Abstract
Aim
The present study aimed to determine the real‐world efficacy and safety of the non‐structural protein (NS)5A inhibitor elbasvir (EBR) combined with the NS3/4A protease inhibitor grazoprevir (GZR) in patients with hepatitis C virus (HCV) genotype 1 (GT1) infection.
Methods
This study retrospectively evaluated the rate of sustained virologic response at 12 weeks post‐treatment (SVR12) and the safety of EBR/GZR treatment in 159 men and 194 women with a median age of 72 years, and it assessed factors associated with the SVR12 rate. The attending physicians were responsible for selecting candidate patients for EBR/GZR in this retrospective study.
Results
Treatment outcomes for EBR/GZR were good in direct‐acting antiviral (DAA)‐naïve patients, of whom 99.4% achieved SVR. Of 353 patients, 10 (2.9%) had treatment failure. Of these patients, eight previously underwent DAA therapy, and the remaining two had NS5A‐L31/Y93 double mutation. The SVR rate was 50% (8/16 patients) in patients who previously underwent DAA therapy, and 18.2% (2/11 patients) in patients with NS5A‐L31/Y93 double mutation. On multivariate logistic regression analysis, NS5A‐Y31/Y93 double mutation (odds ratio 356.3; 95% confidence interval, 23.91–16 940; P < 0.0001) was identified as an independent predictor of treatment failure. No serious adverse events were observed with EBR/GZR therapy.
Conclusions
The SVR rate of EBR/GZR would have been 100% in patients without either a history of DAA therapy or double mutation. This combination of drugs could be safely given and is, thus, considered a highly useful first‐line treatment for DAA‐naïve patients with HCV.
Keywords: elbasvir, genotype 1, grazoprevir, hepatitis C virus, resistance‐associated substitution
INTRODUCTION
Because patients with chronic hepatitis C virus (HCV) infection have high risks for developing cirrhosis, liver failure, and hepatocellular carcinoma (HCC), early eradication of HCV has been considered desirable.1, 2, 3, 4, 5, 6, 7 Since 2014, treatment with direct‐acting antivirals (DAAs) alone has become the standard in Japan, achieving safe and satisfactory outcomes.
Patients with HCV genotype (GT) 1b, the most common type in Japan, were originally treated with the combination of the non‐structural protein (NS)5A inhibitor daclatasvir (DCV) and the NS3/4A protease inhibitor asunaprevir (ASV).8, 9, 10 However, the major drawback of this treatment was that the presence of a resistance‐associated substitution (RAS) at the 168th amino acid of NS3 or the 31st or 93rd amino acid of NS5A markedly decreased the sustained virologic response (SVR) rate.11, 12, 13 Currently, this combination of drugs is excluded as a treatment option.
Later in 2015, the combination of the NS5A inhibitor ledipasvir (LDV) and the NS5B inhibitor sofosbuvir (SOF) was approved in Japan.14, 15 A multicenter study undertaken by the Japanese Red Cross Liver Study Group found the SVR rate of LDV/SOF to be 98.4%.16 In this study, the SVR rate was 100% in patients with neither cirrhosis nor NS5A RAS. In contrast, the rate was 93.0% in patients with cirrhosis and NS5A RAS. Although the Japan Society of Hepatology Guidelines do not require the verification of NS5A RAS prior to LDV/SOF,13 caution could be necessary in patients with cirrhosis who have RAS, because the SVR is slightly lower in this population.16
Combination therapy with the NS5A inhibitor elbasvir (EBR) and NS3/4A protease inhibitor grazoprevir (GZR) for GT1 or GT4 patients was first used in 2016. Unlike LDV/SOF, EBR/GZR can be given to patients with renal dysfunction.17 In a Japanese phase III trial, its SVR rate was 96.5% in chronic hepatitis C patients and 97.1% in cirrhosis patients.18 Moreover, EBR/GZR could be safely given to patients with comorbid chronic kidney disease, as in the reports by Takeuchi et al. and Reddy et al.19, 20
The current study, carried out by the Japanese Red Cross Liver Study Group, investigated the outcomes and safety of EBR/GZR treatment, along with its efficacy, in patients with treatment failure with existing DAAs.
METHODS
Patients
A multicenter cohort study at 25 institutions belonging to the Japanese Red Cross Liver Study Group was carried out. The study population consisted of 353 patients with either GT1 chronic hepatitis C or compensated cirrhosis who underwent 12 weeks of treatment with EBR (50 mg/day) and GZR (100 mg/day) (MSD, Tokyo, Japan) between November 2016 and March 2018. Sustained virologic response was defined as undetectable serum HCV‐RNA 12 weeks after the end of treatment. The attending physicians diagnosed cirrhosis clinically, relying primarily on imaging findings (i.e. presence of collateral circulation, esophageal or gastric varices, and splenomegaly). Patients with decompensated cirrhosis with Child–Pugh grade B or C were excluded.
This study was approved by the ethics committees of the participating institutions and was carried out in accordance with the ethical guidelines specified in the Declaration of Helsinki.
Detection of drug‐resistant substitutions
Amino acid sequences of NS5A‐L31 and NS5A‐Y93 were determined using the Invader assay (BML Laboratory, Tokyo, Japan),21 direct sequencing (SRL Laboratory, Tokyo, Japan or LSI Laboratory, Tokyo, Japan),22 or the Cycleave polymerase chain reaction (SRL Laboratory).23
Resistance‐associated substitution was considered present when it exceeded 10%.
Safety
Adverse events were evaluated in accordance with Common Terminology Criteria for Adverse Events version 4.0, Japan Clinical Oncology Group (National Cancer Institute, Bethesda, MD, USA). The patients were seen approximately every 2 weeks while on the study treatment and approximately every 4–8 weeks thereafter for the evaluation of efficacy and adverse events. Laboratory values, including a complete blood count, alanine aminotransferase, aspartate aminotransferase, total bilirubin, and creatinine were determined before treatment and every 2 weeks after the start of treatment. Serum HCV‐RNA levels were determined every 4 weeks during treatment and 4 and 12 weeks after the end of treatment. At patient visits, the attending physicians evaluated the subjective symptoms, laboratory abnormalities, and other adverse events that had occurred after the start of treatment.
Definition of renal dysfunction
Renal function was assessed using the estimated glomerular filtration rate (eGFR)24 based on pretreatment laboratory data. Chronic kidney disease (CKD) was classified as stage G1 or G2 (eGFR >60 mL/min/1.73 m2), stage G3a or G3b (eGFR = 30–59 mL/min/1.73 m2), and stage G4 or G5 (eGFR <30 mL/min/1.73 m2).25
Statistical analysis
Using JMP version 13.1.0 (SAS Institute, Cary, NC, USA) software for statistical analysis, factors associated with non‐SVR were identified by logistic regression analysis. Results from the risk analysis are expressed as odds ratios (OR) with 95% confidence intervals (CI). All analyses were two‐tailed, and P < 0.05 was considered significant.
RESULTS
The 927 GT1 patients receiving antiviral therapy at a study institution from November 2016 to March 2018 were treated with EBR/GZR (42.0%), DCV/ASV (0.2%), LDV/SOF (10.9%), ombitasvir/paritaprevir/ritonavir (7.3%), or glecaprevir/pibrentasvir (GLE/PIB; 39.6%). The attending physicians were responsible for selecting candidate patients for EBR/GZR in this retrospective study. In total, 389 patients with chronic GT1 hepatitis C who underwent 12‐week treatment with EBR (50 mg/day) and GZR (100 mg/day) were assessed. As 36 patients dropped out or could not be evaluated for SVR at the time of assessment, 353 patients were included in the assessment. The patients’ characteristics are shown in Table 1. The patients’ median age was 72 years, and 40.8% (144/353) of the patients were aged 75 years or older. The study patients included 159 men and 194 women. The proportion of patients with a history of interferon (IFN) therapy was 19.1% (67/351 patients), and the proportion of patients with a history of DAA therapy was 4.7% (16/342 patients). Cirrhosis was identified in 20.5% (68/331 patients), and there was a history of HCC treatment in 10.5% (37 patients). There were 92 patients (26.1%) with CKD stage G3a or G3b and 56 patients (15.9%) with CKD G4 or G5, and 28 of these patients (7.9%) were on dialysis. Data on NS5A RAS could be analyzed in 297 patients, in which 6.0% (18 patients) had NS5A‐L31 RAS, and 15.7% (47 patients) possessed NS5A‐Y93 RAS. The overall SVR12 rate was 97.2% (343/353 patients). Age, sex, presence of cirrhosis, treatment history of HCC, and presence of renal disorder (eGFR<30 mL/min/1.73 m2) were not significantly associated with SVR. However, a history of IFN therapy was a significant univariate predictor of non‐SVR (Fig. 1). A mere 2.9% (10/353 patients) had treatment failure: eight of these patients had a history of DAA therapy, whereas the other two without a history of DAA therapy had NS5A‐L31/‐Y93 double mutation (Table 2). The SVR rate was low, at 50% (8/16 patients), in patients with a history of DAA therapy, and it was extremely poor, at 18.2% (2/11 patients), in patients with NS5A‐L31/‐Y93 double mutation (Fig. 2). In contrast, SVR was achieved in six of seven patients who did not have double mutations at NS5A‐L31/‐Y93, despite a history of DAA therapy. The SVR rate of EBR/GZR would have been 100% in patients without either a history of DAA therapy or double mutation. Univariate logistic regression analysis showed that a history of IFN therapy (P = 0.0044), history of DAA therapy (P < 0.0001), and presence of NS5A‐L31/‐Y93 double mutation (P < 0.0001) were significant factors associated with non‐SVR. Multivariate logistic regression analysis identified NS5A‐Y31/‐Y93 double mutation as an independent predictor of treatment failure (OR 356.3; 95% CI, 23.91–16 940; P < 0.0001) (Table 3).
Table 1.
Baseline characteristics of patients with hepatitis C virus (HCV) genotype 1 treated with elbasvir/grazoprevir
| Characteristic | n = 353 |
|---|---|
| Age, years | 72 (38–90) |
| >75 | 144 (40.8) |
| Male : female, n | 159:194 |
| History of IFN therapy, no/yes (yes %) | 284/67 (19.1) |
| History of DAA therapy, no/yes (yes %) | 326/16 (4.7) |
| History of HCC, no/yes (yes %) | 316/37 (10.5) |
| Cirrhosis, no/yes (yes %) | 263/68 (20.5) |
| NS5A‐L31, wild‐type/mutant (mutant %) | 279/18 (6.1) |
| NS5A‐Y93, wild‐type/mutant (mutant %) | 249/48 (16.2) |
| Platelet count, ×103/μL | 125 (34–684) |
| AST, IU/L | 53 (8–484) |
| ALT, IU/L | 36 (7–470) |
| eGFR, mL/min/1.73 m2 | 62.5 (3.6–112.9) |
| G3a or G3b | 92 (26.1) |
| G4 or G5 | 56 (15.9) |
| M2BPGi, COI | 1.93 (0.25–14.2) |
| FIB‐4 index | 2.97 (0.36–17.2) |
| α‐Fetoprotein, ng/mL | 4 (1–296) |
| HCVRNA, log IU/mL | 6.1 (2.0–7.4) |
Data are shown as n (%) or median (range), unless otherwise indicated.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COI, cut‐off index; DAA, direct‐acting antiviral; eGFR, estimated glomerular filtration rate; FIB‐4, Fibrosis‐4; HCC, hepatocellular carcinoma; IFN, interferon; M2BPGi, Mac‐2 binding protein glycosylation isomer; NS5A, non‐structural protein 5A.
Figure 1.
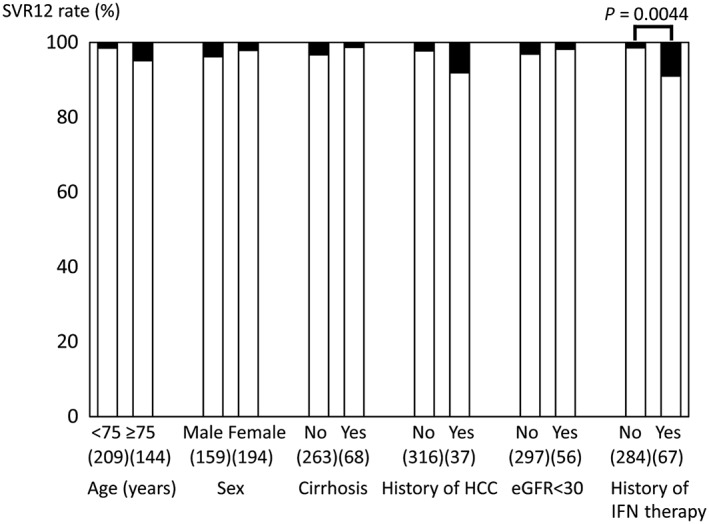
Sustained virologic response rate at 12 weeks after end of treatment (SVR12) with elbasvir/grazoprevir among 353 patients with hepatitis C virus (HCV) genotype 1, grouped by patient characteristics. Although age, sex, presence of cirrhosis, history of hepatocellular carcinoma (HCC), and renal disorder (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) are not significantly associated with SVR, a history of interferon (IFN) therapy significantly decreases the SVR rate.
Table 2.
Patients with hepatitis C virus genotype 1 treated with elbasvir/grazoprevir who failed to achieve sustained virologic response (SVR)
| No. | Age, years | Sex | History of IFN therapy | History of DAA therapy | NS5A‐L31 | NS5A‐Y93 | Cirrhosis | History of HCC | Details of SVR failure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | Yes | DCV/ASV | Mutant | Mutant | No | No | Relapse |
| 2 | 75 | M | Yes | SMV/DCV/ASV | Mutant | Mutant | No | No | Non‐response |
| 3 | 75 | F | Yes | VAN | Mutant | Mutant | No | No | Relapse |
| 4 | 76 | F | No | DCV/ASV | Mutant | Mutant | No | No | Relapse |
| 5 | 76 | M | Yes | DCV/ASV | Mutant | Mutant | No | Yes | Relapse |
| 6 | 79 | F | No | DCV/ASV | Mutant | Mutant | No | No | Relapse |
| 7 | 80 | M | No | DCV/ASV | Wild | Wild | No | Yes | Non‐response |
| 8 | 81 | M | Yes | DCV/ASV | Mutant | Mutant | No | No | Breakthrough |
| 9 | 73 | M | No | No | Mutant | Mutant | Yes | Yes | Relapse |
| 10 | 74 | F | Yes | No | Mutant | Mutant | No | No | Relapse |
ASV, asunaprevir; DAA, direct‐acting antiviral; DCV, daclatasvir; F, female; HCC, hepatocellular carcinoma; IFN, interferon; M, male; NS5A, non‐structural protein 5A; SMV, simeprevir; VAN, vaniprevir.
Figure 2.
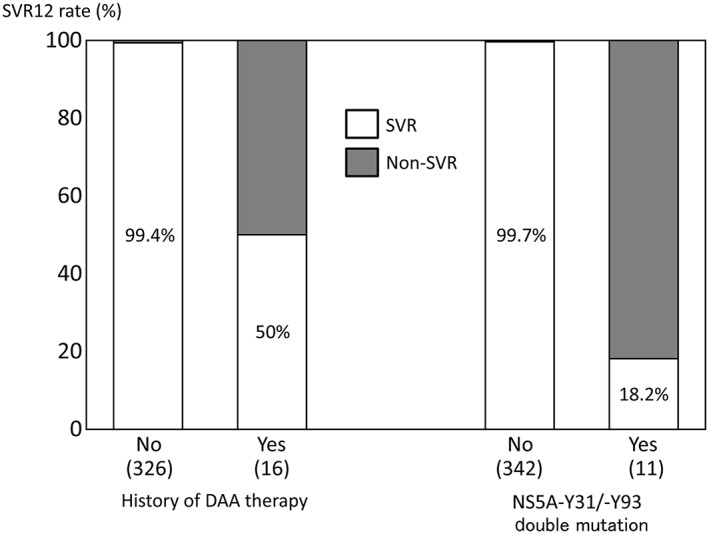
Sustained virologic response rate at 12 weeks after end of treatment (SVR12) with elbasvir/grazoprevir decreased markedly among 353 patients with hepatitis C virus (HCV) genotype 1 when there was a history of direct‐acting antiviral (DAA) therapy or a non‐structural protein (NS)5A double mutation.
Table 3.
Factors associated with non‐sustained virologic response in patients with hepatitis C virus (HCV) genotype 1 after completing antiviral therapy with elbasvir/grazoprevir
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | |
| Age | 1.069 (0.999–1.162) | 0.0538 | — | — |
| Sex, male | 1.819 (0.511–7.222) | 0.3547 | — | — |
| History of IFN therapy, yes | 6.619 (1.837–26.54) | 0.0044 | 4.25 (0.308–104.5) | 0.2706 |
| History of DAA therapy, yes | 162.0 (34.49–1199.2) | <0.0001 | 5.293 (0.161–94.86) | 0.3070 |
| History of HCC, yes | 3.895 (0.811–14.73) | 0.0840 | — | — |
| Cirrhosis, yes | 0.384 (0.021–2.090) | 0.3085 | — | — |
| NS5A‐L31, mutant† | 0.000 (0.000–9.593) | 0.4845 | — | — |
| NS5A‐Y93, mutant‡ | 0.000 (0.000–0.000) | 0.1041 | — | — |
| NS5A‐L31/‐Y93 double mutation, yes | 1278 (153.9–31 220) | <0.0001 | 356.3 (23.91–16 940) | <.0001 |
| Platelet count | 0.954 (0.848–1.048) | 0.3689 | — | — |
| AST | 1.001 (0.978–1.011) | 0.9226 | — | — |
| ALT | 1.001 (0.981–1.011) | 0.8975 | — | — |
| eGFR | 1.013 (0.988–1.043) | 0.3371 | — | — |
| M2BPGi | 0.983 (0.687–1.246) | 0.9054 | — | — |
| FIB‐4 index | 1.047 (0.817–1.257) | 0.6777 | — | — |
| α‐Fetoprotein | 1.001 (0.957–1.015) | 0.9029 | — | — |
| HCV‐RNA | 1.691 (0.735–5.011) | 0.2460 | — | — |
Mutation of L31 alone (double mutation with Y93 is excluded).
Mutation of Y93 alone (double mutation with L31 is excluded).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DAA, direct‐acting antiviral; eGFR, estimated glomerular filtration rate; FIB‐4, Fibrosis‐4; HCC, hepatocellular carcinoma; IFN, interferon; M2BPGi, Mac‐2 binding protein glycosylation isomer; NS5A, non‐structural protein 5A; —, not included
No serious adverse events were observed with EBR/GZR therapy (Table 4). Nine patients were discontinued from treatment. Seven were discontinued for liver damage, one patient was discontinued for virologic breakthrough after being HCV‐RNA‐negative, and one was discontinued at the patient's request. Eight of the nine patients, or all but the patient with virologic breakthrough, achieved SVR. With the discontinuation of treatment, the liver disorders improved quickly.
Table 4.
Adverse events in patients with hepatitis C virus genotype 1 treated with elbasvir/grazoprevir
| Adverse event | n = 353 (%) |
|---|---|
| Elevated aminotransferase levels | 10 (2.8) |
| Fatigue | 5 (1.4) |
| Headache | 2 (0.6) |
| Dizzy | 1 (0.3) |
| Constipation | 1 (0.3) |
| Pharyngitis | 1 (0.3) |
| Stomatitis | 1 (0.3) |
DISCUSSION
Therapy with EBR/GZR achieved a high SVR rate and high tolerability in the present study based on actual clinical practice, similar to a Japanese phase III trial. As in the report by Toyoda et al.,26 the SVR rate was consistent regardless of various factors such as age, sex, eGFR, and HCC treatment history. Patients with chronic renal disorders, including those on dialysis, also showed a comparable SVR rate. On univariate analysis, the SVR rate was significantly lower in patients with a history of IFN therapy, a history of DAA therapy, and in those with NS5A‐Y31/‐Y93 double mutation. On multivariate analysis, NS5A‐Y31/‐Y93 double mutation was identified as an independent factor affecting SVR. The presence of cirrhosis and a history of HCC have been shown to be factors associated with non‐SVR with other DAA treatments;16 however, these were not significant factors associated with SVR with EBR/GZR therapy.
Only 2.9% (10/353) of patients in the current study showed treatment failure, and these patients had a history of DAA therapy or a double mutation at NS5A‐L31/‐Y93. Investigations have found that few patients who fail to respond to DAA treatment respond to EBR/GZR therapy.19, 26 However, SVR was achieved in six of seven patients who had a history of DAA therapy but no double mutation at NS5A‐L31/‐Y93 in the present study. A case report noted that a patient who failed LDV/SOF responded to EBR/GZR, but this patient had no NS5A double mutation.27 Patients who fail DAA therapy often achieve a favorable outcome when subsequently treated with GLE/PIB or velpatasvir/SOF plus ribavarin.28, 29 However, when poor renal function or anemia prevents the use of velpatasvir/SOF plus ribavirin, and GLE/PIB cannot be continued because of rash or another adverse drug reaction, EBR/GZR could be an option, provided the patient lacks an NS5A‐L31/‐Y93 double mutation, although further investigation is required. Two of the treatment failure cases were patients with NS5A double mutation, but without a history of DAA therapy. This indicates the necessity to check, if feasible, for NS5A RAS before EBR/GZR therapy, even in patients without a history of DAA therapy. Furthermore, it is known that SVR cannot be attained with patients who had a deletion of amino acid residue 32 of the HCV‐NS5A region (NS5A‐P32 deletion), even when NS3‐D168 and HCV‐NS5A L31 and Y93 are wild‐type, indicating that this mutation should be determined prior to DAA treatment.30 Although no serious adverse events of EBR/GZR therapy were observed, nine patients discontinued the therapy due to complications such as liver disorders. Nonetheless, SVR was attained in all patients who discontinued the treatment.
There are several limitations to this study. First, due to the retrospective design of this study, the candidates for EBR/GZR were selected at the discretion of their attending physicians in all cases. Furthermore, because the percentage of those who did not achieve SVR was extremely low, the factors associated with treatment failure could not be fully assessed. Resistance‐associated substitutions were analyzed only at NS5A‐L31 and ‐Y93. Analysis of other RAS, such as NS3‐D168 and NS5A‐P32 deletion, was inadequate and could not be undertaken in the present study.
In conclusion, although treatment selection was limited in this retrospective study, EBR/GZR treatment outcomes were good in DAA‐naïve patients, achieving SVR in 99.4%; however, the response was poor, with SVR of 50%, in patients who had previously been treated with DAAs. Moreover, the response was also extremely poor in patients with NS5A‐L31/‐Y93 double mutation, with an SVR rate of 18.2%. Treatment with EBR/GZR would have resulted in an SVR rate of 100% in patients with neither a history of DAA therapy nor a double RAS mutation. This combination of drugs could be given safely to patients and was therefore considered to be extremely useful as first‐line therapy for DAA‐naïve patients. Although DAA failure occurs in only a few patients, because the appearance of RAS greatly affects subsequent treatments, DAA regimens must be selected with care in DAA‐naïve patients.
Acknowledgments
This study was supported by a research grant from the Japan Agency for Medical Research and Development (AMED). The authors wish to express their gratitude to the following doctors for their cooperation and support for the duration of this study: Drs Tomoyuki Yokota, Nobuaki Azemoto, Michiko Aono, Yusuke Okujima, Kaori Sato, and Ryo Yano, of Matsuyama Red Cross Hospital. The authors would like to thank FORTE Science Communications for the English language review.
Mashiba, T. , Joko, K. , Kurosaki, M. , Ochi, H. , Hasebe, C. , Akahane, T. , Sohda, T. , Tsuji, K. , Mitsuda, A. , Kimura, H. , Narita, R. , Ogawa, C. , Furuta, K. , Shigeno, M. , Okushin, H. , Ito, H. , Kusakabe, A. , Satou, T. , Kawanami, C. , Nakata, R. , Kobashi, H. , Tamada, T. , Ide, Y. , Yagisawa, H. , Morita, A. , Matsushita, T. , Okada, K. , and Izumi, N. (2019) Real‐world efficacy of elbasvir and grazoprevir for hepatitis C virus (genotype 1): A nationwide, multicenter study by the Japanese Red Cross Hospital Liver Study Group. Hepatol Res, 49: 1114–1120. 10.1111/hepr.13362.
Conflict of interest: Namiki Izumi has received honoraria from Gilead Sciences and AbbVie. Masayuki Kurosaki has received honoraria from Gilead Sciences, AbbVie and MSD. The other authors have no conflict of interest.
Financial support: This study was supported by a research grant from the Japan Agency for Medical Research and Development (AMED).
References
- 1. Asahina Y, Tsuchiya K, Nishimura T et al α‐Fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology 2013; 58: 1253–1262. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka Y, Hanada K, Mizokami M et al A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA 2002; 99: 15584–15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikeda K, Saitoh S, Arase Y et al Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long‐term observation study of 1643 patients using statistical bias correction with proportional hazard analysis. Hepatology 1999; 29: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 4. Imai Y, Kawata S, Tamura S et al Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Ann Intern Med 1998; 129: 94–99. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida H, Shiratori Y, Moriyama M et al Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med 1999; 131: 174–181. [DOI] [PubMed] [Google Scholar]
- 6. Kasahara A, Hayashi N, Mochizuki K et al Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Hepatology 1998; 27: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa E, Furusyo N, Kajiwara E et al Efficacy of pegylated interferon α‐2b and ribavirin treatment on the risk of hepatocellular carcinoma of patients with chronic hepatitis C: a prospective multicenter study. J Hepatol 2013; 58: 495–501. [DOI] [PubMed] [Google Scholar]
- 8. Chayama K, Takahashi S, Toyota J et al Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b‐infected null responders. Hepatology 2012; 55: 724–728. [DOI] [PubMed] [Google Scholar]
- 9. Kumada H, Suzuki Y, Ikeda K et al Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 2014; 59: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki Y, Ikeda K, Suzuki F et al Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol 2013; 58: 655–662. [DOI] [PubMed] [Google Scholar]
- 11. Izumi N. Efficacy of daclatasvir in hepatitis C virus. Exp Rev Anti‐Infect Ther 2014; 12: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 12. Itakura J, Kurosaki M, Hasebe C et al Complex pattern of resistance‐associated substitutions of hepatitis C virus after daclatasvir/asunaprevir treatment failure. PLoS One 2016; 11: e0165339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Japan Society for Hepatology . JSH guidelines for management of hepatitis C virus infection (version 5.2). 2016. Available at: https://www.jsh.or.jp/medical/guidelines/jsh_guidlines/hepatitis_c. Accessed December 15, 2016.
- 14. Mizokami M, Yokosuka O, Takehara T et al Ledipasvir and sofosbuvir fixed‐dose combination with and without ribavirin for 12 weeks in treatment‐naive and previously treated Japanese patients with genotype 1 hepatitis C: an open‐label, randomized, phase 3 trial. Lancet Infect Dis 2015; 15: 645–653. [DOI] [PubMed] [Google Scholar]
- 15. Afdhal N, Zeuzem S, Kwo P et al Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- 16. Tsuji K, Kurosaki M, Itakura J et al Real‐world efficacy and safety of ledipasvir and sofosbuvir in patients with hepatitis C virus genotype 1 infection: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Gastroenterol 2018; 53: 1142–1150. [DOI] [PubMed] [Google Scholar]
- 17. Bell AM, Wagner JL, Barber KE et al Elbasvir/grazoprevir: a review of the latest agent in the fight against hepatitis C. Int J Hepatol 2016; 2016: 3852126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumada H, Suzuki Y, Karino Y et al The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: a randomized phase II/III study. J Gastroenterol 2017; 52: 520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeuchi Y, Akuta N, Sezaki H et al Efficacy and safety of elbasvir plus grazoprevir combination therapy in Japanese patients infected with hepatitis C virus genotype 1b. Hepatol Res 2018; 49: 256–263. [DOI] [PubMed] [Google Scholar]
- 20. Reddy KR, Roth D, Bruchfeld A et al Elbasvir/grazoprevir does not worsen renal function in patients with hepatitis C virus infection and pre‐existing renal disease. Hepatol Res 2017; 47: 1340–1345. [DOI] [PubMed] [Google Scholar]
- 21. Yoshimi S, Ochi H, Murakami E et al Rapid, sensitive, and accurate evaluation of drug resistant mutant (NS5A‐Y93H) strain frequency in genotype 1b HCV by invader assay. PLoS One 2015; 10: e0130022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki F, Sezaki H, Akuta N et al Prevalence of hepatitis C virus variants resistant to NS3 protease inhibitors or the NS5A inhibitor (BMS‐790052) in hepatitis patients with genotype 1b with genotype 1b. J Clin Virol 2012; 54: 352–354. [DOI] [PubMed] [Google Scholar]
- 23. Uchida Y, Kouyama J, Naiki K, Mochida S. A novel simple assay system to quantify the percent HCV‐RNA levels of NS5A Y93H mutant strains and Y93 wild‐type strains relative to the total HCV‐RNA levels to determine the indication for antiviral therapy with NS5A inhibitors. PLoS One 2014; 9: e112647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuo S, Imai E, Horio M et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 25. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [Google Scholar]
- 26. Toyoda H, Atsukawa M, Takaguchi K et al Real‐world virological efficacy and safety of elbasvir and grazoprevir in patients with chronic hepatitis C virus genotype 1 infection in Japan. J Gastroenterol 2018; 53: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 27. Tadokoro T, Morishita A, Oura K et al Grazoprevir/elbasvir treatment for the relapse of HCV genotype 1b infection after ledipasvir/sofosbuvir: a case report. Exp Ther Med 2018; 16: 1026–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osawa M, Imamura M, Teraoka Y et al Real‐world efficacy of glecaprevir plus pibrentasvir for chronic hepatitis C patient with previous direct‐acting antiviral therapy failures. J Gastroenterol 2019; 54: 291–296. [DOI] [PubMed] [Google Scholar]
- 29. Izumi N, Takehara T, Chayama K et al Sofosbuvir‐velpatasvir plus ribavirin in Japanese patients with genotype 1 or 2 hepatitis C who failed direct‐acting antivirals. Hepatol Int 2018; 12: 356–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumada H, Watanabe T, Suzuki F et al Efficacy and safety of glecaprevir/pibrentasvir in HCV‐infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol 2018; 53: 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]


