Abstract
What is known and objective
The overuse and misuse of antibiotics, especially for viral, and self‐limiting, respiratory tract infections such as sore throat, increases the risk of the development and spread of antimicrobial resistance within communities. Up to 80% of sore throat cases have a viral aetiology, and even when the infection is bacterial, most cases resolve without antibiotics. However, antibiotics are still frequently and often inappropriately prescribed for the treatment of sore throat. Furthermore, topical (local) antibiotics for treatment of sore throat are widely available over the counter. The objective of this systematic review was to establish the evidence for the benefits, risk of harm and antimicrobial resistance associated with topical (local) antibiotics used for patients with sore throat.
Methods
Eligible studies included those in patients with sore throat of any aetiology receiving the topical (local) antibiotics tyrothricin, bacitracin, gramicidin or neomycin where the antibiotic was topically/locally applied via the nasal cavity or throat. Nasal applications were included as these are occasionally used to treat upper respiratory tract infections that may involve sore throat. There was no restriction or requirement regarding comparator. The outcomes of interest included efficacy, safety, and in vitro culture and antimicrobial resistance data.
Results and discussion
This systematic review found sparse and mainly poor‐quality evidence relating to the use of topical (local) antibiotics for sore throat, and it was not possible to establish the benefits, risk of harm or impact of use on antimicrobial resistance.
What is new and conclusions
Further research is necessary to ascertain the risks and benefits of topical (local) antibiotics, their contribution to antimicrobial resistance and the risk of harm. We do, however, question whether it is appropriate and rational to use topical (local) antibiotics for the treatment of sore throat caused by respiratory tract infections in the absence of robust evidence.
Keywords: antimicrobial resistance, benefits, respiratory tract infections, risk of harm, sore throat, topical (local) antibiotics
1. WHAT IS KNOWN AND OBJECTIVE
Increased use of antibiotics, including their misuse and overuse, results in a higher risk of antibiotic resistance developing and spreading within the community.1, 2, 3, 4, 5 Considering that the majority of respiratory tract infections, including sore throat, are caused by self‐limiting viral infections, antibiotic treatment in these patients is a form of antibiotic misuse.1, 6, 7, 8, 9, 10, 11, 12 Specifically, up to 80% of sore throats are viral and the risk of complications from Group A β‐haemolytic streptococcus, which accounts for about 10% of cases in adults, is low.7, 8, 13, 14 Furthermore, most sore throats resolve without the need for antibiotics.15
Difficulties distinguishing between bacterial and viral aetiology may cause healthcare professionals to err on the side of caution, and, consequently, antibiotic use remains high in patients with sore throat.16, 17 Furthermore, topical/local antibiotics for sore throat are widely available over the counter around the world.18 There is less information regarding the sequelae of resistance to topical/local antibiotics compared with their systemic counterparts; however, any inappropriate antibiotic use, whether it is systemic or for topical/local administration, should be discouraged in line with guidance from the World Health Organization (WHO).1
As with all treatments, the potential benefit has to be weighed against the risk of harm, which is exemplified by the withdrawal of the topical/local antibiotic, fusafungine, from the European market in 2016 due to safety concerns.19 Thus, this prompts a timely consideration of whether it is appropriate and rational to have the remaining topical/local antibiotics for sore throat available as over‐the‐counter medications.
The objective of this systematic review was to establish the published evidence for the benefits, risk of harm and antimicrobial resistance associated with topical/local antibiotics (tyrothricin, bacitracin, gramicidin, neomycin) used for patients with sore throat. These four antibiotics all have a WHO Anatomical Therapeutic Chemical Classification System code for throat preparations (ATC R02AB).
2. METHODS
2.1. Literature searches
A literature search of two databases (PubMed and EMBASE) was conducted on 8 August 2017, using Medical Subject Headings terms for antibiotics and pharyngitis (tyrothricin OR bacitracin OR gramicidin OR neomycin OR fusafungine) AND (sore throat OR throat infection OR pharyngitis OR upper respiratory tract infection). The searches were limited to full publications relating to the use of antibiotics in humans. Conference abstracts and posters were excluded. The EMBASE search was conducted using the Emtree search function. No limits were applied to publication date.
2.2. Systematic review
Following deduplication, the abstracts/titles were screened independently by two authors, with a third adjudicating. The full text was obtained for all potentially relevant records, and non‐English language articles were translated and assessed for relevance. Each full‐text record was assessed independently by two authors, with a third adjudicating. No formal data extraction was conducted beyond that required for the current publication. No risk of bias assessment was conducted.
2.3. Inclusion/exclusion criteria
Inclusion criteria included those with sore throat of any aetiology and treatment interventions of either tyrothricin, bacitracin, gramicidin or neomycin where the antibiotic was topically/locally applied via the nose or throat. Nasal applications were included as these are sometimes used to treat upper respiratory tract infections that may involve sore throat. Studies of fusafungine were excluded, following its withdrawal from European markets, as were systemic antibiotics and products that did not contain antibiotics. There was no restriction or requirement regarding comparator: if present, the comparator could be a placebo or any type of active or non‐pharmaceutical therapy. The eligible outcomes of interest included any of the following: (a) efficacy, including, but not limited to, patient‐reported efficacy such as symptom relief and pain relief; (b) safety, for example the absence of adverse effects; and (c) in vitro culture and antimicrobial resistance data, including resistance to the antibiotics themselves and cross‐resistance with other antibiotics.
In vitro studies of bacteria associated with sore throat were also eligible for inclusion.
There were no restrictions on study design, so that any controlled or uncontrolled studies of any duration were eligible, including randomized controlled trials (RCTs) and all observational studies, as well as meta‐analyses or systematic reviews that were specific to the subject of interest.
3. RESULTS
The searches identified 474 unique records (Figure 1), with a further seven records identified from elsewhere (eg from reading bibliographies).20, 21, 22, 23, 24, 25, 26 A total of 63 records underwent full‐text assessment, of which 16 were included in the systematic review.20, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 The 58 records excluded at full‐text stage were excluded mostly due to the use of fusafungine or other ineligible antibiotics, reporting of the use of bacitracin as a diagnostic aid, and ineligibility of patients (Figure 1).
Figure 1.
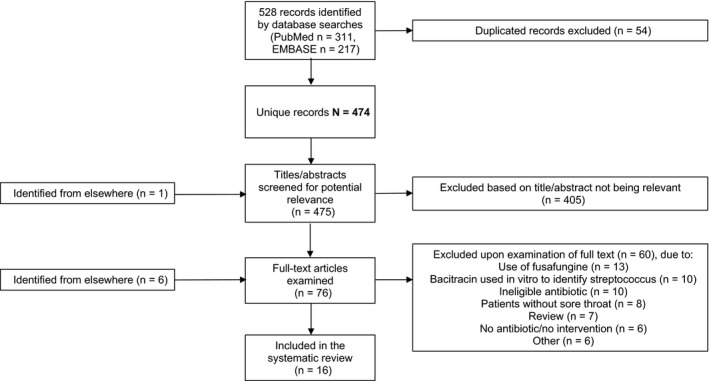
Flow diagram of records through the systematic review
3.1. Characteristics of included studies
Of the 16 included studies, two were RCTs documenting nasal neomycin application28, 31 and a further three RCTs reported buccal bacitracin32, 37 and gramicidin33 use. Seven uncontrolled observational studies describing buccal administration of tyrothricin,34, 41 bacitracin,36 gramicidin38, 39, 40 and a neomycin/tyrothricin combination35 were also included, in addition to a case series describing nasal bacitracin use,27 a case report of buccal tyrothricin use,29 an in vitro study of tyrothricin activity against respiratory viruses20 and a literature review on nasal antimicrobials30 (Table 1).
Table 1.
Characteristics of included studies
| Reference | Study details | Outcomes relevant to sore throat |
|---|---|---|
| Bienen, Raus32 | Randomized comparative study of 100 patients with acute or subacute pharyngitis and/or tonsillitis. Patients received lozenges containing lysozyme, papain and bacitracin or an antiseptic lozenge for use for 5 to 7 d. Symptoms including reddening of the throat, throat swelling, coating of the throat, swelling of lymph nodes, pain in lymph nodes, pain when swallowing and hoarseness were assessed before and after treatment | Both groups showed an improvement in symptoms after treatment. There was a significant improvement in lymph node pain for the bacitracin group compared with the antiseptic group (P < .05); however, differences between the two groups were not significant for outcomes of reddening, throat swelling and pain when swallowing. No antibiotic resistance data were reported. No adverse events were recorded |
| Clark et al27 | Case series of 11 patients with common cold that used local application of aqueous solution of polymyxin B sulphate and bacitracin, 8‐10 drops per nostril, tilting head to distribute it over the nasal cavity and throat, the treatment repeated hourly up to 4 or 5 times then further repeated until cessation of symptoms. Of the 11 case reports, only one (a 64‐y‐old man) is documented with sore throat, described as ‘very large raw spot’ in the back of throat, which he treated | The patient ‘never contracted a cold’, with the experience repeated 1 mo later |
| Cuenant et al28 | Double‐blind, controlled study of 60 patients treated for 11 d with once‐daily endonasal neomycin‐tixocortol pivalate irrigation for chronic allergic and bacterial sinusitis | There were no sore throat‐related outcomes. In patients with bacterial sinusitis, the percentage of nasal deobstruction was greater with tixocortol pivalate‐neomycin treatment (94%) vs neomycin alone (74%), after 11 d. Similarly, in cases of allergic sinusitis, percentage of nasal deobstruction was greater with tixocortol pivalate‐neomycin treatment (69%) versus neomycin treatment (36%). No antibiotic resistance data were reported. No side effects were observed |
| Demols et al29 | This letter to the editor documents one patient taking an average of 6 tyrothricin‐containing oral (buccal) tablets per day for 3 wk for local relief of pharyngitis. The product is probably Tyro‐drops (1 mg tyrothricin per tablet) | The patient was hospitalized with Clostridium difficile‐associated diarrhoea |
| Goh et al30 | Literature review of topical nasal antimicrobial agents | This review did not report anything specific to sore throat, but discusses the use of topical antimicrobials for recurrent staphylococcal nasal colonization. The authors included a statement ‘the use of antimicrobials for nasal/sinus irrigation may lead to increased antibacterial resistance’ but did not elaborate or substantiate |
| Haglind, Gruber33 | Double‐blind, placebo‐controlled study of 468 patients. 233 received lozenges containing gramicidin, cetylpyridinium chloride and 2,4‐dichlorobenzyl alcohol and 235 received placebo lozenges. Both placebo and test lozenges also contained menglytate with reported antiseptic/aesthetic properties. Patients were instructed to take 1 lozenge orally every 2 h for 2 d. Symptoms of throat redness, swollen throat, throat pain, coughing and hoarseness were recorded before and after treatment | The number of symptom‐free patients in the test group was significantly higher than in the placebo group for the symptoms of redness (P < .001), swollen throat (P = .0058) and throat pain (P < .001). No antibiotic resistance data were reported. No safety outcomes were reported |
| Jost34 | Uncontrolled, observational studies conducted in 72 patients with buccopharyngeal disorders. Of these patients, 13 presented with chronic pharyngitis and 1 with acute pharyngitis. Treatment consisted of a mouthwash prepared by mixing 35 drops of 4.4% formaldehyde solution and 25 drops of an alcoholic solution of 2% tyrothricin in half a glass of water. Treatment duration was not specified | Of the 13 patients with chronic pharyngitis, 4 were reported with a ‘good’ outcome following treatment whereas 9 were reported with a ‘poor’ outcome. In the single case of acute pharyngitis, a ‘poor’ outcome was reported. No adverse events were recorded. No antibiotic resistance data were reported |
| Kleinschmidt35 | Clinical study of 111 children mostly admitted due to exudative diathesis with increased susceptibility to infection. Patients were treated with local antibiotic oral tablets containing tyrothricin, neomycin and cetylpyridinium, 1 tablet three times daily in the first week, 1 tablet twice daily during the second week, half a tablet three times daily in the third week and half a tablet daily from the fourth week to day 42. During treatment, children with febrile pharyngeal infections, as well as days where fever was over 37.8°C, were recorded. For comparison, the annual average of the same infections from 1962 (7 treatments with 187 children) without treatment with antibiotic‐based oral tablets was used | The group receiving daily oral antibiotics showed no decrease in the number of febrile pharyngeal infections as well as no reduction in the duration of the illness. No antibiotic resistance data were reported. No safety outcomes were reported |
| Möhr36 | Uncontrolled, observational study of 107 patients with acute pharyngitis, tonsillitis or pharyngolaryngitis. Patients were treated with oral tablets containing lysozyme, papain and bacitracin for up to 9 d. Sore throat outcomes of redness, swelling and pain on swallowing were assessed on days 1 and 6 of treatment | All patients were classified as ‘well improved’ or ‘improved’ following treatment; however, no comparator group was included. No adverse events were recorded. No antibiotic resistance data were reported |
| Raus37 | Double‐blind, randomized controlled trial in 100 patients with the indications for pharyngitis or tonsillitis. Patients were randomized to receive oral tablets containing lysozyme, papain and bacitracin or placebo tablets. Treatment consisted of 8 tablets per day, dissolved slowly in the mouth, for 4 d. Symptoms, including mucus coating, reddening, swelling, pain while swallowing, cough, swelling of the lymph nodes and tenderness of the lymph nodes, were assessed at baseline and on each subsequent day | Significant improvements were observed in the test group, compared with placebo, for swelling after 4 d (P < .05), pain while swallowing after 2 d (P < .01) and after 3 d (P < .001), and tenderness of the lymph nodes after 2 d (P < .05) and after 4 d (P < .001). No distinction between pharyngitis and tonsillitis patients was made when reporting results. No adverse events were recorded. No antibiotic resistance data were reported |
| Schmidbauer20 | In vitro investigation of the antiviral effect of tablets containing tyrothricin, benzalkonium chloride and benzocaine on rhinovirus type 14, respiratory syncytial virus and influenza A virus H1N1. Dissolved tablets were diluted to a range of concentrations between 1:400 (tyrothricin, 1.25 μg/mL; benzalkonium chloride, 2.5 μg/mL; benzocaine 3.8 μg/mL) and 1:64 000 (tyrothricin, 0.008 μg/mL; benzalkonium chloride, 0.016 μg/mL; benzocaine 0.024 μg/mL). Viral inhibition was measured by monitoring viral plaque‐forming units per millilitre | Viral inhibition was observed in a dose‐dependent manner to a maximum of 1:3200 for rhinovirus type 14 (tyrothricin, 0.16 μg/mL; benzalkonium chloride, 0.31 μg/mL; benzocaine 0.48 μg/mL); 1:6400 for respiratory syncytial virus (tyrothricin, 0.08 μg/mL; benzalkonium chloride, 0.16 μg/mL; benzocaine 0.24 μg/mL) and 1:64 000 for influenza A virus H1N1 (tyrothricin, 0.008 μg/mL; benzalkonium chloride, 0.0016 μg/mL; benzocaine 0.024 μg/mL). No antibiotic resistance data were reported |
| Sheikova, Vachev38 | Uncontrolled, observational study in 40 patients with tonsillitis or acute pharyngitis. Treatment consisted of gramicidin (3%) lozenges administered every 2 h for up to 3 d. Bacterial swabs from each patient were also tested for gramicidin susceptibility in vitro | Isolated bacterial cultures appeared susceptible to gramicidin in vitro. Of the 10 patients with pharyngitis, of which all had severe pain when swallowing, red mucous membranes and temperature between 37 and 37.7°C, clinical recovery was achieved following 24‐h application of gramicidin lozenges; however, no comparator group was included. No adverse events were recorded. No antibiotic resistance data were reported |
| Stricker, Ravanelli39 | Uncontrolled, observational study in 214 patients with buccopharyngeal disorders including tonsillitis, pharyngitis, lateral pharyngitis, laryngotracheobronchitis, peritonsillitis, glossitis, gingivitis and stomatitis. Treatment consisted of 1 tablet, containing gramicidin, cetylpyridinium chloride, 2, 4‐dichlorobenzyl alcohol and p‐aminobenzoic acid ethyl ester, dissolved slowly in the mouth every 2 to 3 h, for between 3 and 8 d | The best treatment results were observed in cases of acute tonsillitis and pharyngitis. Difficulty in swallowing as well as inflammation had subsided, or reduced, within 3‐5 d of treatment. No further details were reported and no comparator group was included. No adverse events were recorded. No antibiotic resistance data were reported |
| Sykes et al31 | Double‐blind randomized controlled trial of 50 patients with chronic rhinosinusitis, assessing symptomatic response and improvement in nasal mucociliary clearance, nasal airway resistance, sinus radiographs, and intranasal bacteriology and appearance in response to the addition of neomycin (100 μg/dose) to nasal sprays containing dexamethasone and tramazoline four times daily for 2 wk | There were no sore throat‐related outcomes. Staphylococcus aureus was the only pathogen isolated from nasal swabs, and 8/20 (40%) patients treated with neomycin‐containing nasal spray had a positive culture before treatment and 3/8 (38%) still had a positive culture after treatment. No antibiotic resistance data were reported. No safety outcomes were reported |
| Voberg40 | Uncontrolled, observational study of 160 patients with tonsillitis, pharyngitis laryngitis, stomatitis or gingivitis. Patients were treated with 4‐6 tablets, containing gramicidin, cetylpyridinium chloride, 2, 4‐dichlorobenzyl alcohol and p‐aminobenzoic acid ethyl ester, daily, for an average of 4 d. Throat reddening and swelling were assessed, and patient‐reported descriptions, such as burning and scratching of the throat, feeling of a foreign object, difficulty swallowing and general condition, were noted | Of the 121 patients with pharyngitis, 115 were classified as either a ‘good’ or ‘satisfactory’ improvement following treatment; however, no comparator group was included. No adverse events were recorded. No antibiotic resistance data were reported |
| Willenberg41 | Uncontrolled, observational study of 58 patients with acute infections of the mouth and pharynx with inflammation and mucous congestion. Patients took an average of 5.4 tyrothricin‐containing lozenges daily across an average of 5.3 d. Symptoms were assessed and graded before and after treatment | An average reduction in the ‘strength of complaint’ across all patients was observed following treatment for sore throat‐related outcomes including swallowing complaints (86.5% reduction), throat inflammation (93.4% reduction), swelling of the tonsils (97.0% reduction), feeling of dryness (75.9% reduction), scratching in the throat (86.1% reduction), hoarseness (87.3% reduction) and tickle of the throat (87.4% reduction); however, no comparator group was included. 5 patients suffered nausea and vomiting, 1 patient‐reported burning within the mouth and 1 patient developed allergic dermatitis after 6 d. No antibiotic resistance data were reported |
3.2. Evidence of efficacy
This systematic review found limited or inadequate evidence on the efficacy of tyrothricin, bacitracin, gramicidin or neomycin when used topically/locally for sore throat (Table 1). Haglind and Gruber33 conducted a double‐blind, placebo‐controlled study in 468 patients comparing lozenges containing gramicidin, cetylpyridinium chloride and 2,4‐dichlorobenzyl alcohol against placebo. After 48 hours, a significant improvement in throat redness (P < .001), throat swelling (P = .0058) and throat pain (P < .001) was recorded for patients in the test group compared with placebo. Additionally, an RCT performed by Raus37 compared the efficacy of lozenges containing lysozyme, papain and bacitracin against placebo lozenges in 100 patients with indications of tonsillitis or pharyngitis. Significant improvements were observed in the test group, for sore throat‐related symptoms after 2‐4 days' treatment compared with placebo. These symptoms included throat swelling (P < .05 [4 days]), tenderness of lymph nodes (P < .05 [2 days]; P < .001 [4 days]) and pain while swallowing (P < .01 [2 days]; P < .001 [3 days]) although no distinction between patients with tonsillitis or pharyngitis was made when reporting the results.
In a separate RCT, Beinen and Raus32 also compared lozenges containing lysozyme, papain and bacitracin with antiseptic lozenges in 100 patients. Both groups showed an improvement in sore throat symptoms following 5‐7 days' treatment. Although there was a significant improvement in lymph node pain for the bacitracin group compared with the antiseptic group (P < .05), differences between the groups were not significant for throat redness, throat swelling and pain when swallowing.
Of the seven observational studies included in the review, five36, 38, 39, 40, 41 described varying degrees of symptom improvement following local antibiotic treatment whereas two34, 35 reported poor or no improvement in sore throat‐related outcomes. Evidence from these observational studies is of poor quality, since no comparators were included in the study design. Of the remaining studies, one case series related to the use of topical/local bacitracin in a patient with sore throat was included but few details were reported,27 and several studies included in the review did not report efficacy outcomes of topical/local antibiotics in patients with pharyngitis.28, 29, 30, 31 Finally, one investigation demonstrated an antiviral effect of tyrothricin lozenges against rhinovirus, respiratory syncytial virus and influenza A virus in vitro. 20 The relevance of this finding is uncertain, as contact times were measured in days, which is considerably longer than orally delivered local antibiotics remain in the mouth.
3.3. Evidence of harm
This systematic review found very sparse information relating to harm (adverse events) of tyrothricin, bacitracin, gramicidin or neomycin when used topically/locally for sore throat (Table 1). One case study relating to the use of topical/local tyrothricin in a patient with sore throat was included, which reported Clostridium difficile‐associated diarrhoea,29 and an observational study of 58 patients treating acute infections of the mouth and pharynx with tyrothricin lozenges, reported nausea and vomiting in five patients, a burning sensation in one patient and allergic dermatitis in one patient.41
One RCT37 and five uncontrolled observational studies34, 36, 38, 39, 40 of local antibiotics for buccal‐pharyngeal disorders reported that no adverse events were observed. A further study evaluating endonasal neomycin‐tixocortol pivalate irrigation for chronic allergic and bacterial sinusitis also recorded no adverse events.28
3.4. Evidence of antimicrobial resistance
This systematic review did not find any reports on the risk of antimicrobial resistance when tyrothricin, bacitracin, gramicidin or neomycin is used topically/locally for sore throat (Table 1). Of the 16 studies included, none reported antimicrobial resistance as an outcome.20, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41
4. DISCUSSION
Antibiotic resistance develops as bacteria adapt and grow in the presence of antibiotics, and resistant bacteria can persist in the body for up to 1 year.1, 2, 42 Resistance genes can be transferred from bacteria to bacteria within the host,43 and resistant bacteria in the throat are likely to be swallowed, potentially exposing the whole gut microflora. Resistant bacteria can also spread from person to person,5 and since the respiratory tract is well suited to dispersal of contagious particles (hence the transmissibility of respiratory viruses), it is likely that the spread of antibiotic‐resistant bacteria from the nose and throat could be a considerable risk.
Only a small number of studies were eligible for inclusion in the systematic review. Of the studies that met the inclusion criteria, only five were RCTs28, 31, 32, 33, 37 and two of these reported nasal application.28, 31 The remaining studies included several uncontrolled observational studies,34, 35, 36, 38, 39, 40, 41 a case series,27 a case report,29 a literature review of topical nasal antimicrobials30 and one in vitro antiviral study.20 From this inadequate evidence base, it is not possible to establish the benefits and risk of harm for topical/local antibiotics for respiratory infections, particularly sore throat, or the impact on antimicrobial resistance. Most of the included studies did not report robust efficacy outcomes, limited safety data were reported, and evidence of antimicrobial resistance was not described as an outcome in any of the included studies. This lack of evidence relating to the use of topical/local tyrothricin, bacitracin, gramicidin and neomycin for sore throat is surprising, given their continued and prevalent availability around the world.18 Nevertheless, some countries have acknowledged the lack of benefit of topical/local antibiotics. In France, for example, preparations of the antibiotics bacitracin, fusafungine, gramicidin or tyrothricin, which were administered nasally or via the oropharynx, were withdrawn from the market in 2005 due to a lack of therapeutic efficacy.44
It has been established that systemic antibiotics are only minimally effective for sore throat and laryngitis, despite most studies not differentiating bacterial from viral aetiologies.15, 45 Although three RCTs included in this review did observe significant improvements in sore throat‐related outcomes following local antibiotic use, compared with placebo33, 37 or a non‐antibiotic comparator,32 there are limitations to the evidence. Specifically, the scales used to grade symptom improvement were limited,32, 37 or even binary,33 and do not appear to have been validated. Furthermore, formulations used in each of the studies contained other enzymatic32, 37 or antiseptic33 ingredients, so any effect cannot be attributed to the antibiotic alone.
Additionally, although improvements in sore throat‐related outcomes following local antibiotic use were reported in several observational studies,36, 38, 39, 40, 41 no control groups were included. The use of a comparator is particularly important when assessing a self‐limiting condition such as sore throat, which typically resolves within 7 days without treatment.15, 46
There are also some reports of efficacy of the topical/local antibiotic fusafungine (which is no longer licensed in the European Union), although the available evidence is weak.45, 47, 48, 49 Two studies reported an improvement in patients' sore throat symptoms over a 7‐day study period; again, however, no control groups were included as a comparator.47, 49 A further investigation comparing patients receiving fusafungine or placebo found no significant difference between groups in relation to improvement of pharyngitis symptoms, whereas a trial identified during a meta‐analysis reported the rates of clinical cure after 5 days favoured fusafungine, but no significant differences were observed after 8 or 28 days.45, 48
Fusafungine studies were initially considered for inclusion, but subsequently excluded from this review due to the removal of fusafungine from European markets. Topical/local fusafungine was withdrawn in the European Union following a risk assessment that concluded that the benefits did not outweigh its risks, particularly in regard to the risk of serious allergic reactions.19 Also cited were concerns about the potential for fusafungine to promote antibiotic resistance. While it was acknowledged that there was insufficient evidence to establish that fusafungine can increase the risk of resistance, this risk could not be ruled out.19
Although this systematic review found a lack of evidence of the risk of antimicrobial resistance to topical/local antibiotics when used for respiratory infections like sore throat, such resistance has been documented in other conditions for which topical/local antibiotics are indicated.50, 51 For example, topical erythromycin and clindamycin are used in Europe to treat acne, which has led to antimicrobial‐resistant Propionibacterium acnes and staphylococci strains.50 Moreover, resistance to mupirocin and fusidic acid correlates with increased or unrestricted use of these topical antibiotics for the treatment of minor staphylococcal skin infections.51
Although the causality is not clear, bacitracin resistance has been reported in Streptococcus pyogenes isolated from patients with pharyngitis.52 Additionally, bacitracin‐resistant strains of S pyogenes have been recovered from patients with both invasive and non‐invasive infections, and bacitracin‐resistant strains of Staphylococcus aureus have been described in patients with atopic dermatitis.53, 54, 55 Neomycin‐resistant strains of Escherichia coli have been isolated from patients with urinary tract and gastrointestinal infections, and resistant Pseudomonas aeruginosa has been recovered from ear and skin infections.56 Furthermore, neomycin‐resistant S aureus has been isolated from the skin of eczema and burns patients.56, 57 Although resistance to tyrothricin and gramicidin can be induced in S aureus under laboratory/clinical conditions, reports of resistant strains isolated from patients are absent from the literature.58, 59, 60 Nevertheless, isolation of bacitracin‐resistant and neomycin‐resistant bacterial strains in clinical settings suggests the existence of selection pressures and the overuse of topical/local antibiotics cannot be overlooked.
Therefore, there is an urgent need for further research so as to determine the risks of antibiotic resistance in relation to the use of topical/local antibiotics for sore throat. Additionally, it would be of interest to determine pharmacists' opinions regarding the availability of over‐the‐counter antibiotics for sore throat, which conflicts with their front‐line role in antibiotic stewardship.61
The lack of robust evidence regarding the efficacy of topical/local antibiotics for sore throat suggests it may be sensible to challenge whether it is appropriate to have topical/local antibiotics for sore throat available over the counter. Pharyngitis symptoms can be effectively treated without antibiotics, and given the self‐limiting nature of the majority of cases of sore throat, symptomatic treatment may be more pertinent and beneficial for patients.6, 7, 8, 9, 10, 11, 12, 62, 63, 64, 65 Additionally, for those patients with confirmed bacterial pharyngitis who are at risk of complications, getting progressively worse or who are very unwell, systemic antibiotics are available on prescription.
4.1. Limitations
The limitations of this review include the use of only two databases for the literature search and the exclusion of conference abstracts and posters. The limited number of papers available for inclusion restricted our ability to draw strong conclusions. However, strict rules must be adhered to while performing a systematic review and this article reflects well the situation regarding the availability of evidence relating to the use of topical/local antibiotics for sore throat.
5. WHAT IS NEW AND CONCLUSION
Of the limited number of studies eligible for inclusion in this systematic review, few included robust efficacy outcomes, limited safety data were reported, and evidence of antimicrobial resistance was not described as an outcome in any of the included studies. Consequently, there is a lack of published evidence relating to the use of topical/local tyrothricin, bacitracin, gramicidin and neomycin for sore throat and it was not possible to establish the benefits, risk of harm or effect on antimicrobial resistance. In the absence of robust evidence, it is important to question whether it is appropriate and rational to continue making topical/local antibiotics available for treatment of sore throat.
CONFLICT OF INTEREST
Adrian Shephard is an employee of Reckitt Benckiser Healthcare Ltd UK. John Bell, Douglas S. Burgoyne, Martin Duerden and Sabiha Essack are members of the Global Respiratory Infection Partnership.
AUTHOR CONTRIBUTIONS
All authors contributed to the systematic review and the preparation of this manuscript including its critical review, and have approved the final draft, and accept full responsibility for its content.
ACKNOWLEDGEMENTS
Medical writing assistance was provided by Daniel East and Kim Russell of Elements Communications Ltd, Westerham, UK, which was funded by Reckitt Benckiser Healthcare Ltd, UK.
Essack S, Bell J, Burgoyne DS, Duerden M, Shephard A. Topical (local) antibiotics for respiratory infections with sore throat: An antibiotic stewardship perspective. J Clin Pharm Ther. 2019;44:829–837. 10.1111/jcpt.13012
Funding information
This study was funded by Reckitt Benckiser Healthcare UK, Slough, Berkshire, UK. The Global Respiratory Infection Partnership was convened and funded by an unrestricted educational grant from Reckitt Benckiser Healthcare Ltd UK, Slough, Berkshire, UK.
REFERENCES
- 1. World Health Organization . Global action plan on antimicrobial resistance. 2015. http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/. Accessed June 21, 2019. [DOI] [PubMed]
- 2. Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9(6):e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross‐national database study. Lancet. 2005;365(9459):579‐587. [DOI] [PubMed] [Google Scholar]
- 4. Riedel S, Beekmann SE, Heilmann KP, et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae . Eur J Clin Microbiol Infect Dis. 2007;26(7):485‐490. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Antimicrobial resistance. Fact sheet. 2018. http://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed June 21, 2019.
- 6. Scott JG, Cohen D, DiCicco‐Bloom B, Orzano AJ, Jaen CR, Crabtree BF. Antibiotic use in acute respiratory infections and the ways patients pressure physicians for a prescription. J Fam Pract. 2001;50(10):853‐858. [PubMed] [Google Scholar]
- 7. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA. 2000;284(22):2912‐2918. [DOI] [PubMed] [Google Scholar]
- 8. Worrall GJ. Acute sore throat. Can Fam Physician. 2007;53(11):1961‐1962. [PMC free article] [PubMed] [Google Scholar]
- 9. van Gageldonk‐Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case‐control study of acute respiratory tract infection in general practice patients in the Netherlands. Clin Infect Dis. 2005;41(4):490‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2013;6:Cd000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dasaraju PV, Liu C. Infections of the respiratory system In: Baron S, ed. Medical Microbiology (4th edn). Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 12. Creer DD, Dilworth JP, Gillespie SH, et al. Aetiological role of viral and bacterial infections in acute adult lower respiratory tract infection (LRTI) in primary care. Thorax. 2006;61(1):75‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bisno AL. Acute pharyngitis. N Engl J Med. 2001;344(3):205‐211. [DOI] [PubMed] [Google Scholar]
- 14. Pelucchi C, Grigoryan L, Galeone C, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(suppl 1):1‐28. [DOI] [PubMed] [Google Scholar]
- 15. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;11:Cd000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dekker AR, Verheij TJ, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Fam Pract. 2015;32(4):401‐407. [DOI] [PubMed] [Google Scholar]
- 17. Gulliford MC, Dregan A, Moore MV, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4(10):e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Both L, Botgros R, Cavaleri M. Analysis of licensed over‐the‐counter (OTC) antibiotics in the European Union and Norway, 2012. Eurosurveillance. 2014;20(34):30002. [DOI] [PubMed] [Google Scholar]
- 19. European Medicines Agency . CMDh endorses revocation of authorisations for fusafungine sprays used to treat airway infections. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Fusafungine_31/Position_provided_by_CMDh/WC500203975.pdf. Accessed June 21, 2019.
- 20. Schmidbauer M. Dorithricin acts antiviral (in vitro). Pharm Ztg. 2015;160(38):48‐52. [Google Scholar]
- 21. Bisno AL, Gerber MA, Gwaltney JM Jr, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis. 2002;35(2):113‐125. [DOI] [PubMed] [Google Scholar]
- 22. German‐Fattal M. Fusafungine. An antimicrobial agent for the local treatment of respiratory tract infections. Clin Drug Invest. 1996;12(6):308‐317. [Google Scholar]
- 23. German‐Fattal M. Fusafungine, an antimicrobial with anti‐inflammatory properties in respiratory tract infections. Clin Drug Invest. 2001;9:653‐670. [Google Scholar]
- 24. Thorsson L, Borgâ O, Edsbäcker S. Systemic availability of budesonide after nasal administration of three different formulations: pressurized aerosol, aqueous pump spray, and powder. Br J Clin Pharmacol. 1999;47(6):619‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Misrahi L, Bourrillon A, Lebrun T, Dervaux B. L'antibiothérapie dans la rhinopharyngite de l'enfant en France : entre les recommandations et la pratique quotidienne [Antibiotics for nasopharyngitis in children, in France: the gap between guidelines and daily practice]. Med Mal Infect. 2003;33(11):570‐578. [Google Scholar]
- 26. Gonzales R, Bartlett JG, Besser RE, Hickner JM, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med. 2001;134(6):490‐494. [DOI] [PubMed] [Google Scholar]
- 27. Clark GA. Effective treatment for common colds. Nebr State Med J. 1954;39(11):480‐482. [PubMed] [Google Scholar]
- 28. Cuenant G, Stipon JP, Plante‐Longchamp G, Baudoin C, Guerrier Y. Efficacy of endonasal neomycin‐tixocortol pivalate irrigation in the treatment of chronic allergic and bacterial sinusitis. ORL J Otorhinolaryngol Relat Spec. 1986;48(4):226‐232. [DOI] [PubMed] [Google Scholar]
- 29. Demols A, Van Gossum A, Clevenberg P, Thys JP, Liesnard C. Tyrothricin‐containing oral tablets causing Clostridium difficile‐associated diarrhea. Dig Dis Sci. 1996;41(11):2291. [DOI] [PubMed] [Google Scholar]
- 30. Goh YH, Goode RL. Current status of topical nasal antimicrobial agents. Laryngoscope. 2000;110(6):875‐880. [DOI] [PubMed] [Google Scholar]
- 31. Sykes DA, Wilson R, Chan KL, Mackay IS, Cole PJ. Relative importance of antibiotic and improved clearance in topical treatment of chronic mucopurulent rhinosinusitis: a controlled study. Lancet. 1986;2(8503):359‐360. [DOI] [PubMed] [Google Scholar]
- 32. Bienen H, Raus I. Therapeutic comparison of throat lozenges. MMW Munch Med Wochenschr. 1981;123(18):745‐747. [PubMed] [Google Scholar]
- 33. Haglind K, Gruber P. Testing of the effect of Imposit throat tablets in double‐blind test. Z Allgemeinmed. 1975;51(27):1211‐1214. [PubMed] [Google Scholar]
- 34. Jost G. Local treatment of bucco‐pharyngeal diseases in otorhinolaryngology. Sem Ther. 1964;40(8):479‐481. [PubMed] [Google Scholar]
- 35. Kleinschmidt H. Studies on the effect of a local antibiotic on throat infections in children and the pharyngeal flora. Med Klin. 1964;59:107‐108. [PubMed] [Google Scholar]
- 36. Möhr W. Therapy of tonsillitis and pharyngitis. MMW Munch Med Wochenschr. 1978;120(29‐30):999‐1000. [PubMed] [Google Scholar]
- 37. Raus I. Clinical studies on Frubienzyme in a controlled double‐blind trial. Fortschr Med. 1976;94(28):1579‐1582. [PubMed] [Google Scholar]
- 38. Sheikova T, Vachev G. Utilization of gramicidin dragee in throat inflammation and tonsillitis. Khirurgiia (Sofiia). 1957;10(9):836‐838. [PubMed] [Google Scholar]
- 39. Stricker G, Ravanelli O. Treatment of inflammatory diseases in the mouth and pharynx using Imposit. Ther Ggw. 1975;114(1):71‐72. [PubMed] [Google Scholar]
- 40. Vorberg G. Treatment of inflammatory lesions of the mouth and pharynx using Imposit. Ther Ggw. 1977;116(10):1896‐1904. [PubMed] [Google Scholar]
- 41. Willenberg W. Therapy of acute inflammatory diseases of the mouth and pharyngeal space. ZFA (Stuttgart). 1979;55(10):653‐655. [PubMed] [Google Scholar]
- 42. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ. 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 43. Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization . Bacitracin, Fusafungine, Gramicidin, Tyrothricin ‐ Locally administered products withdrawn. 2005. http://apps.who.int/medicinedocs/en/d/Js8119e/1.3.html#Js8119e.1.3. Accessed June 21, 2019.
- 45. Reveiz L, Cardona AF. Antibiotics for acute laryngitis in adults. Cochrane Database Syst Rev. 2015;5:Cd004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Excellence NIfHaC . Respiratory tract infections (self‐limiting): prescribing antibiotics. Clinical guideline 69. 2008. https://www.nice.org.uk/guidance/cg69. Accessed June 21, 2019.
- 47. Kroslák M. Efficacy and acceptability of fusafungine, a local treatment for both nose and throat infections, in adult patients with upper respiratory tract infections. Curr Med Res Opin. 2002;18(4):194‐200. [DOI] [PubMed] [Google Scholar]
- 48. Pandraud L. Therapeutic efficacy and clinical acceptability of fusafungine in follicular pharyngitis. Curr Med Res Opin. 2002;18(7):381‐388. [DOI] [PubMed] [Google Scholar]
- 49. Samolinski B, Zawisza E, Arcimowicz M, Czapska M, Zmigrodzki B. Influence of fusafungine upon viral and bacterial infections. Med Sci Monit. 1997;3(5):736‐743. [Google Scholar]
- 50. Leccia MT, Auffret N, Poli F, Claudel JP, Corvec S, Dreno B. Topical acne treatments in Europe and the issue of antimicrobial resistance. J Eur Acad Dermatol Venereol. 2015;29(8):1485‐1492. [DOI] [PubMed] [Google Scholar]
- 51. Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017;30(3):827‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Malhotra‐Kumar S, Wang S, Lammens C, Chapelle S, Goossens H. Bacitracin‐resistant clone of Streptococcus pyogenes isolated from pharyngitis patients in Belgium. J Clin Microbiol. 2003;41(11):5282‐5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pires R, Rolo D, Mato R, et al. Resistance to bacitracin in Streptococcus pyogenes from oropharyngeal colonization and noninvasive infections in Portugal was caused by two clones of distinct virulence genotypes. FEMS Microbiol Lett. 2009;296(2):235‐240. [DOI] [PubMed] [Google Scholar]
- 54. York MK, Gibbs L, Perdreau‐Remington F, Brooks GF. Characterization of antimicrobial resistance in Streptococcus pyogenes isolates from the San Francisco Bay area of Northern California. J Clin Microbiol. 1999;37(6):1727‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bessa GR, Quinto VP, Machado DC, et al. Staphylococcus aureus resistance to topical antimicrobials in atopic dermatitis. An Bras Dermatol. 2016;91(5):604‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gad GF, Mohamed HA, Ashour HM. Aminoglycoside resistance rates, phenotypes, and mechanisms of Gram‐negative bacteria from infected patients in upper Egypt. PLoS ONE. 2011;6(2):e17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lowbury EJ, Babb JR, Brown VL, Collins BJ. Neomycin‐resistant Staphylococcus aureus in a burns unit. J Hyg (Lond). 1964;62:221‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bulgakova VG, Grushina VA, Polin AN. Isolation and investigation of Staphylococcus aureus strains resistant to the membrane active antibiotic, gramicidin S. Antibiot Khimioter. 1993;38(8–9):22‐25. [PubMed] [Google Scholar]
- 59. Rammelkamp CH. Observations on resistance of Staphylococcus aureus to action of tyrothricin. Proc Soc Exp Biol Med. 1942;49(3):346‐350. [Google Scholar]
- 60. Shireen T, Singh M, Das T, Mukhopadhyay K. Differential adaptive responses of Staphylococcus aureus to in vitro selection with different antimicrobial peptides. Antimicrob Agents Chemother. 2013;57(10):5134‐5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Essack S, Bell J, Shephard A. Community pharmacists‐Leaders for antibiotic stewardship in respiratory tract infection. J Clin Pharm Ther. 2018;43(2):302‐307. [DOI] [PubMed] [Google Scholar]
- 62. de Looze F, Russo M, Bloch M, Montgomery B, Shephard A, DeVito R. Meaningful relief with flurbiprofen 8.75 mg spray in patients with sore throat due to upper respiratory tract infection. Pain Manag. 2018;8(2):79‐83. [DOI] [PubMed] [Google Scholar]
- 63. Schachtel B, Aspley S, Shephard A, et al. Onset of action of a lozenge containing flurbiprofen 8.75 mg: a randomized, double‐blind, placebo‐controlled trial with a new method for measuring onset of analgesic activity. Pain. 2014;155(2):422‐428. [DOI] [PubMed] [Google Scholar]
- 64. Shephard A, Smith G, Aspley S, Schachtel BP. Randomised, double‐blind, placebo‐controlled studies on flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians' prediction of 'strep throat'. Int J Clin Pract. 2015;69(1):59‐71. [DOI] [PubMed] [Google Scholar]
- 65. Essack S, Pignatari AC. A framework for the non‐antibiotic management of upper respiratory tract infections: towards a global change in antibiotic resistance. Int J Clin Pract Suppl. 2013;180:4‐9. [DOI] [PubMed] [Google Scholar]


