Abstract
The capacity of coral reefs to maintain their structurally complex frameworks and to retain the potential for vertical accretion is vitally important to the persistence of their ecological functioning and the ecosystem services they sustain. However, datasets to support detailed along‐coast assessments of framework production rates and accretion potential do not presently exist. Here, we estimate, based on gross bioaccretion and bioerosion measures, the carbonate budgets and resultant estimated accretion rates (EAR) of the shallow reef zone of leeward Bonaire – between 5 and 12 m depth – at unique fine spatial resolution along this coast (115 sites). Whilst the fringing reef of Bonaire is often reported to be in a better ecological condition than most sites throughout the wider Caribbean region, our data show that the carbonate budgets of the reefs and derived EAR varied considerably across this ~58 km long fringing reef complex. Some areas, in particular the marine reserves, were indeed still dominated by structurally complex coral communities with high net carbonate production (>10 kg CaCO3 m−2 year−1), high live coral cover and complex structural topography. The majority of the studied sites, however, were defined by relatively low budget states (<2 kg CaCO3 m−2 year−1) or were in a state of net erosion. These data highlight the marked spatial heterogeneity that can occur in budget states, and thus in reef accretion potential, even between quite closely spaced areas of individual reef complexes. This heterogeneity is linked strongly to the degree of localized land‐based impacts along the coast, and resultant differences in the abundance of reef framework building coral species. The major impact of this variability is that those sections of reef defined by low‐accretion rates will have limited capacity to maintain their structural integrity and to keep pace with current projections of climate change induced sea‐level rise (SLR), thus posing a threat to reef functioning and biodiversity, potentially leading to trophic cascades. Since many Caribbean reefs are more severely degraded than those found around Bonaire, it is to be expected that the findings presented here are rather the rule than the exception, but the study also highlights the need for similar high spatial resolution (along‐coast) assessments of budget states and accretion rates to meaningfully explore increasing coastal risk at the country level. The findings also more generally underline the significance of reducing local anthropogenic disturbance and restoring framework building coral assemblages. Appropriately focussed local preservation efforts may aid in averting future large‐scale above reef water depth increases on Caribbean coral reefs and will limit the social and economic implications associated with the loss of reef goods and services.
Keywords: Acropora cervicornis, bioerosion, Bonaire, calcification, carbonate budget, Caribbean, climate change, sea‐level rise
This elaborate asset of net accretion rates of the shallow (5 and 10 m depth) leeward coral reefs of Bonaire shows extreme spatial heterogeneity among 115 sites (points). The majority of the fringing reef – which allegedly ranks among the healthiest in the Caribbean – is, however, defined by a limited capacity to maintain structural integrity or to keep pace with projections of sea‐level rise (RCP lines), thus posing a threat to ecological functioning, biodiversity and the ecosystem services it sustains. These observations suggest that only protection and restoration of framework building coral assemblages may avert future large‐scale submergence of Caribbean reefs.

1. INTRODUCTION
In many ecosystems, bioengineered structural habitat complexity (e.g. by corals, trees, grasses or kelp) is a vital component promoting biodiversity by offering more potential niches and facilitating complex ecosystem functioning (Bruno & Bertness, 2001; Estes & Palmisano, 1974; MacArthur & MacArthur, 1961; Risk, 1972). On tropical reefs, the calcium carbonate skeleton of corals provides such architectural heterogeneity (Done, Ogden, & Wiebe, 1996). Continuous accumulation of large volumes of CaCO3 thus underpins the biological success of coral reef ecosystems with regard to such functioning (De Goeij et al., 2013; Graham & Nash, 2013; Kennedy et al., 2013; Perry & Alvarez‐Filip, 2018) and by supporting an extraordinarily rich diversity of reef fish and other reef biota (Connell, 1978; Luckhorst & Luckhorst, 1978; Newman et al., 2015). In addition to this biological advantage, the rigid reef framework facilitates numerous ecosystem goods and services associated with nourishment (i.e. fish proteins), tourism and coastal risk reduction (Moberg & Folke, 1999). By diminishing wave energy, shallow‐water topographically complex reefs have the capacity to act as a natural breakwater, protecting the shoreline to a great extent against erosion and frequent flooding of shores (Lugo‐Fernández, Roberts, & Suhayda, 1998; Sheppard, Dixon, Gourlay, Sheppard, & Payet, 2005). These collective ecosystem services, the maintenance of which are strongly influenced by reef limestone production rates and accretion potential, have allowed human populations to settle and develop tropical coastal areas and are essential concerning current projections of global environmental change (Beetham, Kench, & Popinet, 2017; Ferrario et al., 2014; Harris et al., 2018). However, climate‐driven stressors, such as bleaching, as well as increasing rates of sea‐level rise (SLR), are likely to negatively impact reef communities, and thus represent a direct threat to many tropical coastal areas (Kench, Ford, & Owen, 2018; Storlazzi, Elias, & Berkowitz, 2015). Worldwide, many reefs are currently struggling to maintain net positive carbonate budgets due to environmental disturbances (Eakin, 1996; Perry, Spencer, & Kench, 2008; Perry et al., 2018; Van Heuven et al., 2018), and the combined impacts of resultant reduced vertical growth potential and SLR are increasing the likelihood of increasing water depths above reefs, and thus wave propagation across reefs (Perry et al., 2018).
Such changes have been driven, over the past few decades, by a multitude of global and local stressors associated with human population expansion including warming sea water, ocean acidification, reduced water quality, loss of herbivores and destructive pathogens (Fabricius, De'ath, McCook, Turak, & Williams, 2005; Hughes, 1994; Hughes et al., 2003; Jackson, 2001). The impact of these rapidly changing marine environmental conditions is arguably most evident in the Caribbean, where coral cover has declined substantially on the majority of the coral reefs, largely as a result of thermal stress (i.e. bleaching) and diseases (Bak, Nieuwland, & Meesters, 2005; De Bakker et al., 2017; De'ath, Fabricius, Sweatman, & Puotinen, 2012; Gardner, Côté, Gill, Grant, & Watkinson, 2003; Jackson, Donovan, Cramer, & Lam, 2014). Moreover, the configuration of the coral assemblage on these reefs has generally shifted from historically dominant framework building species (Acropora spp., Orbicella spp.) towards assemblages dominated by small opportunistic species (De Bakker, Meesters, Bak, Nieuwland, & Duyl, 2016; Green, Edmunds, & Carpenter, 2008; Knowlton, 2001; Pandolfi & Jackson, 2006). Recent research efforts have recognized the added value of regarding species‐specific life‐history attributes such as accretion rates and colony morphology when assessing the functional and ecological status of coral reefs as opposed to using coral cover as a sole metric (e.g. González‐Barrios & Álvarez‐Filip, 2018; Perry & Alvarez‐Filip, 2018). Most opportunistic coral species are relatively stress tolerant, yet they lack the capacity to maintain the structural framework necessary to support ecological functioning and biodiversity (Alvarez‐Filip, Carricart‐Ganivet, Horta‐Puga, & Iglesias‐Prieto, 2013; De Bakker et al., 2016; Edinger & Risk, 2000; Mumby et al., 2008; Perry, Murphy, et al., 2015). The unprecedented decline in abundance and cover of calcifying organisms appears to be paralleled by an increase in abundance and activity of biological eroders, which actively break down and weaken the carbonate framework of coral reef (Schönberg, Fang, Carreiro‐Silva, Tribollet, & Wisshak, 2017). Multiple endolithic macroborers and microborers, including excavating sponges, worms and bivalves, appear to thrive in warmer, more eutrophic waters and benefit from the increased availability of substratum following coral mortality (Achlatis et al., 2017; Glynn, 1997; Perry et al., 2014; Schönberg, Fang, & Carballo, 2017; Silbiger et al., 2018; Webb et al., 2017). Such changes in reef configurations have already upset the carbonate balance on many coral reefs, regularly tipping the scale towards net erosion of the reef framework (Glynn, 1997; Perry et al., 2018). The complex carbonate framework of these reefs is disappearing and the height of the reef canopy is gradually reduced (i.e. reef flattening is occurring; sensu Alvarez‐Filip, Dulvy, Gill, Côté, & Watkinson, 2009), changes that may jeopardize reef functionality (Perry & Alvarez‐Filip, 2018).
Whilst widespread ecological changes on reefs are thus evident, it is also reasonable to hypothesize that variations in the ability of coral reefs to maintain vertical accretion and the capacity to keep pace with SLR will actually vary strongly depending on local attributes such as the composition of the coral assemblage, hydrodynamics, coastal morphology, reef‐fronted anthropogenic disturbance and implemented conservation measures (e.g. marine‐protected areas) (Januchowski‐Hartley, Graham, Wilson, Jennings, & Perry, 2017; Perry et al., 2013; Perry, Steneck, et al., 2015). Perry et al. (2013) quantified estimated the reef accretion rate at sites throughout the Western Atlantic and Indian Ocean, demonstrating large regional variations. However, this assessment was based on data from a few discrete sites in each country and our understanding of within‐country variability between reefs or along continuous sections of coastline is very limited, despite this level of data really being necessary to inform management planning and future change projections. In this study, we provide such a high spatial resolution assessment using field data collected from the leeward fringing reefs of Bonaire and Klein Bonaire (southern Caribbean) with the aim of defining the current growth rates of the 5–10 m depth fore‐reef slope habitat. Bonaire's reefs are considered to be amongst the healthiest reefs in the Caribbean relative to historic baselines (Jackson et al., 2014) and available estimates suggest that the reefs around Bonaire, principally on the leeward side, rank among the fastest accreting reefs throughout the wider Caribbean region (Perry et al., 2018). The leeward reefs of Bonaire are, however, known to display a wide range of ecological degradation from almost pristine configurations to reefs that are approaching full functional collapse (De Bakker et al., 2017; Jackson et al., 2014; Steneck, Arnold, & Rasher, 2013). To assess the implications of these variations, we quantified the carbonate budgets and resultant accretion rates of reefs across the shallow leeward reef (~58 km long), and then consider these in the context of spatial variations in implemented conservation measures and anthropogenic activity. The reef‐fronted coastline of Bonaire has several centres of high anthropogenic activity which include the capital (Kralendijk), salt pans in the south and an oil storage facility in the north. Contrastingly, the national marine park surrounding Bonaire, which was established in 1979, includes two no‐dive reserves where entrance by humans is strictly prohibited. It is predicted that the ecological status of a reef site is strongly affected by such variation in the degree of local human impact.
2. MATERIALS AND METHODS
2.1. Study site and data collection
Dedicated surveys to quantify net coral reef carbonate production were carried out on the shallow reefs of the southern Caribbean island of Bonaire (12°9′N, 68°16′W) (Figure 2c). Survey efforts were confined to the leeward side of the island and conducted between September and December 2017. Substantial reef formations are concentrated on this side of the island and there is also a strong gradient in terms of the degree of direct human activity. The general reef profile on the leeward side comprises a terrace (including a lower terrace zone (LT) at ~3 to 7 m depth) that gradually slopes from the shore to the upper drop‐off zone (DO) at approximately 10–15 m depth, after which the reef steeply runs down (usual angle between 20° and 50°) to a depth of 25–50 m towards a second terrace (Bak, 1977; Van Duyl, 1985). Net coral reef carbonate production (in G, where G = kg CaCO3 m−2 year−1) was determined from 115 sites (including the island of Klein Bonaire), separated by a predefined distance of ~500 m and covering the majority of the leeward reef stretch (Figure 2a). Paired transect lines (25 m) at each site were placed in the two shallow zones (LT and DO) positioned in series (starting points 10 m apart) and parallel to the orientation of the reef front (Hill & Wilkinson, 2004).
Figure 2.
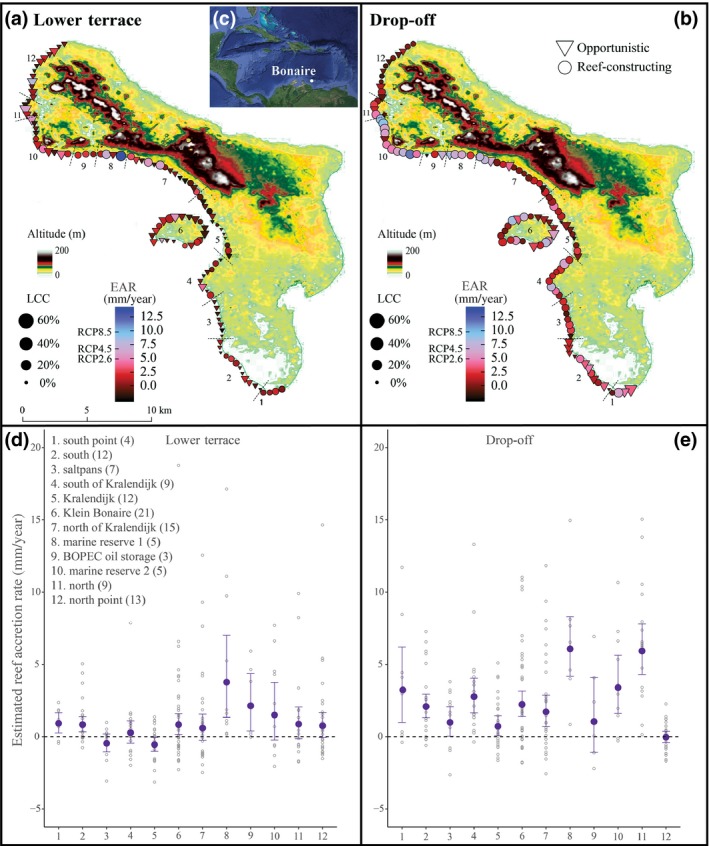
Reef accretion rates of the shallow leeward fringing reef of Bonaire. Top panels (a,b) show the location and the mean reef accretion (EAR in mm/year) of the surveyed sites (n = 115) at each individual site and within each reef zone (lower terrace; drop‐off). EAR is displayed by means of a colour‐coded scheme. IPCC projections for sea‐level rise (RCP 2.6, RCP 4.5, and RCP 8.5) are included on the colour scale as well (in mm/year). Symbol size reflects mean live coral cover (LCC), shape indicates whether a community is dominated (>50% cover) by framework builders or opportunistic coral species. Bottom panels (d,e) show EAR by subregion (1–12), number of sites per subregion is given in brackets. Subregions are defined in the top panels and separated by dotted line insets. Blue points indicate mean EAR, error bars define the 95% confidence interval. Open circles represent EAR on a transect level. The dotted horizontal line indicates the point where EAR equals zero. Altitude maps are adapted from Mücher et al. (2017) [ASTER Global Digital Elevation Model Version 2]. The location of the town of Kralendijk and Klein Bonaire is indicated on the map. Inset in the top left panel (c) specifies the position of Bonaire in the southern Caribbean Sea
2.2. Assessment of net carbonate production
Data to determine gross and net carbonate production and erosion were collected following procedures as outlined by Perry et al. (2012) in the ReefBudget methodology. Some minor adaptations were implemented, which we expand on below. In the ReefBudget approach, net coral reef carbonate production is estimated as the cumulative CaCO3 produced by coral and calcifying algae minus the chemical dissolution and mechanical removal of CaCO3 through bioeroding activities. Bioeroding organisms include parrotfish, sea urchins, boring macrofauna and microborers. The percentage cover of all biotic and abiotic groups was measured to the nearest cm directly below the transect line and following the full contour of the reef. To avoid too much spatial correlation between transects, the two benthic transects per depth at each site were positioned at the end of the two transect lines (from 15 to 25 m), separating them by a distance of 40 m. Scleractinian corals were visually identified to species level. The difference between the contour‐following length and the horizontal main transect line (10 m) was used to approximate reef rugosity (Risk, 1972). As a second complexity measure, the maximum reef height, measured from the bottom, was determined every 2 m (within 2 m of the transect line), yielding six values for reef elevation for each main 10 m benthic transect.
Coral CaCO3 production was calculated based on the measured cover for each species and species‐specific growth rates and skeletal density. Because growth rates and skeletal density vary with depth, different constants were applied for the two reef zones (ReefBudget, Perry et al., 2012 and references therein). The sum of the production rates for each encountered colony of a certain species resulted in a gross coral carbonate production (kg m−2 year−1) per transect. Carbonate production by crustose coralline algae (CCA) was calculated using cover and a standard calcification rate derived from published Caribbean data (Perry et al., 2012 and references therein). Total carbonate removed by parrotfish was estimated based on the erosion potential of each individual fish encountered within a 5 m wide belt following the main 25 m transect lines. Individual erosion was calculated using fish biomass (derived from fork length), life stage (juvenile, initial, terminal) bite rate, ratio of bites leaving scars and mass eroded per bite. The sum of erosion by all observed fish resulted in an estimate of gross carbonate removal by parrotfish. Parrotfish erosion was set to zero when no hard substratum was available within the transect (i.e. only sand). The contribution of sponges to gross bioerosion was determined using species‐specific erosion rates for chemical and mechanical erosion per unit of infested substrate as defined by De Bakker et al. (2018). Cover for all excavating sponges was measured within 50 cm on both sides of the transect line (10 m2 in total). The impacts of urchins and microborers were determined as described (Perry et al., 2012). Final estimates for net coral reef carbonate production for each transect were then converted to an estimated accretion rate (EAR in mm/year) following Perry, Steneck, et al. (2015) and Perry et al. (2013), by applying corrections for sediment infill (25% of the sediment produced by parrotfish and 50% of the sediment produced by other bioeroding organisms) and framework porosity (30% for head and massive coral dominated assemblages, 70% for branched and tabular dominated assemblages and 50% for mixed coral assemblages). We also included an estimate for natural framework export through background (non event‐driven) physical processes (20% for sheltered sites and 50% for exposed sites). EAR was subsequently assessed against various projections of SLR (2018 IPCC report Representative Concentration Pathway scenarios RCP2.6, 4.5 and 8.5). Mean EAR was defined for each a priori‐defined subregion of the leeward fringing reef of Bonaire. Subregions were defined based on the distance to major centres of local anthropogenic stress, being Kralendijk, the BOPEC oil storage facility and the salt pans and lack of stress being sites in both marine reserves (Figure 2).
To elucidate any observed heterogeneity in EAR, we assessed the effect of various local geomorphological and exposure variables (coastal height [m], terrace width [m] and wind exposure [J m3]). In order to express possible anthropogenic impact on EAR, a variable was defined which ranges from minimal disturbance to extreme disturbance and was based on human coastal activity, distance to outflow of land‐based water sources, diving activity, exposure and implemented conservation strategy at each individual site (for an overview of the impact factors on each site, see Table S1). The relation between EAR and the various local environmental factors was modelled by means of regression analysis and analysis of covariance. Assumptions for linear regression were confirmed visually (Zuur, Ieno, & Smith, 2007). All descriptive data were fourth‐root transformed prior to analyses. Data on the environmental parameters terrace width and wind exposure were acquired from van Duyl (1985) and the FORCE project (http://webgis.force-project.eu/forcewebgis.html) respectively. Estimates for coastal height were derived from digital surface model (DSM, ALOS World 3D) captured by the National Aeronautics and Space Administration (NASA).
2.3. Reef accretion rates versus sea‐level rise
Generalized additive modelling (GAM) was applied to predict the minimum coral cover needed to support a net reef accretion rate able to cope with the various projections for rising sea levels. The GAM used a Gaussian error distribution, log‐transformed EAR values and fourth‐root transformed coral cover. A model including both zones separately explained less deviance (74.3% compared to 76.4% for the model combining both zones) and individually fitted responses were virtually identical (ANOVA, F = 5.52, p = .72). Inclusion of a separation between coral assemblages dominated by framework building or opportunistic coral species (classification sensu De Bakker et al., 2016), however, significantly improved the model (80% deviance explained compared to 76.4% for the model without assemblage separation; ANOVA, F = 4.6, p < .001). Exploration of the model residuals with variogram plots indicated that there was no need to account for spatial autocorrelation. Data processing and statistical analyses were conducted in the R programming environment v3.4.1 (R Core Team, 2013) using the following packages: mgcv (Wood, 2012) and stats (R Core Team, 2002). Since the aim of this study was to determine the reef accretion rates for the entire leeward reef and larger stretches of reef and not for individual sites, four transects were assessed per site (two in each zone) instead of six as proposed in the ReefBudget methodology. As such, the statistical power of the analyses lies not in the number of transects per site, but in the total number of surveyed reef sites.
3. RESULTS
Mean net carbonate production along the leeward coast of Bonaire was 0.22 G (95% CI: −0.24 to 0.72), on the LT and 1.99 G (95% CI: 1.41 to 2.60) and in the DO zone (Figure 1b,f). Considerable variation was observed across sites, with G ranging from −5.72 to 21.12 (DO) and −5.98 to 26.48 (LT) on a transect level (Table S2). Almost 50% (n = 113) of all transects in the LT zone had a negative carbonate budget. In the DO, this was 32% (n = 73). Eight LT transects had 100% cover of sand. In contrast, 23 DO and 12 LT transects had a net production higher than 10 G. In four of these LT transects, A. cervicornis colonies accounted for the majority of the carbonate production (87%–93%). In virtually all DO sites with a production greater than 5 kg m−2 year−1, Orbicella spp. contributed on average one‐third to gross production. Evaluation of the individual budget constituents (Table S2) reveals that at present, visible CCA contribute marginally (on average <1%) to the gross carbonate production on the leeward reefs of Bonaire. CaCO3 production by CCA, which largely depends on their presence, was found to be markedly low on disturbed reefs. It is unsurprising that overall the bulk of CaCO3 is produced by corals. Parrotfish were the main eroders of carbonate structure on these reefs (responsible for at least 50% of gross erosion in 88% of all transects). The contribution of sponge erosion to total erosion was 4.3% (95% CI: 3.6–5.0) and appeared to increase with increasing human disturbance, with a mean contribution of 7.0%, (95% CI: 4.7–10.1) at extremely impacted sites compared to 2.0% (95% CI: 0.9–4.1) at minimal‐impacted sites (see Table S2). Micro‐bioerosion accounted on average for 7.3% (95% CI: 0.9–4.1) of total erosion largely depending on the availability of substratum (F = 44.9, p < .001). Erosion by sea urchins was negligible along the entire shallow reef as densities were extremely low (on average 0.08 urchins m2).
Figure 1.

Reef characteristics by subregion. Main descriptive reef characteristics related to reef accretion for each subregion (1:12, see Figure 2 for the location of each region). Points represent the mean of all transects within each cluster, segments define the 95% confidence limits. Mean coral cover (a, e) shows the percentage of the total three‐dimensional transect length covered by live coral (including Millepora spp.). Net carbonate (b, f) production (gross production minus gross erosion), given in G (kg CaCO3 m−2 year−1). Measures for rugosity (c, g) are based on the chain and link technique (Risk, 1972). Mean reef height (d, h) from the bottom (in cm) was collected every 2 m along each transect line. Overall means for each zone are given as ‘Mean’ within each panel
Comparing general reef characteristics across subregions and zones (LT and DO) revealed considerable variation across the leeward fringing reef of Bonaire (Figure 1; Table S3). Within both reef zones, and particularly in the DO zone (Figure 1e–h), reefs are overall markedly well developed in the northern region of Bonaire. High coral cover (>25%) and architectural complexity (R > 1.8) generally characterize reefs within the drop‐off zone of this region, with the exception of the highly exposed north point sites (subregion 12) and multiple sites in front of the BOPEC oil storage facility. The relatively well‐developed reefs north of Kralendijk and at the south point (subregion 1) of the island also generally have low sand cover (Table S3). Highest net carbonate production was found within Reserve 1 (Figures 1b and 2a,b). Here, mean net production was 10.94 G (95% CI: 7.22–15.94) and 13.56 G (95% CI: 10.38–17.41) in the LT and drop‐off zone respectively. This could largely be attributed to a still considerable mean coral cover of 25% (LT) and 30% (DO) (Figure 1a,e). The coral community in the no‐dive reserve was dominated by Orbicella spp. colonies that have created extraordinarily rugose reefs in both zones. Rugosity within the reserve resulted in a surface enlargement of 32%–66% (LT) and 95%–133% (DO) (Figure 1c,g), with a mean reef elevation from the bottom of 24.4 and 104.1 cm respectively (Figure 1d,h). Such reef characteristics were also found, although to a lesser extent, in Reserve 2 (Figure 1). Reefs on the southern side of Bonaire were generally characterized by the absence of well‐developed structural complexity, generally low live coral cover, little hard substratum and substantial sandy areas (Tables S2 and S3). In these subregions, many sites are currently already in a state of net erosion or are defined by a net budget state of less than 2 G (Figure 1b,f). This is particularly evident for the reef in front of Kralendijk and in front of the saltpans where almost all LT sites are net eroding. Reefs in the northern most subregion (top north) were defined by low‐complexity reefs with minimal net production; however, in contrast to the southern clusters, these more exposed reefs had substantially fewer sandy patches (Tables S2 and S3).
The estimated mean reef accretion (EAR) of the shallow reef was 1.33 mm/year (95% CI: 1.09–1.58), but showed remarkable variation among zones and sites (Figure 2). Mean EAR for the LT zone was 0.67 mm/year (95% CI: 0.38–0.97) and was substantially lower than in the DO zone (2.05 mm/year, 95% CI: 1.67–2.45). The vast majority of the LT sites had an EAR considerably below the most conservative SLR estimates (RCP2.6). Vertical accretion were found to be lowest on the extremely disturbed LT subregions – Kralendijk and Salt pans – although the entire southern and central sections of the LT zone could be characterized as only marginally growing (Figures 2d and 4). In both reef zones, relatively high EAR was observed in subregion 1 and the area ranging from halfway between subregion 7 to subregion 11 (Figure 2). Relatively high accretion rates were found for several reef sites on Klein Bonaire as well. On the LT – the zone more prominently involved in reducing wave energy – only nine sites have maintained a reef accretion rate capable of tracking the most optimistic projections for SLR (RCP 2.6: 4.9 mm SLR per year), four sites have rates that would currently track the RCP 4.5 (6.9 mm SLR per year) rate and only two sites the RCP 8.5 (9.4 mm SLR per year) rate (Figure 4). For the DO zone, these relationships are 23, 12, and 1 sites, respectively. Highest EAR was found in marine reserve 1 and was estimated to be 6.07 mm/year (95% CI: 4.19–8.31) for the DO and 3.77 mm/year (95% CI: 1.33–7.01) for the LT (Figure 2d,e).
Figure 4.
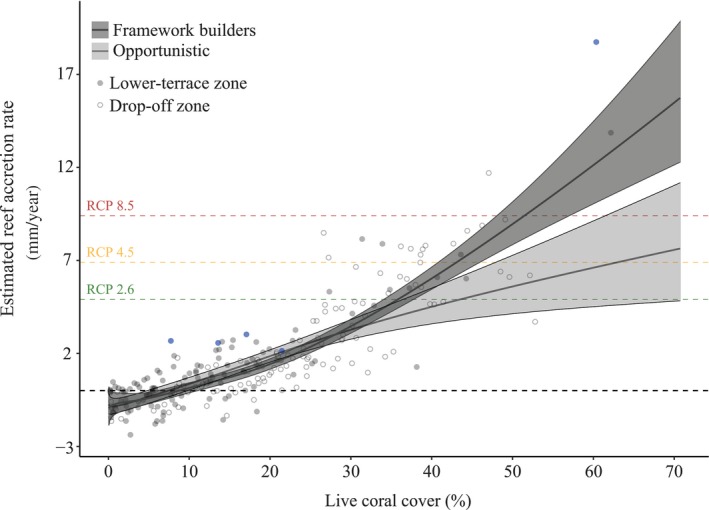
Relation between estimates accretion rate (EAR) and the percentage of live coral cover on the shallow reef of Bonaire. The black curve represents the fitted response (EAR) versus live coral cover for coral assemblages dominated by framework builders (dark grey) or dominated by opportunistic coral species (light grey). Points represent the measured means for each site (lower terrace sites as solid circles, drop‐off sites as open circles). Grey bands represent the predicted 95% confidence interval. The horizontal black dashed line represents an EAR of zero. The point where this line intercepts the fitted curve can tentatively be regarded as the approximate minimum required coral cover percentage to maintain the current depth elevation of the reef framework. Coloured dashed lines indicate the estimated accretion rates necessary to match IPCC‐projected sea‐level rise scenarios for Bonaire: RCP 2.6 (green, 4.9 mm SLR per year), RCP 4.5 (yellow, 6.9 mm SLR per year) and RCP 8.5 (red, 9.4 mm SLR per year; Slangen et al., 2014). Blue points indicate sites containing Acropora cervicornis stands (all lower terrace)
The capacity of reefs to achieve vertical accretion showed a strong correlation with the degree of anthropogenic disturbance (Figure 3, F = 14.8, p < .001). Whilst considerable heterogeneity exists among transects, in particular at sites with low to moderate anthropogenic disturbance, lowest accretion rates were observed in sites exposed to extreme human activity, that is, at Kralendijk, the salt pans, oil storage and point courses of terrestrial water input (Figure 3). In contrast, sites with minimal negative human interference (marine reserves) displayed much higher EAR, particularly in the LT zone (Figure 3). None of the environmental variables, that is, coastal height, wind exposure and terrace width, displayed significant correlation to the capacity of reefs to achieve vertical accretion if not in combination with anthropogenic impact (Linear regression, p > .05).
Figure 3.
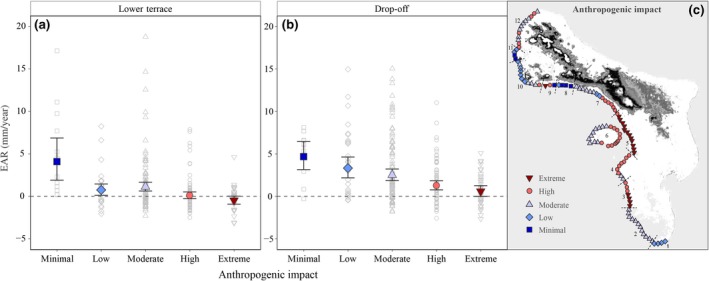
Relation between the degree of anthropogenic disturbance and estimated reef accretion. The degree of anthropogenic impact is classified from minimal to extreme (colours and symbols). Left and middle panels (a, b) display mean EAR (mm/year) per impact level (large coloured symbols) for both reef zones, error bars indicate the 95% confidence intervals. Grey open symbols indicate EAR for the individual transects. Right panel (c) shows the map of Bonaire with the degree of disturbance for each site given in colours and symbols corresponding to those of the other two panels. Dotted lines and numbers (1–12) define the coastal subregions
3.1. EAR and sea‐level rise
The fitted response curves indicate that the threshold for live coral cover at which EAR on the shallow leeward reef of Bonaire (LT and DO) becomes negative lies at approximately 7.4% cover for coral assemblages dominated by opportunistic species and 8.7% cover for framework building assemblages (Figure 4). Below this level, depth of the water column overlying the reef will start to increase with time even without taking into account SLR and the effects of extreme events that might increase physical framework loss rates. The model also suggests that the coral communities dominated by framework building corals need ~36% coral cover for these fore‐reef habitats to maintain their present depth configurations under the most conservative SLR prediction for Bonaire (RCP 2.6). Coral cover would have to be roughly 43.5% (RCP 4.5) or 51% (RCP 8.5) to follow more pessimistic, but likely more realistic, SLR projections (Figure 4). The cover estimates for sites where the majority of the corals species are opportunistic lies substantially higher at 44%, 63% and 90% cover respectively. 97% of the transects with an EAR above the RCP 2.6 level are found north of Kralendijk between subregions 7 and 11, South point (subregion 1) and Klein Bonaire (subregion 6; Figure 2a,b). Several of the few LT sites that ranged above RCP 2.6 projections had such EAR values largely due to the presence of A. cervicornis stands (Figure 4, blue points). All LT sites with A. cervicornis cover had a positive EAR, even with relatively low coral cover (Figure 4). Whilst currently LT reefs are mainly dominated by sand or turf covered substratum (Table S2), with some small opportunistic coral colonies, these sites were formerly characterized by extensive fields of highly productive A. cervicornis (Figure 5a).
Figure 5.
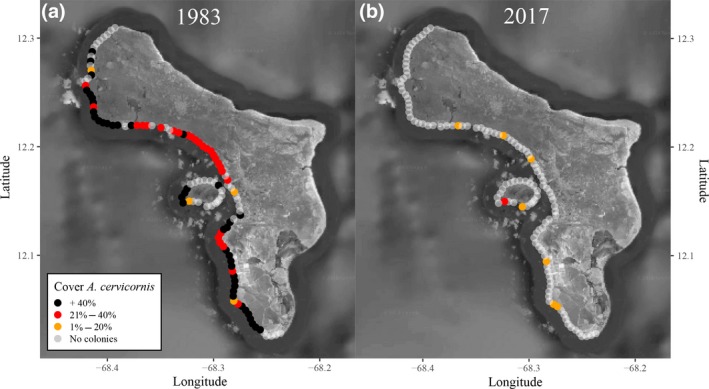
Decline in Acropora cervicornis since the 1980s. Estimated percentage cover of A. cervicornis along the leeward coast of Bonaire and Klein Bonaire based on data collected between 1981 and 1983 (a) (Van Duyl, 1985) and in 2017 (b) (this study). Dots show the 115 sites as surveyed in 2017 (this study), colours represent ranges in percentage cover of the total benthic community. Source of map: Google Earth v7.3
4. DISCUSSION
The majority of the shallow reef of Bonaire is at present hardly growing, and in many places, our data suggest that it is in a net erosional phase. Current estimates of carbonate production also show that only a few sections of the leeward fringing reef appear to have the accretion capacity to maintain their present depth configurations under projections of future SLR. Furthermore, our high spatial resolution datasets show that observed patterns of heterogeneity in estimated reef accretion rate (EAR) can be largely attributed to variations in the degree of terrestrial impact on the reefs. At sites that experience high impact from land‐based activity (e.g. Kralendijk, salt pans, sites close to water inlets), the historically low rates of gross carbonate production are strongly linked to the substantial loss in coral cover that has occurred over the past decades (Bak et al., 2005; De Bakker et al., 2017; Sommer, Harrison, Brooks, & Scheffers, 2011; this study). Bonaire's coral reefs are generally reported to have experienced less ecological decline in relation to other Caribbean reefs (Jackson et al., 2014; Kramer, 2003; Steneck et al., 2013, but see De Bakker et al., 2016, 2017). However, mean live coral cover was generally low in the LT zone (9.5%) and reasonable in the drop‐off zone (21%). On the entire shallow reef, stony corals covered, on average, only 12% of the substratum. This is in range with, but slightly below, the Caribbean average of approximately 16% (Jackson et al., 2014) and many sites close to point sources of human disturbance around Bonaire have low accretion rates. In contrast, sites that have experienced little local anthropogenic impact, such as those within the marine reserves, retain accretion rates close to SLR projections under the different RCP scenarios. These sites that have maintained higher accretion rates, albeit being only few in number, were defined by remarkable high live coral cover (Figures 1 and 2; Table S2), with the coral community at these sites dominated by large framework building species such as Orbicella spp. or Acropora spp. Relating EAR to projected trends of SLR indicated that high cover of coral alone can be a misleading predictor (Figure 4). Whilst more opportunistic coral species that have life‐history traits that may cope better with environmental stress may still achieve appreciable cover, they contribute less obviously to vertical reef accretion due to a limited carbonate production per unit area covered (Alvarez‐Filip et al., 2013; De Bakker et al., 2016). Sites at which the coral assemblage is dominated by opportunistic corals will thus require a considerably higher cover to sustain accretion rates in line with most SLR projections.
Some structurally complex DO reefs dominated by framework building Orbicella spp. do still exist around Bonaire, mostly in the northern region (subregions 8–11). These reefs were characterized by high cover of stony corals and are comparable to the relatively ‘good’ reefs as described by Steneck et al. (2013) for Bonaire. Several of these near‐pristine Orbicella reefs are found to have vertical accretion rates that can track the more pessimistic SLR projections (Figure 4). These reefs were mostly found north of Kralendijk (subregions 8–11), a region – with the exception of diver activity – characterized by relatively little local human disturbance (Bonaire National Marine Park Management Plan 2006). Particularly high levels of gross carbonate production are found in the marine reserve, where entrance by humans is prohibited (subregion 8). Arguably, the marine reserve is located in a region with generally favourable conditions, yet accretion rates, live coral cover and rugosity were higher in the reserve compared to the directly adjacent reef sites. Although well‐developed reefs were an exception rather than rule, their existence does indicate the potential of these reefs to continue to prevail under the currently unfavourable global environmental conditions when harmful local anthropogenic interference is minimized. Conversely, the reefs in front of Kralendijk, and explicitly the LT, receive extensive direct impact through a variety of anthropogenic stressors including run‐off, sedimentation and sewage drainage. The continuous detrimental impact of this centre of human activity has led to a near‐complete functional collapse of the reef system in this area, most notably on the LT. These findings underline the importance of understanding heterogeneity in local conditions and support the claim (e.g. Knowlton & Jackson, 2008) that locally focussed conservation efforts, including the restoration of framework building coral assemblages with high growth potential, will be essential to ensure reef functioning and net accretion rates that match future projections of SLR.
Currently, the average estimated net CaCO3 production on the shallow reef habitat of Bonaire is 1.1 kg m2 year−1. This is below previous (1970s and 1980s) estimates on Caribbean reefs (Scoffin, 1980; Stearn, Scoffin, & Martindale, 1977), but comparable to some more recent estimates from sites around Florida and the Greater Antilles (Perry et al., 2018, 2013). In pilot work, Perry and colleagues found the average production rates across a few Bonaire reef sites to range from −1.74 to 15.25 G on the LT and 2.62 to 14.46 G in the DO zone. These rates are comparable to our findings, but our data provide a unique insight into how these rates (and the processes driving them) vary along the coast, and how these are correlated to local disturbance pressures. In this study, this understanding is also extended to estimates of reef accretion, which show that the lowest accretion rates were found on the southern reefs of Bonaire and around the densely populated capital (Kralendijk), a region that has received less attention than in previous studies. The observed heterogeneity in carbonate production and estimated accretion rate across the 115 sites is thus striking. Carbonate production defined at such high spatial resolution, varying from near‐pristine sites approaching pre‐industrial accretion rates to sites with considerable net erosion rates, reveals that transect placement or the inclusion of too few sites can strongly affect the outcome of survey studies. Indeed, insufficient sampling density could easily lead to incorrect conclusions about the state of a reef, which in turn can result in inadequate management efforts.
Overall, mean vertical accretion (EAR) for the shallow reef of Bonaire estimated to be 1.33 mm/year, and considerably lower for the LT (0.67 mm/year) in comparison to the drop‐off zone (2.01 mm/year). Mean rates in both zones were thus well below even the most optimistic projections for average mean SLR (~4.9 mm/year, source: IPCC 2018, https://www.ipcc.ch/). The latter implies that the majority (~73%) of the fore‐reef habitats on the Bonaire reefs will not be able to accrete vertically at the same rate at SLR. As reef accretion for a given depth zone lags behind SLR, the depth of the water body overlaying that area of the reef will gradually increase (Van Woesik, Golbuu, & Roff, 2015). Whilst the immediate impacts of such changes across the 5–10 m deep fore‐reef sites studied here will be less severe for coastal protection functions we predict, based on reported ecological status in the shallowest reef zones (between 0 and 5 m depth), that similar limited reef accretion rates may also apply in these areas. This is because these sites are nowadays often mostly covered by sand and rubble, and with sparse coral cover that is limited to opportunistic corals, rather than the formerly dominant dense communities of Acropora palmata and A. cervicornis (Bak, 1977; Van Duyl, 1985). The loss of these fast‐growing corals, caused largely by white band disease (Bak & Criens, 1981; Gladfelter, 1982), has especially profound implications because they contribute disproportionally to reef accretion rates (Figures 4 and 5) and structural complexity (Alvarez‐Filip, Côté, Gill, Watkinson, & Dulvy, 2011a; Alvarez‐Filip, Dulvy, Côté, Watkinson, & Gill, 2011b). Whilst there thus appears to be a widespread perception that the Bonaire coral reefs are relatively ‘healthy’, our data show that, taking into account the loss of these principal calcifiers, declining coral cover and the current low EAR, this might be a statement, that at least to some degree, is subjected to the ‘shifting baseline syndrome’ (Johnson & Jackson, 2015; McClenachan, O'Connor, Neal, Pandolfi, & Jackson, 2017; Pauly, 1995).
The actual or relative submergence of the reef canopy and the loss of a complex carbonate framework have severe implications for reef ecological functioning and biodiversity (Kennedy et al., 2013). Simultaneously, declining structural complexity in combination with SLR is likely to have considerable social and economic implications for the island (i.e. caused by frequent inundation of coastal areas, declining tourism and reduced fish stocks) (Ferrario et al., 2014; Pratchett, Hoey, & Wilson, 2014; Storlazzi et al., 2018). Several recent studies point out the augmented risk of annual flooding and coastal erosion following climatological change (Harris et al., 2018; Perry et al., 2018; Storlazzi et al., 2018; Vitousek et al., 2017). They predict that under the current conditions of reduced reef accretion in combination with SLR and changing storm patterns, many low‐lying tropical areas will already become uninhabitable in the coming decades (Hopkinson, Lugo, Alber, Covich, & Bloem, 2008; Quataert, Storlazzi, Rooijen, Cheriton, & Dongeren, 2015; Slangen et al., 2014; Storlazzi et al., 2018; Vitousek et al., 2017). The shore of southern Bonaire, including Kralendijk, which only rises approximately 1 m or less above mean sea level (Mücher, Suomalainen, Stuiver, & Meesters, 2017; Van Duyl, 1985), is therefore especially susceptible to inundation and erosion. Worryingly, the reefs that are supposed to help diminish wave energy in this area are currently in a particularly poor state with a negligible reef accretion (<1 mm/year) and widespread loss of structural 3D‐complexity. Of relevance, we note that Bonaire has already been experiencing repeated flooding in this region during the passing of storms and hurricanes (https://www.youtube.com/watch?v=YvZuAhJ-7pg).
Whilst the issue of climate change induced SLR should be addressed on a global scale, the findings presented in this study underline the importance of local conservation implementations. It should be pointed out that the present study does not specifically quantify the expected changes in reef functioning or reef services following a reduction in accretion potential. Nonetheless, the concerns raised here have significant importance for local management policies. Considering projections of increased frequency and intensity of physical disturbances (storms and large swell events) (Hoeke et al., 2013; Hopkinson et al., 2008), restoring reefs for natural coastal protection may be substantially more sustainable and cost‐efficient than artificial coastal protection efforts. Based on our findings, we recommend management practices on Bonaire and other Caribbean islands focus on reducing negative local anthropogenic impacts and restoring and preserving the carbonate production potential and general resilience of Acropora and Orbicella reefs. Reefs dominated by these framework builders actually have the potential to retain accretion rates necessary to ensure proper reef functioning and protect sensitive low‐lying coastal areas. On Bonaire, this would be the region from the town of Kralendijk to the southern tip of the island where the coastal shore zone is already suffering from the effects of the loss of natural coral reef wave breakers and where socio‐economic implication will be greatest. It is to be expected that these findings are rather the rule than the exception for low‐lying coastal areas of tropical Western Atlantic islands, especially since many Caribbean reefs are more severely degraded than those found along the island of Bonaire and Klein Bonaire.
4.1. Methodological considerations
The methods applied in this study (ReefBudget and EAR) provide current best estimates of the present‐day rates and processes. Naturally, these methodologies rely largely on rate data in the available literature. The effect of sediment transportation, incorporation and dissolution (Eyre et al., 2018; Yates, Zawada, Smiley, & Tiling‐Range, 2017), rubble fields preventing sand transportation and rubble deposition onto shore (Engel et al., 2012), deposition and the geochemical and microbiological processes of lithification or the impact of sub‐rubble communities are at present very hard to parameterize due to a lack of published data. Sub‐rubble communities, for instance, are often dominated by CCA (Gischler, 1997; Meesters et al., 1991). Whilst such overlooked constituents can have an important role in net reef accretion and sea floor elevation change (Yates et al., 2017) it is unlikely that including these would significantly change the conclusions drawn here on the state of some of the “best” reefs in the Caribbean. We do, however, encourage the integration of such relevant aspects into existing methodologies to quantify reef accretion. Furthermore, by excluding the most shallow reef zones (<5 m depth), it is not possible to directly predict the impacts on wave breaking and coastal protection (Harris et al., 2018). However, the Acropora palmata colonies have largely disappeared and coral cover in the most shallow reef zone is generally considered to be minimal compared to the zones included in this study. Hence, we believe that it is reasonable to assume that the wave energy diminishing capacity of these reefs will be substantially declining at many sites as well.
Supporting information
ACKNOWLEDGEMENTS
We want to extend our gratitude to Sil Piek, Yun Scholten, Sarah Veillat and Roger Meijs for their efforts in collecting the data. We sincerely thank C. Eckrich for her support in the field. We also acknowledge the invaluable logistical assistance of the STINAPA Bonaire National Parks Foundation. This study has been funded by the Ministry of Economic Affairs for the purposes of the Policy Supporting Research Theme ‘Analyse fotomateriaal koraalrif/fase 1' (Project No. BO‐11‐019.02‐038) and the Wageningen University. The Royal Netherlands Institute for Sea Research provided funding of expenses and in‐kind support. Finally, we extend our gratitude to the various reviewers for their constructive review of the manuscript.
de Bakker DM, van Duyl FC, Perry CT, Meesters EH. Extreme spatial heterogeneity in carbonate accretion potential on a Caribbean fringing reef linked to local human disturbance gradients. Glob Change Biol. 2019;25:4092–4104. 10.1111/gcb.14800
REFERENCES
- Achlatis, M. , van der Zande, R. M. , Schönberg, C. H. , Fang, J. K. , Hoegh‐Guldberg, O. , & Dove, S. (2017). Sponge bioerosion on changing reefs: Ocean warming poses physiological constraints to the success of a photosymbiotic excavating sponge. Scientific Reports, 7(1), 10705 10.1038/s41598-017-10947-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Filip, L. , Carricart‐Ganivet, J. P. , Horta‐Puga, G. , & Iglesias‐Prieto, R. (2013). Shifts in coral‐assemblage composition do not ensure persistence of reef functionality. Scientific Reports, 3, 3486 10.1038/srep03486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Filip, L. , Côté, I. M. , Gill, J. A. , Watkinson, A. R. , & Dulvy, N. K. (2011a). Region-wide temporal and spatial variation in Caribbean reef architecture: Is coral cover the whole story? Global Change Biology, 17(7), 2470–2477. 10.1111/j.1365-2486.2010.02385.x. [DOI] [Google Scholar]
- Alvarez‐Filip, L. , Dulvy, N. K. , Côté, I. M. , Watkinson, A. R. , & Gill, J. A. (2011b). Coral identity underpins architectural complexity on Caribbean reefs. Ecological Applications, 21(6), 2223–2231. 10.1890/10-1563.1 [DOI] [PubMed] [Google Scholar]
- Alvarez‐Filip, L. , Dulvy, N. K. , Gill, J. A. , Côté, I. M. , & Watkinson, A. R. (2009). Flattening of Caribbean coral reefs: region‐wide declines in architectural complexity. Proceedings of the Royal Society B: Biological Sciences, 276(1669), 3019–3025. 10.1098/rspb.2009.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak, R. P. M. (1977). Coral reefs and their zonation in Netherlands Antilles: Modern and ancient reefs. AAPG Studies in Geology, 4, 3–16. [Google Scholar]
- Bak, R. P. M. , & Criens, S. R. (1981) Survival after fragmentation of colonies of Madracis mirabilis, Acropora palmata and A. cervicornis (Scleractinia) and the subsequent impact of a coral disease. Proceedings of the 4th International Coral Symposium, 2, 221–227. [Google Scholar]
- Bak, R. P. M. , Nieuwland, G. , & Meesters, E. H. (2005). Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curaçao and Bonaire. Coral Reefs, 24(3), 475–479. 10.1007/s00338-005-0009-1 [DOI] [Google Scholar]
- Beetham, E. , Kench, P. S. , & Popinet, S. (2017). Future reef growth can mitigate physical impacts of sea‐level rise on atoll islands. Earth's Future, 5, 1002–1014. 10.1002/2017EF000589 [DOI] [Google Scholar]
- Bruno, J. F. , & Bertness, M. D. (2001). Habitat modification and facilitation in benthic marine communities In Bertness M. D., Gaines S. D., & Hay M. E. (Eds.), Marine community ecology (pp. 201–218). Sunderland, MA: Sinauer. [Google Scholar]
- Connell, J. H. (1978). Diversity in tropical rain forests and coral reefs. Science, 199(4335), 1302–1310. 10.1126/science.199.4335.1302 [DOI] [PubMed] [Google Scholar]
- De Bakker, D. M. , Meesters, E. H. , Bak, R. P. M. , Nieuwland, G. , & Van Duyl, F. C. (2016). Long‐term shifts in coral communities on shallow to deep reef slopes of Curaçao and Bonaire: Are there any winners? Frontiers in Marine Science, 3, 247 10.3389/fmars.2016.00247 [DOI] [Google Scholar]
- De Bakker, D. M. , Van Duyl, F. C. , Bak, R. P. M. , Nugues, M. M. , Nieuwland, G. , & Meesters, E. H. (2017). 40 years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: The rise of slimy cyanobacterial mats. Coral Reefs, 36(2), 355–367. 10.1007/s00338-016-1534-9 [DOI] [Google Scholar]
- De Bakker, D. M. , Webb, A. E. , van den Bogaart, L. A. , van Heuven, S. M. A. C. , Meesters, E. H. , & van Duyl, F. C. (2018). Quantification of chemical and mechanical bioerosion rates of six Caribbean excavating sponge species found on the coral reefs of Curaçao. PLoS ONE, 13(5), e0197824 10.1371/journal.pone.0197824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goeij, J. M. , Van Oevelen, D. , Vermeij, M. J. , Osinga, R. , Middelburg, J. J. , de Goeij, A. F. , & Admiraal, W. (2013). Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science, 342(6154), 108–110. 10.1126/science.1241981 [DOI] [PubMed] [Google Scholar]
- De'ath, G. , Fabricius, K. E. , Sweatman, H. , & Puotinen, M. (2012). The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences of the United States of America, 109, 17995–17999. 10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done, T. J. , Ogden, J. C. , & Wiebe, W. J. (1996). Biodiversity and ecosystem function of coral reefs In Mooney H. A., Cushman J. H., Medina E., Sala O. E., & Schulze E. D. (Eds.), Functional roles of biodiversity: A global perspective (pp. 393–429). Chichester, UK: John Wiley & Sons. [Google Scholar]
- Eakin, C. M. (1996). Where have all the carbonates gone? A model comparison of calcium carbonate budgets before and after the 1982–1983 El Nino at Uva Island in the eastern Pacific. Coral Reefs, 15(2), 109–119. 10.1007/bf01771900 [DOI] [Google Scholar]
- Edinger, E. N. , & Risk, M. J. (2000). Reef classification by coral morphology predicts coral reef conservation value. Biological Conservation, 92(1), 1–13. 10.1016/s0006-3207(99)00067-1 [DOI] [Google Scholar]
- Engel, M. , Brückner, H. , Messenzehl, K. , Frenzel, P. , May, S. M. , Scheffers, A. , … Kelletat, D. (2012). Shoreline changes and high‐energy wave impacts at the leeward coast of Bonaire (Netherlands Antilles). Earth, Planets and Space, 64(10), 9 10.5047/eps.2011.08.011 [DOI] [Google Scholar]
- Estes, J. A. , & Palmisano, J. F. (1974). Sea otters: Their role in structuring nearshore communities. Science, 185(4156), 1058–1060. 10.1126/science.185.4156.1058 [DOI] [PubMed] [Google Scholar]
- Eyre, B. D. , Cyronak, T. , Drupp, P. , De Carlo, E. H. , Sachs, J. P. , & Andersson, A. J. (2018). Coral reefs will transition to net dissolving before end of century. Science, 359(6378), 908–911. 10.1126/science.aao1118 [DOI] [PubMed] [Google Scholar]
- Fabricius, K. , De'ath, G. , McCook, L. , Turak, E. , & Williams, D. M. B. (2005). Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Marine Pollution Bulletin, 51(1–4), 384–398. 10.1016/j.marpolbul.2004.10.041 [DOI] [PubMed] [Google Scholar]
- Ferrario, F. , Beck, M. W. , Storlazzi, C. D. , Micheli, F. , Shepard, C. C. , & Airoldi, L. (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nature Communications, 5, 3794 10.1038/ncomms4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, T. A. , Côté, I. M. , Gill, J. A. , Grant, A. , & Watkinson, A. R. (2003). Long‐term region‐wide declines in Caribbean corals. Science, 301(5635), 958–960. 10.1126/science.1086050 [DOI] [PubMed] [Google Scholar]
- Gischler, E. (1997). Cavity dwellers (coelobites) beneath coral rubble in the Florida reef tract. Bulletin of Marine Science, 61(2), 467–484. [Google Scholar]
- Gladfelter, W. B. (1982). White‐band disease in Acropora palmata: Implications for the structure and growth of shallow reefs. Bulletin of Marine Science, 32(2), 639–643. [Google Scholar]
- Glynn, P. W. . (1997). Bioerosion and coral-reef growth: A dynamic balance In Birkeland C. (Ed.), Life and death of coral reefs (pp. 68–95). New York, NY: Chapman and Hall. [Google Scholar]
- González‐Barrios, F. J. , & Alvarez‐Filip, L. (2018). A framework for measuring coral species‐specific contribution to reef functioning in the Caribbean. Ecological Indicators, 95, 877–886. 10.1016/j.ecolind.2018.08.038 [DOI] [Google Scholar]
- Graham, N. A. J. , & Nash, K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs, 32(2), 315–326. 10.1007/s00338-012-0984-y [DOI] [Google Scholar]
- Green, D. H. , Edmunds, P. J. , & Carpenter, R. C. (2008). Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Marine Ecology Progress Series, 359, 1–10. 10.3354/meps07454 [DOI] [Google Scholar]
- Harris, D. L. , Rovere, A. , Casella, E. , Power, H. , Canavesio, R. , Collin, A. , … Parravicini, V. (2018). Coral reef structural complexity provides important coastal protection from waves under rising sea levels. Science Advances, 4(2), eaao4350 10.1126/sciadv.aao4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, J. , & Wilkinson, C. (2004). Methods for ecological monitoring of coral reefs. Townsville,Qld: Australian Institute of Marine Science. 117. [Google Scholar]
- Hoeke, R. K. , McInnes, K. L. , Kruger, J. C. , McNaught, R. J. , Hunter, J. R. , & Smithers, S. G. (2013). Widespread inundation of Pacific islands triggered by distant‐source wind‐waves. Global and Planetary Change, 108, 128–138. 10.1016/j.gloplacha.2013.06.006 [DOI] [Google Scholar]
- Hopkinson, C. S. , Lugo, A. E. , Alber, M. , Covich, A. P. , & Van Bloem, S. J. (2008). Forecasting effects of sea‐level rise and windstorms on coastal and inland ecosystems. Frontiers in Ecology and the Environment, 6(5), 255–263. 10.1890/070153 [DOI] [Google Scholar]
- Hughes, T. P. (1994). Catastrophes, phase shifts, and large‐scale degradation of a Caribbean coral reef. Science, 265(5178), 1547–1551. 10.1126/science.265.5178.1547 [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , Baird, A. H. , Bellwood, D. R. , Card, M. , Connolly, S. R. , Folke, C. , … Roughgarden, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science, 301(5635), 929–933. 10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- Jackson, J. B. C. (2001). What was natural in the coastal oceans? Proceedings of the National Academy of Sciences, 98(10), 5411–5418. 10.1073/pnas.091092898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, J. B. C. , Donovan, M. , Cramer, K. , & Lam, V. (Eds.). (2014). Status and trends of Caribbean coral reefs: 1970–2012. Washington, DC: Global Coral Reef Monitoring Network, International Union for the Conservation of Nature Global Marine and Polar Program. [Google Scholar]
- Januchowski‐Hartley, F. A. , Graham, N. A. , Wilson, S. K. , Jennings, S. , & Perry, C. T. (2017) Drivers and predictions of coral reef carbonate budget trajectories. Proceedings of the Royal Society of London B: Biological Sciences, 284(1847), 20162533 10.1098/rspb.2016.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. E. , & Jackson, J. B. (2015). Fisher and diver perceptions of coral reef degradation and implications for sustainable management. Global Ecology and Conservation, 3, 890–899. 10.1016/j.gecco.2015.04.004 [DOI] [Google Scholar]
- Kench, P. S. , Ford, M. R. , & Owen, S. D. (2018). Patterns of island change and persistence offer alternate adaptation pathways for atoll nations. Nature Communications, 9, 605 10.1038/s41467-018-02954-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, E. V. , Perry, C. T. , Halloran, P. R. , Iglesias‐Prieto, R. , Schönberg, C. H. L. , Wisshak, M. , … Mumby, P. J. (2013). Avoiding coral reef functional collapse requires local and global action. Current Biology, 23(10), 912–918. 10.1016/j.cub.2013.04.020 [DOI] [PubMed] [Google Scholar]
- Knowlton, N. (2001). The future of coral reefs. Proceedings of the National Academy of Sciences of the United States of America, 98(10), 5419–5425. 10.1073/pnas.091092998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton, N. , & Jackson, J. B. C. (2008). Shifting baselines, local impacts, and global change on coral reefs. PLoS Biology, 6(2), e54 10.1371/journal.pbio.0060054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, P. A. (2003). Synthesis of coral reef health indicators for the western Atlantic: Results of the AGRRA program (1997–2000). Atoll Research Bulletin, 496, 1–57. 10.5479/si.00775630.496-3.1 [DOI] [Google Scholar]
- Luckhurst, B. E. , & Luckhurst, K. (1978). Analysis of the influence of substrate variables on coral reef fish communities. Marine Biology, 49(4), 317–323. 10.1007/bf00455026 [DOI] [Google Scholar]
- Lugo‐Fernández, A. , Roberts, H. H. , & Suhayda, J. N. (1998). Wave transformations across a Caribbean fringing‐barrier coral reef. Continental Shelf Research, 18(10), 1099–1124. 10.1016/S0278-4343(97)00020-4 [DOI] [Google Scholar]
- MacArthur, R. H. , & MacArthur, J. W. (1961). On bird species diversity. Ecology, 42(3), 594–598. 10.2307/1932254 [DOI] [Google Scholar]
- McClenachan, L. , O'Connor, G. , Neal, B. P. , Pandolfi, J. M. , & Jackson, J. B. (2017). Ghost reefs: Nautical charts document large spatial scale of coral reef loss over 240 years. Science Advances, 3, e1603155 10.1126/sciadv.1603155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meesters, E. , Knijn, R. , Willemsen, P. , Pennartz, R. , Roebers, G. , & van Soest, R. V. (1991). Sub‐rubble communities of Curaçao and Bonaire coral reefs. Coral Reefs, 10(4), 189–197. 10.1007/BF00336773 [DOI] [Google Scholar]
- Moberg, F. , & Folke, C. (1999). Ecological goods and services of coral reef ecosystems. Ecological Economics, 29(2), 215–233. 10.1016/s0921-8009(99)00009-9 [DOI] [Google Scholar]
- Mücher, S. , Suomalainen, J. , Stuiver, J. , & Meesters, E. H. (2017). Hyperspectral Coral Reef Classification of Bonaire (No. C062/17). Retrieved from https://library.wur.nl/WebQuery/wurpubs/fulltext/422722
- Mumby, P. J. , Broad, K. , Brumbaugh, D. R. , Dahlgren, C. P. , Harborne, A. R. , Hastings, A. , … Sanchirico, J. N. (2008). Coral reef habitats as surrogates of species, ecological functions, and ecosystem services. Conservation Biology, 22(4), 941–951. 10.1111/j.1523-1739.2008.00933.x [DOI] [PubMed] [Google Scholar]
- Newman, S. P. , Meesters, E. H. , Dryden, C. S. , Williams, S. M. , Sanchez, C. , Mumby, P. J. , & Polunin, N. V. C. (2015). Reef flattening effects on total richness and species responses in the Caribbean. Journal of Animal Ecology, 84(6), 1678–1689. 10.1111/1365-2656.12429 [DOI] [PubMed] [Google Scholar]
- Pandolfi, J. M. , & Jackson, J. B. C. (2006). Ecological persistence interrupted in Caribbean coral reefs. Ecology Letters, 9(7), 818–826. 10.1111/j.1461-0248.2006.00933.x [DOI] [PubMed] [Google Scholar]
- Pauly, D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology & Evolution, 10(10), 430 10.1016/S0169-5347(00)89171-5 [DOI] [PubMed] [Google Scholar]
- Perry, C. T. , & Alvarez‐Filip, L. (2018). Changing geo‐ecological functions of coral reefs in the Anthropocene. Functional Ecology, 1–13. 10.1111/1365-2435.13247 [DOI] [Google Scholar]
- Perry, C. T. , Alvarez‐Filip, L. , Graham, N. A. J. , Mumby, P. J. , Wilson, S. K. , Kench, P. S. , … Macdonald, C. (2018). Loss of coral reef growth capacity to track future increases in sea level. Nature, 558(7710), 396–400. [DOI] [PubMed] [Google Scholar]
- Perry, C. T. , Edinger, E. N. , Kench, P. S. , Murphy, G. N. , Smithers, S. G. , Steneck, R. S. , & Mumby, P. J. (2012). Estimating rates of biologically driven coral reef framework production and erosion: A new census‐based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs, 31(3), 853–868. 10.1007/s00338-012-0901-4 [DOI] [Google Scholar]
- Perry, C. T. , Murphy, G. N. , Graham, N. A. , Wilson, S. K. , Januchowski‐Hartley, F. A. , & East, H. K. (2015). Remote coral reefs can sustain high growth potential and may match future sea‐level trends. Scientific Reports, 5, 18289 10.1038/srep18289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C. T. , Murphy, G. N. , Kench, P. S. , Edinger, E. N. , Smithers, S. G. , Steneck, R. S. , & Mumby, P. J. (2014). Changing dynamics of Caribbean reef carbonate budgets: Emergence of reef bioeroders as critical controls on present and future reef growth potential. Proceedings of the Royal Society B: Biological Sciences, 281(1796), 20142018 10.1098/rspb.2014.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C. T. , Murphy, G. N. , Kench, P. S. , Smithers, S. G. , Edinger, E. N. , Steneck, R. S. , & Mumby, P. J. (2013). Caribbean‐wide decline in carbonate production threatens coral reef growth. Nature Communications, 4, 1402 10.1038/ncomms2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C. T. , Spencer, T. , & Kench, P. S. (2008). Carbonate budgets and reef production states: A geomorphic perspective on the ecological phase‐shift concept. Coral Reefs, 27(4), 853–866. 10.1007/s00338-008-0418-z [DOI] [Google Scholar]
- Perry, C. T. , Steneck, R. S. , Murphy, G. N. , Kench, P. S. , Edinger, E. N. , Smithers, S. G. , & Mumby, P. J. (2015). Regional‐scale dominance of non‐framework building corals on Caribbean reefs affects carbonate production and future reef growth. Global Change Biology, 21(3), 1153–1164. 10.1111/gcb.12792 [DOI] [PubMed] [Google Scholar]
- Pratchett, M. S. , Hoey, A. S. , & Wilson, S. K. (2014). Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Current Opinion in Environmental Sustainability, 7, 37–43. 10.1016/j.cosust.2013.11.022 [DOI] [Google Scholar]
- Quataert, E. , Storlazzi, C. , Rooijen, A. , Cheriton, O. , & Dongeren, A. (2015). The influence of coral reefs and climate change on wave‐driven flooding of tropical coastlines. Geophysical Research Letters, 42(15), 6407–6415. 10.1002/2015GL064861 [DOI] [Google Scholar]
- R Core Team . (2002) The R stats package. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org [Google Scholar]
- R Core Team . (2013) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3‐900051‐07‐0. [Google Scholar]
- Risk, M. J. (1972). Fish diversity on a coral reef in the Virgin Islands. Atoll Research Bulletin, 153, 1–6. 10.5479/si.00775630.153.1 [DOI] [Google Scholar]
- Schönberg, C. H. , Fang, J. K. H. , & Carballo, J. L. (2017). Bioeroding sponges and the future of coral reefs In Bell J. J. & Carballo J. L. (Eds.), Climate change, ocean acidification and sponges (pp. 179–372). Berlin, Germany: Springer. [Google Scholar]
- Schönberg, C. H. , Fang, J. K. , Carreiro‐Silva, M. , Tribollet, A. , & Wisshak, M. (2017). Bioerosion: The other ocean acidification problem. ICES Journal of Marine Science, 74(4), 895–925. 10.1093/icesjms/fsw254 [DOI] [Google Scholar]
- Scoffin, T. P. (1980). Calcium carbonate budget of a fringing reef on the west coast of Barbados. Part II – Erosion, sediments and internal structure. Bulletin of Marine Science, 30, 475–508. [Google Scholar]
- Sheppard, C. R. , Dixon, D. J. , Gourlay, M. , Sheppard, A. , & Payet, R. (2005). Coral mortality increases wave energy reaching shores protected by reef flats: Examples from the Seychelles. Estuarine, Coastal and Shelf Science, 64(2–3), 223–234. 10.1016/j.ecss.2005.02.016 [DOI] [Google Scholar]
- Silbiger, N. J. , Nelson, C. E. , Remple, K. , Sevilla, J. K. , Quinlan, Z. A. , Putnam, H. M. , … Donahue, M. J. (2018). Nutrient pollution disrupts key ecosystem functions on coral reefs. Proceedings of the Royal Society B: Biological Sciences, 285(1880), 20172718 10.1098/rspb.2017.2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slangen, A. B. A. , Carson, M. , Katsman, C. A. , Van de Wal, R. S. W. , Köhl, A. , Vermeersen, L. L. A. , & Stammer, D. (2014). Projecting twenty‐first century regional sea‐level changes. Climatic Change, 124(1–2), 317–332. 10.1007/s10584-014-1080-9 [DOI] [Google Scholar]
- Sommer, B. , Harrison, P. L. , Brooks, L. , & Scheffers, S. R. (2011). Coral community decline at Bonaire, southern Caribbean. Bulletin of Marine Science, 87(3), 541–565. 10.5343/bms.2010.1046 [DOI] [Google Scholar]
- Stearn, C. W. , Scoffin, T. P. , & Martindale, W. (1977). Calcium carbonate budget of a fringing reef on the West Coast of Barbados Part I – Zonation and productivity. Bulletin of Marine Science, 27(3), 479–510. [Google Scholar]
- Steneck, R. S. , Arnold, S. N. , & Rasher, D. B. (2013). Status and trends of Bonaire's reefs in 2013: Causes for optimism. University of Maine, School of Marine Sciences; 124. Retrieved from https://www.researchgate.net/publication/279513150 [Google Scholar]
- Storlazzi, C. D. , Elias, E. P. , & Berkowitz, P. (2015). Many atolls may be uninhabitable within decades due to climate change. Scientific Reports, 5, 14546 10.1038/srep14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlazzi, C. D. , Gingerich, S. B. , van Dongeren, A. , Cheriton, O. M. , Swarzenski, P. W. , Quataert, E. , … McCall, R. (2018). Most atolls will be uninhabitable by the mid‐21st century because of sea‐level rise exacerbating wave‐driven flooding. Science Advances, 4(4), eaap9741 10.1126/sciadv.aap9741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyl, F. C. (1985). Atlas of the living reefs of Curaçao and Bonaire (Netherlands Antilles). Utrecht, The Netherlands: Foundation for Scientific Research in Surinam and the Netherlands Antilles. 117. [Google Scholar]
- van Heuven, S. M. , Webb, A. E. , de Bakker, D. M. , Meesters, E. , van Duyl, F. C. , Reichart, G.‐J. , & de Nooijer, L. J. (2018). In‐situ incubation of a coral patch for community‐scale assessment of metabolic and chemical processes on a reef slope. PeerJ, 6, e5966 10.7717/peerj.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woesik, R. , Golbuu, Y. , & Roff, G. (2015). Keep up or drown: Adjustment of western Pacific coral reefs to sea‐level rise in the 21st century. Royal Society Open Science, 2, 150181 10.1098/rsos.150181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek, S. , Barnard, P. L. , Fletcher, C. H. , Frazer, N. , Erikson, L. , & Storlazzi, C. D. (2017). Doubling of coastal flooding frequency within decades due to sea‐level rise. Scientific Reports, 7(1), 1399 10.1038/s41598-017-01362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, A. E. , van Heuven, S. M. A. C. , de Bakker, D. M. , van Duyl, F. C. , Reichart, G.‐J. , & de Nooijer, L. J. (2017). Combined effects of experimental acidification and eutrophication on reef sponge bioerosion rates. Frontiers in Marine Science, 4, 311 10.3389/fmars.2017.00311 [DOI] [Google Scholar]
- Wood, S. (2012). mgcv: Mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation. R package Version 1.7‐17. Retrieved from https://cran.r-project.org/web/packages/mgcv/index.html
- Yates, K. K. , Zawada, D. G. , Smiley, N. A. , & Tiling‐Range, G. (2017). Divergence of seafloor elevation and sea level rise in coral reef ecosystems. Biogeosciences, 14(6), 1739 10.5194/bg-14-1739-2017 [DOI] [Google Scholar]
- Zuur, A. , Ieno, E. N. , & Smith, G. M. (2007). Analyzing ecological data (Zuur A., Ieno E. N., & Smith G. M., Eds.). New York, NY: Springer Science & Business Media. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


