Abstract
Background and Aims
Our understanding of the long‐term safety of prenatal exposure to opioid maintenance treatment (OMT) is insufficient. We compared childhood morbidity (0–3 years) between OMT‐exposed and relevant comparison groups.
Design
Nation‐wide, registry‐based cohort study. Registries on reproductive health, addiction treatment, hospitalization and death were linked using identification numbers.
Setting
The Czech Republic (2000–14).
Participants
Children with different prenatal exposure: (i) mother in OMT during pregnancy (OMT; n = 218), (ii) mother discontinued OMT before pregnancy (OMT‐D; n = 55), (iii) mother with opioid use disorder, but not in OMT during pregnancy (OUD; n = 85) and (iv) mother in the general population (GP) (n = 1 238 452)
Measurements
Episodes of hospitalization were observed as outcomes. Information on in‐patient contacts, length of stay and diagnoses (International Classification of Diseases version 10) were assessed. Binary logistic regressions were conducted to estimate the associations between OMT exposure and the outcomes, crude and adjusted for the socio‐economic status and smoking.
Findings
No significant differences were found in the overall proportion of hospitalization among OMT‐exposed children, children of OMT‐D and children of women with OUD [54.1%, 95% confidence interval (CI) = 47.3–60.1%; 47.3%, 95% CI = 33.9–61.1%; 51.8%, 95% CI = 40.7%–62.6%], while the proportion was significantly lower (35.8%, 95% CI = 35.7–35.8%) in the GP. There were no significant differences in risk of specific diagnoses between OMT‐exposed children, children of OMT‐D and children of women with OUD. In the adjusted analyses, differences between OMT‐exposed and children in the GP were still present for infections and parasitic diseases (OR = 2.0, 95% CI = 1.4–2.7), diseases of the digestive system (OR = 1.7, 95% CI = 1.2–2.6) and diseases of the skin and subcutaneous tissue (OR = 1.9, 95% CI = 1.2–3.2).
Conclusion
This study did not find clear evidence for an increase in risk of morbidity during the first 3 years of life in children with prenatal opioid maintenance treatment exposure compared with children of women who discontinued such treatment before pregnancy or suffered from opioid use disorder without this treatment. Compared the general population, there appears to be an increased risk of hospitalizations for infectious, gastrointestinal and skin diseases.
Keywords: Buprenorphine, child morbidity, health registries, hospitalization, long‐term effects, methadone, opioid maintenance treatment, prenatal exposure
Introduction
Opioid maintenance therapy (OMT) is the recommended treatment for opioid dependence during pregnancy 1. Patients in OMT receive long‐acting opioid agonists (e.g. methadone or buprenorphine) in order to reduce craving for illicit opioids and to prevent relapse. Studies on the safety of these drugs for the unborn child focus mainly on birth parameters and short‐term outcomes. Offspring exposed to OMT in utero have been shown to have lower growth parameters at birth and higher rate of neonatal abstinence syndrome (NAS) when compared to the general population 2, 3, 4.
A few studies have attempted to address the effects of prenatal OMT exposure on the child's health beyond the perinatal period. Inconsistent findings regarding children's mental development following in‐utero OMT exposure have been reported 5, 6, 7, 8.
Even fewer studies have investigated the general morbidity and mortality among OMT‐exposed children 9, 10, 11. In a study from Western Australia, health outcomes during the first 5 years of life of children exposed to methadone, buprenorphine or naltrexone in utero were compared with a control group selected from the general population. Overall, the rates of hospital admissions were elevated both in children exposed to the two opioid agonists (methadone and buprenorphine) and the opioid antagonist (naltrexone) 10.
Previous studies on childhood morbidity after prenatal opioid exposure have focused mainly on children with NAS. NAS is a serious adverse event that could potentially influence the child's future health. Prenatal OMT exposure results in NAS in approximately 60–80% of neonates 12. Maternal use of other substances that cause drug dependence may also result in NAS in the newborn 13. The studies focusing on NAS are therefore not purely studies of consequences of OMT treatment. Generally, studies of children experiencing NAS found significantly increased rates of hospitalization during childhood compared to children in the general population 14, 15, 16.
In previous studies, OMT‐exposed children or children with NAS have been compared to children in the general population. The OMT‐exposed children are not only exposed to OMT drugs, but also to several other risk factors, such as maternal smoking and somatic and psychiatric illnesses as well as other unfavourable life‐style factors. When studying OMT exposure, appropriate comparison groups are therefore needed to disentangle the effect of the drug from the effect of other risk factors and to avoid influence of unmeasured confounding. Children of pregnant women with indications for OMT but who were not in OMT during pregnancy could serve as such comparison group.
In the Czech Republic, nation‐wide health registries with compulsory registration exist 17. Using unique personalized identification numbers, it is possible to link data from the registries on an individual level. This approach gives us the opportunity to study large, unselected populations of women with opioid use disorders in or out of treatment without loss to follow‐up 17.
The aims of this study were to examine morbidity in the first 3 years of life. Specifically, comparisons were made between the following groups.
-
1
Children prenatally exposed to OMT and:
children of women who had used OMT before, but not during pregnancy (OMT discontinuers; OMT‐D);
children of women with opioid use disorders (OUD), who were not in OMT during pregnancy; and
children of women in the general population of pregnant women (GP), without indications of opioid use disorders.
-
2
Children prenatally exposed to buprenorphine versus those exposed to methadone.
The hypothesis in the study was that OMT‐exposed children will not have higher morbidity than the two relevant comparison groups, but higher morbidity than the general population. In addition, buprenorphine‐exposed children might have lower morbidity compared to methadone‐exposed children.
Methods
Data from nation‐wide health registries were used to investigate in‐patient childhood morbidity. Linkage of data between the registries on reproductive health, addiction treatment, hospitalization and death was based on the personal identification numbers assigned to all individuals in the Czech Republic 17, 18. Identification numbers are assigned by the Municipal Registry Office shortly after the birth. It is used as an essential tool for the identification of the citizens across the public administration.
Data sources
In the Czech Republic, physicians are obliged by law to report data to the national health registries.
The National Register of reproduction health (NRRH)
The NRRH holds information about maternal health, life‐style during pregnancy, demographic and socio‐economics and information about delivery and the neonate, including birth parameters, congenital malformations and death.
The National Register of addiction treatment (NRAT)
The NRAT includes information about patients who receive OMT, e.g. date of initiation and termination of treatment and type of OMT drug.
OMT became available for treatment in Czech Republic in the late 1990s; methadone became available in 1997, buprenorphine in 2000 and a buprenorphine–naloxone combination in 2008 19. Methadone is provided only at specialized OMT clinics free of charge, while buprenorphine and buprenorphine–naloxone can be prescribed by all physicians irrespective of their specialization, and are dispensed in pharmacies and typically fully paid for by the patients.
The National Register of in‐patient treatment (NRIT)
The NRIT includes information on every episode of all types of hospitalizations, including information on dates of admission and discharge from hospital. Transfer to a different department during the same hospital stay is recorded as a separate hospitalization. Diagnoses in the discharge summary are coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD‐10).
Hospitals represent secondary health‐care level. The primary level is represented by general practitioners for children and adolescents—each child is registered to one specific general practitioner. The general practitioners act as gate‐keepers for in‐patient treatment. Outside the general practitioners for children and adolescents’ working hours, out‐patient emergency units in hospitals refer patients to in‐patient departments. Nearly all hospitals offering acute care have paediatric departments. The NRIT does not have information for patients who are only in contact with primary health‐care services.
The information system on deaths (ISZEM)
The ISZEM is a general mortality register of the Czech Republic, holding time and cause of death for people with a permanent or long‐term residence in the Czech Republic.
Exposure to OMT drugs during pregnancy
The start and end of pregnancy was assessed for women registered in NRRH. This information was linked to the NRAT to identify use of OMT drugs (methadone, buprenorphine or buprenorphine–naloxone) during pregnancy. Children were defined as prenatally exposed to OMT if their mother had received one of the OMT drugs during pregnancy (OMT‐exposed group). None of the pregnant women switched between different OMT drugs during pregnancy.
Comparison groups
Children prenatally exposed to OMT were compared with three groups. The two most relevant comparison groups were children of pregnant women with indications for OMT but who were not in OMT during pregnancy. More specifically, group 1, ‘OMT discontinuers (OMT‐D)’, were defined as children of women who used an OMT drug during the 360‐day period before pregnancy start, but not during pregnancy, and group 2, ‘opioid use disorders (OUD)’, were defined as children of women hospitalized with a diagnosis of mental or behavioural disorder due to opioid use (ICD‐10 code F11, all subcodes) during pregnancy, but who were not in OMT either 360 days before or during pregnancy. The third group 3 was represented by children of pregnant women without indications of opioid use disorders in the general population (GP).
Outcomes
Hospitalizations were used as a measure of morbidity. Information about hospitalizations of children was assessed for the time‐period from discharge from the hospital after birth until the age of 3 years. Data from NRIT were used to assess information on all in‐patient contacts, length of stay, primary and secondary ICD‐10 diagnoses (chapter level I–XXI) at discharge. Where diagnoses were recorded for three or fewer cases, the data were not reported.
Study population and study period
The study population included all children born in the Czech Republic during the study period, 2000–14. Of these, 331 were children in the OMT group, 81 were in the OMT‐D group and 104 in the OUD group. Children of women from the GP with no recorded history of opioid use disorder were the largest group (n = 1 556 583). Some of the children were born late in the study period and were excluded because they did not reach the age of 3 years (Fig. 1). Children who died by the age of 3 were also excluded, including two (0.6%) in the OMT group, none in the OMT‐D and OUD groups and 997 (0.1%) in the GP group. Both of the OMT children who died during follow‐up were exposed to buprenorphine and lived just below 1.5 years; only one of them was hospitalized for diseases of respiratory system (ICD‐10 code J03). The final study population consisted of 218 children in the OMT group, 55 in the OMT‐D group, 85 in the OUD group and 1 238 452 in the GP group.
Figure 1.
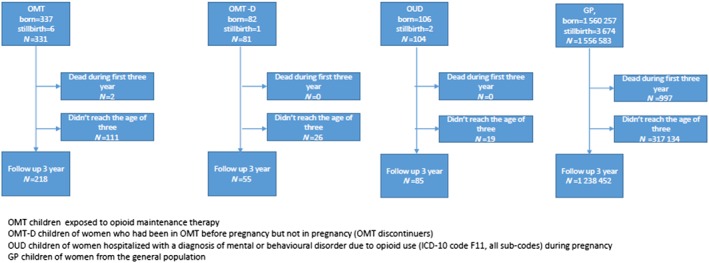
Children included in the study. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Other variables
Background characteristics of the pregnant women were obtained from NRRH as described above 18.
Analysis strategy and statistics
Descriptive statistics [mean, median and interquartile range (IQR)] were used to present the proportion of hospitalized children, frequency of hospitalizations, length of hospital stay and number of diagnoses (primary and secondary diagnoses) per child in each group for children who reached age of 3. Negative binominal regression analysis was used to calculate the risk for hospitalization among OMT‐exposed children compared to children of OMT‐D women, women with OUD and women from the GP. For the risk associated with the exposure to methadone versus buprenorphine and buprenorphine–naloxone, binary logistic regression with the outcome of the child being hospitalized (yes/no) was performed.
Next, the proportion of children hospitalized with different ICD‐10 chapter diagnoses during the period after discharge following birth and until the age of 3 years was calculated. The population of children who reached 3 years of age was used as the denominator. Confidence intervals (CI) for proportion were calculated using the continuity correlated with the score interval method 20. To control for relevant maternal background characteristics, binary logistic regression for the categorical dependent variables (diagnoses yes/no) for each diagnosis chapter separately was performed. Unadjusted and adjusted odds ratio (aOR) with 95% CI were presented for the OMT group compared to the OMT‐D, OUD and GP groups. Only significant comparisons from unadjusted analyses were adjusted for maternal age, marital status, education and smoking.
The level of statistical significance for all analyses was set at P < 0.05 using two‐tailed comparisons. Statistical analyses were conducted using SPSS for Windows, version 23, and STATA 14.
Significant associations were further examined in subanalyses stratified by gender and the presence of diseases originating in perinatal period.
Ethics
The study was approved by the Institutional Review Board of the General University Hospital in Prague (IRB00002705).
Results
Background characteristics
Table 1 illustrates maternal background characteristics in the OMT, OMT‐D, OUD and GP groups. OMT, OMT‐D and OUD were similar in all characteristics, with the exception of the OUD women being markedly younger. However, each of these groups had more unfavourable life‐style characteristics than the GP.
Table 1.
Socio‐economic characteristics of pregnant women in opioid maintenance therapy compared to the OMT‐D, OUD and GP groups in the Czech Republic.
| Buprenorphine | Methadone | OMT | OMT‐D | OUD | GP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| Total number | 91 | 127 | 218 | 55 | 85 | 1 238 452 | ||||||||||||
| Age, years | ||||||||||||||||||
| ≤ 24 | 31 | 34.1 | 24.7–44.8 | 43 | 33.9 | 25.9–42.9 | 74 | 33.9 | 27.8–40.7 | 19 | 34.5 | 22.6–48.7 | 61 | 71.8 | 60.8–80.7 | 264 822 | 21.4 | 21.3–21.5 |
| 25–29 | 37 | 40.7 | 30.6–51.5 | 53 | 41.7 | 33.2–50.8 | 90 | 41.3 | 34.7–48.1 | 25 | 45.5 | 32.2–59.3 | 16 | 18.8 | 11.5–29.1 | 469 583 | 37.9 | 37.8–38.0 |
| 30–34 | 22 | 24.2 | 16.1–34.5 | 25 | 19.7 | 13.4–27.9 | 47 | 21.6 | 16.4–27.7 | 9 | 16.4 | 8.2–29.3 | 4 | 4.7 | 1.5–12.3 | 363 229 | 29.3 | 29.2–29.4 |
| ≥ 35 | 1 | 1.1 | 0.1–6.8 | 6 | 4.7 | 1.9–10.4 | 7 | 3.2 | 1.4–6.8 | 2 | 3.6 | 0.6–13.6 | 2 | 2.4 | 0.4–9.0 | 129 678 | 10.5 | 10.4–10.5 |
| Marital status | ||||||||||||||||||
| Not married | 70 | 76.9 | 66.7–84.8 | 100 | 78.4 | 70.4–85.3 | 170 | 78.0 | 71.8–83.2 | 40 | 72.7 | 58.8–83.5 | 69 | 81.2 | 70.9–88.5 | 388 288 | 31.4 | 31.3–31.4 |
| Married | 15 | 16.5 | 9.8–26.1 | 25 | 19.7 | 13.4–27.9 | 40 | 18.3 | 13.6–24.3 | 10 | 18.2 | 9.5–31.4 | 8 | 9.4 | 4.4–18.2 | 822 761 | 66.4 | 66.4–66.5 |
| Unknown | 6 | 6.6 | 2.7–14.3 | 2 | 1.6 | 0.3–6.1 | 8 | 3.7 | 1.7–7.4 | 5 | 9.1 | 3.4–20.7 | 8 | 9.4 | 4.4–18.2 | 27 403 | 2.2 | 2.2–2.2 |
| Education | ||||||||||||||||||
| Primary | 41 | 45.1 | 34.7–55.8 | 74 | 58.3 | 49.2–66.8 | 115 | 52.8 | 45.9–59.5 | 33 | 60.0 | 45.9–72.7 | 48 | 56.5 | 45.3–67.1 | 137 688 | 11.1 | 11.1–11.2 |
| Secondary | 45 | 49.5 | 38.9–60.1 | 48 | 37.8 | 29.5–46.9 | 93 | 42.7 | 36.1–49.5 | 18 | 32.7 | 21.0–46.8 | 33 | 38.8 | 28.6–50.0 | 848 295 | 70.9 | 70.8–71.0 |
| University | 3 | 3.3 | 0.9–10.0 | 0 | 0.0 | 0.0–2.9 | 3 | 1.4 | 0.4–4.3 | 1 | 1.8 | 0.1–11.0 | 0 | 0.0 | 0.0–4.2 | 185 951 | 15.0 | 15.0–15.1 |
| Unknown | 2 | 2.2 | 0.4–8.5 | 5 | 3.9 | 1.5–9.4 | 7 | 3.2 | 1.4–6.8 | 3 | 5.5 | 1.4–16.1 | 4 | 4.7 | 1.5–12.3 | 66 518 | 5.4 | 5.3–5.4 |
| Using of addictive substances during pregnancy | ||||||||||||||||||
| Smoking | 33 | 36.3 | 26.6–47.1 | 54 | 42.5 | 33.9–51.6 | 87 | 39.9 | 33.4–46.8 | 23 | 41.8 | 28.9–55.9 | 32 | 37.6 | 27.6–48.9 | 73 575 | 5.9 | 5.9–6.0 |
| Alcohol | 3 | 3.3 | 0.9–10.0 | 5 | 3.9 | 1.5–9.4 | 8 | 3.7 | 1.7–7.4 | 2 | 3.6 | 0.6–13.6 | 6 | 7.1 | 2.9–15.3 | 1626 | 0.1 | 0.1–0.1 |
| Illicit drugs | 35 | 38.5 | 28.6–49.3 | 52 | 40.9 | 32.4–50.0 | 87 | 39.9 | 33.4–46.8 | 22 | 40.0 | 27.3–54.1 | 36 | 42.4 | 31.9–53.5 | 1976 | 0.2 | 0.2–0.2 |
OMT = women in opioid maintenance therapy;
OMT‐D= women who had been in OMT before pregnancy but not in pregnancy (OMT discontinuers);
OUD = women hospitalized with a diagnosis of mental or behavioural disorder due to opioid use (ICD‐10 code F11, all subcodes) during pregnancy;
GP = pregnant women from the general population; CI = confidence interval.
Hospitalization
By 3 years of age, 54.1% of OMT‐exposed children, 47.3% of children of OMT‐D and 51.8% of children of women with OUD had been hospitalized at least once, compared to 35.8% of children in the GP (Table 2). Regarding the number of hospitalizations, length of stay and number of diagnoses, OMT‐exposed children and children in the OMT‐D and OUD groups were similar, but they had longer stays and more diagnoses than children in the GP. The children born by OMT‐D had worse outcomes than the OMT‐exposed children regarding the number of hospitalizations (P = 0.001) and length of stay (P < 0.001).
Table 2.
Hospital admissions in children (0–3 years) of women in opioid maintenance therapy compared to the OMT‐D, OUD and GP groups in the Czech Republic.
| OMT | OMT‐D | OUD | OMT versus OMT‐D | OMT versus OUD | GP | OMT versus GP | ||
|---|---|---|---|---|---|---|---|---|
| Children who reached age of 3 years, n | 218 | 55 | 85 | 1 238 452 | ||||
| ORa (95% CI) | ORa (95% CI) | ORa (95% CI) | ||||||
| Hospitalized children, n (%, CI) | 118 (54.1, 47.3–60.1) | 26 (47.3, 33.9–61.1) | 44 (51.8, 40.7–62.6) | 1.3 (0.7–2.4) | 1,1 (0.7–1.8) | 442 862 (35.8, 35.7–35.8) | 2.1 (1.6–2.8) | |
| adjb 1.3 (0.7–2.3) | adjb 1.2 (0.7–2.1) | adj b 1.6 (1.2–2.1) |
| P c | P c | P c | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of hospitalizations, mean, median, IQR | 2.0, 2.0, 1.0–2.3 | 3.5, 1.5, 1.0–3.0 | 2.2, 2.0, 1.0–2.8 | 0.001 | 0.400 | 1.8, 1.0, 1.0–2.0 | 0.100 | |
| Length of stay in days, mean, median, IQR | 17.4, 10.0, 4.0–21.0 | 41.8, 8.0, 4.0–23.5 | 14.4, 7.7, 4.0–16.5 | < 0.001 | 0.297 | 8.6, 4.0, 2.0–9.0 | < 0.001 | |
| Number of all diagnoses | ||||||||
| mean, median, IQR | 3.9, 3.0, 2.0–5.0 | 4.5, 3.0, 1.8–5.0 | 4.1, 3.0, 2.0–5.0 | 0.378 | 0.672 | 3.0, 2.0, 1.0–4.0 | < 0.001 | |
Excluded ICD 10 codes Z37 and Z38 diagnoses and birth hospitalization.
OMT = children exposed to opioid maintenance therapy;
OMT‐D = children of women who had been in OMT before pregnancy but not in pregnancy (OMT discontinuers);
OUD = children of women hospitalized with a diagnosis of mental or behavioural disorder due to opioid use (ICD‐10 code F11, all subcodes) during pregnancy;
GP = children of women from the general population;
CI = confidence interval;
IQR = interquartile range.
Odds ratios (ORs) from binary logistic regression of the child being hospitalized;
adj = adjusted for maternal age, education and smoking status during pregnancy;
P‐value from negative binominal regression analyses.
When the OMT‐exposed group was compared to the GP, the risk of hospitalization was increased for the OMT‐exposed (aOR = 1.6, 95% CI = 1.2–2.1). Significant differences were also found for length of stay (17.4 versus 8.6 mean days, P < 0.001) and number of diagnoses (3.9 versus 3.0, P < 0.001) (Table 2).
The risk of hospitalization was lower for buprenorphine‐exposed children compared to methadone‐exposed (aOR = 0.6, 95% CI = 0.3–1.0) (Table 3). The buprenorphine‐exposed children also had significantly shorter hospital stays compared to children prenatally exposed to methadone.
Table 3.
Hospital admissions in children (0–3 years) of women in opioid maintenance therapy using buprenorphine during pregnancy compared compared to the women using methadone in the Czech Republic.
| Buprenorphinea | Methadone | Buprenorphinea versus methadone | |
|---|---|---|---|
| Children who reached age of 3 years, n | 91 | 127 | |
| ORb (95% CI) | |||
| Hospitalized children, n (%, CI) | 40 (44.0, 33.7–54.3) | 78 (61.4, 52.3–69.8) | 0.5 (0.3–0.9) |
| adj c 0.6 (0.3–1.0) |
| P d | |||
|---|---|---|---|
| Number of hospitalizations, mean, median, IQR | 1.9, 2.0, 1.0–2.0 | 2.1, 2.0, 1.0–3.0 | 0.497 |
| Length of stay in days, mean, median, IQR | 12.0, 6.5, 3.0–15.8 | 20.2, 11.0, 4.8–27.3 | 0.008 |
| Number of all diagnoses mean, median, IQR | 3.7, 3.0, 2.0–4.0 | 4.0, 3.0, 2.0–5.0 | 0.496 |
Excluded ICD 10 codes Z37 and Z38 diagnoses and birth hospitalization.
CI = confidence interval;
IQR = interquartile range.
Buprenorphine (n = 82) and buprenorphine–naloxone combination (n = 9).
Odds ratios (ORs) from binary logistic regression of the child being hospitalized;
adj = adjusted for maternal age, education and smoking status during pregnancy;
P‐value from negative binominal regression analyses.
Diagnoses
Table 4 shows the proportions of children in the different groups who had received the different diagnoses until the age of 3. The proportions of children in the OMT‐exposed group and the OMT‐D and OUD groups with diagnoses were higher than in the general population for almost all diagnosis chapters (Table 4). The most common diagnosis chapter was diseases of the respiratory system (chapter X) and certain infections and parasitic diseases (chapter I). The proportion receiving these diagnoses in the OMT exposed children were 24.3 and 21.6%, respectively, compared to 16.3 and 8.9% in the GP.
Table 4.
Binary logistic regression comparing children of women in opioid maintenance therapy compared to the OMT‐D, OUD and GP groups in the Czech Republic.
| Chapter of ICD‐10 diagnoses | OMT (n = 218) | OMT‐D (n = 55) | OUD (n = 85) | GP (n = 1 238 452) | OMT versus OMT‐D (ref.) | OMT versus OUD (ref.) | OMT versus GP (ref.) | |
|---|---|---|---|---|---|---|---|---|
| Cases n (%) | Cases n (%) | Cases n (%) | Cases n (%) | OR unadjusted (95% CI) | OR unadjusted (95% CI) | OR unadjusted (95% CI) | OR adjusteda (95% CI) | |
| I. Certain infectious and parasitic diseases (A00–B99) | 47 (21.6) | 9 (16.4) | 14 (16.5) | 110 383 (8.9) | 1.4 (0.6–3.1) | 1.4 (0.7–2.7) | 2.8 (2.0–3.9) | 2.0 (1.4–2.7) |
| III. Diseases of the blood, blood‐forming organs and certain disorders involving the immune mechanisms (D50–D89) | 13 (6.0) | 2 (3.6) | 5 (5.9) | 34 680 (2.8) | 1.7 (0.4–7.7) | 1.0 (0.4–2.9) | 2.2 (1.3–3.9) | 1.4 (0.8–2.4) |
| IV. Endocrine, nutritional and metabolic diseases (E00–E90) | 7 (3.2) | 3 (5.5) | 5 (5.9) | 50 140 (4.0) | 0.6 (0.1–2.3) | 0.5 (0.2–1.7) | 0.8 (0.4–1.7) | b |
| VII. Diseases of the eye and adnexa (H00–H59) | 7 (3.2) | 1 (1.8) | 2 (2.4) | 18 013 (1.5) | 1.8 (0.2–14.9) | 1.4 (0.3–6.8) | 2.2 (1.1–4.8) | 1.6 (0.7–3.3) |
| VIII. Diseases of the ear and mastoid process (H60–H95) | 8 (3.7) | 1 (1.8) | 7 (8.2) | 27 323 (2.2) | 2.1 (0.3–16.8) | 0.4 (0.2–1.2) | 1.7 (0.8–3.3) | b |
| X. Diseases of the respiratory system (J00–J99) | 53 (24.3) | 12 (21.8) | 23 (27.1) | 201 825 (16.3) | 1.2 (0.6–2.3) | 0.9 (0.5–1.5) | 1.7 (1.2–2.2) | 1.1 (0.8–1.5) |
| XI. Diseases of the digestive system (K00–K93) | 30 (13.8) | 4 (7.3) | 10 (11.8) | 77 917 (6.3) | 2.0 (0.7–6.0) | 1.2 (0.6–2.6) | 2.4 (1.6–3.5) | 1.7 (1.2–2.6) |
| XII. Diseases of the skin and subcutaneous tissue (L00–L99) | 18 (8.3) | 5 (9.1) | 4 (4.7) | 35 017 (2.8) | 0.9 (0.3–2.5) | 1.8 (0.6–5.6) | 3.1 (1.9–5.0) | 1.9 (1.2–3.2) |
| XIV. Diseases of the genitourinary system (N00–N99) | 8 (3.7) | 3 (5.5) | 5 (5.9) | 44 674 (3.6) | 0.7 (0.2–2.6) | 0.6 (0.2–1.9) | 1.0 (0.5–2.1) | 0.9 (0.5–1.9) |
| XVI. Certain conditions originating in the perinatal period (P00‐P96) | 32 (14.7) | 6 (10.9) | 11 (12.9) | 35 882 (2.9) | 1.4 (0.6–3.6) | 1.2 (0.6–2.4) | 5.8 (4.0–8.4) | 4.6 (3.1–6.7) |
| XVII. Congenital malformations, deformations and chromosomal abnormalities (Q00–Q99) | 13 (6.0) | 5 (9.1) | 6 (7.1) | 43 355 (3.5) | 0.6 (0.2–1.9) | 0.8 (0.3–2.3) | 1.7 (1.0–3.1) | b |
| XVIII. Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified (R00–R99) | 40 (18.3) | 8 (14.5) | 9 (10.6) | 118 551 (9.6) | 1.3 (0.6–3.0) | 1.9 (0.9–4.1) | 2.1 (1.5–3.0) | 1.5 (1.1–2.1) |
| XIX. Injury, poisoning and certain other consequences of external causes (S00–T98) | 21 (9.6) | 4 (7.3) | 5 (5.9) | 79 796 (6.4) | 1.4 (0.4–4.1) | 1.7 (0.6–4.7) | 1.5 (1.0–2.4) | b |
| XXI. Factors influencing health status and contact with health services (Z00–Z99) | 19 (8.7) | 5 (9.1) | 7 (8.2) | 56 426 (4.6) | 1.0 (0.3–2.7) | 1.1 (0.4–2.6) | 2.0 (1.2–3.2) | 1.5 (0.9–2.4) |
OMT = children exposed to opioid maintenance therapy;
OMT‐D children of women who had been in OMT before pregnancy but not in pregnancy (OMT discontinuers);
OUD = children of women hospitalized with a diagnosis of mental or behavioural disorder due to opioid use (ICD‐10 code F11, all subcodes) during pregnancy;
GP = children of women from the general population;
CI = confidence interval.
OR 95% CI = odds ratio with 95% confidence interval.
Adjusted for maternal age, education and smoking status during pregnancy;
adjusted analyses not performed because there were no significant results in the unadjusted analyses.
The unadjusted logistic regression analysis showed no statistically significant differences between the OMT‐exposed and OMT‐D and OUD groups (Table 4). For the majority of diagnoses there were differences in risk in the unadjusted analysis when comparing OMT‐exposed to the GP. After adjustment (a), there were still increased ORs of infectious and parasitic diseases (aOR = 2.0, 95% CI = 1.4–2.7), diseases of the digestive system (aOR = 1.7, 95% CI = 1.2–2.6) and diseases of the skin and subcutaneous tissue (aOR = 1.9, 95% CI = 1.2–3.2). The risk of having a diagnosis in the diagnosis chapter of certain conditions originating in the perinatal period and in symptoms, signs and abnormal clinical laboratory findings were also significantly increased.
When comparing diagnoses, there were significant differences between children prenatally exposed to buprenorphine and methadone only for certain conditions originating in the perinatal period (Table 5).
Table 5.
Binary logistic regression comparing children of women using buprenorphine compared to women using methadone in the Czech Republic.
| Chapter of ICD‐10 diagnoses | Buprenorphinea (n = 91) | Methadone (n = 127) | Buprenorphinea versus methadone (ref.) |
|---|---|---|---|
| Cases n (%, 95% CI) | Cases n (%, 95% CI) | OR unadjusted (95% CI) | |
| I. Certain infectious and parasitic diseases (A00–B99) | 16 (17.6, 10.7–27.3) | 31 (24.4, 17.4–33.0) | 0.7 (0.3–1.3) |
| III. Diseases of the blood, blood‐forming organs and certain disorders involving the immune mechanisms (D50–D89) | 6 (6.6, 2.7–14.4) | 7 (5.5, 2.4–11.4) | 1.2 (0.4–3.7) |
| IV. Endocrine, nutritional and metabolic diseases (E00–E90) | 3 (3.3, 0.9–10.0) | 4 (3.1, 1.0–8.4) | 1.0 (0.2–4.8) |
| VII. Diseases of the eye and adnexa (H00–H59) | 1 (1.1, 0.01–6.8) | 6 (4.7, 1.9–10.4) | 0.2 (0.0–2.9) |
| VIII. Diseases of the ear and mastoid process (H60–H95) | 0 (0.0, 0.0–4.0) | 8 (6.3, 3.0–12.4) | b |
| X. Diseases of the respiratory system (J00–J99) | 18 (19.8, 12.5–29.7) | 35 (27.6, 20.2–36.3) | 0.6 (0.3–1.2) |
| XI. Diseases of the digestive system (K00–K93) | 13 (14.3, 8.1–23.6) | 17 (13.4, 8.2–20.8) | 1.1 (0.5–2.3) |
| XII. Diseases of the skin and subcutaneous tissue (L00–L99) | 6 (6.6, 2.7–14.4) | 12 (9.4, 5.2–16.3) | 0.7 (0.2–1.9) |
| XIV. Diseases of the genitourinary system (N00–N99) | 3 (3.3, 0.9–10.0) | 5 (3.9, 1.5–9.4) | 0.8 (0.2–3.6) |
| XVI. Certain conditions originating in the perinatal period (P00–P96) | 8 (8.8, 4.2–17.1) | 24 (18.9, 12.7–27.0) | 0.4 (0.2–1.0) c |
| XVII. Congenital malformations, deformations and chromosomal abnormalities (Q00–Q99) | 5 (5.5, 2.0–12.9) | 8 (6.3, 3.0–12.4) | 0.9 (0.3–2.7) |
| XVIII. Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified (R00–R99) | 13 (14.3, 8.1–23.6) | 27 (21.3, 14.7–29.6) | 0.6 (0.3–1.3) |
| XIX. Injury, poisoning and certain other consequences of external causes (S00–T98) | 7 (7.7, 3.4–15.7) | 14 (11.0, 6.4–18.1) | 0.7 (0.3–1.7) |
| XXI. Factors influencing health status and contact with health services (Z00–Z99) | 7 (7.7, 3.4–15.7) | 12 (9.4, 5.2–16.3) | 0.8 (0.3–2.1) |
OR 95% CI = odds ratio with 95% confidence interval.
Buprenorphine (n = 82) and buprenorphine naloxone combination (n = 9);
zero cases;
adjusted OR = 0.4 (0.2–1.0), P = 0.049, adjusted for maternal age, education and smoking status during pregnancy.
Results of the subanalyses of diagnoses generally supported our main findings. In the analysis stratified on either gender or presence of conditions originating in the perinatal period, the results were in the same direction as in the main analysis (Supporting information, Table S1).
Discussion
There was no increased risk of morbidity for the OMT‐exposed children compared to children in the OMT‐D and OUD groups, as measured by hospitalization and the prevalence of ICD‐10 diagnoses. When compared to the GP group, children in the OMT group had a higher risk of hospitalization and received more diagnoses by the age of 3. More specifically, the OMT group had a higher risk of infectious, digestive diseases, diseases of the skin and subcutaneous tissue, as well as conditions originating in the perinatal period, compared to the GP group.
The strong and unique aspects of our study reside in using relevant comparison groups. This study compared OMT‐exposed children not only to children in the GP but also to OMT discontinuers and to children of women with OUD. These women are more similar to women in OMT treatment regarding their socio‐demographic characteristics and life‐style than to women in the general population. Comparison among similar groups may contribute to increased control over unmeasured residual confounding. The comparable risk found in children with different prenatal OMT exposure suggests that it is not OMT treatment itself that is associated with the increased morbidity in OMT‐exposed children. Kelty et al.'s previous finding, that both prenatal exposure to opioid agonists and antagonists increase the risk of morbidity, also points in the direction that the association is not caused by OMT drugs 10. The implications of the study findings might be that that other risk factors could be associated with opioid use disorders as opposed to use of OMT drugs during pregnancy, which result in a higher risk of morbidity in all the groups of women with opioid use disorders (OMT, OMT‐D and OUD) compared to the GP.
In concordance with this study, Kelty et al. also reported a higher risk of hospitalization for skin and subcutaneous diseases in OMT‐exposed children 10. A study focusing on children with NAS 15 also found an increased risk of these ICD‐10 diagnostic chapters 15.
Increased morbidity in OMT‐exposed children, as well as in children in the comparison groups, can be explained by multiple factors. A possibility is that the excess of infectious, digestive and skin diseases could be attributed to a higher risk of infections in general due either to increased exposure to microbial pathogens or a weak immune system. A weaker immune system might be due to a direct effect of opioids on the immune cells or to poorer maternal health compared to the general pregnant population 21. Findings from studies on the opioid effects on the immune system have been inconclusive, nor is it clear if there is an effect on the developing immune system of the fetus 22. Our findings did not support a direct influence on the immune system of OMT drugs, as there was no difference between the OMT group and the OMT discontinuers. Higher morbidity in children born to substance‐using mothers due to epigenetic mechanism during intrauterine development has also been suggested as an explanation for the weaker immune systems of exposed children 22.
Aside from prenatal exposure to opioid drugs, we also cannot ignore the importance of pre‐ and postnatal risk factors, including poor nutrition, hygiene, parenting and other drug use. Psychosocial stress can also contribute to higher vulnerability in the context of allostatic load during pregnancy 23, 24.
Not surprisingly, our study found a higher risk of conditions originating in the perinatal period in the OMT‐exposed group when compared to the GP. It is well documented that OMT‐exposed newborns have a higher risk of preterm birth, growth retardation and NAS compared to the general population.
The risk for conditions originating in the perinatal period was lower among OMT children exposed to buprenorphine compared to those exposed to methadone. This is in contrast to our previous findings regarding no differences in neonatal outcomes in children prenatally exposed to buprenorphine versus methadone 18. The diagnostic chapter of conditions originating in the perinatal period includes more disorders than were included in our previous study, and some of these can be diagnosed retrospectively. In addition, children exposed to methadone had longer hospital stays than buprenorphine‐exposed children, which can be partly explained by longer treatment of more severe NAS, as previously reported by others in newborns exposed to methadone 25.
The few existing studies on long‐term outcomes found an increased frequency of mental and behavioural disorders among NAS children or children of OMT‐exposed women 10, 15. It was not possible to examine mental and behavioural disorders in this study, as these conditions are typically diagnosed at a later stage of the child's development.
Methodological consideration
By accessing national registries on reproductive health, addiction treatment, hospitalization and death, it was possible to establish a national cohort and examine their longitudinal data. Therefore, selection bias is less of a problem than in many clinical samples. In addition, health registries identify larger samples than can feasibly be included in clinical studies.
Using information from the registries reduces the risk of recall bias. However, some important information can be under‐reported or reported in insufficient format in the registries, e.g. use of alcohol and illicit drugs by pregnant women. Further, information on nutrition or infections during pregnancy is lacking. An important limitation is the lack of information on NAS in the newborn, which could be a mediator of the observed association between OMT exposure and childhood morbidity.
Mortality was slightly higher among OMT‐exposed children than among children in comparison groups (0.6 versus 0% in the observation period). It is unknown whether the cause of death could be linked to in‐utero exposure to OMT drugs, but hospital data do not suggest that any of the children had serious disease prior to death. Nevertheless, the mortality rate was so low that it would not influence the estimates.
While opioid‐dependent women in the Czech Republic can receive methadone free of charge, they must pay a substantial price for buprenorphine. Bias is likely, as women who are able to pay for buprenorphine probably belong to a higher socio‐economic class and/or they are under‐dosed in order to limit the expenses. Their background characteristics might suggest that the buprenorphine‐using women had higher education and smoked to a lesser extent than the methadone‐using women, but the results were not significant. Unfortunately, the registry does not contain information concerning the OMT drugs dose.
The current study used data from the hospitalization registry on frequency and diagnosis set by the physicians at hospital discharge reports to describe morbidity of the children prenatally exposed to OMT, from birth to 3 years of age. Out‐patient morbidity, which probably represents a substantial proportion of morbidity, is missing in our analysis. Nonetheless, the more serious health problems that required hospitalization are included.
Conclusion
In this nation‐wide cohort of pregnant women with opioid use disorders, no statistically significant differences were observed in childhood morbidity between children of women in OMT during pregnancy, OMT discontinuers or women with OUD during pregnancy. Compared to children in the general population, OMT‐exposed children had higher risk of infections, digestive diseases and diseases of the skin and subcutaneous tissue, but the risk estimates are probably confounded by unmeasured life‐style factors associated with opioid use disorders. These findings need to be replicated in other countries, preferably in larger study samples.
Declaration of interests
None.
Supporting information
Table S1 Binary logistic regression comparing children of women in opioid maintenance therapy to the general population in the Czech Republic. Stratified analyses on gender and certain conditions originating in the perinatal period.
Acknowledgements
This study was supported by the Ministry of Health of the Czech Republic, grant no. 16‐28157A.
Skurtveit, S. , Nechanská, B. , Handal, M. , Mahic, M. , Mravčík, V. , and Gabrhelík, R. (2019) Hospitalization of children after prenatal exposure to opioid maintenance therapy during pregnancy: a national registry study from the Czech Republic. Addiction, 114: 1225–1235. 10.1111/add.14576.
[Correction added on 7 May 2019, after first online publication: The funder information has been added in this version.]
[The copyright line for this article was changed on 4 November 2019 after original online publication.]
References
- 1. World Health Organization (WHO) . Guidelines for Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 2. Farid W. O., Dunlop S. A., Tait R. J., Hulse G. K. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr Neuropharmacol 2008; 6: 125–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones H. E., O'Grady K. E., Malfi D., Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict 2008; 17: 372–386. [DOI] [PubMed] [Google Scholar]
- 4. Norgaard M., Nielsson M. S., Heide‐Jorgensen U. Birth and neonatal outcomes following opioid use in pregnancy: a Danish population‐based study. Subst Abuse Res Treat 2015; 9: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunt R. W., Tzioumi D., Collins E., Jeffery H. E. Adverse neurodevelopmental outcome of infants exposed to opiate in‐utero . Early Hum Dev 2008; 84: 29–35. [DOI] [PubMed] [Google Scholar]
- 6. Kaltenbach K., O'Grady K. E., Heil S. H., Salisbury A. L., Coyle M. G., Fischer G. et al Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend 2018; 185: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konijnenberg C., Melinder A. Prenatal exposure to methadone and buprenorphine: a review of the potential effects on cognitive development. Child Neuropsychol 2011; 17: 495–519. [DOI] [PubMed] [Google Scholar]
- 8. Konijnenberg C., Sarfi M., Melinder A. Mother–child interaction and cognitive development in children prenatally exposed to methadone or buprenorphine. Early Hum Dev 2016; 101: 91–97. [DOI] [PubMed] [Google Scholar]
- 9. Kivisto K., Tupola S., Kivitie‐Kallio S. Prenatally buprenorphine‐exposed children: health to 3 years of age. Eur J Pediatr 2015; 174: 1525–1533. [DOI] [PubMed] [Google Scholar]
- 10. Kelty E., Hulse G. A retrospective cohort study of the health of children prenatally exposed to methadone, buprenorphine or naltrexone compared with non‐exposed control children. Am J Addict 2017; 26: 845–851. [DOI] [PubMed] [Google Scholar]
- 11. Kelly L. E., Rieder M. J., Bridgman‐Acker K., Lauwers A., Madadi P., Koren G. Are infants exposed to methadone in utero at an increased risk for mortality? J Popul Ther Clin Pharm 2012; 19: e160–e165. [PubMed] [Google Scholar]
- 12. Gomez‐Pomar E., Finnegan L. P. The epidemic of neonatal abstinence syndrome, historical references of its origins, assessment, and management. Front Pediatr 2018; 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lind J. N., Petersen E. E., Lederer P. A., Phillips‐Bell G. S., Perrine C. G., Li R. et al Infant and maternal characteristics in neonatal abstinence syndrome—selected hospitals in Florida, 2010–2011. Morb Mortal Wkly Rep 2015; 64: 213–216. [PMC free article] [PubMed] [Google Scholar]
- 14. Patrick S. W., Burke J. F., Biel T. J., Auger K. A., Goyal N. K., Cooper W. O. Risk of hospital readmission among infants with neonatal abstinence syndrome. Hospital Pediatr 2015; 5: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uebel H., Wright I. M., Burns L., Hilder L., Bajuk B., Breen C. et al Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatrics 2015; 136: e811–e820. [DOI] [PubMed] [Google Scholar]
- 16. Witt C. E., Rudd K. E., Bhatraju P., Rivara F. P., Hawes S. E., Weiss N. S. Neonatal abstinence syndrome and early childhood morbidity and mortality in Washington state: a retrospective cohort study. J Perinatol 2017; 37: 1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gabrhelik R, Nechanska B, Mravcik V, Skurtveit S, Lund IO, Handal M. A unique opportunity to study short and long term consequences in children prenatally exposed to illicit drugs and opioid maintenance treatment using Czech and Scandinavian registers. Cent Eur J Public Health 2016;24:248–251. [DOI] [PubMed] [Google Scholar]
- 18. Nechanska B., Mravcik V., Skurtveit S., Lund I. O., Gabrhelik R., Engeland A. et al Neonatal outcomes after fetal exposure to methadone and buprenorphine: national registry studies from the Czech Republic and Norway. Addiction 2018; 113: 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popov P. Metadonová substituční léčba v České republice [in Czech; Methadone substitution treatment in the Czech Republic]. Adiktologie 2002; 2: 25–31. [Google Scholar]
- 20. Vollset S. E. Confidence intervals for a binomial proportion. Stat Med 1993; 12: 809–824. [DOI] [PubMed] [Google Scholar]
- 21. Al‐Hashimi M., Scott S. W., Thompson J. P. et al Opioids and immune modulation: more questions than answers. Br J Anaesth 2013; 111: 80–88. 10.1093/bja/aet153 [DOI] [PubMed] [Google Scholar]
- 22. Liang X., Liu R., Chen C., Ji F., Li T. Opioid system modulates the immune function: a review. Transl Perioper Pain Med 2016; 1: 5–13. [PMC free article] [PubMed] [Google Scholar]
- 23. Loomans E. M., van Dijk A. E., Vrijkotte T. G., van Eijsden M., Stronks K., Gemke R. J. et al Psychosocial stress during pregnancy is related to adverse birth outcomes: results from a large multi‐ethnic community‐based birth cohort. Eur J Public Health 2013; 23: 485–491. [DOI] [PubMed] [Google Scholar]
- 24. Premji S. Perinatal distress in women in low‐ and middle‐income countries: allostatic load as a framework to examine the effect of perinatal distress on preterm birth and infant health. Matern Child Health J 2014; 18: 2393–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez S., Bruni T., Bishop D. V., Turuba R., Olibris B., Jumah N. A. Differences in hospital length of stay between neonates exposed to buprenorphine versus methadone in utero: a retrospective chart review. Paediatrics & Child Health 2017; 22: e22–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Binary logistic regression comparing children of women in opioid maintenance therapy to the general population in the Czech Republic. Stratified analyses on gender and certain conditions originating in the perinatal period.


