFGF)‐2 in combination with deproteinized bovine bone mineral (DBBM) compared with rhFGF‐2 alone, in the treatment of intrabony periodontal defects.
Materials and Methods
Patients with periodontitis who had received initial periodontal therapy and had intrabony defects of ≥ 3 mm in depth were enrolled. Sites were randomly assigned to receive a commercial formulation of 0.3% rhFGF‐2 + DBBM (test) or rhFGF‐2 alone (control). Clinical parameters and a patient‐reported outcome measure (PROM) were evaluated at baseline and at 3 and 6 months postoperatively.
Results
Twenty‐two sites in each group were evaluated. A significant improvement in clinical attachment level (CAL) from baseline was observed in both groups at 6 months postoperatively. CAL gain was 3.16 ± 1.45 mm in the test group and 2.77 ± 1.15 mm in the control group, showing no significant difference between groups. Radiographic bone fill was significantly greater in the test group (47.2%) than in the control group (29.3%). No significant difference in PROM between groups was observed.
Conclusions
At 6 months, no significant difference in CAL gain or PROM between the two treatments was observed, although combination therapy yielded an enhanced radiographic outcome.
Keywords: bone graft, deproteinized bovine bone mineral, FGF‐2, patient‐reported outcome, periodontal regeneration, periodontitis
1. INTRODUCTION
Thus far, many regenerative approaches have been introduced as therapy for periodontal defects. Of these, guided tissue regeneration (GTR) and treatment with enamel matrix derivative (EMD) are widely performed and have shown considerable success (Heden & Wennstöm, 2006; Kao, Nares, & Reynolds, 2015; Rösing, Aass, Mavropoulos, & Gjermo, 2005; Tonetti et al., 2004).
Guided tissue regeneration using a collagen barrier membrane and deproteinized bovine bone mineral (DBBM) as a scaffold has been reported to yield significantly greater regeneration of periodontal tissue, compared with that achieved by using each material alone (Camelo et al., 2001; Nevins, Camelo, Nevins, Schenk, & Lynch, 2003). We previously reported favourable clinical outcomes at up to 2.5 years after combination therapy in the treatment of intrabony defects (Irokawa, Okubo, et al., 2017; Irokawa, Takeuchi, et al., 2017). However, such procedure involving the use of a barrier membrane is technique‐sensitive and difficult to implement in some clinical situations. Notably, limitations have also been reported in the predictability of regenerative therapy, including bone grafts and GTR (Kao et al., 2015; Lin, Rios, & Cochran, 2015).
Attempts have been made to use bone substitutes with biological agents, without barrier membranes. Clinical and histological analyses in humans have revealed that treatment using recombinant human platelet‐derived growth factor (rhPDGF)‐BB with bone allograft yielded regeneration of periodontal tissues in intrabony defects and Class II furcations at 9 months (Nevins et al., 2003). In a large multicenter randomized controlled trial (RCT), the use of beta‐tricalcium phosphate (β‐TCP) with rhPDGF‐BB showed superior clinical outcomes, compared with those achieved using β‐TCP alone, at 6 months (Nevins et al., 2005). Three‐year extension results from a multicenter RCT showed that use of rhPDGF‐BB with β‐TCP yielded long‐term clinical and radiographic improvements (Nevins et al., 2013). Furthermore, a systematic review indicated that EMD used with bone graft materials may yield additional clinical improvements, as measured by gains in clinical attachment (CAL) and reductions in probing depth, compared with those achieved using EMD alone (Matarasso et al., 2015).
Fibroblast growth factor (FGF)‐2 exerts mitogenic and angiogenic effects on mesenchymal cells in the periodontal ligament and has been shown to regenerate periodontal tissue in pre‐clinical models (Ishii et al., 2013; Murakami et al., 2003; Oi, Ota, Yamamoto, Shibukawa, & Yamada, 2009; Takayama, Murakami, Shimabukuro, Kitamura, & Okada, 2001). Clinical trials have shown that trafermin, a form of recombinant human FGF‐2 (rhFGF‐2) is a safe and effective regenerative therapy in patients with periodontitis (Kitamura et al., 2008, 2011, 2016). Notably, a novel regenerative therapy using 0.3% rhFGF‐2 received a formal pharmaceutical approval for clinical use in Japan in December 2016.
A comparative controlled clinical trial showed that, at 1 year postoperatively, the use of combination therapy, comprising EMD and DBBM, yielded greater improvements in clinical and radiographical outcomes than achieved using EMD alone (Zucchelli, Amore, Montebugnoli, & De Sanctis, 2003). Additionally, treatment using either EMD with DBBM or collagen membrane with DBBM showed similar results with respect to non‐contained intrabony defects at 1 year postoperatively (Iorio‐Siciliano et al., 2014). However, no information is available regarding the effect of combined use of rhFGF‐2 with DBBM on periodontal healing.
This randomized, prospective study aimed to assess the use of rhFGF‐2 in combination with DBBM, compared with rhFGF‐2 alone, in the treatment of intrabony periodontal defects after an observation period of 6 months.
2. MATERIALS AND METHODS
2.1. Study design
This prospective, parallel‐arm, single‐blind RCT, which involved periodontal regenerative therapy in patients with periodontitis, was conducted at two centres: Tokyo Dental College Suidobashi Hospital (Tokyo, Japan) and Tokyo Dental College Chiba Hospital (Chiba, Japan). This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Tokyo Dental College (No.747). This RCT was registered (UMIN 000025257) and followed Consolidated Standards of Reporting Trials (CONSORT) guidelines.
2.2. Participants
Study participants, aged 20–79 years, were recruited consecutively between January 2017 and February 2018 from among patients with moderate to severe chronic periodontitis (Armitage, 1999; Page & Eke, 2007), all of whom had completed initial periodontal therapy (IP). All participants consented to enrolment in this study.
The inclusion criteria were as follows: presence of an intrabony defect depth of ≥ 3 mm in inter‐proximal areas of teeth, inter‐proximal sites with probing pocket depth (PPD) ≥ 4 mm, and a good level of plaque control [mean Plaque Control Record (O'Leary, Drake, & Naylor, 1972) ≤ 20%]. A sufficient level of keratinized gingiva had to be present for complete tissue coverage.
Exclusion criteria were the presence of diabetes mellitus; respiratory and cardiovascular diseases; immunodeficiency; oral cancers and other cancers requiring treatment within the past 5 years; an ongoing smoking habit; allergy to medication; and/or previous or concurrent bisphosphonate or corticosteroid therapy. We excluded patients who were pregnant/lactating, as well as those with general contraindications for surgical therapy.
2.3. Clinical examination
After obtaining systemic and dental histories, the following examinations were performed by calibrated examiners who were blinded to treatment assignment: PPD was measured using a periodontal probe with a constant force applier (Gram Probe #2, YDM, Tokyo, Japan). PPD and gingival recession (GR) were measured in 0.5 mm increments. CAL was determined by adding PPD values to GR values. Bleeding on probing (BOP) was assessed dichotomously, and tooth mobility (TM) was also evaluated (Miller, 1950). These parameters were re‐evaluated at 3 and 6 months postoperatively.
In an investigator meeting that was held before initiation of the study, six non‐study volunteers participated in a calibration exercise. Intra‐examiner reproducibility was assessed as the standard deviation (SD) of the difference in triplicate measurements. All examiners achieved an SD of ≤ 0.4 mm for PPD. Inter‐examiner variability was assessed as the difference from the gold standard examiner. The kappa value ranged from 0.75 to 0.80 for PPD.
2.3.1. Radiographic examination
Periapical radiographs were taken using customized film holders as described previously (Irokawa, Okubo, et al., 2017). Prior to surgery, the depth of intrabony defects was estimated on radiographs; this was confirmed during surgery.
Assessment of radiographic changes (Schei et al., 1959) at the surgical sites was performed using a method previously described (Seshima et al., 2017). The difference in tooth axis height between the cemento‐enamel junction and the defect bottom was defined as linear bone growth (LBG). The percentage of radiographic bone fill was determined by dividing the LBG by the baseline defect depth.
2.3.2. Patient‐reported outcome measure
An oral health‐related quality of life (QoL) instrument, the OHRQL (Japanese version) (Saito et al., 2010, 2011), was used. Scoring was performed as previously described (Saito et al., 2010, 2011).
2.4. Sample size estimation
Two treatment modalities were compared; a combined application of rhFGF‐2 + DBBM (test) and rhFGF‐2 alone (control). A sample size of 20 defects in each group was needed to achieve 80% power for detection of a clinically relevant difference in CAL gain of 1.1 mm between treatment groups, assuming a SD of 1.2 mm (Losada, González, Garcia, Santos, & Nart, 2017) with an alpha level 0.05 (two‐tailed). An overquotation of 10% was calculated to compensate for possible sample dropout. Thus, total sample size was 22 sites per group.
The unstratified design was used to calculate a two‐sided 95% CI for the difference in CAL gain 6 months following surgery. The non‐inferiority of test to control was accepted if the lower limit of the 95% CI for between‐group difference was ‐1.1 mm or greater. Superiority of the test treatment was tested only when non‐inferiority was established.
2.5. Randomization, allocation concealment, and blinding
Periodontal defects were randomly assigned (1:1 ratio) to one of the two treatments, using a permuted block method by an individual who was not involved in other aspects of the study. Allocation to test or control groups was concealed from periodontists until the time of surgery. The assignment was performed using a sealed‐envelope method, and patients were blinded to their assigned treatment. If a patient had more than one defect, all defects received the same treatment assignment.
2.6. Surgical procedures
Following local infiltration anaesthesia, defects were accessed using the papilla preservation techniques (Cortellini, Pini Prato, & Tonetti, 1995, 1999). After debridement, defect morphology was recorded, and the depth and width of the intrabony defect were measured. Then, root surfaces underwent scaling and root planing, and the sites were rinsed with sterile saline. In the test group, 0.3% rhFGF‐2 [REGROTH® Dental Kit, 600 μg or 1200 μg in hydroxypropyl cellulose (HPC), Kaken Pharmaceutical, Tokyo, Japan] with DBBM (Geistlich Bio‐Oss®, 0.25–1.0 mm granules, Geistlich Pharma AG, Wolhusen, Switzerland) was applied to the defect. Prior to the application, the rhFGF‐2 solution was thoroughly mixed with DBBM in a sterile disposable dish. In the control group, rhFGF‐2 alone was applied to the defect. Immediately after application, the flaps were repositioned for complete closure and sutured with modified vertical mattress or interrupted sutures. Representative treatment cases are shown in Figure 1.
Figure 1.
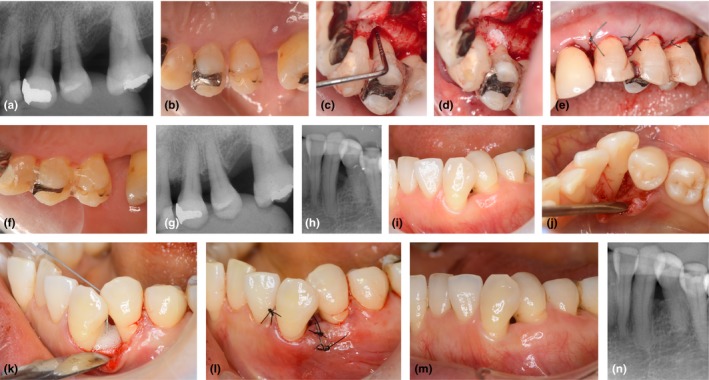
Surgical procedure and outcomes. (a–g) 60‐year‐old female patient who received recombinant human fibroblast growth factor‐2 (rhFGF‐2) with deproteinized bovine bone mineral (DBBM; test group). (a) Preoperative radiograph. (b) Baseline clinical view (palatal). Probing pocket depth (PPD) at the mesial aspect of tooth #24 was 7 mm. (c) Intra‐operative view. Defect depth 3 mm, width 5 mm. (d) After debridement and rinsing, the defect of #24 was filled with rhFGF‐2 formulation as well as DBBM that had been pre‐saturated with rhFGF‐2. (e) Suturing. (f) Six‐month follow‐up view; PPD = 2 mm. (g) Six‐month follow‐up radiograph. (h‐n) 53‐year‐old female patient who received rhFGF‐2 alone (control group); (h) Preoperative radiograph. (i) Baseline clinical view. PPD at the distal aspect of tooth #33 was 7 mm. (j) Intra‐operative view. Defect depth 5 mm, width 3 mm. (k) After debridement and rinsing, the defect of #33 was filled with rhFGF‐2. (l) Suturing. (m) Six‐month follow‐up view. PPD = 3 mm. (n) Six‐month follow‐up radiograph
2.7. Postsurgical care
Patients received antimicrobials (amoxicillin 750 mg/day or cefdinir 300 mg/day) for 4 days. Standard pain medications were prescribed as needed. Patients used a mouthwash twice per day. They gently cleaned the treated area with an ultrasoft toothbrush, beginning 1 day postoperatively and continued for 4 weeks.
Sutures were removed after 10–14 days. The patients were then placed in supportive care programs.
2.8. Statistical analysis
Fisher's exact test was used to assess categorical variables. The Mann–Whitney U test was used to compare other non‐categorical demographic and baseline parameters between the two groups.
The primary outcome was the change in CAL at 6 months postoperatively. Sites were compared with baseline, as well as between treatments. The data from the two centres were pooled for the present analysis. The Friedman test with post hoc analysis was used to compare intra‐group data over time. For intra‐group comparisons of OHRQL scores over time, repeated measures analysis of variance (ANOVA) with Tukey–Kramer post hoc test was used to compare intra‐group data over time, after testing on normal distribution of data.
Correlations between postoperative CAL gains and baseline variables were determined by Spearman correlation coefficient. Statistical software (InStat 3.10, GraphPad, La Jolla, CA, USA) was used. A p value of 0.05 was considered to indicate statistical significance.
3. RESULTS
3.1. Participant demographics and clinical parameters
A total of 34 patients were assessed for eligibility at two study centres; 44 sites in 32 patients were randomized between the two treatment groups (Figure S1 flowchart).
Table 1 shows the participant demographics and baseline clinical parameters. Five patients contributed more than one defect site. There were no significant differences in participant demographics and baseline parameters between groups.
Table 1.
Participant demographics and baseline parameters
| rhFGF‐2 | rhFGF‐2 + DBBM | Difference | |
|---|---|---|---|
| Age (years; mean ± SD) | 50.0 ± 10.9 (range, 28–69) | 52.3 ± 10.1 (range, 37–76) | N.S. |
| Gender | |||
| Men | 6 | 7 | N.S.a |
| Women | 10 | 9 | |
| No. of teeth (mean ± SD) | 25.7 ± 3.9 | 26 ± 2.2 | N.S. |
| Clinical attachment level (CAL) (mm; mean ± SD) | |||
| Full‐mouth | 3.30 ± 0.59 | 3.30 ± 0.63 | N.S. |
| Reference siteb | 7.07 ± 1.56 | 7.57 ± 1.64 | N.S. |
| Probing pocket depth (PPD) (mm; mean ± SD) | |||
| Full‐mouth | 2.86 ± 0.50 | 3.00 ± 0.53 | N.S. |
| Reference siteb | 6.02 ± 1.33 | 6.32 ± 1.25 | N.S. |
| Gingival recession (GR) (mm; mean ± SD) | |||
| Reference siteb | 1.23 ± 1.51 | 1.25 ± 1.38 | N.S. |
| Bleeding on probing (BOP) positive (%) | |||
| Reference siteb | 72.7 | 77.3 | N.S.a |
| Tooth mobility (TM) (mean ± SD) | |||
| Reference toothb | 0.18 ± 0.50 | 0.22 ± 0.43 | N.S. |
Differences were assessed by the Mann–Whitney U test (aFisher's exact test). b n = 22 per group.
3.2. Clinical outcome
In each group, 22 sites of 16 patients completed the study. Healing was uneventful for all participants. There were no notable adverse events.
Characteristics of intrabony defects in the participants are summarized in Table 2. Overall, there were no significant differences in maxillary versus mandibular sites, tooth type, defect morphology, or defect depth between groups. However, there was a statistically significant difference in the defect width (p = 0.007). The test group had significantly wider defects.
Table 2.
Distribution and configuration of intrabony defects
| Intrabony defect | rhFGF‐2 | rhFGF‐2 + DBBM |
|---|---|---|
| Position [n (%)] | ||
| Maxilla | 9 (41) | 10 (45.5) |
| Mandible | 13 (59) | 12 (54.5) |
| Anterior teeth | 7 (31.8) | 2 (9.1) |
| Premolars | 6 (27.3) | 6 (27.3) |
| Molars | 9 (40.9) | 14 (63.6) |
| Morphology [n (%)] | ||
| 1‐wall | 3 (13.6) | 2 (9.1) |
| 2‐wall | 5 (22.7) | 5 (22.7) |
| 3‐wall | 8 (36.4) | 7 (31.8) |
| Combination | 6 (27.2) | 8 (36.4) |
| Depth (mm; mean ± SD) | 4.66 ± 1.76 (range, 3.0–11.0) | 4.70 ± 1.08 (range, 3.0–6.5) |
| Width (mm; mean ± SD) | 2.80 ± 0.75 (range, 2.0–5.0) | 3.89 ± 1.81a (range, 2.0–10.0) |
p = 0.007, Mann–Whitney U test.
At 3 and 6 months postoperatively, marked improvements in CAL and PPD from baseline (post‐IP) were noted in both groups (p < 0.001) (Table 3).
Table 3.
Clinical and radiographic outcomes of treated sites (Total n = 44; n = 22 per group)
| Variable/Group | Baseline | 3 months | Change from baseline to 3 months | 6 months | Change from baseline to 6 months | Change from 3 to 6 months |
|---|---|---|---|---|---|---|
| CAL (mm) | ||||||
| rhFGF‐2 | 7.07 ± 1.56 (6.38–7.76) | 4.75 ± 1.24 (4.20–5.30) | p < 0.001 | 4.29 ± 1.33 (3.70–4.89) | p < 0.001 | N.S. |
| rhFGF‐2 + DBBM | 7.57 ± 1.64 (6.80–8.39) | 4.50 ± 1.59 (3.80–5.20) | p < 0.001 | 4.41 ± 1.40 (3.80–5.03) | p < 0.001 | N.S. |
| Difference | N.S. | N.S. | N.S. | |||
| PPD (mm) | ||||||
| rhFGF‐2 | 6.02 ± 1.33 (5.43–6.61) | 3.05 ± 0.80 (2.69–3.40) | p < 0.001 | 2.73 ± 0.84 (2.35–3.10) | p < 0.001 | N.S. |
| rhFGF‐2 + DBBM | 6.32 ± 1.25 (5.76–6.87) | 2.84 ± 0.82 (2.48–3.21) | p < 0.001 | 2.77 ± 0.72 (2.45–3.09) | p < 0.001 | N.S. |
| Difference | N.S. | N.S. | N.S. | |||
| GR (mm) | ||||||
| rhFGF‐2 | 1.23 ± 1.51 (0.56–1.90) | 1.57 ± 1.38 (0.96–2.18) | N.S. | 1.39 ± 1.34 (0.80–1.98) | N.S. | N.S. |
| rhFGF‐2 + DBBM | 1.25 ± 1.38 (0.64–1.86) | 1.68 ± 1.41 (1.06–2.31) | N.S. | 1.64 ± 1.33 (1.05–2.23) | N.S. | N.S. |
| Difference | N.S. | N.S. | N.S. | |||
| BOP positive (%) | ||||||
| rhFGF‐2 | 72.7 | 22.7 | p = 0.002 | 9.1 | p < 0.001 | N.S. |
| rhFGF‐2 + DBBM | 77.3 | 4.5 | p = 0.001 | 4.5 | p < 0.001 | N.S. |
| Differencea | N.S. | N.S. | N.S. | |||
| TM | ||||||
| rhFGF‐2 | 0.18 ± 0.50 (0–0.40) | 0.09 ± 0.29 (0–0.22) | N.S. | 0.09 ± 0.29 (0–0.22) | N.S. | N.S. |
| rhFGF‐2 + DBBM | 0.22 ± 0.43 (0.09–0.04) | 0.05 ± 0.21 (0–0.14) | N.S. | 0.05 ± 0.21 (0–0.14) | N.S. | N.S. |
| Difference | N.S. | N.S. | N.S. | |||
| RBF (%) | ||||||
| rhFGF‐2 | – | 21.8 ± 11.9 | – | 29.3 ± 13.3 | – | p = 0.001 |
| rhFGF‐2 + DBBM | – | 39.3 ± 17.6 | – | 47.2 ± 16.0 | – | p < 0.001 |
| Difference | p = 0.001 | p = 0.001 | ||||
Data shown as mean ± SD (95% Confidence Interval) (except for BOP and RBF). Inter‐group difference at each time point was assessed by the Mann–Whitney U test. Intra‐group difference over time was assessed by the Friedman test with Dunn's post‐test (aCategorical data were assessed by Fisher's exact test).
CAL: clinical attachment level; PPD: probing pocket depth; GR: gingival recession; BOP: bleeding on probing; TM: tooth mobility; RBF: radiographic bone fill.
At 6 months postoperatively, CAL gains were 3.16 ± 1.45 mm in the test group and 2.77 ± 1.15 mm in the control group (Figure 2a). Non‐inferiority of the test treatment to the control can be claimed, because the lower limit of the 95% CI for the difference in CAL gain was 0.39 ± 1.73 [95% CI (−0.38 to 1.15) was greater than −1.1 mm. No statistically significant inter‐group difference was observed. In the test group, 22.7% of sites (n = 5) showed CAL gains of >4 mm; only 4.5% of sites (n = 1) in the control group showed this level of CAL gain (Table S1).
Figure 2.
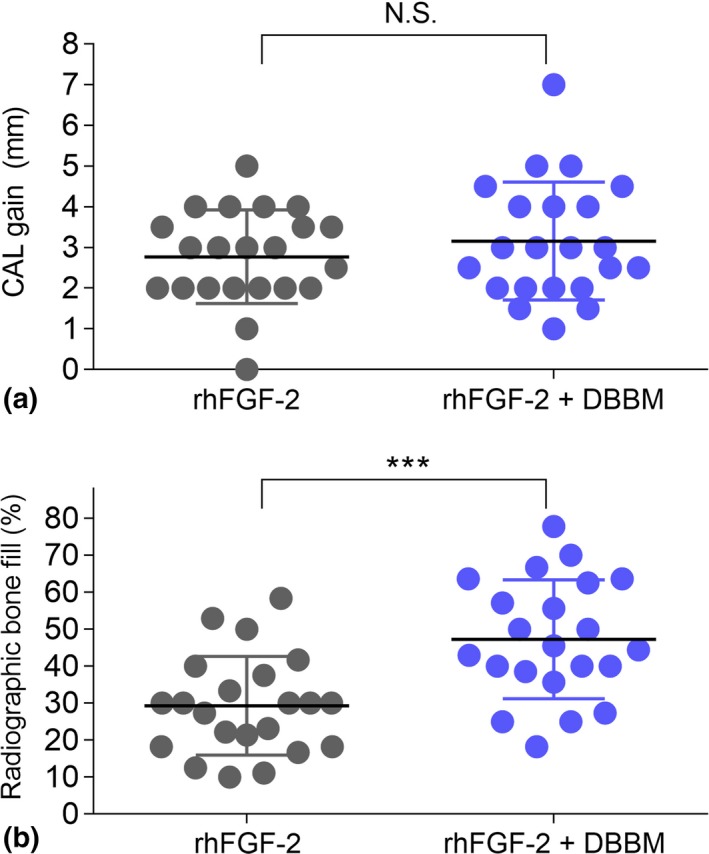
Clinical attachment level (CAL) gain (a) and radiographic bone fill (RBF) (b) at 6 months. Scatter plots showing individual data with mean (middle line) and standard deviation (error bars).***p = 0.001 by Mann–Whitney U test
Reductions in PPD were 3.55 ± 1.35 mm in the test group and 3.30 ± 1.20 mm in the control group; these did not significantly differ between groups.
3.3. Correlation between CAL gain and baseline parameters
In a secondary analysis, relationships were assessed between postoperative CAL gains at 6 months postoperatively and baseline (post‐IP) variables. CAL gain at 6 months and baseline CAL or PPD values showed significantly positive correlations in both groups (Table S2). In the control group, a significant positive correlation was noted between CAL gain and the number of teeth at baseline. There was no significant correlation with other baseline variables in either group.
3.4. Radiographic outcome
In both groups, a significant increase was observed in radiographic bone fill from 3 months to 6 months postoperatively (Table 3). At 6 months, the mean value for the radiographic bone fill was significantly greater in the test group (47.2%) than in the control group (29.3%) (p = 0.013) (Figure 2b).
3.5. Effect of defect configurations
When 6‐month postoperative CAL gains were compared between 1–2‐wall and 3‐wall defects, no significant differences were found in either treatment group (Table 4). Similarly, no significant differences in radiographic bone fill were found between 1–2‐wall defects and 3‐wall defects in either group.
Table 4.
Clinical attachment gain and radiographic bone fill at 6 months postoperatively, based on defect configuration
| Defect | rhFGF‐2 | Difference | rhFGF‐2 + DBBM | Difference | |
|---|---|---|---|---|---|
| CAL gain (mm) | 3‐wall | 2.78 ± 0.79 | N.S. | 3.43 ± 2.09 | N.S. |
| 1‐2‐wall | 2.77 ± 0.38 | 3.11 ± 1.11 | |||
| RBF (%) | 3‐wall | 29.4 ± 11.2 | N.S. | 41.5 ± 13.0 | N.S. |
| 1‐2‐wall | 29.2 ± 15.1 | 49.9 ± 17.0 |
Data are shown as mean ± SD.
CAL: clinical attachment level; RBF: radiographic bone fill.
3.6. Patient‐reported outcome measure (PROM)
In both groups, IP yielded a significant improvement in total OHRQL score (Figure 3). Compared with baseline (post‐IP) total OHRQL score, no significant changes were observed at 3 or 6 months postoperatively. At each timepoint, no significant difference in total OHRQL score was observed between groups.
Figure 3.
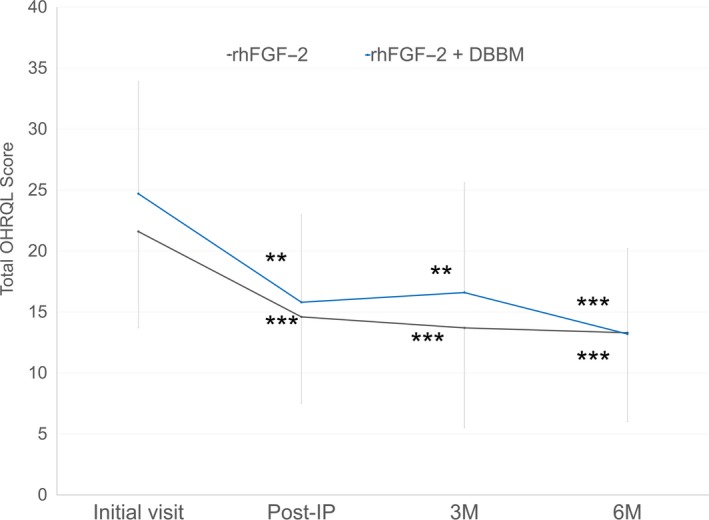
Change in total OHRQL scores over time. **p < 0.01, ***p < 0.001, significantly different from those at initial visit, by the repeated measures analysis of variance with Tukey–Kramer post hoc test. No significant difference was noted between the two different treatment groups at any time point. IP: initial periodontal therapy
4. DISCUSSION
To the best of our knowledge, this is the first RCT to evaluate the use of rhFGF‐2 with DBBM, compared with rhFGF‐2 alone in the treatment of intrabony defects, using both clinical and patient‐centred outcomes. We have shown no significant difference in CAL gain between the two groups at 6 months postoperatively. The combination therapy yielded a greater radiographic bone fill than rhFGF‐2 alone. Furthermore, no significant difference in PROM between groups was noted, as assessed by total OHRQL scores.
In clinical trials regarding treatment of periodontal defects, the use of rhFGF‐2 alone has yielded clinically favourable outcomes (Kitamura et al., 2008, 2011, 2016). This is remarkable considering that, as in the present study, prior investigators used 0.3% rhFGF‐2 with HPC, a gel‐like base material, without the support of other scaffolds such as bone graft materials (Li et al., 2017). To further explore the regenerative potential of rhFGF‐2, the effects of its combined use with β‐TCP have been studied. Oi et al. (2009) reported that, in dogs, rhFGF‐2 + β‐TCP treatment yielded greater levels of new bone and cementum formation compared with treatment with rhFGF‐2‐alone. In a previous study, we used rhFGF‐2 + β‐TCP for root coverage in a dog model (Ishii et al., 2013); we found that the combination therapy enhanced formation of new bone and cementum. Thus, we speculated that β‐TCP provided a local environment that was suitable for periodontal regeneration. Moreover, Anzai et al. (2010) reported that the use of FGF‐2 with β‐TCP increased the bone mineral contents of 1‐wall defects in a dog model, compared with the use of β‐TCP alone. In a multicenter RCT evaluating the treatment of intrabony periodontal defects, the use of 0.3% and 0.4% rhFGF‐2 with β‐TCP showed an improved success rate (based on changes in CAL and LBG) at 6 months postoperative, compared with β‐TCP alone (Cochran et al., 2016). The reported CAL gain achieved using 0.3% rhFGF‐2 with β‐TCP was 3.0 ± 1.4 mm at 6 months; this was comparable to our present findings using rhFGF‐2 with DBBM (3.2 ± 1.5 mm). However, the participants in the prior study exhibited considerably greater baseline mean CAL (8.3 mm) and PPD (7.9 mm) values, compared with those (CAL 7.6 mm, PPD 6.3 mm) in the present study. In this study, the CAL gain was significantly positively correlated with baseline CAL or PPD values, which is consistent with the previous reports (Cortellini, Pini Prato, & Tonetti, 1993; Irokawa, Okubo, et al., 2017; Saito, Nanbu, Nagahata, & Yamada, 2008). This indicates that CAL gain after regenerative therapy depends on the initial level of destruction, which has to be taken into account when comparing data from different studies.
Regarding the effect of the combined use of rhFGF‐2 with demineralized bone matrix (DBM), Hagino, Hamada, and Amemiya (2001) showed that the use of 200 μg of rhFGF‐2 with DBM yielded early new bone formation in segmental bone defects in a rabbit model. Notably, these authors suggested that when DBM was used as a carrier, a single local administration of rhFGF‐2 at an appropriate concentration may be effective for bone formation; moreover, the release of FGF‐2 over an extended period may not be necessary. When DBBM was used with EMD in vitro, the investigators observed enhanced attachment, proliferation and differentiation of osteoblasts and periodontal ligament cells on DBBM granules (Miron et al., 2012). In a study using concentrated growth factors with DBBM, persistent releases of cytokines, including FGF‐2, was observed over a period of 28 days (Yu, Wang, Liu, & Qiao, 2018). These data indicate the potential for use of DBBM as a scaffold or carrier for growth factors. The adsorption of rhFGF‐2 to DBBM, as well as specific cell behaviours on DBBM with rhFGF‐2 should be further explored.
There are multiple opinions within the literature regarding the effect of bone substitutes in combination regenerative therapy. In a multicenter RCT, Jepsen et al. (2008) reported the treatment of intrabony defects using EMD with alloplast or EMD alone; both approaches showed similar results after 6 months. The similar results were confirmed at 36 months after treatment (Hoffmann, Al‐Machot, Meyle, Jervøe‐Storm, & Jepsen, 2016). Pietruska et al. (2012) also evaluated clinical outcomes following treatment of intrabony defects using EMD with synthetic bone graft or EMD alone; they concluded that, in 2‐ and 3‐wall defects, combination therapy did not show any advantage after 4 years. In contrast, a systematic review revealed that the use of EMD with bone grafts may enhance clinical outcomes (Matarasso et al., 2015). According to a clinical practice guideline of the Japanese Society of Periodontology (JSP, 2016), there is no clear evidence of an additional effect when bone graft is applied in combination with GTR or EMD. The guideline states that careful consideration should be given to this type of application. In the present study, the addition of DBBM to rhFGF‐2 therapy did not yield a significantly greater CAL gain at 6 months (the primary endpoint). Of concern was the statistically significant difference (p = 0.007) in baseline defect width for the test (3.89 mm) and the control (2.80 mm). This may be one reason for no significant difference in CAL gains found between groups. However, the radiographic bone fill (a secondary endpoint) in the test group was significantly greater than in the control group. The influence of difference in the initial defect width on the clinical outcome remains to be clarified.
Tonetti et al. (2002) reported that defect morphology (i.e. number of bone walls) influenced the clinical results of regenerative therapy with EMD. Trombelli and Farina (2008) reported that EMD does not maintain a space itself when used in non‐contained defects. According to an evidence‐based decision tree by Cortellini and Tonetti (2000), the combined use of supportive or filling materials is recommended to treat wide defects and/or non‐contained defects. Because the rhFGF‐2 formulation in the present study used HPC, a gel‐like base material, its ability for space maintenance may also be limited. Therefore, we initially anticipated that the use of rhFGF‐2 with DBBM might provide superior clinical results, compared with rhFGF‐2 alone, especially in more compromised sites (i.e. non‐contained defects). However, when we compared the results in 3‐wall defects and 1–2‐wall defects, no significant difference was noted in CAL gain or radiographic bone fill. There are two possible reasons for this phenomenon: (1) rhFGF‐2 alone is clinically effective for non‐contained defects; (2) the present study was underpowered to detect this difference. Considering that 1‐wall defects comprised only 14% and 9% of sites in the test and control groups, respectively, both possibilities should be examined.
Subjective oral health‐related QoL assessments are considered true endpoints when evaluating the effect of periodontal treatment (Hujoel, 2004). In the present study, oral health‐related QoL was assessed as a PROM. Consistent with the findings of our previous study (Makino‐Oi et al., 2016), patients’ oral health‐related QoL scores were significantly improved after IP. Regenerative therapy using rhFGF‐2 alone or rhFGF‐2 combined with DBBM yielded no significant changes in oral health‐related QoL, indicating that neither surgical intervention positively or negatively affected patients’ oral health‐related QoL. Furthermore, patients’ perceptions of the rhFGF‐2 treatment and healing process may not have been impaired by the addition of DBBM.
There were limitations in this study. For example, the test sites had wider defects at baseline, but our statistical analysis did not include an adjustment for this. Because the number of non‐contained defects was limited, the impact of the number of residual walls on treatment outcomes may not have been appropriately evaluated. Moreover, because the observation period of 6 months is relatively short, a longer follow‐up is needed.
In conclusion, treatment of intrabony defects with rhFGF‐2, with or without DBBM, yielded significant improvements in periodontal parameters at 6 months, relative to baseline measurements. No significant difference in CAL gain was observed between groups, although combination therapy yielded an enhanced radiographic outcome. There was no significant difference in PROM between groups. Further longitudinal investigation with a larger number of participants should identify the characteristics of cases that would benefit most from combined treatment using rhFGF‐2 and DBBM.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in connection with this article.
5.
Clinical Relevance.
Scientific rationale for the study: Currently, information is limited regarding the clinical effect of combined use of rhFGF‐2 and bone graft on periodontal healing.
Principal findings: Treatment of intrabony defects using 0.3% rhFGF‐2 with DBBM or rhFGF‐2 alone demonstrated comparable values of clinical attachment level gain at 6 months postoperatively. The combination therapy yielded a greater radiographic bone fill than rhFGF‐2 alone. No significant difference in PROM between groups was observed.
Practical implications: A significant improvement in periodontal parameters can be expected by treatment using rhFGF‐2, with or without DBBM. Further investigations are necessary to clarify the true benefit of adding bone graft to rhFGF‐2 therapy.
Supporting information
Saito A, Bizenjima T, Takeuchi T, et al. Treatment of intrabony periodontal defects using rhFGF‐2 in combination with deproteinized bovine bone mineral or rhFGF‐2 alone: A 6‐month randomized controlled trial. J Clin Periodontol. 2019;46:332–341. 10.1111/jcpe.13086
Funding information
This study was supported in part by a grant from Osteology Foundation (Advanced Researcher Grant No.17‐136) and a grant from Multidisciplinary Research Center for Jawbone Disease (MRCJD), Tokyo Dental College (a MEXT Private University Research Branding Project).
Trial registration: The University Hospital Medical Information Network‐Clinical Trials Registry (UMIN‐CTR) number UMIN 000025257
REFERENCES
- Anzai, J. , Kitamura, M. , Nozaki, T. , Nagayasu, T. , Terashima, A. , Asano, T. , & Murakami, S. (2010). Effects of concomitant use of fibroblast growth factor (FGF)‐2 with beta‐tricalcium phosphate (β‐TCP) on the beagle dog 1‐wall periodontal defect model. Biochemical and Biophysical Research Communications, 403, 345–350. 10.1016/j.bbrc.2010.11.032 [DOI] [PubMed] [Google Scholar]
- Armitage, G. C. (1999). Development of a classification system for periodontal diseases and conditions. Annals of Periodontology, 4, 1–6. 10.1902/annals.1999.4.1.1 [DOI] [PubMed] [Google Scholar]
- Camelo, M. , Nevins, M. L. , Lynch, S. E. , Schenk, R. K. , Simion, M. , & Nevins, M. (2001). Periodontal regeneration with an autogenous bone‐Bio‐Oss composite graft and a Bio‐Gide membrane. International Journal of Periodontics & Restorative Dentistry, 21, 109–119. [PubMed] [Google Scholar]
- Cochran, D. L. , Oh, T. J. , Mills, M. P. , Clem, D. S. , McClain, P. K. , Schallhorn, R. A. , … Takemura, A. (2016). A randomized clinical trial evaluating rh‐FGF‐2/β‐TCP in periodontal defects. Journal of Dental Research, 95, 523–530. 10.1177/0022034516632497 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Pini Prato, G. , & Tonetti, M. S. (1993). Periodontal regeneration of human infrabony defects I. Clinical measures. Journal of Periodontology, 64, 254–260. 10.1902/jop.1993.64.4.254 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Pini Prato, G. , & Tonetti, M. S. (1995). The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. Journal of Periodontology, 66, 261–266. 10.1902/jop.1995.66.4.261 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Pini Prato, G. , & Tonetti, M. S. (1999). The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. International Journal of Periodontics & Restorative Dentistry, 19, 589–599. [PubMed] [Google Scholar]
- Cortellini, P. , & Tonetti, M. S. (2000). Focus on intrabony defects: guided tissue regeneration. Periodontology 2000, 22, 104–132. 10.1034/j.1600-0757.2000.2220108.x [DOI] [PubMed] [Google Scholar]
- Hagino, T. , Hamada, Y. , & Amemiya, T. (2001). Fibroblast growth factor‐2 stimulates new bone formation on allografts of demineralized bone matrix in segmental bone defects in rabbits. Yamanashi Medical Journal, 16, 9–14. [Google Scholar]
- Heden, G. , & Wennstöm, J. L. (2006). Five‐year follow‐up of regenerative periodontal therapy with enamel matrix derivative at sites with angular bone defects. Journal of Periodontology, 77, 295–301. 10.1902/jop.2006.050071 [DOI] [PubMed] [Google Scholar]
- Hoffmann, T. , Al‐Machot, E. , Meyle, J. , Jervøe‐Storm, P. M. , & Jepsen, S. (2016). Three‐year results following regenerative periodontal surgery of advanced intrabony defects with enamel matrix derivative alone or combined with a synthetic bone graft. Clinical Oral Investigations, 20, 357–364. 10.1007/s00784-015-1522-4 [DOI] [PubMed] [Google Scholar]
- Hujoel, P. P. (2004). Endpoints in periodontal trials: the need for an evidence‐based research approach. Periodontology 2000, 36, 196–204. 10.1111/j.1600-0757.2004.03681.x [DOI] [PubMed] [Google Scholar]
- Iorio‐Siciliano, V. , Andreuccetti, G. , Blasi, A. , Matarasso, M. , Sculean, A. , & Salvi, G. E. (2014). Clinical outcomes following regenerative therapy of non‐contained intrabony defects using a deproteinized bovine bone mineral combined with either enamel matrix derivative or collagen membrane. Journal of Periodontology, 85, 1342–1350. 10.1902/jop.2014.130420 [DOI] [PubMed] [Google Scholar]
- Irokawa, D. , Okubo, N. , Nikaido, M. , Shimizu, H. , Konobu, H. , Matsui, T. , … Saito, A. (2017). Periodontal regenerative therapy of intrabony defects using deproteinized bovine bone mineral in combination with collagen barrier membrane: a multicenter prospective case‐series study. International Journal of Periodontics & Restorative Dentistry, 37, 393–401. 10.11607/prd.2888 [DOI] [PubMed] [Google Scholar]
- Irokawa, D. , Takeuchi, T. , Noda, K. , Goto, H. , Egawa, M. , Tomita, S. , … Saito, A. (2017). Clinical outcome of periodontal regenerative therapy using collagen membrane and deproteinized bovine bone mineral: a 2.5‐year follow‐up study. BMC Rsearch Notes, 10(1), 102 10.1186/s13104-017-2426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, Y. , Fujita, T. , Okubo, N. , Ota, M. , Yamada, S. , & Saito, A. (2013). Effect of basic fibroblast growth factor (FGF‐2) in combination with beta tricalcium phosphate on root coverage in dog. Acta Odontologica Scandinavica, 71, 325–332. 10.3109/00016357.2012.680906 [DOI] [PubMed] [Google Scholar]
- Japanese Society of Periodontology (2016). JSP clinical practice guideline for the periodontal treatment, 2015 (53 pp). Tokyo, Japan: Ishiyaku Publishers. [Google Scholar]
- Jepsen, S. , Topoll, H. , Rengers, H. , Heinz, B. , Teich, M. , Hoffmann, T. , … Jervøe‐Storm, P. M. (2008). Clinical outcomes after treatment of intra‐bony defects with an EMD/synthetic bone graft or EMD alone: a multicentre randomized‐controlled clinical trial. Journal of Clinical Periodontology, 35, 420–428. 10.1111/j.1600-051X.2008.01217.x [DOI] [PubMed] [Google Scholar]
- Kao, R. T. , Nares, S. , & Reynolds, M. A. (2015). Periodontal regeneration ‐ intrabony defects: a systematic review from the AAP Regeneration Workshop. Journal of Periodontology, 86(2 Suppl), S77–S104. 10.1902/jop.2015.130685 [DOI] [PubMed] [Google Scholar]
- Kitamura, M. , Akamatsu, M. , Kawanami, M. , Furuichi, Y. , Fujii, T. , Mori, M. , … Murakami, S. (2016). Randomized placebo‐controlled and controlled non‐inferiority Phase III trials comparing trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. Journal of Bone and Mineral Research, 31, 806–814. 10.1002/jbmr.2738 [DOI] [PubMed] [Google Scholar]
- Kitamura, M. , Akamatsu, M. , Machigashira, M. , Hara, Y. , Sakagami, R. , Hirofuji, T. , … Murakami, S. (2011). FGF‐2 stimulates periodontal regeneration: results of a multi‐center randomized clinical trial. Journal of Dental Research, 90, 35–40. 10.1177/0022034510384616 [DOI] [PubMed] [Google Scholar]
- Kitamura, M. , Nakashima, K. , Kowashi, Y. , Fujii, T. , Shimauchi, H. , Sasano, T. , … Murakami, S. (2008). Periodontal tissue regeneration using fibroblast growth factor ‐2: randomized controlled phase II clinical trial. PLoS ONE, 3, e2611 10.1371/journal.pone.0002611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Yu, F. , Xu, X. , Li, C. , Huang, D. , Zhou, X. , … Zheng, L. (2017). Evaluation of recombinant human FGF‐2 and PDGF‐BB in periodontal regeneration: a systematic review and meta‐analysis. Scientific Reports, 7(1), 65 10.1038/s41598-017-00113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Z. , Rios, H. F. , & Cochran, D. L. (2015). Emerging regenerative approaches for periodontal reconstruction: A systematic review from the AAP Regeneration Workshop. Journal of Periodontology, 86(2 Suppl), S134–S152. 10.1902/jop.2015.130689 [DOI] [PubMed] [Google Scholar]
- Losada, M. , González, R. , Garcia, À. P. , Santos, A. , & Nart, J. (2017). Treatment of non‐contained infrabony defects with enamel matrix derivative alone or in combination with biphasic calcium phosphate bone graft: a 12‐month randomized controlled clinical trial. Journal of Periodontology, 88, 426–435. 10.1902/jop.2016.160459 [DOI] [PubMed] [Google Scholar]
- Makino‐Oi, A. , Ishii, Y. , Hoshino, T. , Okubo, N. , Sugito, H. , Hosaka, Y. , … Saito, A. (2016). Effect of periodontal surgery on oral health‐related quality of life in patients who have completed initial periodontal therapy. Journal of Periodontal Research, 51, 212–220. 10.1111/jre.12300 [DOI] [PubMed] [Google Scholar]
- Matarasso, M. , Iorio‐Siciliano, V. , Blasi, A. , Ramaglia, L. , Salvi, G. E. , & Sculean, A. (2015). Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta‐analysis. Clinical Oral Investigations, 19, 1581–1593. 10.1007/s00784-015-1491-7 [DOI] [PubMed] [Google Scholar]
- Miller, S. C. (1950) Textbook of periodontia, 3rd ed Philadelphia, PA: Blakiston. [Google Scholar]
- Miron, R. J. , Bosshardt, D. D. , Hedbom, E. , Zhang, Y. , Haenni, B. , Buser, D. , & Sculean, A. (2012). Adsorption of enamel matrix proteins to a bovine‐derived bone grafting material and its regulation of cell adhesion, proliferation, and differentiation. Journal of Periodontology, 83, 936–997. 10.1902/jop.2011.110480 [DOI] [PubMed] [Google Scholar]
- Murakami, S. , Takayama, S. , Kitamura, M. , Shimabukuro, Y. , Yanagi, K. , Ikezawa, K. , … Okada, H. (2003). Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. Journal of Periodontal Research, 38, 97–103. 10.1034/j.1600-0765.2003.00640.x [DOI] [PubMed] [Google Scholar]
- Nevins, M. , Camelo, M. , Nevins, M. L. , Schenk, R. K. , & Lynch, S. E. (2003). Periodontal regeneration in humans using recombinant human platelet‐derived growth factor‐BB (rhPDGF‐BB) and allogenic bone. Journal of Periodontology, 74, 1282–1292. 10.1902/jop.2003.74.9.1282 [DOI] [PubMed] [Google Scholar]
- Nevins, M. , Giannobile, W. V. , McGuire, M. K. , Kao, R. T. , Mellonig, J. T. , Hinrichs, J. E. , … Lynch, S. E. (2005). Platelet‐Derived Growth Factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. Journal of Periodontology, 76, 2205–2215. 10.1902/jop.2005.76.12.2205 [DOI] [PubMed] [Google Scholar]
- Nevins, M. , Kao, R. T. , McGuire, M. K. , McClain, P. K. , Hinrichs, J. E. , McAllister, B. S. , … Giannobile, W. V. (2013). Platelet‐derived growth factor promotes periodontal regeneration in localized osseous defects: 36‐month extension results from a randomized, controlled, double‐masked clinical trial. Journal of Periodontology, 84, 456–464. 10.1902/jop.2012.120141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi, Y. , Ota, M. , Yamamoto, S. , Shibukawa, Y. , & Yamada, S. (2009). β‐tricalcium phosphate and basic fibroblast growth factor combination enhances periodontal regeneration in intrabony defects in dogs. Dental Materials Journal, 28, 162–169. 10.4012/dmj.28.162 [DOI] [PubMed] [Google Scholar]
- O'Leary, T. J. , Drake, R. B. , & Naylor, J. E. (1972). The plaque control record. Journal of Periodontology, 43, 38–38. 10.1902/jop.1972.43.1.38 [DOI] [PubMed] [Google Scholar]
- Page, R. C. , & Eke, P. I. (2007). Case definitions for use in population‐based surveillance of periodontitis. Journal of Periodontology, 78, 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Pietruska, M. , Pietruski, J. , Nagy, K. , Brecx, M. , Arweiler, N. B. , & Sculean, A. (2012). Four‐year results following treatment of intrabony periodontal defects with an enamel matrix derivative alone or combined with a biphasic calcium phosphate. Clinical Oral Investigations, 16, 1191–1197. 10.1007/s00784-011-0611-2 [DOI] [PubMed] [Google Scholar]
- Rösing, C. K. , Aass, A. M. , Mavropoulos, A. , & Gjermo, P. (2005). Clinical and radiographic effects of enamel matrix derivative in the treatment of intrabony periodontal defects: A 12‐month longitudinal placebo‐controlled clinical trial in adult periodontitis patients. Journal of Periodontology, 76, 129–133. 10.1902/jop.2005.76.1.129 [DOI] [PubMed] [Google Scholar]
- Saito, A. , Hosaka, Y. , Kikuchi, M. , Akamatsu, M. , Fukaya, C. , Matsumoto, S. , … Nakagawa, T. (2010). Effect of initial periodontal therapy on oral health–related quality of life in patients with periodontitis in Japan. Journal of Periodontology, 81, 1001–1009. 10.1902/jop.2010.090663 [DOI] [PubMed] [Google Scholar]
- Saito, A. , Nanbu, Y. , Nagahata, T. , & Yamada, S. (2008). Treatment of intrabony periodontal defects with enamel matrix derivative in private practice: a long‐term retrospective study. The Bulletin of Tokyo Dental College, 49, 89–96. 10.2209/tdcpublication.49.89 [DOI] [PubMed] [Google Scholar]
- Saito, A. , Ota, K. , Hosaka, Y. , Akamatsu, M. , Hayakawa, H. , Fukaya, C. , … Nakagawa, T. (2011). Potential impact of surgical periodontal therapy on oral health‐related quality of life in patients with periodontitis: a pilot study. Journal of Clinical Periodontology, 38, 1115–1121. 10.1111/j.1600-051X.2011.01796.x [DOI] [PubMed] [Google Scholar]
- Schei, O. , Waerhaug, J. , Lovdal, A. , & Arno, A. (1959). Alveolar bone loss as related to oral hygiene and age. Journal of Periodontology, 30, 7–16. 10.1902/jop.1959.30.1.7 [DOI] [Google Scholar]
- Seshima, F. , Aoki, H. , Takeuchi, T. , Suzuki, E. , Irokawa, D. , Makino‐Oi, A. , … Saito, A. (2017). Periodontal regenerative therapy with enamel matrix derivative in the treatment of intrabony defects: a prospective 2‐year study. BMC Research Notes, 10(1), 256 10.1186/s13104-017-2572-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, S. , Murakami, S. , Shimabukuro, Y. , Kitamura, M. , & Okada, H. (2001). Periodontal regeneration by FGF‐2 (bFGF) in primate models. Journal of Dental Research, 80, 2075–2079. 10.1177/00220345010800121001 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Cortellini, P. , Lang, N. P. , Suvan, J. E. , Adriaens, P. , Dubravec, D. , … Zybutz, M. (2004). Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. Journal of Clinical Periodontology, 31, 770–776. 10.1111/j.1600-051X.2004.00562.x [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Lang, N. P. , Cortellini, P. , Suvan, J. E. , Adriaens, P. , Dubravec, D. , … Walkamm, B. (2002). Enamel matrix proteins in the regenerative therapy of deep intrabony defects: A multicentre randomized controlled clinical trial. Journal of Clinical Periodontology, 29, 317–325. 10.1034/j.1600-051X.2002.290407.x [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , & Farina, R. (2008). Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. Journal of Clinical Periodontology, 35, 117–135. 10.1111/j.1600-051X.2008.01265.x [DOI] [PubMed] [Google Scholar]
- Yu, M. , Wang, X. , Liu, Y. , & Qiao, J. (2018). Cytokine release kinetics of concentrated growth factors in different scaffolds. Clinical Oral Investigations, in press. 10.1007/s00784-018-2582-z [DOI] [PubMed] [Google Scholar]
- Zucchelli, G. , Amore, C. , Montebugnoli, L. , & De Sanctis, M. (2003). Enamel matrix proteins and bovine porous bone mineral in the treatment of intrabony defects : a comparative controlled clinical trial. Journal of Periodontology, 74, 1725–1735. 10.1902/jop.2003.74.12.1725 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


