Abstract
An estimated 9 million individuals are chronically infected with hepatitis B virus (HBV) and hepatitis C virus (HCV) across the European Union/European Economic Area (EU/EEA), many of which are yet to be diagnosed. We performed a systematic review to identify interventions effective at improving testing offer and uptake in the EU/EEA. Original research articles published between 1 January 2008 and 1 September 2017 were retrieved from PubMed and EMBASE. Search strings combined terms for HBV/HCV, intervention, testing and geographic terms (EU/EEA). Out of 8331 records retrieved, 93 studies were selected. Included studies reported on testing initiatives in primary health care (9), hospital (12), other healthcare settings (31) and community settings (41). Testing initiatives targeted population groups such as migrants, drug users, prisoners, pregnant women and the general population. Testing targeted to populations at higher risk yielded high coverage rates in many settings. Implementation of novel testing approaches, including dried blood spot (DBS) testing, was associated with increased coverage in several settings including drug services, pharmacies and STI clinics. Community‐based testing services were effective in reaching populations at higher risk for infection, vulnerable and hard‐to‐reach populations. In conclusion, our review identified several successful testing approaches implemented in healthcare and community settings, including testing approaches targeting groups at higher risk, community‐based testing services and DBS testing. Combining a diverse set of testing opportunities within national testing strategies may lead to higher impact both in terms of testing coverage and in terms of reduction, on the undiagnosed fraction.
Keywords: Europe, hepatitis B, hepatitis C, screening, testing
Abbreviations
- DBS
dried blood spot
- DBS
dried blood spot
- GPs
general practitioners
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- MSM
men who have sex with men
- OST
opioid substitution therapy
- PLHIV
people living with HIV
- PWID
people who inject drugs
- RCT
randomized controlled trial
- SIGN
Scottish Intercollegiate Guidelines Network
- STI
sexually transmitted infection
1. INTRODUCTION
Across the European Union/European Economic Area (EU/EEA), an estimated 4.7 million people are chronically infected with hepatitis B virus (HBV) and 3.9 million are chronically infected with hepatitis C virus (HCV).1 HBV and HCV can both cause acute and chronic hepatitis, potentially leading to the development of cirrhosis, liver cancer or death of infected patients.2, 3 Early disease and development of liver damage are often asymptomatic,4, 5, 6 meaning that HBV and HCV infection may go undetected for many years7 and many infected people remain undiagnosed.8
Transmission of HBV and HCV can occur sexually, through blood‐to‐blood contact or vertically (mother‐to‐child). Over the past decades, there have been shifts in the patterns of transmission in Europe, due to various factors, including improvements in blood transfusion and healthcare safety standards, HBV vaccination programmes, harm reduction programmes targeting injecting drug use, as well as significant changes in patterns of injecting drug use and immigration. However, a number of population groups are still potentially at high risk or have a high burden of HBV/HCV in EU/EEA countries, including people who inject drugs (PWID), men who have sex with men (MSM), people living with HIV (PLHIV), people in prison and migrants from countries of high endemicity.9, 10, 11
As highly effective treatment options have become available for HBV and HCV,12, 13 it is crucial that an informed public health response is in place tailored to the local epidemiological situation, which will ensure that those infected are diagnosed and linked to care. Estimates of the undiagnosed fraction in the general population in EU/EEA countries range from 40% to 85% for HBV and 20% to 91% for HCV, with large variability between countries.11 The WHO has formulated an action plan to eliminate viral hepatitis as a public health threat in the European region by 2030, setting targets of 50% of people with chronic infection diagnosed by 2020 and 90% by 2030.14 To this end, testing programmes must be scaled up in order to reduce the undiagnosed fraction.
Testing can occur through a number of methods and in various settings, depending on the population group targeted and the local epidemiology and healthcare infrastructure. Testing in health care may take place across a range of different settings in primary health care and hospitals as well as other healthcare settings such as sexually transmitted infection (STI) clinics, antenatal services and pharmacies. Outside of formal healthcare facilities, settings within the community, such as homeless shelters, migrant services and community‐based drugs services can offer testing services which are adapted, targeted and made accessible to the populations that frequent them.15 Outreach testing, for example using mobile units, street outreach by community health workers, or satellite services based at other agencies can be used to reach people who are not in contact with other health services.16 Common testing strategies for hepatitis B and C include universal screening, birth cohort testing or testing targeted towards those at increased risk. In recent years, new technologies such as self‐testing kits, and strategies for testing implementation have been developed, which may be considered for incorporation in countries' national testing policies and programmes. Evidence around the effectiveness of different interventions in relation to uptake and positivity rates could help inform countries in deciding which strategic approaches to incorporate in national testing programmes.
A systematic review covering HBV and HCV testing studies in key populations in the European region until June 2013 found that, although a large number of studies on testing existed, these were unevenly distributed across Europe and that large gaps existed in certain key populations including migrants, people in prison and MSM.17 Previous systematic reviews on testing interventions focused solely on either HBV or HCV,18, 19, 20, 21, 22, 23 specific key populations or settings,19, 21, 22, 24 targeted testing interventions18, 23 or included comparative studies only.25 Finally, a comprehensive report on hepatitis testing policies and activities in EU/EEA countries revealed substantial gaps in testing coverage and testing offers targeting specifically higher risk groups across the region.8
The scope of this study was to provide an overview of different effective testing strategies for hepatitis B and C and their outcomes in the EU/EEA, covering all relevant population groups and settings. A systematic review was performed to collect, synthesize and analyse available data on HBV/HCV testing outcomes and acceptability measures from EU/EEA countries. This study was conducted as part of a larger project to develop an integrated European testing guidance for HBV, HCV and HIV, coordinated by the European Centre for Disease Prevention and Control (ECDC).
2. METHODS
2.1. Search strategy and selection criteria
Original research articles were retrieved from PubMed and EMBASE databases on 1 September 2017. PICO questions (Patient/problem, Intervention, Comparison and Outcome26) were formulated (listed in Supporting information). Search strategies combined controlled (MeSH/Emtree terms) and natural vocabulary on terms for HBV and HCV with terms for intervention and testing and geographic terms (EU/EEA) (detailed in Supporting Information). The search strategy employed also included terms for linkage to care, however, for the purposes of this article, methods and results applying to approaches to improve testing coverage are presented only. Only studies published between 1 January 2008 and 1 September 2017 were included in the search. Articles in all EU/EEA languages were included.
Only articles reporting data from EU/EEA countries were included. Publications were included which described approaches to improve coverage of testing and reported any of the following outcomes of interest: offer of test, uptake and coverage of testing, positivity rate, acceptability and feasibility outcomes. Studies on unlinked anonymous testing to determine prevalence and studies describing the sensitivity/specificity of laboratory tests were excluded. The full inclusion and exclusion criteria are listed in Table S3. Only original research articles (ie not reviews) and conference abstracts were included in this review; however, the reference lists of relevant systematic reviews retrieved in the literature search were checked manually for additional original articles not captured by the literature search. Conference abstracts from the International Liver Congress or retrieved in the systematic literature search, reporting relevant quantitative data and published since 2015, were included as grey literature. Additional publications captured were subject to the same inclusion and exclusion criteria listed above.
Two reviewers reviewed titles and abstracts of retrieved publications. Initially, a random sample of 5% was screened in duplicate; the results were then compared and used to refine the inclusion and exclusion criteria. Further rounds of duplicate review were conducted until a level of concordance of more than 95% was achieved, after which the remaining publications were divided between reviewers and screening continued in EndNote. The full texts of selected articles were subsequently screened by two reviewers, of which a random sample of 20% was screened in duplicate and these reached more than 95% concordance. The remaining 80% of publications were divided between the reviewers and screened. Articles were included in case of uncertainty about inclusion or exclusion if this was not resolved after discussion between the reviewers.
2.2. Definitions
Primary health care was defined as health care provided by general practitioners (GPs). Hospital settings included all hospital departments including inpatients, outpatients, medical admissions units and infectious disease units. Other healthcare settings included any formal healthcare settings outside of primary health care or hospital departments, for example STI clinics, pharmacies and prisons. Community‐based testing was defined as any programme or service offering HBV/HCV testing outside of formal health facilities. Drugs services were defined as services offering prevention, support, detox and treatment for addiction to drugs or alcohol, either embedded in healthcare settings or set in the community. Outreach activities were defined as testing activities taking place in the community without a fixed‐site facility, including mobile units or vans, street outreach by community health workers and regular satellite services based at other agencies.
Testing offer rate was defined as the proportion of people targeted by a testing programme who were offered testing, while testing coverage was defined as the percentage of people targeted by a testing programme that received testing. Positivity rate was defined as the percentage of people testing positive for HBsAg and anti‐HCV for HBV and HCV, respectively. When other markers were reported instead, these data were extracted, and the marker was specified in tables.
2.3. Data extraction and quality assessment
Relevant data were extracted from included articles and recorded in a data extraction file in Microsoft Excel. A predefined set of variables covering study characteristics, study population details and outcomes was extracted per study. The complete list of variables is provided in Table S5. The unit for data extraction was study, not article. A study is defined as a report of data on a testing approach or linkage to care approach for HBV or HCV, in a defined country, over a discrete period of time. Therefore, one article may contain more than one study. If a study was captured by two different articles, the study was extracted once and the article with the most detail used as a reference.
The quality of included peer‐reviewed literature was assessed using checklists developed by the Scottish Intercollegiate Guidelines Network (SIGN)27 that include the most important criteria on publication quality from the PRISMA and STROBE guidelines. Using these checklists, an overall quality score was assigned per study: high (++), acceptable (+) and low (−). As some of the included publications concerned study designs for which no checklists exist, a list (provided in Table S6) was compiled of relevant aspects from standard checklists, regarding the level of detail and clarity of the study, appropriateness of study population, data collection and denominator, and the representativeness of the sample, which were answered with yes or no per study. Using the checklist, it was not possible to calculate an overall quality score for these studies; therefore, all relevant articles were included regardless of their quality, but the results of the assessment were taken into consideration in interpreting the results. Articles were excluded, however, when the methods and/or results provided an insufficient level of details making it not possible to accurately extract data. No quality assessment was performed on included grey literature publications.
A set of detailed summary tables were developed per setting (provided in Tables S7‐S11), containing the following information: study reference, country, study period, study design, study population and specific setting, sample size, outcomes (test offer, coverage and positivity rate, acceptance rate and patient and provider indicators of acceptability and feasibility), critical appraisal and comments. Within each table, findings are ordered by virus, study population, country and year of publication.
3. RESULTS
The literature search retrieved 8331 unique publications, of which 370 were selected based on title and abstract and were assessed in full text for eligibility. Of these, 62 articles were retrieved that formed the evidence base for the effectiveness of testing initiatives and interventions (Figure 1). Reasons for exclusion of publications are listed in Table S4. The included publications comprised 93 studies in total, each detailing an intervention designed to improve coverage of HBV or HCV testing in a certain setting. A total of 78 studies were from peer‐reviewed publications and 15 concerned conference abstracts. A formal quality assessment was performed for 19 peer‐reviewed studies; the remainder had study designs which precluded this. Detailed information of each included study is provided in Tables S7‐S11.
Figure 1.
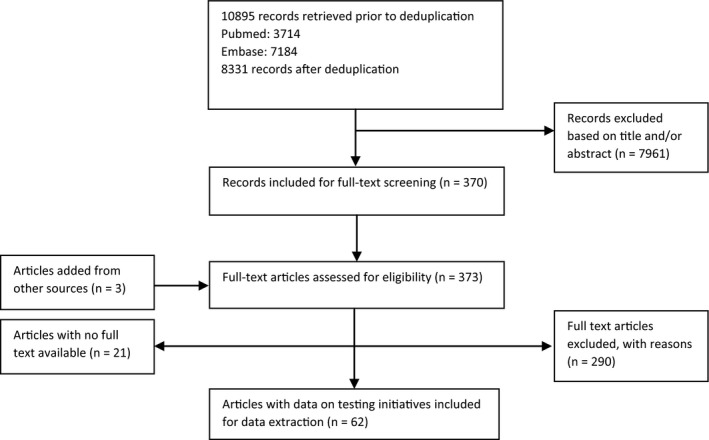
PRISMA flow diagram for the systematic review
3.1. Testing initiatives in primary healthcare settings
Nine studies that reported outcomes on testing initiatives performed in primary healthcare settings were retrieved28, 29, 30, 31, 32, 33, 34, 35, 36 (Table 1). HBV/HCV test offer rates, coverage and positivity rates, where reported, ranged widely between studies. The highest offer rate and coverage reported was 70% and 100%, respectively, in a study targeting migrants.30 Very high positivity rates for HCV were reported in initiatives targeting PWID (70%) and homeless people (26%).29, 33 Two initiatives targeting migrants reported HBV and HCV positivity rates of 0%.30, 32
Table 1.
Evidence base for the effectiveness of testing initiatives in primary healthcare settings
| Intervention |
Studies included Quality of evidence |
Target population | Test offer | Coverage/number of tests/testedb | Positivity rate |
|---|---|---|---|---|---|
| Risk group testing | HBV | ||||
|
Quality: 3 studies NA |
Migrants (n = 3) (sample size: 47‐560a) | 100% |
Number tested/tests: 2223 Coverage: 2.3% and 70% |
0%‐6.7% |
|
| HCV | |||||
|
Quality: 1 study low 3 studies NA |
PWID (n = 1) (sample size: intervention 485, control NR) | 52% intervention | Coverage: 24.8% intervention, 0.3% control | Comparative study: 70% intervention, 22% control | |
| Homeless (n = 1) (sample size: NR) | Number tested/tests: 460 |
26% 9.6% newly diagnosed |
|||
| Migrants (n = 2) (sample size: 47 and 560) | 100% | Coverage: 2.3% and 70% | 0% | ||
| Birth cohort testing | HCV | ||||
|
N = 1 study28 Quality: Acceptable |
30‐ to 54‐year‐olds (n = 1) (sample size: Intervention 584, control NR) | Comparative study: 72% intervention; 0% control | Comparative study: coverage: 20% intervention; 0% control | Comparative study: 13% intervention; NA control | |
| Novel testing | HCV | ||||
|
Quality: 2 studies NA |
General pop (n = 1) (sample size: 600‐29 600) | Coverage (oral): 9.1%‐22% | 0.4%‐4.5% | ||
| Homeless (n = 1) (sample size: NR) | Number tested/tests (oral): 460 |
26% 9.6% newly diagnosed |
|||
| Education | HBV | ||||
|
N = 1 study30 Quality: NA |
Targeted at GPs Migrants (n = 1) (sample size: 47) |
100% |
Coverage: 70% |
0% | |
| HCV | |||||
|
Quality: 1 study low 1 study NA |
Targeted at GPs Migrants (n = 1); general pop. (n = 1) (sample size: 47a) |
100% |
Coverage: 70% Comparative study: number tested: campaign + education (intervention): 57 tests before; 172 during; campaign (control): 86 tests before; 118 during OR of the increase intervention vs control: 2.2 (95% CI 1.5‐3.3) |
0% Comparative study: campaign + education (intervention): 0% before; 1.7% during; campaign (control): 1.7% before; 0.8% during |
|
| Campaign | HCV | ||||
|
Quality: 1 study low 1 study NA |
Targeted at public General pop. (n = 1) (no sample) |
Comparative study: number tested/tests: campaign + education (intervention): 57 tests before; 172 during; campaign (control): 86 tests before; 118 during OR of the increase intervention vs control: 2.2 (95% CI 1.5‐3.3) |
Comparative study HCV: campaign + education (intervention): 0% before; 1.7% during; campaign (control): 1.7% before; 0.8% during | ||
|
Targeted at GPs/risk groups General pop. (n = 1) (no sample) |
Comparative study: number HCV tested/tests: before 5421 tests; during 10 117 tests | Comparative study HCV: before 9.6%; during 6.8% | |||
Abbreviations: HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; NA: not applicable; NR: not reported; oral: oral sampling; PWID: people who inject drugs; TB: tuberculosis
Denominator not reported for all studies.
Number of tests is only reported here if no data on coverage are available.
One comparative study on HCV risk group testing in PWID reported testing coverage of 24.8% among PWID in GP practices exposed to the intervention, compared to 0.3% of PWID tested in comparable control practices.29 In a comparative study on birth cohort testing for HCV testing in an area of high prevalence of HCV and intravenous drug use, 72% of 30‐ to 54‐year‐olds were offered testing, compared to 0% in a control practice in the same area where birth cohort testing was not implemented.28 In an additional comparative study, the number of tests performed increased from 5421 before to 10 117 during a national testing programme which involved awareness‐raising activities for GPs and those at higher risk.34 Another comparative study found a significant increase in the number tested when a public awareness campaign was combined with additional educational brochures and training for GPs, which resulted in three times more people being tested, compared to a control group in which only the campaign was implemented and testing rates increased by 1.4 times.31
Two studies provided data on the acceptability and feasibility of testing in primary healthcare settings. Testing acceptance rates were 56% among PWID29 and 70% among migrants.30 Among PWID interviewed about a testing intervention, all responded positively about the acceptability of provider‐initiated HCV testing. Staff viewed the intervention as an opportunity to facilitate identification and subsequent referral.29
3.2. Testing initiatives in hospital settings
Twelve studies were identified on the effectiveness of testing initiatives and interventions in hospital settings34, 35, 37, 38, 39, 40, 41 (Table 2). Test offer rates, coverage and HBV/HCV positivity rates varied with high offer rates (83% and 100%) in testing initiatives targeted at migrants and psychiatric patients.37, 40, 41 Coverage was highest (88.4%) in a study reporting on a universal testing initiative conducted at a single emergency department.38 A separate universal testing initiative conducted in multiple emergency departments, however, yielded a much lower overall coverage of 27%, although with variations among testing sites.39 Positivity rates for HBV and HCV were higher in studies reporting on initiatives targeted at key populations (2.2%‐7.8% for HBV and 0.3%‐8.7% for HCV) than those aimed at the general population.37, 40, 41
Table 2.
Evidence base for the effectiveness of testing initiatives in hospital settings
| Intervention |
Studies included Quality of evidence |
Setting; target population | Test offer | Coverage/number of tests/testeda | Positivity rate |
|---|---|---|---|---|---|
| Risk group testing | HBV | ||||
|
Quality: 3 studies NA |
Other hospital departments (n = 3) (sample size: 105‐3226) Migrants (n = 2); psychiatric patients (n = 1) |
Migrants: 100% Psychiatric patients: 83% |
Coverage migrants: 28.7% and 61% Coverage psychiatric patients: 54% |
Migrants: 2.2% and 7.8% Psychiatric patients: 7.0% |
|
| HCV | |||||
|
Quality: 3 studies NA |
Other hospital departments (n = 3) (sample size: 105‐3226) Migrants (n = 2); psychiatric patients (n = 1) |
Migrants: 100% Psychiatric patients: 83% |
Coverage migrants: 28.7% and 61% Coverage psychiatric patients: 54% |
Migrants: 0.3% and 3.6% Psychiatric patients: 8.7% |
|
| Universal testing | HBV | ||||
|
Quality: 2 studies NA |
ED only (n = 2) (sample size: 7807 and 10 000) General population (n = 2) |
Coverage: 27% and 88.4% |
0.5% and 0.7% 0.2% and 0.5% newly diagnosed |
||
| HCV | |||||
|
Quality: 2 studies NA |
ED only (n = 2) (sample size: 7807 and 10 000) General population (n = 2) |
Coverage: 27% and 88.4% |
1.8% and 5% 0.6% and 0.7% newly diagnosed |
||
| Novel testing | HCV | ||||
|
N = 1 study35 Quality: NA |
Other hospital departments (n = 1 (sample size: 600‐29 600) General population (n = 1) |
Comparative study: coverage (oral): 9.1% (2011) 14% (2012) 16.9% (2013) 22% (2014) |
Comparative study: 0.5% (2011) 0.5% (2011) 0.4% (2013) 4.5% (2014) |
||
| Education | HBV | ||||
|
N = 1 study40 Quality: NA |
Targeted at migrants Migrants (n = 1) (sample size: 3226) |
100% | Coverage: 28.7% |
2.2% |
|
| HCV | |||||
|
N = 1 study40 Quality: NA |
Targeted at migrants Migrants (n = 1) (sample size: 3226) |
100% | Coverage: 28.7% | 0.3% | |
| Campaign | HBV | ||||
|
Quality: 3 studies NA |
Targeted at patients General pop. (n = 1) (sample size: 7807) |
Coverage: 27% |
0.7% 0.5% newly diagnosed |
||
|
Targeted at‐risk groups Migrants (n = 1) (sample size: 3226) |
100% | Coverage: 28.7% | 2.2% | ||
| HCV | |||||
|
Quality: 3 studies NA |
Targeted at patients General pop. (n = 1) (sample size: 7807) |
Coverage: 27% |
1.8% 0.7% newly diagnosed |
||
|
Targeted at‐risk groups General pop. (n = 1) (sample size: NR) Migrants (n = 1) (sample size: 3226) |
100% |
Comparative study: before 10 536 tests; during 12 170 Coverage: 28.7% |
Comparative study: 9.9% before; 7.9% during 0.3% |
||
Abbreviations: ED, emergency department; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NA, not applicable; NR, not reported; oral, oral sampling.
Number of tests is only reported here if no data on coverage are available.
One comparative study reported on implementation of oral sampling for HCV testing between 2011 and 2014.35 Over this period, coverage increased from 9.1% to 22% and positivity rates increased from 0.5% to 4.5%. In another comparative study, a national programme involving awareness‐raising activities was associated with an increase in number of tests taken in hospitals from 10 536 tests before and 12 170 during the intervention.34
3.3. Testing initiatives in other healthcare settings
Thirty‐one studies were included relating to other healthcare settings34, 35, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 (Table 3), which included antenatal services, clinics for people with no health insurance, drug services (embedded in health services), migrant clinics, pharmacies, prisons, public health clinics and STI clinics. Testing coverage during or after interventions were found to vary widely between and within settings, with the highest coverage levels reported by studies in Italian migrant clinics (87%‐91.4%),48, 54 clinics for people with no health insurance (71%‐98.2%)44, 57 and public health clinics (90% and 98%).51 Four studies on novel testing initiatives yielded high coverage levels when dried blood spot (DBS) sampling or rapid tests were used (up to 98.2% for rapid testing and up to 96.6% for DBS).44, 50, 64 The highest positivity rates for both diseases were reported in studies targeting drug users or PWID (up to 48% anti‐HBc positive for HBV and up to 61% for HCV).43, 51, 52, 59, 62, 64 No studies conducted in other healthcare settings reported test offer rates.
Table 3.
Evidence base for the effectiveness of testing initiatives in other health care settings
| Intervention |
Studies included Quality of evidence |
Setting/target population | Coverage/number of tests/testedc | Positivity rate |
|---|---|---|---|---|
| Risk group testing | HBV | |||
|
N = 6 studies48, 54, 56, 57, 61, 62 Quality: 6 studies NA |
Migrant clinic (n = 2) (sample size: 516 and 4078) | Coverage: 87% and 91.4% | 6.0% and 7.7% | |
| Prisons (n = 2) (sample size: 3468a) |
Number tested: 160 Coverage: 65.3% |
0% and 4.4% 1.5% newly diagnosed |
||
| Drug services (n = 1) (sample size: 287 and 2024) | Coverage: 34% and 69% | 33% and 48% (anti‐HBc) | ||
| Clinic for people with no health insurance (n = 1) (sample size: NR) | Coverage: 90% when screening is proposed during a prevention interview; 71% without interview |
6.9% |
||
| HCV | ||||
|
N = 15 studies43, 45, 47, 48, 49, 51, 52, 56, 57, 58, 59, 61, 62, 63, 64 Quality: 3 studies acceptable 1 study low 11 studies NA |
STI clinics (n = 1) (sample size: 3365) MSM (n = 1) |
Coverage: 69% | 0.65% | |
| Prisons (n = 3) (sample size: 3468 ‐ 3600a) |
Number tested: 160 Coverage: 64.6% Comparative study (DBS): ORs for effect of the intervention on testing rate: OR: 0.86; 95% CI: 0.71‐1.06; P = 0.153 |
22.8% and 33.8% 1.5% newly diagnosed |
||
| Drug services (n = 3) (sample size: 287‐2566) | Coverage: 53%‐84.2% | 26%‐61% | ||
| Antenatal services (n = 1) (sample size: 4369) | 28.3% received targeted screening | Comparative study: 1.3% (compared to 1.7% universal screening; difference between the two NS) | ||
| Migrant clinic (n = 1) (sample size: 4078) | Coverage: 90.8% | 3.6% | ||
| Pharmacies (n = 2) (sample size: 143 intervention, 561 control; 244 conventional care pathway; 262 pharmacist care pathway) |
Comparative study (DBS): 30% intervention; 13% control. OR: 2.25 (95% CI 1.48‐3.42) Comparative study: 24% coverage conventional care pathway; 36% pharmacist care pathway |
Comparative study: 25.9% conventional care pathway 26.5%; pharmacist care pathway | ||
| Drug clinics and prisons (n = 1) (sample size: 6550 intervention, 5800 control) |
Comparative study coverage (DBS) intervention: 8.4% before, 20.6% during intervention control: 7.7% before, 5.4% during intervention |
32% (overall) | ||
| Public health clinic (n = 1) (sample size: 81 and 497) | Coverage: 90% and 98% |
60% (overall) |
||
| Specialist servicesb (n = 1) (sample size: 1322) | 55% | |||
| Clinic for people with no health insurance (n = 1) (sample size: NR) |
Number tested/tests: 1196 Coverage: 90% when screening is proposed during a prevention interview; 71% without interview |
5.8% | ||
| Universal testing | HCV | |||
|
N = 1 study47 Quality: NA |
Antenatal services (n = 1) (No sample size) | Number tested: 4222 |
Comparative study: 1.7% (compared to 1.3% targeted screening; difference between the two NS) |
|
| Novel testing | HBV | |||
|
Quality: 1 study high 1 study acceptable 1 study NA |
Clinic for people with no health insurance (n = 1) (sample size: 162 intervention, 162 control) | Coverage: 64.2% (serology); 98.2% (RT) | Comparative study: 9.6% (serology); 8.1% (RT) | |
| Antenatal services (n = 1) (Sample size: 41 pre‐intervention; 58 post‐intervention; 91 pre‐control; 68 post‐control) |
Comparative study: Coverage (DBS): 62% pre‐intervention; 97% post‐intervention; 40% pre‐control; 39% post‐control |
Comparative study: 0%‐3% pre‐intervention; 3%‐22% post‐intervention; 6%‐8% pre‐control; 2%‐9% post‐control |
||
| Prisons (n = 1) (sample size: NR) | Number tested (DBS): 160 | 0% | ||
| HCV | ||||
|
N = 11 studies35, 42, 43, 44, 45, 49, 52, 56, 58, 59, 64 Quality: 1 study high 4 studies acceptable 1 study low 5 studies NA |
Clinic for people with no health insurance (n = 1) (sample size: 162 intervention, 162 control) | Coverage: 64.2% (serology); 98.2% (RT) | Comparative study: 3.8% (serology); 2.5% (RT) | |
| Drug services (n = 3) (sample size: 25‐1123) |
Number tested/tests (DBS): 266 Coverage (FibroScan): 20% Coverage (DBS): 84.2% |
31.2% and 35% |
||
| Prisons (n = 2) (sample size: 3600a) |
Number tested (DBS): 160 Comparative study (DBS): ORs for effect of the intervention on testing rate: 0.86; 95% CI: 0.71‐1.06; P = 0.153 |
33.8% | ||
| Drug clinics and prisons (n = 1) (sample size: 6550 intervention, 5800 control) |
Comparative study coverage (DBS) intervention: 8.4% before, 20.6% during intervention control: 7.7% before, 5.4% during intervention |
32% (overall) | ||
| STI clinics and GP practices (n = 1) (sample size: 29 600) | Coverage (oral): 15.2% |
0.6% |
||
| Specialist servicesb (n = 1) (sample size: 1322) | 55% | |||
| Pharmacies (n = 2) (sample size: 143 intervention, 561 control; 244 conventional care pathway; 262 pharmacist care pathway) |
Comparative study coverage (DBS): 30% intervention; 13% control. OR: 2.25 (95% CI 1.48‐3.42) Comparative study coverage (DBS): 24% conventional care pathway; 36% pharmacist care pathway |
Comparative study: 25.9% conventional care pathway; 26.5% pharmacist care pathway | ||
| Education | HCV | |||
|
N = 1 study42 Quality: High |
Targeted at‐risk groups Drug services (n = 1) (sample size: 52) |
Comparative study coverage: 7% control; 20% intervention (NS) Willingness for HCV screening: Control: 89%; 56%; 67% (baseline; after 1 mo; after 3 mo) Intervention: 86%; 96%; 100%; 77%; 100% (baseline; after info; after 1 mo; after 3 mo; after FibroScan) |
||
| Campaign | HCV | |||
|
Quality: 2 studies NA |
Targeted at general pop. Mixed settings (n = 1), STI clinic/Prison (n = 1) (sample size: 4200 and 33 667a) |
Coverage from mixed settings: 2.3%‐3.7% |
Comparative study: prison: 44.4% before, 27.0% during intervention STI clinic: 5.2% before, 3.5% during intervention 45%‐53% newly diagnosed |
|
| Guideline | HCV | |||
|
N = 1 study53 Quality: NA |
STI clinics (n = 1) (sample size: NR) |
Comparative study: Coverage: 4.7% before, 13.6% after intervention |
||
|
Clinical decision‐making tools Computer‐assisted self/ personal interviewing (CASI/CAPI) vs paper & pen (PAPI, control) |
HBV | |||
|
N = 1 study60 Quality: High |
STI clinic (n = 1) (sample size: 2318) |
Comparative study: Coverage: PAPI: 16%; CAPI: 24%; CASI: 17% |
Comparative study: Any STI: PAPI: 10%; CAPI: 11%; CASI: 10% |
|
| HCV | ||||
|
N = 1 study60 Quality: High |
STI clinic (n = 1) (sample size: 2318) |
Comparative study: Coverage: PAPI: 3%; CAPI: 9%; CASI: 3% |
Comparative study: Any STI: PAPI: 10%; CAPI: 11%; CASI: 10% |
|
Abbreviations: BBV, bloodborne virus; CASI, computer‐assisted self‐interviewing; CAPI, computer‐assisted personal interviewing; CI, confidence interval; DBS, dried blood spot; GP, general practitioner; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; mo, months; MSM, men who have sex men; NA, not applicable; NS, not significant; OR, odds ratio; PAPI, pen‐and‐paper interviewing; RT, rapid test; STI, sexually transmitted infection.
Denominator not reported for all studies.
specialist services mainly targeting PWID.
Number of tests is only reported here if no data on coverage are available.
Eleven comparative studies reported on interventions in other healthcare settings. In pharmacies, HCV testing coverage was 30% among drug users receiving opioid substitution therapy (OST) when DBS testing was offered, compared to 13% in pharmacies which did not offer DBS. Furthermore, DBS testing coverage was 36% in pharmacies in which pharmacist‐led care pathways were used compared to 24% when conventional care pathways were used.58, 59 One randomized controlled trial (RCT) found that offering DBS testing for HCV in drug clinics and prisons significantly increased coverage rates in intervention sites with a 14.5% increase compared to control sites49; however, another RCT in which DBS testing for HCV was offered in prisons found an OR of 0.86 (95% CI: 0.71‐1.06) for the effect of the intervention on testing rate.45 Another comparative study reported a doubling in the number of tests performed in prisons and STI clinics during a national testing programme which involved awareness‐raising activities.34 Point‐of‐care rapid testing in clinics for people without healthcare coverage was found to significantly increase testing coverage to 98.2%, compared to standard serology testing performed elsewhere which had 64.2% coverage.44 One study which implemented testing of household contacts of HBV‐positive pregnant women through nurse‐led at‐home DBS testing reported a significant increase in coverage from 62% to 97% in antenatal services in which the intervention was implemented, compared to control sites where there was no increase observed in the same time frame.50 Another comparative study compared universal and risk‐based HCV screening for pregnant women in antenatal services and reported a positivity rate of 1.7% for universal screening compared to 1.3% for targeted screening; the difference was not significant.47 Information sessions and peer education increased coverage from 7% to 20% in a drug clinic, although this difference was not significant.42 Introduction of a clinic‐specific guideline in an STI clinic led to an increase in coverage from 4.7% to 13.6%.53 One comparative study reported on a clinical decision‐making tool initiative which included computer‐assisted self/personal interviewing vs paper‐and‐pen interviews in STI clinics. Both HBV and HCV testing coverage were highest (24% and 9%, respectively) after computer‐assisted personal interviewing.60
Acceptability and feasibility of testing initiatives in other healthcare settings were reported by three studies.44, 58, 60 One study on rapid DBS testing for HCV in pharmacies showed that patients found the pharmacy a good place to be tested, but some patients were suspicious when offered testing due to previous experience of discrimination at pharmacies.58 Among pharmacy staff, rapid DBS testing for HCV was found to be simple to perform. Rapid testing in a clinic for people with no health insurance was preferred over serological tests by 76% of the participants, with reasons including less stress with same‐day results and more practical use. Half of the clinical staff said that rapid testing simplified their consultation.44 A study that compared computer‐assisted interviewing to paper‐and‐pen interviews in STI clinics found that computer‐assisted interviewing encouraged the disclosure of sexual risk‐taking behaviour more.60
3.4. Testing initiatives in community settings
Forty‐one studies were retrieved that formed the evidence base for the effectiveness of testing initiatives and interventions in community settings.34, 43, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 Results of initiatives performed in community settings are presented in Table 4. Results for all intervention types are presented first, stratified by setting type.
Table 4.
Evidence base for the effectiveness of testing initiatives in community settings
| Intervention |
Studies included Quality of evidence |
Target population | Test offer | Coverage/number of tests/testedc |
Positivity rate |
|---|---|---|---|---|---|
| All per setting | HBV | ||||
|
N = 18 studies 65, 66, 69, 72, 73, 74, 75, 76, 78, 79, 82, 83, 84, 85, 86, 87, 88, 89 Quality: 3 studies acceptable 15 studies NA |
Community‐based testing sites (n = 2) (sample size: 512 and 744) | Coverage: 37% and 71.1% | 8.3% and 9.4% | ||
| Outreach (n = 11) (sample size: 30‐86 000) | 100% |
Number tested/tests: 299‐1090 Coverage: 9.8%‐76.2% Comparative study coverage: 1.5% control; 42.8% intervention (outreach education + testing at health centre); 59.7% intervention (outreach education + onsite test) Comparative study: Coverage: 53.3% DBS postal kit; 83.3% nurse‐led sauna outreach testing; 100% standard STI clinic testing |
0%‐12.4% Comparative study: 0% control; 2.1% intervention (outreach education + testing at health centre); 4.8% intervention (outreach education + onsite test) |
||
| Drug services/ harm reduction programmes (n = 2) (sample size: intervention 27, control 28; 391) |
Coverage: 49% Comparative study coverage: Intervention (oral) 92.6%; control (referral) 7.4% |
20% (anti‐HBc) | |||
| Online tools (n = 3) (sample size: 265 and 1400a) |
Coverage: 16.2% 4305 self‐sampling kits requested; 48% of requested kits returned Comparative study coverage: 43.9% intervention (behavioural tailoring plus cultural tailoring) (OR 0.94 95% CI 0.69‐1.26); 43.5% intervention (behavioural tailoring) (OR 0.88 95% CI 0.65‐1.19) 46.0% control |
0% and 0.2% | |||
| HCV | |||||
|
N = 23 studies34, 43, 64, 65, 67, 68, 69, 70, 71, 72, 73, 74, 75, 77, 78, 79, 80, 81, 82, 83, 88, 89, 90 Quality: 3 studies acceptable 20 studies NA |
Community‐based testing sites (n = 3) (sample size: 32‐744) |
Number tested/tests: 95 Coverage: 37% and 71.1% |
0%‐6.3% | ||
| Outreach (n = 7) (sample size: 30‐65 000) | 100% |
Number tested/tests: 491‐520 Coverage: 9.8%‐76.2% Comparative study coverage: 1.5% control; 42.8% intervention (outreach education + testing at health centre); 59.7% intervention (outreach education + onsite test) Comparative study coverage: Coverage: 53.3% DBS postal kit; 83.3% nurse‐led sauna outreach testing; 100% standard STI clinic testing |
0.8%‐37.6% Comparative study: 0% control; 3.2% intervention (outreach education + testing at health centre); 2.8% intervention (outreach education + onsite test) Comparative study: HBV/HCV active; cleared infection 6.25%; 25% DBS postal kits 0%; 4% nurse‐led sauna outreach testing 0%; 0% standard STI clinic testing |
||
| Drug services/harm reduction programmes (n = 8) (sample size: 27‐5399) |
Comparative study: 67 tests before 973 during Coverage: 42%‐84.2% Comparative study coverage: Intervention (oral) 100%; control (referral) 7.4% Comparative study: 44% intervention; 51% control |
18%‐53% Comparative study: 15% intervention; 14% control Comparative study: 19.4% before; 38.1% during |
|||
| Online tools (n = 2) (sample size: 265 and 9653) | 15.3% | Coverage: 4.4% and 16.2% | 4.5% and 4.6% | ||
| Combined settings (n = 3) (sample size: 240 and 564a) | 73% |
Number tested/tests: 266 Coverage: 37% and 72% |
4.8%‐35% | ||
| Risk group testing | HBV | ||||
|
N = 16 studies65, 66, 69, 72, 73, 75, 76, 78, 79, 82, 83, 84, 85, 86, 88, 89 Quality: 3 studies acceptable 13 studies NA |
Drug users (n = 3) (sample size: intervention 27, control 28; 391a) | 100% |
Coverage: 49%‐76.2% Comparative study coverage: intervention (oral) 92.6%; control (referral) 7.4% |
0% 20% (anti‐HBc) |
|
| Homeless (n = 1) (sample size: NR) | Number tested/tests: 491 | 12.4% | |||
| Migrants (n = 9) (sample size: 744‐65 000a) |
Number tested/tests: 299‐1090 Coverage: 9.8%‐71.1% Comparative study coverage: 43.9% intervention (behavioural tailoring plus cultural tailoring) (OR 0.94 95% CI 0.69‐1.26); 43.5% intervention (behavioural tailoring) (OR 0.88 95% CI 0.65‐1.19) 46.0% control |
0.6%‐8.7% | |||
| MSM (n = 2) (sample size: 30‐265) |
Coverage: 16.2% Comparative study: coverage: 53.3% DBS postal kit; 83.3% outreach sauna nurse; 100% standard STI clinic |
0% Comparative study: HBV/HCV active; cleared infection 6.25%; 25% DBS postal kits 0%; 4% nurse‐led sauna outreach testing 0%; 0% standard STI clinic testing |
|||
| Underprivileged peopleb (n = 1) (sample size: 811 control; 222 intervention; 243 intervention + onsite test | Comparative study coverage: 1.5% control; 42.8% intervention (outreach education + testing at health centre); 59.7% intervention (outreach education + onsite test) | Comparative study: 0% control; 2.1% intervention (outreach education + testing at health centre); 4.8% intervention (outreach education + onsite test) | |||
| HCV | |||||
|
N = 20 studies 43, 64, 65, 67, 68, 69, 70, 71, 72, 73, 75, 76, 78, 79, 80, 81, 82, 83, 89, 90 Quality: 2 studies acceptable 18 studies NA |
Drug users (n = 6) (sample size: 27‐9653) | 100% |
Number tested/tests: 266 Coverage: 49%‐84.2% Comparative study coverage: intervention (oral) 100%; control (referral) 7.4% |
31.2%‐41% |
|
| PWID (n = 3) (sample size: 240 and 3463a) | 73% |
Number tested/tests: 24‐202 Coverage: 42% and 72% |
18% and 20.3% | ||
| Homeless (n = 2) (sample size: NR) | Number tested/tests: 95‐491 | 6.3% and 13% | |||
| Migrants (n = 5) (sample size: 744‐65 000) |
Number tested/tests: 520 Coverage: 9.8%‐71.1% |
0.3%‐4.7% | |||
| MSM (n = 2) (sample size: 30‐265) |
Coverage: 16.2% Comparative study: coverage: 53.3% DBS postal kit; 83.3% outreach sauna nurse; 100% standard STI clinic |
4.6% Comparative study: HBV/HCV active; cleared infection 6.25%; 25% DBS postal kits 0%; 4% nurse‐led sauna outreach testing 0%; 0% standard STI clinic testing |
|||
| Underprivileged peopleb (n = 1) (sample size: 811 control; 222 intervention; 243 intervention + onsite test | Comparative study coverage: 1.5% control; 42.8% intervention (outreach education + testing at health centre); 59.7% intervention (outreach education + onsite test) | Comparative study: 0% control; 3.2% intervention (outreach education + testing at health centre); 2.8% intervention (outreach education + onsite test) | |||
| Risk groups (n = 1) (sample size: 9653) | 15.3% | Coverage: 4.4% | 4.5% | ||
| Novel testing | HBV | ||||
|
N = 8 studies 65, 69, 73, 74, 75, 85, 87, 88 Quality: 1 study acceptable 7 studies NA |
Drug users (n = 3) (sample size: intervention 27, control 28; 391a) | 100% |
Coverage (DBS): 49% Coverage (FibroScan): 76.2% Comparative study coverage: intervention (oral) 92.6%; control (referral) 7.4% |
0% 20% (anti‐HBc) |
|
| Migrants (n = 2) (sample size: 21 000 and 65 000) | Number tested/tests (DBS): 229‐1126 | 8.7% | |||
| MSM (n = 1) (sample size: 30 per group) | Comparative study: coverage: 53.3% DBS postal kit; 83.3% outreach sauna nurse; 100% standard STI clinic |
Comparative study: HBV/HCV active; cleared infection 6.25%; 25% DBS postal kits 0%; 4% nurse‐led sauna outreach testing 0%; 0% standard STI clinic testing |
|||
| Students (n = 1) (sample size: 512) | Coverage (DBS): 37% | 9.4% | |||
| General pop (n = 1) (sample size: NR) | 4305 self‐sampling kits requested; 48% of requested kits returned | 0.2% | |||
| HCV | |||||
|
N = 13 studies34, 43, 64, 65, 67, 68, 69, 73, 74, 75, 80, 81, 88 Quality: 1 study acceptable 12 studies NA |
Drug users (n = 7) (Sample size: 27‐1123) |
Number tested/tests (DBS): 266 Coverage (DBS): 13%‐49% Coverage (FibroScan): 76.2% Comparative study coverage: Intervention (oral) 100%; control (referral) 7.4% Comparative study (DBS): 3‐fold increase in testing (RR = 3.5, P < 0.001) |
27.5%‐53% Comparative study (DBS): 12‐fold increase in positives (RR = 12.1, P < 0.001) |
||
| PWID (n = 2) (sample size: 240a) | 73% (oral) |
Number tested/tests (DBS): 202 Coverage (oral): 72% |
20.3% (oral) | ||
| Homeless (n = 1) (sample size: NR) | Number tested/tests (DBS): 95 | 6.3% | |||
| Migrants (n = 1) (sample size: 65 000) | Number tested/tests (DBS): 520 | 0.8% | |||
| MSM (n = 1) (sample size: 30 per group) | Comparative study: coverage: 53.3% DBS postal kit; 83.3% outreach sauna nurse; 100% standard STI clinic |
Comparative study: HBV/HCV active; cleared infection 6.25%; 25% DBS postal kits 0%; 4% nurse‐led sauna outreach testing 0%; 0% standard STI clinic testing |
|||
| Students (n = 1) (sample size: 512) | Coverage (DBS): 37% | 0% | |||
| Education | HBV | ||||
|
Quality: 1 study acceptable 2 studies NA |
Targeted at‐risk groups Underprivileged peopleb (n = 1) (sample size: 811 control; 222 intervention; 243 intervention + onsite test) Migrants (n = 2) (sample size: 1500 and 6337 |
Coverage: 11.2% and 31% Comparative study coverage: 1.5% control; 42.8% intervention (outreach education + testing at health centre); 59.7% intervention (outreach education + onsite test) |
1.1% and 2.8% Comparative study 0% control; 2.1% intervention (outreach education + testing at health centre); 4.8% intervention (outreach education + onsite test) |
||
| HCV | |||||
|
Quality: 1 study acceptable 3 studies NA |
Targeted at‐risk groups Underprivileged peopleb (n = 1) (sample size: 811 control; 222 intervention; 243 intervention + onsite test) Migrants (n = 2) (sample size: 1500 and 6337) PWID (n = 1) (sample size: 88) |
Coverage: 11.2% and 31% Comparative study coverage: 1.5% control; 42.8% intervention (outreach education + testing at health centre); 59.7% intervention (outreach education + onsite test) Comparative study: 78% control; 85% intervention |
0.3% and 2.4% Comparative study HCV: 0% control; 3.2% intervention (outreach education + testing at health centre); 2.8% intervention (outreach education + onsite test) Comparative study: 14% control; 15% intervention |
||
| Campaign | HBV | ||||
|
Quality: 4 studies NA |
Targeted at‐risk groups Migrants (n = 4) (sample size: 6337‐29 000) |
Number tested/tests: 299 and 1090 Coverage: 11.2% and 15.3% |
2.8%‐8.7% 2.9% newly diagnosed |
||
| HCV | |||||
|
Quality: 3 studies NA |
Targeted at‐risk groups Migrants (n = 1) (sample size: 6337) |
Coverage: 11.2% | 0.3% | ||
| Targeted at GPs and those at risk General pop. (n = 1) (sample size: 5339) | Comparative study number tested/tests: 67 before; 973 during | Comparative study: 19.4% before; 38.1% during | |||
| Targeted at public General pop. (n = 1) (sample size: 9653) |
15.3% website visitors offered testing. Coverage: 4.4% |
4.5% | |||
| National programme | HCV | ||||
|
Quality: 2 studies NA |
PWID (n = 1) (sample size: 3463) |
Coverage: 42% |
18% |
||
| General population (n = 1) (sample size: 5399) | Comparative study: coverage before and after programme: 26% (in 2000); 62% (in 2008) | Comparative study: 19.4% before; 38.1% during | |||
| Communication & technology | HBV | ||||
|
Quality: 1 study acceptable 1 study NA |
MSM (n = 1) (sample size: 265) |
Coverage: 16.2% |
0% |
||
| Migrants (n = 1) (HCV n = 0, HBV n = 1 sample:432‐496) |
Comparative study coverage: 43.9% BCT; 43.5% BT; 46.0% GI ORs (95%CI): BCT vs GI: 0.94 (0.69‐1.26) BT vs GI: 0.88 (0.65‐1.19) |
||||
| HCV | |||||
|
Quality: 2 studies NA |
MSM (n = 1) (sample size: 265) |
Coverage: 16.2% | 4.6% | ||
| General population (n = 1) (sample size: 9653) |
15.3% website visitors offered testing. Coverage: 4.4% |
4.5% | |||
Abbreviations: anti‐HBC, antibody to the hepatitis B core antigen; BCT, behavioural plus cultural tailoring; BT, behavioural tailoring; CI, confidence interval; DBS, dried blood spot; GI, generic information; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MSM, men who have sex with men; NA, not applicable; OR, odds ratio; PWID, people who inject drugs; STI, sexually transmitted infection; TB, tuberculosis.
Denominator not reported by all studies.
Unemployed, social assistance beneficiaries and seekers of political asylum living in long‐term shelters.
Number of tests is only reported here if no data on coverage is available.
Coverage rates above 80% were reported by two testing initiatives conducted in community drugs services,64, 65 although the other six studies conducted in this setting reported lower coverage. HCV positivity rates were high in this setting.34, 64, 70, 75, 77, 81 Outreach testing activities and testing initiatives conducted in fixed community sites yielded coverage rates of up to 83.3% and 71.1%, respectively.66, 69, 72, 73, 74, 76, 79, 80, 82, 83, 85, 86, 88, 89 In general, online testing initiatives reported somewhat lower coverage rates relative to other settings (4.4% and 16.2%),78, 90 as well as low positivity rates for HBV (0% and 0.2%). Across all settings, novel testing initiatives yielded relatively high coverage rates in general, with eight out of fourteen studies that reported coverage testing more than 50% of the targeted population.34, 43, 64, 65, 67, 68, 69, 73, 74, 75, 80, 81, 85, 87, 88
Two comparative studies reported on novel testing initiatives targeted to risk groups. In community drug services, onsite oral sampling of drug users had a reported coverage of 100%, compared to 7.4% for standard serological testing which was performed offsite, at an STI clinic.65 Another study compared nurse‐delivered outreach screening at a sauna and self‐sampled DBS postal testing kits to standard screening at an STI clinic for MSM and reported coverage rates for the first 30 users of each service: 83.3% for outreach, 53.3% for DBS postal kits and 100% for standard screening. Positivity rates were higher for outreach (4% had a cleared HBV or HCV infection) and DBS postal kits (25% had a cleared HBV or HCV infection), compared to 0% for those tested in STI clinics. In addition, almost all STI clinic users had been tested previously, compared to just over half of sauna and postal kit users.88
An RCT compared outreach testing in shelters for underprivileged people, involving group education sessions and individual consultations during which subjects were offered testing, either at a health centre or onsite. Coverage was 42.8% at healthcare centres and 59.7% onsite. Coverage in a control group that received no intervention during the same time period was 1.5%.79 Educational sessions for PWID attending harm reduction centres contributed to an increased coverage from 44% to 85%, compared to an increase of 51 to 78% in a control group that received no educational intervention.77 During an HCV action plan in Scotland, implementation of DBS testing and awareness activities resulted in a 3‐fold increase in HCV testing coverage in community drug services (RR 3.5, P < 0.001) and a 12‐fold increase in positive test results (RR = 12.1, P < 0.001).In England, a national framework of activities to improve prevention, diagnosis and treatment of HCV led to an increase from 26% coverage in 2000 (prior to the national programme) to 62% coverage in 2008.34, 70 Lastly, a communication and technology HBV testing initiative involving Internet‐based recruitment of migrants for screening yielded coverage of 43.5%‐46.0%, although comparison between different strategies (behavioural tailoring, behavioural plus cultural tailoring or generic information) showed no differences.84
Eight studies provided data on the feasibility and acceptability of testing in community settings. Test acceptance rates ranged from 28.4% to 98.2%68, 69, 74, 90 with the highest rates reported in two studies which described outreach initiatives targeting drug users, one of which used oral sampling.68, 69 Another study reporting on an outreach testing initiative targeted at Chinese migrants reported that 100% of interviewed recipients found an information session prior to testing useful and just 5% responded that the test caused discomfort.85 A study describing an oral sampling initiative at a homeless shelter reported that 91% of the participants would agree to screening or thought an oral swab test was acceptable form of testing.80 An online platform offering home sampling kits for HBV, HIV and STIs reported that of the samples returned, 15% were provided insufficient blood and 39% of participants reported difficulties taking blood samples. However, 95% said they would use the online service again and 93% would recommend it to family and friends.87 A testing initiative targeted at university students with an acceptance rate of 37% reported that there was no indication or reports from students or staff that the testing offer and process were stigmatizing or undesirable.74 Lastly, a culturally targeted screening project involving campaigns and educational meetings for Turkish migrants was considered good and understandable by 97% of the participants.76
3.5. Testing initiatives in multiple/unspecified settings
In addition, two studies were retrieved which provided data on initiatives conducted in multiple settings or did not specify results per setting. One study examined the outcomes of a French national HCV prevention programme implemented in 1999 and reported that testing increased from 2000 to 2005 by 45% but decreased by 10% in 2006. Positivity rates decreased from 4.3% to 2.9%.91 A further study describing a national testing programme which involved awareness‐raising activities reported outcomes across all settings; 19 058 tests were taken before; and 29 045 tests were taken during the programme and positivity rates decreased from 9.6% to 6.8%.34
4. DISCUSSION
We undertook a comprehensive systematic review to collect, synthesize and analyse available data on HBV/HCV testing strategies across the EU/EEA. Available evidence on testing strategies was analysed qualitatively across primary health care, hospital settings, other healthcare settings and community settings.
Primary health care is usually the first point of contact that patients will have with the health care system. Therefore, it is an important setting for testing a number of population groups that may not present to other settings and may be especially important for specific population groups including ex‐PWID.92 However, according to a UK study, of all HCV diagnostic tests performed in a pool of sentinel laboratories, only 16% were requested by GPs.93 Overall, the evidence retrieved on the effectiveness of testing interventions to improve HBV and HCV testing coverage in primary health care was limited, largely restricted to Western European countries, and focused mainly on targeted test offer to risk groups such as migrants, PWID and homeless. A previous meta‐analysis assessing the effectiveness of targeted HCV testing interventions found these strategies to be effective in diagnosing cases and increasing uptake of treatment, particularly when strategies involved practitioners.18 In this review, limited evidence indicated that educational interventions targeting GPs and campaigns targeting the public, GPs or risk groups may have benefit, particularly when education and campaigns are combined, and that offering HCV testing in this setting was acceptable to patients. A recent EU‐funded project investigated options to further expand testing opportunities for migrant communities in this setting and has produced a useful toolkit that may support its implementation.94
Hospitals are another important location for testing, with more than half of all HCV tests performed in UK sentinel laboratories requested by hospital‐based clinicians, especially hepatology and infectious disease departments.93 However, the evidence on testing interventions aimed at improving HBV and HCV testing in hospital settings retrieved was relatively limited. In many studies, testing for HBV and HCV was performed as part of a BBV screening test including HIV testing. Specific hospital departments could be strategic settings to capture patients belonging to certain risk groups and encourage and facilitate testing. Targeted testing to individuals from risk groups (migrants and psychiatric patients) was implemented in hospital departments with generally high positivity rates and fairly high coverage levels in studies involving hospital inpatients or outpatients attending the hospital for reasons other than testing, although uptake was lower when participants were invited to come to the hospital for the sole purpose of testing. This emphasizes the importance of practitioners considering opportunistic test offer for patients that may be at risk. Two studies on universal testing in emergency departments reported lower positivity rates compared to other strategies; however, this could be an important strategy for case finding in areas that are known to have a high prevalence or burden of disease, or where injecting drug use is prevalent.
Evidence relating to other healthcare settings found similar variation in coverage and positivity rates which were often high when risk groups were targeted. This suggests that offering testing to groups at high risk of infection at locations they specifically attend for health purposes, such as health clinics for migrants, could be an effective method for case finding. Locations frequently visited by certain groups, where staff are medically trained, present an opportunity that can be exploited for testing. For example, individuals receiving OST access pharmacies on a regular basis to receive medication. People receiving OST were found to be more likely to accept DBS testing at the pharmacy than testing from other providers.58 Furthermore, pharmacies in some countries can provide treatment for those found to be infected.59 Novel testing approaches such as rapid testing and DBS were frequently employed in testing initiatives in the various healthcare settings and were found to be effective in increasing coverage in a number of comparative settings, as well as being highly acceptable among users and testing staff. Rapid testing allows point‐of‐care testing, simplifying the testing process as those tested do not have to visit outside testing centres or wait to pick up results, although it is crucial to ensure that individuals testing positive are linked to ongoing care. Another strategy identified that may help to improve testing coverage in other healthcare settings included implementation of clinic‐specific guidelines.53 These may be particularly useful in countries where national hepatitis testing guidelines are not available.8
Community‐based testing services, both fixed‐site and outreach‐based, represent potential sites to target specific population groups and individuals who may be at increased risk of infection and who are not in contact with formal health services. Testing in community settings can be adapted and made accessible for these groups in order to maximize testing uptake15; however, a gap in policy on community and outreach testing was reported by some EU countries suggesting that it is not always implemented at scale.8 Yet, we identified considerable evidence supporting the implementation of community‐based testing services. According to the evidence, testing services in community settings often resulted in high coverage and high positivity rates, particularly when targeting MSM, people with migration background and drug users, although influenced by the underlying epidemiology. Outreach activities, including short‐term testing facilities, mobile testing services and street‐based outreach, were also shown to be highly effective in targeting these population groups, while being highly adaptable. Available evidence also suggested that use of oral sampling and DBS increase testing coverage and oral sampling was considered acceptable in community‐based services. Compared to other infectious diseases, the market for HBV and HCV rapid tests is less abundant, and the scale of implementation is comparatively limited.8 However, DBS and rapid tests are recommended by the WHO to facilitate testing in settings where no laboratory is available.95 Finally, educational interventions, campaigns and national programmes were also found to increase community‐based testing coverage for HBV and HCV, including among migrant communities. An earlier systematic review on chronic HBV testing in community settings identified common features of successful screening interventions that included strategies to increase community awareness and knowledge and incorporated the target population's values in the design and implementation of the programme and were able to provide low cost or free access to care.21
Contact tracing and partner notification for HBV and HCV have recognized public health benefits, such as controlling the spread, reducing morbidity and mortality, reaching people with asymptomatic infection and people who do not present for diagnosis, counselling and treatment96 and have been shown to be effective.50 Partner notification may be implemented in different ways,97 and approaches designed to meet diverse structural, societal and legal barriers. However, our study identified very limited evidence on this approach, possibly due to its suboptimal implementation in EU/EEA, as reported in a previous study.98
A recent WHO guideline recommended that HCV screening in birth cohorts of older persons at higher risk of infection and morbidity may be applied in populations with overall lower general prevalence, where indicated by the local epidemiology.95, 99 Recent epidemiological studies to assess the relevance of such an approach have been performed in some EU/EEA countries, demonstrating the growing interest in this testing strategy and suggesting a possible role of this approach in the elimination effort.100, 101, 102, 103 The evidence resulting from our study, however, indicated that the utility of birth cohort screening is dependent on the local epidemiology and context of the screening.
Self‐sampling and self‐testing are relatively new testing modalities that have the potential to increase testing coverage. For HIV, use of self‐sampling or self‐testing kits is already authorized in a limited number of countries.104 For HIV, self‐testing has been associated with increased testing uptake among men in a number of RCTs and is recommended as a testing approach by the WHO.105 We identified very limited evidence on self‐sampling kits and no evidence on self‐testing for HBV/HCV. Rather than indicating a lack of interest or limited applicability, this may reflect the lack of technology availability for HBV/HCV self‐testing. However, as these technologies become available in the future, self‐testing may become a potentially important approach to expand access to testing.106 Transferability of the evidence and relevance of these approaches from the HIV field may contribute to this process.
Barriers to testing exist at individual, healthcare provider and institutional levels which can impede case‐finding efforts. In general, the asymptomatic course of HBV/HCV, low levels of knowledge and awareness and fear of stigma and discrimination may prevent people from seeking testing or accepting test offer.32, 58, 107, 108, 109, 110, 111, 112 For vulnerable populations, health and social problems, unstable or unstructured lives and poverty can be barriers, for PWID venous access can be an issue and for people with a migration background, culture, faith and language and their perceptions and understanding of the health care system may present barriers to testing.29, 58, 109, 110, 111, 113 The proximity of health care services to individuals, low awareness among health care professionals and forgetting to test are barriers that may exist at the testing provider level.58, 109, 113 Barriers specific to health care include administrational limitations, time limitations and the GP's relationship with their patients.29, 32, 113 Potential barriers specific to community include inconvenient testing facilities and lack of advocacy and promotion.109, 112 At an institutional level, pressures, capacity and funding shortages in primary healthcare sectors and community organizations can present barriers to testing.109, 110 Implementing certain testing strategies may help to alleviate many of these barriers; for example, educational initiatives, campaigns and other health promotional activities could help to raise awareness and knowledge within certain populations, the wider public and healthcare professionals. Using novel techniques such as DBS or oral sampling can bypass any issues with venous access. Demedicalizing services and bringing them out into the community could circumvent barriers which exist within healthcare settings. Implementing testing activities in a range of settings could lessen the impact of setting‐specific barriers.
The comparability of data retrieved in the systematic review was limited by the large degree of heterogeneity between studies in the outcomes measured, the specific populations targeted, recruitment and length of interventions, and whether testing initiatives were combined with health promotional activities, all of which could influence the success of the intervention. In addition, the lack of a threshold for sufficient levels of testing uptake or coverage precluded quantitative analysis. The majority of studies did not use a comparator to assess effectiveness of interventions. Furthermore, none of the studies retrieved assessed the long‐term outcomes of interventions such as the impact of testing on prevalence and incidence over time. Few studies were deemed to be of high quality and the majority had a study design which precluded formal quality assessment. For these studies, the most common quality issues noted were a lack of clarity or detail in methodology (eg data collection methods), limited description of the study population and unclear or inappropriate denominator. A significant proportion (16%) of the evidence base of the review was from included conference abstracts, that is non‐peer‐reviewed literature for which methods and results were often extremely limited and quality assessment was not possible.
In conclusion, evidence was retrieved for successful testing approaches applied in primary health care, hospital and other healthcare settings and community settings, although within most settings the evidence was fairly limited. Testing approaches targeting population groups at high risk of HBV/HCV were found to be viable in various settings, and there was evidence that other interventions such as awareness campaigns, education and the implementation of testing in the context of a national strategy may improve coverage by helping to overcome some of the barriers to testing. DBS was also associated with increased testing coverage in several different settings and other potentially effective testing strategies include contact tracing, home sampling and birth cohort testing. Further research and an understanding of local factors including the epidemiology are needed to provide policymakers with a clearer overview of which initiatives will be most successful in improving testing uptake and yielding high positivity rates. However, combining a diverse set of testing opportunities within national testing strategies may lead to higher impact in terms of both testing coverage and reduction of the undiagnosed fraction. Elimination of viral hepatitis in Europe by 2030, according to the WHO regional goal, will require diagnosing those infected and ensuring linkage to appropriate prevention, care, treatment and support services.14, 95 Implementation of diversified set of effective, evidence‐based testing strategies, particularly among vulnerable and hard‐to‐reach populations is a vital step in realizing this goal.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHORS’ CONTRIBUTIONS
LT, ED, LM, IV, EB, AAG contributed to the design of the project and the development of the study protocol, LT coordinated the study. LM, AA, EB, LT performed the systematic review, including data collection and data analysis. All authors contributed to data interpretation, manuscript drafting and review. LM drafted the first version of the manuscript.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank the ECDC library for their technical support with the systematic review search. The authors would also like to acknowledge all the ECDC colleagues who helped translating and screening the retrieved records published in languages other than English, Spanish and French. This work was supported by the European Centre for Disease Prevention and Control (framework contract number ECDC/2016/027) and is part of a wider undertaking to develop European guidance on integrated testing for HBV, HCV and HIV.
Mason LMK, Veldhuijzen IK, Duffell E, et al. Hepatitis B and C testing strategies in healthcare and community settings in the EU/EEA: A systematic review. J Viral Hepat. 2019;26:1431–1453. 10.1111/jvh.13182
REFERENCES
- 1. Hofstraat SHI, Falla AM, Duffell EF, et al. Current prevalence of chronic hepatitis B and C virus infection in the general population, blood donors and pregnant women in the EU/EEA: a systematic review. Epidemiol Infect. 2017;145:2873‐2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21‐29. [PubMed] [Google Scholar]
- 3. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529‐538. [DOI] [PubMed] [Google Scholar]
- 4. Memon M, Memon M. Hepatitis C: an epidemiological review. J Viral Hepat. 2002;9:84‐100. [DOI] [PubMed] [Google Scholar]
- 5. Seeff LB. Natural history of chronic hepatitis C. Hepatology (Baltimore, MD). 2002;36(5B):s35‐s46. [DOI] [PubMed] [Google Scholar]
- 6. Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112‐125. [DOI] [PubMed] [Google Scholar]
- 7. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546‐1555. [DOI] [PubMed] [Google Scholar]
- 8. European Centre for Disease Prevention and Control . Hepatitis B and C testing activities, needs and priorities in the EU/EEA Stockholm. Stockholm, Sweden: ECDC; 2017. [Google Scholar]
- 9. Easterbrook PJ. Who to test and how to test for chronic hepatitis C infection–2016 WHO testing guidance for low‐and middle‐income countries. J Hepatol. 2016;65:S46‐S66. [DOI] [PubMed] [Google Scholar]
- 10. European Centre for Disease Prevention and Control . Systematic review on hepatitis B and C prevalence in the EU/EEA 2016. TQ‐02‐16‐837‐EN‐N. [Google Scholar]
- 11. European Centre for Disease Prevention and Control . Hepatitis B and C epidemiology in selected population groups in the EU/EEA. Stockholm: ECDC; 2018. [Google Scholar]
- 12. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631‐640. [DOI] [PubMed] [Google Scholar]
- 13. Lampertico P, Agarwal K, Berg T, et al. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370‐398. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization . Action plan for the health sector response to viral hepatitis in the WHO European Region [Draft ]. Geneva: WHO; 2016. [Google Scholar]
- 15. Reyes‐Uruena J, Breveglieri M, Furegato M, Fernandez‐Lopez L, Agusti C, Casabona J. Heterogeneity of community‐based voluntary, counselling and testing services for HIV in Europe: the HIV‐COBATEST survey. Int J STD AIDS. 2017;28:28‐38. [DOI] [PubMed] [Google Scholar]
- 16. European Centre for Disease Prevention and Control and European Monitoring Centre for Drugs and Drug Addiction . Prevention and control of infectious diseases among people who inject drugs. 2011, Stockholm: ECDC; 2011. [Google Scholar]
- 17. Lazarus JV, Sperle I, Spina A, Rockstroh JK. Are the testing needs of key European populations affected by hepatitis B and hepatitis C being addressed? A scoping review of testing studies in Europe. Croat Med J. 2016;57:442‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aspinall EJ, Doyle JS, Corson S, et al. Targeted hepatitis C antibody testing interventions: a systematic review and meta‐analysis. Eur J Epidemiol. 2015;30:115‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int J Drug Policy. 2017;47:34‐46. [DOI] [PubMed] [Google Scholar]
- 20. Chou R, Cottrell EB, Wasson N, Rahman B, Guise JM. Screening for hepatitis C virus infection in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:101‐108. [DOI] [PubMed] [Google Scholar]
- 21. Robotin MC, George J. Community‐based hepatitis B screening: what works? Hepatol Int. 2014;8:478‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vedio A, Liu E, Lee A, Salway S. Improving access to health care for chronic hepatitis B among migrant Chinese populations: A systematic mixed methods review of barriers and enablers. J Viral Hepat. 2017;24:526‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuure FR, Urbanus AT, Langendam MW, et al. Outcomes of hepatitis C screening programs targeted at risk groups hidden in the general population: a systematic review. BMC Public Health. 2014;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rumble C, Pevalin DJ, O'Moore É. Routine testing for blood‐borne viruses in prisons: a systematic review. Eur J Public Health. 2015;25:1078‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou K, Fitzpatrick T, Walsh N, et al. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta‐analyses. Lancet Infect Dis. 2016;16:1409‐1422. [DOI] [PubMed] [Google Scholar]
- 26. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guidelines International Network . Critical appraisal: Notes and checklists. http://www.sign.ac.uk/checklists-and-notes.html., 2015. [Google Scholar]
- 28. Anderson EM, Mandeville RP, Hutchinson SJ, et al. Evaluation of a general practice based hepatitis C virus screening intervention. Scottish Med J. 2009;54:3‐7. [DOI] [PubMed] [Google Scholar]
- 29. Cullen BL, Hutchinson SJ, Cameron SO, et al. Identifying former injecting drug users infected with hepatitis C: an evaluation of a general practice‐based case‐finding intervention. J Public Health (Oxford, England). 2012;34:14‐23. [DOI] [PubMed] [Google Scholar]
- 30. Hargreaves S, Seedat F, Car J, et al. Screening for latent TB, HIV, and hepatitis B/C in new migrants in a high prevalence area of London, UK: a cross‐sectional study. BMC Infect Dis. 2014;14:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Helsper CW, van Essen GA, Bonten MJ, de Wit NJ. A support programme for primary care leads to substantial improvements in the effectiveness of a public hepatitis C campaign. Fam Pract. 2010;27:328‐332. [DOI] [PubMed] [Google Scholar]
- 32. Kunkel J, Sweeney L, Falla A, Veldhuijzen I, Foster GR. Screening for viral hepatitis among migrants in the EU: What lessons can we learn from poor response to a pilot trial using GP registers? J Hepatol. 2015;62:S838. [Google Scholar]
- 33. Lambert JS, Murphy C, O'Carroll A, et al. The Dublin hepcheck study: Community based testing of HCV by point of care oraquick® HCV saliva test in homeless populations. J Hepatol. 2016;64:S726. [Google Scholar]
- 34. McLeod A, Weir A, Aitken C, et al. Rise in testing and diagnosis associated with Scotland's Action Plan on Hepatitis C and introduction of dried blood spot testing. J Epidemiol Commun Health. 2014;68:1182‐1188. [DOI] [PubMed] [Google Scholar]
- 35. Parisi MR, Soldini L, Vidoni G, et al. Point‐of‐care testing for HCV infection: recent advances and implications for alternative screening. New Microbiol. 2014;37:449‐457. [PubMed] [Google Scholar]
- 36. Roudot‐Thoraval F, Rosa‐Hézode I, Trompette M, Costes L, Chousterman M. Successful management of precarious population after systematic HBV testing in france: A prospective cohort study. J Hepatol. 2015;62:S825‐S826. [Google Scholar]
- 37. Aparicio C, Mourez T, Simoneau G, et al. [Proposal of HIV, HBV and HCV targeted screening: short period feasibility study in a free‐access outpatient medical structure]. Presse Med. 2012;41:e517‐523. [DOI] [PubMed] [Google Scholar]
- 38. O'Connell S, Lillis D, Cotter A, et al. Hepatitis C diagnosis and linkage to care rates in an urban emergency department blood borne virus screening programme. J Hepatol. 2016;64:S525. [Google Scholar]
- 39. Orkin C, Flanagan S, Wallis E, et al. Incorporating HIV/hepatitis B virus/hepatitis C virus combined testing into routine blood tests in nine UK Emergency Departments: The "Going Viral" campaign. HIV Med. 2016;17:222‐230. [DOI] [PubMed] [Google Scholar]
- 40. Richter C, Ter beest G, Gisolf EH, et al. Screening for chronic hepatitis B and C in migrants from Afghanistan, Iran, Iraq, the former Soviet Republics, and Vietnam in the Arnhem region, The Netherlands. Epidemiol Infect. 2014;142:2140‐2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanger C, Hayward J, Patel G, et al. Acceptability and necessity of HIV and other blood‐borne virus testing in a psychiatric setting. Br J Psychiatry. 2013;202:307‐308. [DOI] [PubMed] [Google Scholar]
- 42. Arain A, De Sousa J, Corten K, et al. Pilot study: combining formal and peer education with FibroScan to increase HCV screening and treatment in persons who use drugs. J Subst Abuse Treat. 2016;67:44‐49. [DOI] [PubMed] [Google Scholar]
- 43. Bishton E, Oluboyede F, Grylls E, Woods L, Thomas S. Screening for hepatitis C in injecting and ex‐injecting drug users in North East Essex. Public Health. 2014;128:1036‐1038. [DOI] [PubMed] [Google Scholar]
- 44. Bottero J, Boyd A, Gozlan J, et al. Simultaneous human immunodeficiency virus‐hepatitis B‐hepatitis C point‐of‐care tests improve outcomes in linkage‐to‐care: results of a randomized control trial in persons without healthcare coverage. Open Forum Infect Dis. 2015;2:ofv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Craine N, Whitaker R, Perrett S, Zou L, Hickman M, Lyons M. A stepped wedge cluster randomized control trial of dried blood spot testing to improve the uptake of hepatitis C antibody testing within UK prisons. Eur J Public Health. 2015;25:351‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Defossez G, Verneau A, Ingrand I, Silvain C, Ingrand P, Beauchant M. Evaluation of the French national plan to promote screening and early management of viral hepatitis C, between 1997 and 2003: a comparative cross‐sectional study in Poitou‐Charentes region. Eur J Gastroenterol Hepatol. 2008;20:367‐372. [DOI] [PubMed] [Google Scholar]
- 47. Diab‐Elschahawi M, Dosch V, Honsig C, et al. Evaluation of a universal vs a targeted hepatitis C virus screening strategy among pregnant women at the Vienna University Hospital. Am J Infect Control. 2013;41:459‐460. [DOI] [PubMed] [Google Scholar]
- 48. El‐Hamad I, Pezzoli MC, Chiari E, et al. Point‐of‐care screening, prevalence, and risk factors for hepatitis B infection among 3,728 mainly undocumented migrants from non‐EU countries in northern Italy. J Travel Med. 2015;22:78‐86. [DOI] [PubMed] [Google Scholar]
- 49. Hickman M, McDonald T, Judd A, et al. Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial. J Viral Hepat. 2008;15:250‐254. [DOI] [PubMed] [Google Scholar]
- 50. Keel P, Edwards G, Flood J, et al. Assessing the impact of a nurse‐delivered home dried blood spot service on uptake of testing for household contacts of hepatitis B‐infected pregnant women across two London trusts. Epidemiol Infect. 2016;144:2087‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lindenburg CEA, Lambers FAE, Urbanus AT, et al. Hepatitis C testing and treatment among active drug users in Amsterdam: results from the DUTCH‐C project. Eur J Gastroenterol Hepatol. 2011;23:23‐31. [DOI] [PubMed] [Google Scholar]
- 52. McAllister G, Innes H, Mcleod A, et al. Uptake of hepatitis C specialist services and treatment following diagnosis by dried blood spot in Scotland. J Clin Virol. 2014;61:359‐364. [DOI] [PubMed] [Google Scholar]
- 53. Murira J, Monteiro E. Hepatitis C screening by country of birth in a genitourinary medicine clinic‐how much are we missing? Sex Transm Infect. 2016;92:A21. [Google Scholar]
- 54. Nosotti L, Petrelli A, Rossi A, et al. HBV infection prevalence and vaccination in an immigrant population in Rome. Digest Liver Dis. 2016;48:e130. [Google Scholar]
- 55. op de Coul EL, van Weert JW, Oomen PJet al. [Antenatal screening in the Netherlands for HIV, hepatitis B and syphilis is effective]. Ned Tijdschr Geneeskd. 2010;154:A2175. [PubMed] [Google Scholar]
- 56. Patel S, Clarke B, Bird G. Hepatitis B and hepatitis c virus case finding in a medium security UK prison. Can J Gastroenterol Hepatol. 2016;2016:108. [Google Scholar]
- 57. Pauti MD, Simonnot N, Estecahandy P. [Development of actions for the prevention of HIV, hepatitis and sexually transmitted infections among immigrants consulting in the doctors of the world "Missions France"]. Medecine et Maladies Infectieuses. 2009;39:191‐195. [DOI] [PubMed] [Google Scholar]
- 58. Radley A, Melville K, Tait J, Stephens B, Evans J, Dillon JF. A quasi‐experimental evaluation of dried blood spot testing through community pharmacies in the Tayside region of Scotland. Frontline Gastroenterol. 2017;8:221‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Radley A, Tait J, Dillon JF. DOT‐C: A cluster randomised feasibility trial evaluating directly observed anti‐HCV therapy in a population receiving opioid substitute therapy from community pharmacy. Int J Drug Policy. 2017;47:126‐136. [DOI] [PubMed] [Google Scholar]
- 60. Richens J, Copas A, Sadiq ST, et al. A randomised controlled trial of computer‐assisted interviewing in sexual health clinics. Sex Transm Infect. 2010;86:310‐314. [DOI] [PubMed] [Google Scholar]
- 61. Sagnelli E, Starnini G, Sagnelli C, et al. Blood born viral infections, sexually transmitted diseases and latent tuberculosis in italian prisons: a preliminary report of a large multicenter study. Eur Rev Med Pharmacol Sci. 2012;16:2142‐2146. [PubMed] [Google Scholar]
- 62. Schreuder I, van der Sande MAB, de Wit M, et al. Seroprevalence of HIV, hepatitis b, and hepatitis c among opioid drug users on methadone treatment in the netherlands. Harm Reduct J. 2010;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scott C, Day S, Low E, Sullivan A, Atkins M, Asboe D. Unselected hepatitis C screening of men who have sex with men attending sexual health clinics. J Infect. 2010;60:351‐353. [DOI] [PubMed] [Google Scholar]
- 64. Tait JM, Stephens BP, McIntyre PG, Evans M, Dillon JF. Dry blood spot testing for hepatitis C in people who injected drugs: Reaching the populations other tests cannot reach. Frontline Gastroenterol. 2013;4:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Apoola A, Brunt L. A randomised controlled study of mouth swab testing versus same day blood tests for HIV infection in young people attending a community drug service. Drug Alcohol Rev. 2011;30:101‐103. [DOI] [PubMed] [Google Scholar]
- 66. Coenen S, van Meer S, Vrolijk JM, et al. Clinical impact of five large‐scale screening projects for chronic hepatitis B in Chinese migrants in the Netherlands. Liver Int. 2016;36:1425‐1432. [DOI] [PubMed] [Google Scholar]
- 67. Craine N, Parry J, O'Toole J, D'Arcy S, Lyons M. Improving blood‐borne viral diagnosis; clinical audit of the uptake of dried blood spot testing offered by a substance misuse service. J Viral Hepat. 2009;16:219‐222. [DOI] [PubMed] [Google Scholar]
- 68. Fernandez‐Lopez L, Folch C, Majo X, Gasulla L, Casabona J. Implementation of rapid HIV and HCV testing within harm reduction programmes for people who inject drugs: a pilot study. AIDS Care. 2016;28:712‐716. [DOI] [PubMed] [Google Scholar]
- 69. Foucher J, Reiller B, Jullien V, et al. FibroScan used in street‐based outreach for drug users is useful for hepatitis C virus screening and management: a prospective study. J Viral Hepat. 2009;16:121‐131. [DOI] [PubMed] [Google Scholar]
- 70. Hope V, Parry JV, Marongui A, Ncube F. Hepatitis C infection among recent initiates to injecting in England 2000–2008: Is a national hepatitis C action plan making a difference? J Viral Hepat. 2012;19:55‐64. [DOI] [PubMed] [Google Scholar]
- 71. Hope VD, McVeigh J, Smith J, et al. Low levels of hepatitis C diagnosis and testing uptake among people who inject image and performance enhancing drugs in England and Wales, 2012–15. Drug Alcohol Depend. 2017;179:83‐86. [DOI] [PubMed] [Google Scholar]
- 72. Jafferbhoy H, Miller MH, McIntyre P, Dillon JF. The effectiveness of outreach testing for hepatitis C in an immigrant Pakistani population. Epidemiol Infect. 2012;140:1048‐1053. [DOI] [PubMed] [Google Scholar]
- 73. McPherson S, Valappil M, Moses SE, et al. Targeted case finding for hepatitis B using dry blood spot testing in the British‐Chinese and South Asian populations of the North‐East of England. J Viral Hepat. 2013;20:638‐644. [DOI] [PubMed] [Google Scholar]
- 74. Okpo E, Corrigan H, Gillies P. Blood borne virus (BBV) testing in a university setting in North‐East Scotland: a pilot initiative. Public Health. 2015;129:825‐827. [DOI] [PubMed] [Google Scholar]
- 75. O'Sullivan M, Williams H, Jones AM, Verma S. Project ITTREAT (integrated community based test‐stage‐treat) HCV service for people who inject drugs (PWID). Hepatology (Baltimore, MD). 2016;63:385A. [Google Scholar]
- 76. Richter C, Beest GT, Sancak I, et al. Hepatitis B prevalence in the Turkish population of Arnhem: implications for national screening policy? Epidemiol Infect. 2012;140:724‐730. [DOI] [PubMed] [Google Scholar]
- 77. Roux P, Castro DR, Ndiaye K, et al. Increased uptake of HCV testing through a community‐based educational intervention in difficult‐to‐reach people who inject drugs: results from the ANRS‐AERLI study. PLoS ONE. 2016;11:e0157062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ruutel K, Lohmus L, Janes J. Internet‐based recruitment system for HIV and STI screening for men who have sex with men in Estonia, 2013: analysis of preliminary outcomes. Eurosurveillance. 2015;20(15):21094 [PubMed] [Google Scholar]
- 79. Sahajian F, Bailly F, Vanhems P, et al. A randomized trial of viral hepatitis prevention among underprivileged people in the Lyon area of France. J Public Health (Oxford, England). 2011;33:182‐192. [DOI] [PubMed] [Google Scholar]
- 80. Selvapatt N, Harrison L, Brown A. A pilot study of outreach testing for hepatitis C and linkage to care in a London centre for homeless persons. Gut. 2015;64:A109. [Google Scholar]
- 81. Selvapatt N, Ward T, Harrison L, et al. The cost impact of outreach testing and treatment for hepatitis C in an urban Drug Treatment Unit. Liver Int. 2017;37:345‐353. [DOI] [PubMed] [Google Scholar]
- 82. Story A, Hayward A, Aldridge R. Co‐infection with hepatitis C, hepatitis B, HIV and latent TB infection among homeless people in London. J Hepatol. 2016;64:S455‐S456. [Google Scholar]
- 83. Tafuri S, Prato R, Martinelli D, et al. Prevalence of hepatitis B, C, HIV and syphilis markers among refugees in Bari, Italy. BMC Infect Dis. 2010;10:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van der Veen YJ, van Empelen P, de Zwart O, Visser H, Mackenbach JP, Richardus JH. Cultural tailoring to promote hepatitis B screening in Turkish Dutch: a randomized control study. Health Promot Int. 2014;29:692‐704. [DOI] [PubMed] [Google Scholar]
- 85. Vedio AB, Ellam H, Rayner F, et al. Hepatitis B: report of prevalence and access to healthcare among Chinese residents in Sheffield UK. J Infect Public Health. 2013;6:448‐455. [DOI] [PubMed] [Google Scholar]
- 86. Veldhuijzen IK, Wolter R, Rijckborst V, et al. Identification and treatment of chronic hepatitis B in Chinese migrants: Results of a project offering on‐site testing in Rotterdam, the Netherlands. J Hepatol. 2012;57:1171‐1176. [DOI] [PubMed] [Google Scholar]
- 87. Williams S, Scholfield C, Nadarzynski T, Symonds Y, Kidsley S. Acceptability, uptake and impact of online home‐sampling for stis in Hampshire, UK: A service evaluation. Sex Transm Infect. 2017;93:A6. [Google Scholar]
- 88. Wood M, Elks R, Grobicki M. Outreach sexual infection screening and postal tests in men who have sex with men: How do they compare with clinic‐based screening? HIV Med. 2014;15:32. [DOI] [PubMed] [Google Scholar]
- 89. Zuure FR, Bouman J, Martens M, et al. Screening for hepatitis B and C in first‐generation Egyptian migrants living in the Netherlands. Liver Int. 2013;33:727‐738. [DOI] [PubMed] [Google Scholar]
- 90. Zuure FR, Davidovich U, Coutinho RA, et al. Using mass media and the internet as tools to diagnose hepatitis C infections in the general population. Am J Prevent Med. 2011;40:345‐352. [DOI] [PubMed] [Google Scholar]
- 91. Delarocque‐Astagneau E, Meffre C, Dubois F, et al. The impact of the prevention programme of hepatitis C over more than a decade: the French experience. J Viral Hepat. 2010;17:435‐443. [DOI] [PubMed] [Google Scholar]
- 92. Bechini A, Levi M, Falla A, et al. The role of the general practitioner in the screening and clinical management of chronic viral hepatitis in six EU countries. J Prevent Med Hyg. 2016;57:E51‐60. [PMC free article] [PubMed] [Google Scholar]
- 93. Brant LJ, Hurrelle M, Balogun MA, Klapper P, Ramsay ME. Where are people being tested for anti‐HCV in England? Results from sentinel laboratory surveillance. J Viral Hepat. 2008;15:729‐739. [DOI] [PubMed] [Google Scholar]
- 94. HEPscreen Toolkit. http://hepscreen.eu/. Accessed April 5, 2019. [Google Scholar]
- 95. World Health Organization . Guidelines on hepatitis B and C testing. Geneva: WHO; 2017. [Google Scholar]
- 96. European Centre for Disease Prevention and Control . Public health benefits of partner notification for sexually transmitted infections and HIV. Stockholm: ECDC; 2013. [Google Scholar]
- 97. World Health Organization . Guidelines on HIV self‐testing and partner notification. Supplement to consolidated guidelines on HIV testing services. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 98. European Centre for Disease Prevention and Control . Public health benefits of partner notification for sexually transmitted infections and HIV. Stockholm: ECDC; 2013. [Google Scholar]
- 99. Easterbrook PJ, Group WHOGD . Who to test and how to test for chronic hepatitis C infection ‐ 2016 WHO testing guidance for low‐ and middle‐income countries. J Hepatol. 2016;2016(65):S46‐66. [DOI] [PubMed] [Google Scholar]
- 100. Mena A, Moldes L, Meijide H, et al. Seroprevalence of HCV and HIV infections by year of birth in Spain: impact of US CDC and USPSTF recommendations for HCV and HIV testing. PLoS ONE. 2014;9:e113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chlibek R, Smetana J, Sosovickova R, et al. Prevalence of hepatitis C virus in adult population in the Czech Republic ‐ time for birth cohort screening. PLoS ONE. 2017;12:e0175525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walewska‐Zielecka B, Religioni U, Juszczyk G, et al. Anti‐hepatitis C virus seroprevalence in the working age population in Poland, 2004 to 2014. Euro Surveill. 2017;22(2):30441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Deuffic‐Burban S, Huneau A, Verleene A, et al. Assessing the cost‐effectiveness of hepatitis C screening strategies in France. J Hepatol. 2018;69:785‐792. [DOI] [PubMed] [Google Scholar]
- 104. European Centre for Disease Prevention and Control . HIV testing ‐ Monitoring implementation of the Dublin Declaration on Partnership to fight HIV/AIDS in Europe and Central Asia: 2017 progress report. Stockholm: ECDC; 2017. [Google Scholar]
- 105. Johnson CC, Kennedy C, Fonner V, et al. Examining the effects of HIV self‐testing compared to standard HIV testing services: a systematic review and meta‐analysis. J Int AIDS Soc. 2017;20:21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hepatitis B and C testing: Why? Who? How? A guidance paper on testing in community and harm reduction settings. Correlation network 2016. Amsterdam. 2016. [Google Scholar]
- 107. Cochrane A, Collins P, Horwood JP. Barriers and opportunities for hepatitis B testing and contact tracing in a UK Somali population: a qualitative study. Eur J Public Health. 2016;26:389‐395. [DOI] [PubMed] [Google Scholar]
- 108. Harris M, Ward E, Gore C. Finding the undiagnosed: a qualitative exploration of hepatitis C diagnosis delay in the United Kingdom. J Viral Hepat. 2016;23:479‐486. [DOI] [PubMed] [Google Scholar]
- 109. Seedat F, Hargreaves S, Friedland JS. Engaging new migrants in infectious disease screening: a qualitative semi‐structured interview study of UK migrant community health‐care leads. PLoS ONE. 2014;9:e108261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sweeney L, Owiti JA, Beharry A, et al. Informing the design of a national screening and treatment programme for chronic viral hepatitis in primary care: qualitative study of at‐risk immigrant communities and healthcare professionals. BMC Health Serv Res. 2015;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van der Veen YJJ, de Zwart O, Voeten HA, et al. Hepatitis B screening in the Turkish‐Dutch population in Rotterdam, the Netherlands; Qualitative assessment of socio‐cultural determinants. BMC Public Health. 2009;9:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zuure FR, Heijman T, Urbanus AT, Prins M, Kok G, Davidovich U. Reasons for compliance or noncompliance with advice to test for hepatitis C via an internet‐mediated blood screening service: a qualitative study. BMC Public Health. 2011;11:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Datta S, Horwood J, Hickman M, Sharp D. Case‐finding for hepatitis C in primary care: a mixed‐methods service evaluation. Br J Gen Pract. 2014;64:e67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


