Abstract
Emulsifiers are common components of processed foods consumed as part of a Western diet. Emerging in vitro cell‐line culture, mouse model and human intestinal tissue explant studies have all suggested that very low concentrations of the food emulsifier polysorbate 80 may cause bacterial translocation across the intestinal epithelium, intestinal inflammation and metabolic syndrome. This raises the possibility that dietary emulsifiers might be factors in conditions such as coronary artery disease, type 2 diabetes and Crohn's disease. The potential mechanism behind the observed effects of this emulsifier is uncertain but may be mediated via changes in the gut microbiota or by increased bacterial translocation, or both. It is also unknown whether these effects are generalisable across all emulsifiers and detergents, including perhaps the natural emulsifier lecithin or even conjugated bile acids, particularly if the latter escape reabsorption and pass through to the distal ileum or colon. A major objective of the Medical Research Council (MRC)‐funded Mechanistic Nutrition in Health (MECNUT) Emulsifier project is therefore to investigate the underlying mechanisms and effects of a range of synthetic and natural emulsifiers and detergents in vitro and in vivo, and to determine the effects of a commonly consumed emulsifier (soya lecithin) on gut and metabolic health through a controlled dietary intervention study in healthy human volunteers – the FADiets study. This report provides an overview of the relevant literature, discussing the impact of emulsifiers and other additives on intestinal and metabolic health, and gives an overview of the studies being undertaken as part of the MECNUT Emulsifier project.
Keywords: bacterial translocation, emulsifiers, food additives, gut microbiota, intestinal health and inflammation, metabolic syndrome
Introduction
Permitted food additives in the Western diet
Food additives are substances which are purposely added to food and drink products to perform certain functions, such as to colour, sweeten and/or stabilise and preserve (Mepham 2011). The use of food additives has increased dramatically since they were intentionally used for food preservation in the early 1800s (Fennema 1987; Carocho et al. 2014; Zinöcker & Lindseth 2018). Today, when grocery shopping, it is nearly impossible to avoid processed foods, particularly in the consumption of a typical Western diet – a modern dietary pattern that is characterised by low intake of fruit, legume and vegetable fibre and high intake of red meat, dairy, eggs and refined grains, saturated fat, sugar and salt along with increased exposure to additives due to their use in processed foods (Slimani et al. 2009; Adams & White 2015; Zhong et al. 2018). Some processed foods can form part of a healthy, balanced diet (e.g. wholemeal bread; low‐sugar, high‐fibre breakfast cereals), whilst others may be considered more detrimental for health (e.g. processed meats, high‐fat dairy and bakery products, confectionery, foodstuffs containing hydrogenated oils and high fructose corn syrups) (Carocho et al. 2014; Zinöcker & Lindseth 2018). Today, there are over 2500 permitted additives that are included in foods to enhance appearance, smell, texture and taste, and/or to extend shelf‐life (Branen et al. 2001; Carocho et al. 2014). In the European Union, these are classified into 26 functional classes (Table 1), with some of the most commonly consumed additives being sweeteners, colourants and emulsifiers/surfactants (Huvaere et al. 2012; Roberts et al. 2013; Stevens et al. 2014). Data acquired through surveys from a number of populations (including the UK, mainland Europe, the US, Canada, New Zealand and South America) have suggested consumption of additive‐containing processed food products can contribute to between 25 and 50% of total daily energy intake (Slimani et al. 2009; Moubarac et al. 2013; Adams & White 2015; Costa Louzada et al. 2015; Steele et al. 2016; Zhong et al. 2018; Cediel et al. 2018), whilst total intake of food additives per person in industrial countries has been suggested to be 7‐8 kg per annum (Mepham 2011), although this represents only 0.7‐0.8% of the total food intake of a US adult consuming ~ 996 kg per annum (National Geographic/FAOSTAT 2011).
Table 1.
Functional classes and examples of additives in foods – adapted from European Parliament (2008)
| Functional class | Description | Example additive (E number*) |
|---|---|---|
| Acidity regulators | Alter or control the acidity or alkalinity pH of a foodstuff |
E325 Sodium lactate |
| Acids | Increase the acidity of a foodstuff and/or impart a sour taste to it |
E507 Hydrochloric acid |
| Anti‐caking agents | Reduce the tendency of individual particles of a foodstuff to adhere to one another |
E341 Calcium phosphate |
| Anti‐foaming agents | Prevent or reduce foaming |
E905a Mineral oil |
| Antioxidants | Prolong the shelf‐life of foods by protecting them against deterioration caused by oxidation, such as fat rancidity and colour changes |
E300 Ascorbic Acid |
| Bulking agents | Contribute to the volume of a foodstuff without contributing significantly to its available energy value |
E336 Potassium tartrates |
| Carriers | Dissolve, dilute, disperse or otherwise physically modify a food additive or a flavouring, food enzyme, nutrient and/or other substance added for nutritional or physiological purposes to a food without altering its function (and without exerting any technological effect themselves) to facilitate its handling, application or use |
E1200 Polydextrose |
| Colours | Add or restore colour in a food, and include natural constituents of foods and natural sources, which are normally not consumed as foods as such and not normally used as characteristic ingredients of food |
E100 Curcumin |
| Emulsifiers | Make it possible to form or maintain a homogenous mixture of two or more immiscible phases such as oil and water in a foodstuff |
E322 Lecithin |
| Emulsifying salts | Convert proteins contained in cheese into a dispersed form and thereby bring about homogenous distribution of fat and other components |
E325 Sodium lactate |
| Firming agents | Make or keep tissues of fruit or vegetables firm or crisp, or interact with gelling agents to produce or strengthen a gel |
E333 Calcium citrates |
| Flavour enhancers | Enhance the existing taste and/or odour of a foodstuff |
E620 Glutamic acid |
| Flour treatment agents | Added to flour or dough to improve its baking quality |
E927b Carbamide |
| Foaming agents | Make it possible to form a homogenous dispersion of a gaseous phase in a liquid or solid foodstuff |
E999 Quillaia extract |
| Gelling agents | Give a foodstuff texture through formation of a gel |
E441 Gelatine |
| Glazing agents | When applied to the external surface of a foodstuff, impart a shiny appearance or provide a protective coating |
E901 Bees wax |
| Humectants | Prevent foods from drying out by counteracting the effect of an atmosphere having a low degree of humidity, or promote the dissolution of a powder in an aqueous medium |
E965 Maltitol |
| Modified starches | Obtained by one or more chemical treatments of edible starches, which may have undergone a physical or enzymatic treatment, and may be acid or alkali thinned or bleached |
E1404 Oxidised starch |
| Packaging gases | Gases other than air, introduced into a container before, during or after the placing of a foodstuff in that container |
E938 Argon |
| Preservatives | Prolong the shelf‐life of foods by protecting them against deterioration caused by micro‐organisms and/or which protect against growth of pathogenic micro‐organisms |
E200 Sorbic acid |
| Propellants | Gases other than air that expel a foodstuff from a container |
E942 Nitrous oxide |
| Raising agents | Substances or combinations of substances that liberate gas and thereby increase the volume of a dough or a batter |
E500 Sodium carbonate |
| Sequestrants | Form chemical complexes with metallic ions |
E385 Calcium disodium ethylene diamine tetraacetate |
| Stabilisers | Make it possible to maintain the physico‐chemical state of a foodstuff |
E415 Xanthan gum |
| Sweeteners | Impart a sweet taste to foods or in table‐top sweeteners |
E955 Sucralose |
| Thickeners | Increase the viscosity of a foodstuff |
E1400 Dextrin |
FSA (2018a).
There have been reports of associations between ‘ultra‐processed’ foods and adverse health outcomes in populations around the world, including allergic and autoimmune disorders, some types of cancer, cardiovascular diseases and metabolic disorders, such as type 2 diabetes and obesity (Csáki 2011; Fardet 2018; Srour et al. 2019). ‘Ultra‐processed’ foods have been defined by researchers in South America as industrial formulations ‘…made from processed substances extracted or refined from whole foods… are typically energy dense; have a high glycaemic load; are low in dietary fibre, micronutrients, and phytochemicals; and are high in unhealthy types of dietary fat, free sugars, and sodium’ (Monteiro et al. 2013) and ‘formulations made mostly or entirely from substances derived from foods and additives, with little if any intact…food’ (Monteiro et al. 2017). Furthermore, the researchers state that ‘intense palatability’ achieved by high content of fat, sugar, salt and cosmetic and other additives (along with other factors such as marketing) encourages overconsumption of such foods (Monteiro et al. 2013) and that ‘classes of additives found only in ‘ultra‐processed’ products include those used to imitate or enhance the sensory qualities of foods or to disguise unpalatable aspects of the final product. These additives include dyes and other colours, colour stabilizers; flavours, flavour enhancers, non‐sugar sweeteners; and processing aids such as carbonating, firming, bulking and anti‐bulking, de‐foaming, anti‐caking and glazing agents, emulsifiers, sequestrants and humectants (Monteiro et al. 2017). Suggested examples of ‘ultra‐processed’ foods include ice creams, cake mix, powered soups, reconstituted meat products, packaged ‘instant’ noodles and pre‐prepared meat, fish, vegetable and cheese dishes (Monteiro et al. 2013; Monteiro et al. 2017). It should be noted, however, that the definition of ‘ultra‐processed’ foods, and indeed examples of foods within this category, is highly variable and open to interpretation, highlighting that foods are perhaps better categorised based on their nutrient value rather than level of processing and presence of particular ingredients including additives (Gibney 2018). Regulatory bodies ensure that food additives are rigorously tested for safety and additives continue to undergo long‐term monitoring for their effects on chronic health conditions. Food additives that pass these safety tests are given an ‘E’ number which must be listed on packaging (FSA 2018b).
Widespread use of emulsifiers in the Western diet
Whilst consumption of some food additives (e.g. artificial sweeteners such as sucralose) can be limited through food choice, it may be much more difficult to avoid ingestion of emulsifiers (also known as surfactants or detergents) because they are commonly added to a wide variety of foods within the modern Western diet (see Table 2). Whilst regulatory bodies can define limits on amounts that can be added to food products, information regarding actual content within foods is lacking on food labels, limiting our knowledge of levels consumed and our ability to avoid consumption of a large, diverse array of surfactant compounds used in foods (Halmos et al. 2019). The term ‘emulsifier’ is commonly used for surfactants that are used in both the food and pharmaceutical industries, whilst the term ‘detergent’ is more commonly used to refer to specific surfactants used in household and cleaning products (e.g. washing liquids, shampoos, toothpastes). A wide range of surfactants is available, both those that are man‐made (e.g. polysorbates, derived from polyethoxylated sorbitan and oleic acid, also known as Tween) and natural (e.g. lecithin), many of which can also be modified chemically to alter their properties (Table 2). Surfactants have the common property of being amphophilic [i.e. with a molecular structure that includes both a hydrophile (water‐loving, polar) and a lipophile (fat‐loving) component]. Lipophilic components tend to be similar, but hydrophilic components vary and form the basis for the classification of surfactants as non‐ionic, anionic, cationic and amphoteric. Within the food industry, synthetic non‐ionic polysorbates were introduced in the 1930s, initially incorporated into margarines and then used extensively in the baking industry as preservatives to prevent staling, and enhance firmness and volume of bakery goods (Langhans & Thalheimer 1971; Hasenhuettl & Hartel 2008). Polysorbates, and other synthetic emulsifiers, are frequently incorporated into dietary products, either singly or in combination, usually at doses of 0.2‐0.5% of flour weight (Csáki 2011). Blended with other emulsifiers, such as natural and synthesised sources of mono‐ and diglycerides, polysorbates aid the formation of stable oil‐in‐water emulsions needed for margarines, sauces and dressings, to hold the fat in ice creams and to retard fat bloom (separation of cocoa butter) in chocolate products. In many cases, the same synthetic emulsifiers are used in pharmaceutical products as absorption enhancers (Hasenhuettl & Hartel 2008). Data on the gastrointestinal fate of many emulsifiers are not readily available, although a recent review has highlighted the likely metabolic process for some key surfactants and thickening agents (Halmos et al. 2019). Natural emulsifiers such as lecithin (phosphatidylcholine) are broken down to choline‐rich nutrients on passage through the small intestine by intestinal lipases (Szuhaj 1989; JECFA 1974a) and then acted upon by bacteria to produce triethylamine (Tang et al. 2013). There is a greater resistance to breakdown by digestion of synthetic emulsifiers, such as the polysorbate series of surfactants, as seen for polysorbate 80 where the fatty acid moieties are effectively metabolised but the sorbitol part of the molecule is seen to be highly resistant to digestion in the intestine (JECFA 1974b; Singh et al. 2009). Likewise, carboxymethylcellulose is a non‐digestible polysaccharide polymer, hence its common use as a thickening agent and stabilizer in food emulsions (Halmos et al. 2019). Citric acid esters of mono‐ and diglycerides used to stabilise emulsions in food and infant formulas were thought be completely hydrolysed in the gut into constituent free fatty acids, glycerol and citric acid, and however, recent evidence suggests that the ester bond between citric acid and glycerol is likely not fully hydrolysable (Amara et al. 2014). More work needs to be undertaken in this area.
Table 2.
Common emulsifiers (compound name, ‘E’ number, food and other uses)
| Category | Compound | ‘E’ number* | Food uses | Other uses |
|---|---|---|---|---|
| 1. Synthesised emulsifiers | Mono‐ and diglycerides of fatty acids (glyceryl monostearate, glyceryl distearate) | E471 | Bread, cakes, desserts, margarines, spreads, ice cream, chewing gum | |
| Diacetyl tartaric acid ester of mono‐ and diglycerides (DATEM) | E472e | Baked goods, beverage whiteners, cream, chewing gum, processed meat and poultry, sauces, coffee | ||
| Sodium stearoyl‐2‐lactylate | E481 | Bread, cake, flour products, ice cream, coffee, soft drinks, creams, cookies, crackers, pasta | ||
|
Sucrose esters of fatty acids: Sucrose monolaurate Sucrose monodecanoate Sucrose octaacetate |
E473 | Sauces, salad dressings, candy, chocolate, baked goods, icing and fillings, ice cream, soft drinks, cream, baby food, soups, chewing gum, desserts | Animal feed, pharmaceutical tablets | |
|
Polysorbates (Tweens): Polyoxyethene sorbitan monolaurate (PS20) Polyoxyethene sorbitan monooleate (PS80) Polyoxyethene sorbitan monopalmitate (PS40) Polyoxyethene sorbitan monostearate (PS60) Polyoxyethene (20) sorbitan tristearate (PS65) |
E432
E433
E434
E435
E436 |
Whipped toppings, salad dressings, cakes, cake mixes, edible oils, cake icings and filling, dairy product substitutes, chocolate syrups, ice creams, coffee | Cosmetics, shampoos, floor cleaner, vaccines, drug formulations | |
|
Sorbitans: Sorbitan monostearate (Span 60) Sorbitan tristearate (Span 65) Sorbitan monolaurate (Span 20) Sorbitan monooleate (Span 80) Sorbitan monopalmitate (Span 40) Sorbitan trioleate (Span 85) |
E491 E492 E493 E494 E495 E496 |
Bread, baked goods, active dry yeast, beverages, dairy products, non‐dairy alternatives, margarine and spreads, chocolate, confectionary |
Leather brighteners, plastics, synthetics, creams, pesticides, cosmetics | |
| Methylcellulose | E461 | Ice cream, nutritional supplements, vegetarian products, restructured seafood, batters | Shampoos, toothpaste, lubricant, treatment for constipation, construction materials, glue, paper and textiles, movie special effects | |
| 2. Natural emulsifiers |
Lecithin: Egg yolk Soya (powder/granules) Sunflower |
E322 | Ice cream, margarine, candy, chocolate, soft and hard caramels, chewing gum, cakes, pastries, bread, biscuits, baby formula, hot chocolate, protein powder | Drug formulations (particularly IV), moisturisers, lipstick, foundations, shampoo, soap, creams and lotions, dying leather and textiles, paints and inks, biocides, animal feed |
| Sucrose acetate isobutyrate | E444 | Cocktail mixers, beer, malt beverages | Cosmetics/skin care, fragrance fixative, hair care/styling products | |
| 3. Household detergents | Dodecyltrimethylammonium bromide (DDTMA) | Paint strippers, bactericidal lotions, antiseptics, soaps, purifying or cleansing agents | ||
| Sodium dodecyl sulphate/sodium lauryl sulphate (SDS) | E487 | Whipped products | Household and car cleaning products, laxatives and drug formulation, topical microbicide |
FSA (2018a). IV, intravenous.
Potential concerns regarding the use of emulsifiers in the Western diet
Emerging evidence suggests that permitted dietary emulsifiers may impact on gut health through impairing intestinal barrier function, thus increasing antigen exposure, and/or by modulating the microbiota, thus potentially increasing the incidence of inflammatory bowel disease (IBD) and metabolic syndrome (Roberts et al. 2010; Csáki 2011; Chassaing et al. 2015; Cani & Everard 2015). We have highlighted significant correlations between emulsifier consumption per capita and Crohn’s disease incidence across countries/continents, particularly in Japan, where there has been a particularly marked recent increase in Crohn’s disease (Roberts et al. 2013). Other key food stabilisers and additives, including maltodextrin, have been associated with increased early life intestinal stress, damage and inflammation in animal studies (Arnold & Chassaing 2019). For example, mice consuming a maltodextrin‐rich diet (5% w/v in drinking water over a period of 45 days) displayed an increased susceptibility to intestinal damage and endoplasmic reticulum stress (where improper folding and secretion of intestinal epithelial cell proteins leads to impairment of the intestinal barrier and activation of inflammatory responses in the host) (Laudisi et al. 2019). Likewise, in mice, ingestion of drinking water containing the emulsifier/thickener carboxymethylcellulose (a 2% w/v solution for 3 weeks) induced changes to their intestinal structure and promoted leukocyte migration to the intestinal lumen (Swidsinski et al. 2009). Exposure to carboxymethylcellulose in this study though [∼66 mg/kg bodyweight/day based on a 30g mouse (Vo et al. 2019)] is 2‐3 times higher than the estimated mean daily exposure seen in the US population (Shah et al. 2017). Potential effects of food additives on the gut microbiome have generally been overlooked; however, emerging evidence, mainly from animal studies, suggests that several common food additives, not just emulsifiers, can induce microbiota‐mediated adverse effects (see Table 3). Taken together, the emerging effects on intestinal inflammation and gut microbiota are consistent with those observed in IBD. Food exclusion diets for Crohn’s disease, which encourage the avoidance of additive‐rich ‘processed foods’, have been observed to induce remission, although lots of other dietary factors may be involved (Sigall‐Boneh et al. 2014; Lee et al. 2018).
Table 3.
Common food additives present in a Western diet and their suggested impact on the gut microbiota and/or host physiology (not an exhaustive list)
| Type of additive | Additive and dose | Model | Effect on microbiota | Effect on host physiology | Reference |
|---|---|---|---|---|---|
| Colour | Titanium dioxide (2.3 x 105–2.3 x 109 particles/ml) | Human colon cells | Not determined | Decrease in absorptive microvilli, decreased nutrient uptake | Guo et al. (2017) |
| Emulsifier | Carboxymethylcellulose (2% w/v within drinking water for 3 weeks) | Mice (Il10−/−) | Bacterial overgrowth | Intestinal (small bowel) inflammation | Swidsinski et al. (2009) |
| Emulsifier | Carboxymethylcellulose, or Polysorbate 80 (0.1 to 1% v/v within drinking water for 12 weeks) | Mice (Il10−/−, Tlr5−/− & C57BL/6) | Microbiota encroachment, altered species composition, increased pro‐inflammatory potential | Colitis, metabolic syndrome | Chassaing et al. (2015) |
| Emulsifier | Carboxymethylcellulose, or Polysorbate 80 (0.1 to 1% v/v faecal suspension culture) | M‐SHIME human colon model | Not determined | Increased levels of bioactive flagellin (increased pro‐inflammatory potential) | Chassaing et al. (2017) |
| Emulsifier | Polysorbate 80 (1% v/v per kg bodyweight via gavage, daily for 4 weeks) | Mice (C57BL/6) | Altered microbiota composition | Intestinal inflammation, obesity, impaired glycaemic tolerance, liver dysfunction | Singh et al. (2016) |
| Emulsifier | Polysorbate 80 (1% w/v in drinking water for 8 weeks) | Mice (C57BL/6J) | Altered microbiota composition | Enhanced indomethacin‐induced intestinal damage | Furuhashi et al. (2019) |
| Emulsifier | Glycerol monolaurate (basal diet supplemented with 150 mg/kg ingested daily for 8 weeks) | Mice (C57BL/6) | Altered microbiota composition | Metabolic syndrome, systemic low‐grade inflammation | Jiang et al. (2018) |
| Emulsifier | Methylcellulose (150 g/kg in chow for 7 days) | Mice (Rag1‐/‐ & C57BL/6J) | Not determined | Increased severity of colitis | Llewellyn et al. (2018) |
| Preservatives | Silver nanoparticles (0, 11.4, 114 and 1140 μg Ag NP/kg bodyweight/day for 28 days) | Mice (C57BL/6) | Altered microbiota composition | Not determined | Van Den Brûle et al. (2016) |
| Sweetener | Sucralose (by oral gavage 100, 300, 500, or 1000 mg/kg/day for 12 weeks) | Rats (Sprague Dawley) | Altered microbiota composition | Not determined | Abou‐Donia et al. (2008) |
| Sweetener | Sucralose (0.1 mg/ml within drinking water for 6 months) | Mice (C57BL/6J) | Altered microbiota composition | Altered bile acids, elevated pro‐inflammatory gene expression in the liver | Bian et al. (2017b) |
| Sweetener | Sucralose (1.08, 3.5 and 35 mg/ml within drinking water for 6 weeks) | Mice (SAMP, AKR, and C57BL/6J) | Altered microbiota composition | Increased ileal tissue myeloperoxidase activity | Rodriguez‐Palacios, et al. (2018) |
| Sweetener | Saccharin (0.1 mg/ml within drinking water for 5 weeks) | Mice (C57BL/6) and humans | Altered microbiota composition (mice only, humans not studied) | Glucose intolerance (mice and humans) | Suez et al. (2014) |
| Sweetener | Saccharin (0.3 mg/ml within drinking water for 6 months) | Mice (C57BL/6J) | Altered microbiota composition | Liver inflammation | Bian et al. (2017c) |
| Sweetener | Aspartame (5–7 mg/kg/day for 10 weeks) | Rats (WT) | Altered microbiota composition | Glucose intolerance | Palmnäs et al. (2014) |
| Sweetener | Acesulfame K (37.5 mg/kg/day for 4 weeks) | Mice (CD‐1) | Altered microbiota composition | Weight gain (male mice only) | Bian et al. (2017a) |
| Thickener | Maltodextrin (1 to 5% w/v within drinking water over a period of 45 days) | Mice (Balb/c) | No effect on microbiota composition | Altered mucus barrier, increased intestinal inflammation | Laudisi et al. (2019) |
Adapted from Zinöcker and Lindseth (2018). Ag NP, silver nanoparticles.
Importance of intestinal microbiota in the pathogenesis of human disease
Recent advances in next‐generation sequencing technology have allowed for a greater expansion in our knowledge of the gut microbiota (Simpson & Campbell 2015; Malla et al. 2018). The human gut microbiota, established early in life and becoming stable by around 2‐3 years of age, is influenced by numerous factors including diet, exposure to antibiotics, inflammation and exercise throughout the life course (Arrieta et al. 2014). Perturbations in microbiota composition and activity have been associated with inflammation and with various other conditions including obesity, metabolic syndrome (associated with the risk of developing cardiovascular disease and type 2 diabetes), IBD and colorectal cancer (CRC) (Arrieta et al. 2014; Simpson & Campbell 2015).
Broadly, similar differences in microbiota composition are commonly observed in the faeces, and more importantly, in the mucosa‐associated intestinal microbiota which promotes low‐grade inflammation in both IBD and metabolic syndrome (Everard & Cani 2013; Schaubeck & Haller 2015; Michalak et al. 2016). In IBD, common changes include a reduction in key Gram‐positive bacteria from within the phylum Firmicutes and an increase in Gram‐negative Proteobacteria, especially Enterobacteriaceae such as Escherichia coli pathovars associated with patient bowel lesions and which have been demonstrated to induce intestinal inflammation and inflammation‐associated CRC in mice (Arthur et al. 2012; Merga et al. 2014). In a mouse model of metabolic syndrome, high‐fat diet‐induced diabetes is preceded by an increase in mucosa‐associated Enterobacteriaceae, including E. coli that are actively translocated into mesenteric fat and to the blood (Amar et al. 2011). Human studies have since confirmed that faecal transplantation from lean donors can improve the insulin sensitivity of the recipients, supporting the role of intestinal microbiota composition as a contributor to the development of metabolic syndrome (Vrieze et al. 2012; Nieuwdorp et al. 2014). We, and others, have reported an increase in mucosally associated E. coli in both Crohn’s disease and CRC (Swidsinski et al. 1998; Darfeuille‐Michaud et al. 2004; Martin et al. 2004). M (microfold) cells overlying ileal Peyer’s patches and smaller lymphoid follicles in the colon are the likely portal of entry for Crohn’s disease E. coli (Chassaing et al. 2011; Dogan et al. 2014; Prorok‐Hamon et al. 2014).
What are the potential mechanisms behind the effects of synthetic food emulsifiers on health and are they generalisable to all emulsifiers?
Remarkably, there has been little study of the potential harmful effects of ingested detergents or emulsifiers in humans. Whilst investigating the impact of dietary components on bacterial–epithelial interactions relevant to IBD and CRC, we explored the hypothesis that increases in intestinal epithelial barrier permeability to bacteria might result from ingestion of emulsifiers or detergents (Roberts et al. 2010). We showed that the presence of permitted food emulsifier polysorbate 80, at low concentrations (0.01‐0.1% v/v) that might plausibly be present in the distal ileum of someone consuming a Western‐style diet, markedly increased translocation of mucosa‐associated E. coli across epithelial cell monolayers and across human ileal mucosa explants cultured in Ussing chambers (Roberts et al. 2010). This occurred across both M cells and across villous epithelium which would not normally allow entry of bacteria in healthy individuals (Fig. 1). At these low concentrations, bacterial translocation was transcellular (i.e. through cells) and not via the paracellular route (i.e. through increased ‘leakiness’ of intercellular tight junctions).
Figure 1.
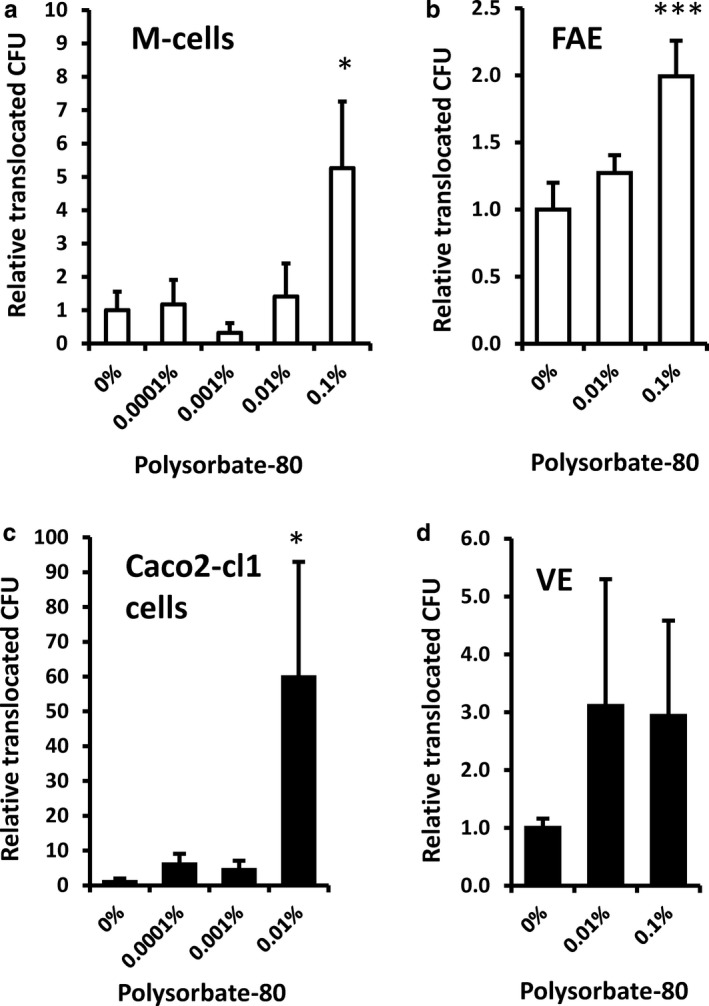
Dietary emulsifier polysorbate 80 increases translocation of E. coli across intestinal epithelial cell cultures (a and c) and intestinal ileum epithelium mounted in Ussing chambers (b and d). M (microfold)‐cell (Caco2‐cl1/Raji B cell co‐culture) model (a), Caco2‐cl1 intestinal cell monolayers (c), human ileal villous epithelium (VE) (d) or follicle‐associated epithelium (FAE) overlying Peyer’s patches (b). *, p < 0.05; **, p < 0.01; Kruskal–Wallis analysis of variance (ANOVA) corrected for multiple comparisons; n = 4‐8). Reproduced from Roberts et al. (2010) with permission from BMJ Publishing Group Ltd – Copyright clearance center Licence number 4638800129868. CFU, colony‐forming unit
Benoit Chassaing and colleagues undertook in vivo animal studies where ingestion by mice of polysorbate 80 at higher concentrations [up to 1% v/v in their drinking water for 13 weeks; about equivalent to 2500 mg/kg bodyweight/day (see Vo et al. 2019)] caused depletion of the mucus barrier, allowing for closer apposition between luminal bacteria and the intestinal epithelium. More severe inflammation was observed in colitis‐susceptible interleukin‐10 knockout (Il10‐/‐) mice (Chassaing et al. 2015). Although ingestion of emulsifiers did not alter the total bacterial load in the faeces, it did significantly increase the number of bacteria adherent to the colon in both wild‐type and Il10‐/‐ mice and altered the overall composition of the microbiota, including increasing the predominance of potentially inflammation‐promoting Proteobacteria. Intriguingly, they also found that mice fed polysorbate 80 developed low‐level inflammation and metabolic syndrome (Fig. 2). These changes, including a weakened mucus layer, were not seen in germ‐free mice lacking a microbiota and but were observed upon transfer of faeces from emulsifier‐fed mice to germ‐free recipients. Similar effects were seen in mice consuming diets containing carboxymethylcellulose (Chassaing et al. 2015), arguably a food thickener rather than a true emulsifier, but which has been shown previously in other mouse studies to induce inflammation in the small intestine (Swidsinski et al. 2009). Subsequent mouse studies showed that both polysorbate 80 and carboxymethylcellulose also potentiated intestinal inflammation associated with CRC (Viennois et al. 2017).
Figure 2.
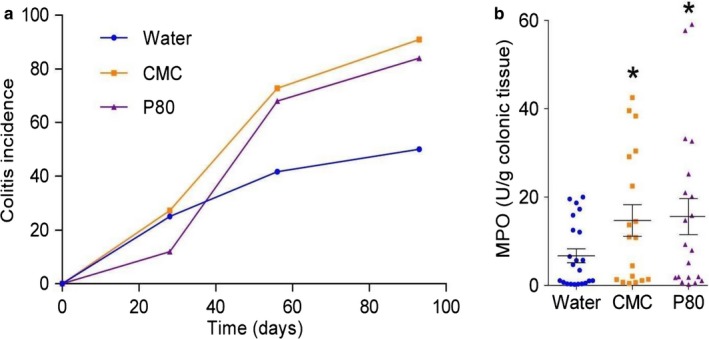
Emulsifiers polysorbate 80 (P80) and carboxymethylcellulose (CMC) administered to drinking water (1.0% v/v for 12 weeks) promote colitis (incidence of epithelial damage and inflammatory infiltrate, as determined by histology, in colonic tissues over time) (a) and increase colonic tissue myeloperoxidase (MPO) (b) in Il10‐/‐ mice, and low‐grade intestinal inflammation in wild‐type mice (not shown). Points are from individual mice. *p < 0.05 compared to water‐treated group, using one‐way ANOVA corrected for multiple comparisons. Reproduced from Chassaing et al. (2015) with permission from Springer Nature – Copyright clearance center Licence number 4638801338133 [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Chassaing and colleagues also recently showed that emulsifiers can cause striking changes in the microbiota. When added to a dynamic in vitro slurry that mimicked a human colonic microbial culture, both polysorbate 80 and carboxymethylcellulose induced gene expression profile changes in the bacterial slurry, including an increase in bacterial flagellin expression. When administered to mice by gavage, these slurries with emulsifier‐altered expression profiles induced low‐grade inflammation and metabolic syndrome, whereas a similar slurry not treated with emulsifiers did not (Chassaing et al. 2017). However, to date these effects have not been confirmed in humans. Comparison with estimated dietary exposure to polysorbate 80 and carboxymethylcellulose suggests that researchers conducting these animal studies have used far higher levels of exposure than would typically be seen for the US population (Shah et al. 2017; Vo et al. 2019). Using maximum‐use levels obtained from publicly available sources, it has been estimated that lecithin and mono‐ and diglycerides have the highest mean exposures among consumers (between 60 and 80 mg/kg bodyweight/day), whereas the exposure to carboxymethylcellulose is half to one‐third less, and the exposure to polysorbate 80 is approximately half that of carboxymethylcellulose, with no additional evidence available to suggest that levels have increased since 2010 (Shah et al. 2017).
Emulsifiers, or surfactants/detergents, are also useful in reducing surface tension between two different substances and hence are commonly used as dishwashing detergents. It is highly plausible that contamination of food by washing detergents that have not been fully rinsed from cutlery and crockery could also be harmful if ingested (Roberts et al. 2013; Rhodes 2018). These detergents are often used in shampoos and toothpaste, including sodium dodecyl (lauryl) sulphate. The potential harm of washing detergents is seen in one early study which reported that dogs given regular intravenous injections of the non‐ionic detergent Triton WR‐1339 over 4‐5 months all died, with evidence of early atheroma (Scanu et al. 1961). A less drastic study showed that rodents ingesting washing detergent, at low levels that could plausibly be ingested by human infants, had increased permeability and irreversible atrophy of the intestinal villi (Mercurius‐Taylor et al. 1984).
The most extensively consumed emulsifier is the phospholipid lecithin, a natural zwitterionic surfactant present in all plant and animal cell walls (Kinyanjui et al. 2003). It is typically commercially sourced from soybeans and sunflowers (an alternative source increasingly used in industry as it does not need to be avoided by people with a soya allergy), but perhaps best known as a key component of egg yolks, accounting for their emulsifier properties used to make foodstuffs such as mayonnaise. Daily intake of lecithin from food sources in a typical Western diet averages about 3.6 g/day but can be up to 7 g/day, with a single egg yolk typically containing around 1.8 g of lecithin (Canty & Zeisel 1994; Palacios & Wang 2005). In contrast, total polysorbate intake is only around 10‐100 mg/day, although synthetic detergents are more resistant to breakdown by digestion (Singh et al. 2009). Lecithin contains varying amounts of phospholipids: phosphatidylcholine (egg lecithin 80% w/w; soy lecithin 20‐30%); phosphatidylethanolamine (egg lecithin 12%; soy lecithin 20‐30%); and phosphatidylinositol (egg lecithin 5%; soy lecithin 20%) (Palacios & Wang 2005; American Lecithin Company 2009). There are, as yet, no published studies of the impact of lecithin on either the bacterial translocation or the microbiota. Also, a considerable quantity of lecithin enters the human intestine in bile (1.4‐8.1 g/l), which, with bile secretion at around 0.75 l/day, amounts to ~ 1‐6 g/day of phosphatidylcholine entering the human intestine daily (Boyer 2013). The presence of phosphatidylcholine in bile is beneficial, helping to prevent cholesterol gallstone formation and reduce toxicity of bile acid micelles (Tompkins et al. 1970).
Ingestion of lecithin at high dosage in healthy human volunteers (22‐83 g/day for 2‐4 months) has shown no obvious ill effects, though a lowering of plasma triglyceride levels has been reported (Cobb et al. 1980). Therefore, it may have been considered unnecessary to define a safe limit, although the European Food Safety Authority (EFSA) does propose an ‘adequate intake’ of dietary choline (a quaternary amine, mainly present in lecithin as phosphatidylcholine and released during digestion by intestinal lipases) as 400 mg/day for adults (JECFA 1974a; EFSA NDA Panel 2016). Indeed, egg yolk, soy lecithin and lecithin components such as phosphatidylinositol have been shown in short‐term dietary supplementation studies to induce potentially beneficial elevations in high‐density lipoprotein (HDL)‐cholesterol (Burgess et al. 2005; Blesso et al. 2013) and a reduction in serum low‐density lipoprotein (LDL)‐cholesterol (Mourad et al. 2010), and encapsulated phosphatidylcholine designed for colonic delivery has shown promise in treatment of ulcerative colitis (Karner et al. 2014). However, dietary lecithin, or more specifically phosphatidylcholine, has been indicated as a possible risk factor for coronary artery disease, likely a consequence of its conversion of choline by the intestinal microbiota to the pro‐atherogenic metabolite trimethylamine‐N‐oxide (Tang et al. 2013).
The other widely consumed group of food emulsifiers is the mono‐ and diglycerides of free fatty acids (Moonen & Bas 2004). Commercially, these are semi‐synthetic, largely manufactured by enzymatic hydrolysis of triglycerides although they are also thought to occur naturally by the hydrolysis of triglycerides by lipase. There are no published data yet on their interactions with the mammalian microbiota or intestinal epithelium.
The health effects of conjugated bile acids, which are another variety of powerful detergents/emulsifiers that our intestines are continuously exposed to on a daily basis, should also be considered. We have speculated that detergents, such as bile acids, may cause harm only if they co‐exist with bacteria particularly in the terminal small intestine where, unlike the colon, there is no continuous mucus barrier (Johansson et al. 2014) and where, even in healthy individuals, there is substantial backwash of bacteria through the ileocaecal valve (Vince et al. 1972; Simon & Gorbach 1984), with a consequent mucosal colonisation that is more marked in Crohn’s disease (Gevers et al. 2014). The highly effective ileal reabsorption of bile acids under healthy conditions, which starts to occur at least 100 cm proximal to the ileocaecal valve (Ung et al. 2002), may mean that relatively little conjugated bile acid remains in the gut lumen by the distal 20 cm or so of the ileum. Conjugated bile acids, like other detergents and emulsifiers, can form micelles and thus facilitate fat absorption but also like other detergents, have a potential for cell toxicity (Raimondi et al. 2008). Dihydroxy bile acids (e.g. chenodeoxycholic and deoxycholic acid), formed by microbial dehydroxylation (i.e. loss of the 7α‐hydroxyl group on the bile salt nucleus), are known to enhance permeability and uptake of bacteria across the human colonic mucosa (Münch et al. 2007; Münch et al. 2011).
It has long been known that bacterial translocation from the intestine into the blood occurs in very sick individuals (e.g. sepsis patients in intensive care) (Quigley 2011), but it is becoming apparent that this may be much more common and particularly relevant to the pathogenesis of a number of diseases. For example, significant increases in circulating bacterial DNA have been reported in venous samples from patients with cardiovascular disease (Dinakaran et al. 2014), type 2 diabetes (Sato et al. 2014) and Crohn’s disease (Gutiérrez et al. 2014). In Crohn’s disease, the presence of circulating bacterial DNA has also been shown to be highly predictive of subsequent relapse (Gutiérrez et al. 2016). The mouse studies examining the effect of ingestion of emulsifiers polysorbate 80 and carboxymethylcellulose (Chassaing et al. 2015), although not directly assessing bacterial translocation across the intestine into the circulation, did report an increase in circulating anti‐lipopolysaccharide and anti‐flagellin antibody in mice consuming emulsifiers, suggesting an altered intestinal permeability and an increased exposure to bacteria‐derived molecules. Further studies by the same group showed that these emulsifiers did affect the microbiota (Chassaing et al. 2017), and changes in the composition of the microbiota can lead to increased bacterial translocation, as has been shown dramatically with antibiotics (Knoop et al. 2016). Dietary exposure to the natural emulsifier lecithin in the human diet is far higher than that seen for either polysorbate 80 or carboxymethylcellulose (Shah et al. 2017). Our own preliminary study in mice has suggested that ingestion of 0.1% w/v egg lecithin for 4 days in their drinking water can enhance bacterial translocation to the systemic circulation and distant organs, with levels of total bacteria measured being much greater than those we observed in mice ingesting 0.1% v/v polysorbate 80 (unpublished observations). Further evidence in mice suggests that dietary soya lecithin can enhance acute fatty acid absorption across the intestine (Couedelo et al. 2015) and induce inflammation and hypertrophy of white adipose tissue (Lecomte et al. 2016). Adipose tissue inflammation can occur as a result of enhanced lipopolysaccharide translocation across the intestine (Kim et al. 2012). Whether lecithin consumption in humans increases systemic levels of bacterial DNA is yet to be determined.
Objectives of the MECNUT Emulsifier project and the FADiets study
The Mechanistic Nutrition in Health (MECNUT) Emulsifier project, incorporating the human Food Additives – do processed Diets impact on gut and metabolic health (FADiets) study, is funded by the Medical Research Council (MRC) (from November 2018 to October 2020) to answer two main questions:
What impact do different emulsifiers have on the mucosal barrier, particularly in respect to bacterial translocation and inflammation?
Does ingestion of the dietary emulsifier lecithin in controlled diets induce bacterial translocation and affect selected biomarkers of gut and metabolic health in healthy volunteers?
An overview of the proposed approach is presented in Figure 3.
Figure 3.
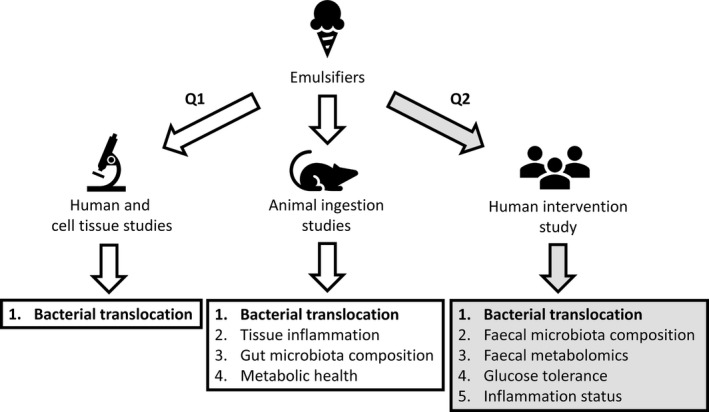
Overview of the approach to study the effects of emulsifiers on bacterial translocation, intestinal inflammation and metabolic health. We propose to use human intestinal cell cultures, 3‐D ‘mini‐gut’ organoid cultures and human ileal tissue explants, and mouse models to investigate Question (Q) 1: ‘What impact do different food emulsifiers have on the mucosal barrier particularly in respect to bacterial translocation and inflammation?’ A human volunteer study has been designed to answer Q2: ‘Does ingestion of the dietary emulsifier lecithin in controlled diets induce bacterial translocation and affect selected biomarkers of gut and metabolic health in healthy volunteers?’
Objective 1: To assess the impact of a wide range of commercially‐used food emulsifiers, dihydroxyl bile salts (as a source of natural detergents) and synthetic dish washing detergents on the mucosal barrier, particularly in respect of bacterial translocation and inflammation
The MECNUT Emulsifier study will implement a three‐step model approach, examining the effect of treatment or ingestion of a wide range of permitted food emulsifiers, bile salts and household dishwashing detergents (see Table 2), using the following:
in vitro human cell lines of the intestinal epithelium, an M‐cell model of the follicle‐associated epithelium and intestinal crypt stem‐cell derived 3‐dimensional (3‐D) ‘mini‐gut’ organoid cultures;
ex vivo human distal ileum tissue explants mounted in Ussing chambers; and
in vivo mouse studies.
In vitro cell‐line studies
Expanding on our previous research (Roberts et al. 2010), to explore how a wide range of emulsifiers influence bacterial translocation across the intestinal epithelium, we will use three well‐characterised human intestinal epithelial cell‐line cultures: fully differentiated Caco2 (modelling small intestinal enterocytes), HT29 (colonocytes) (Martin et al. 2004) and Caco2‐Raji B lymphocyte co‐cultures [an M‐cell model of the follicle‐associated epithelium (Roberts et al. 2010)]. These will be grown as monolayers on Millicell™ membrane culture plate inserts to allow treatment and sampling of the apical and basolateral aspects. As a primary endpoint, the ability of emulsifiers to affect transcellular movement of bacteria (i.e. entry through cells within the monolayer) will be monitored over 4 hours. Emulsifiers will be added apically to cell monolayers for 30 minutes prior to addition of E. coli or Salmonella, along with low molecular weight (3 kDa) dextran to monitor paracellular permeability (i.e. ‘leakiness’ of cell–cell tight junctions). Transepithelial electrical resistance will also be monitored to provide additional information about the integrity of the tight junctions formed between the polarised cells of the monolayers throughout all treatment stages. Emulsifiers and detergents will be used at concentration ranges found within a typical Western diet, as well as below and up to those that would start to disrupt cell–cell tight junctions (Münch et al. 2007; Roberts et al. 2010; Münch et al. 2011). For polysorbates, such as polysorbate 80, known to show resistance to digestion (Singh et al. 2009), we previously calculated that 0.01% v/v would be realistic (Roberts et al. 2010). Based on the acceptable daily intake of 25 mg/kg bodyweight (JECFA 1974b), a level of 0.01% would represent a persistence of 6.7% into the terminal ileum of a typical 60 kg human, assuming 1l of intestinal contents per day passing to the caecum. Assuming 1 g/ml [approximately correct for lecithin based on daily output of biliary phosphatidyl choline plus daily dietary intake of lecithin (JECFA 1974a; Boyer 2013)] then for lecithin, this would be ~ 6% v/v entering the caecum assuming 1 l/day of intestinal contents entering caecum – but this would of course allow for no breakdown of lecithin during digestion (Szuhaj 1989), so study levels up to 5% w/v likely would be appropriate. For dishwashing detergents, Mercurius‐Taylor and colleagues calculated intake in adults of ~ 1 mg/kg/day arising from residue left on detergent washed (5 ml detergent in 2 l tap water), unrinsed crockery and glassware (Mercurius‐Taylor et al. 1984) – again assuming 1 l/day entering caecum and no breakdown or absorption proximally, this would amount to 7 mg/100 ml (i.e. 0.007% v/v). For bile salts, levels to be tested are as defined by Münch et al. (2007, 2011).
Study of some of the most commercially used non‐ionic series of emulsifiers [such as the 6 fatty acid ester series of sorbitan (Span 20 to 85) and their ethoxylated derivatives (polysorbates 20 to 85)] may enable us to correlate the effect of the head group and hydrophobic tail of the surfactants with their functional behaviour (Tadros 2005). We have already shown differences in the ability of polysorbate 60 and polysorbate 80 to promote bacterial translocation through intestinal epithelial cells and M cells in culture without impacting on membrane paracellular permeability (i.e. a transcytotic effect), with polysorbate 60 showing less marked ability to effect translocation (Roberts et al. 2010).
Key emulsifiers/detergents identified as enhancing epithelial transcytosis of bacteria in the human cell‐line studies will be further studied using ‘mini‐gut’ organoid cultures. Generated from intestinal crypt tissue stem cells, organoids (both those from the small and large intestine) mimic the complex 3D architecture of the epithelium, with all differentiated cell types being present (Nigro et al. ). Emulsifiers will be microinjected along with enhanced green fluorescent protein (EGFP)‐expressing E. coli and a paracellular permeability dye to monitor organoid epithelium integrity. These ‘mini‐gut’ models will serve to bridge the in vitro and in vivo work. Experiments will be carried out using emulsifiers both in solution (where possible) and as emulsions, in combination with low and high‐fat levels, to resemble more closely food structures as influenced by process and ingredient interactions, and interactions taking place within the intestinal lumen.
Ex vivo human tissue studies
Emulsifiers/detergents identified as affecting translocation of bacteria in the in vitro and ex vivo models, also including egg and soy lecithin, and polysorbate 80 as comparators (Roberts et al. 2010), will be assayed for their ability to enhance translocation of EGFP‐expressing E. coli and/or Salmonella across villous epithelium isolated from fresh, macro/microscopically normal, human distal ileum tissue removed during routine surgery (e.g. for colon cancer) and mounted in Ussing chambers as previously described (Roberts et al. 2010; Chassaing et al. 2011). Again, transepithelial electrical resistance will be monitored to assess any changes in paracellular permeability.
In vivo mouse studies
Impact of ingestion (either in drinking water or incorporated within the diet, short‐ and long‐term) of five emulsifiers [polysorbate 80, lecithin and sodium dodecyl (lauryl) sulphate plus two selected from the in vitro studies] will be studied in wild‐type C57BL/6J and Il‐10‐/‐ mice (a colitis‐susceptible model relevant to human IBD). The primary endpoint will be detection of venous blood bacterial DNA as a marker of microbiota translocation from the intestinal lumen to the circulation and also to the systemic organs such as the liver, spleen and kidneys. Evidence of altered intestinal barrier function (Williams et al. 2013), histological intestinal inflammation, bacterial DNA in venous blood, systemic organs and community alterations in the intestinal microbiota [using total bacteria, phyla‐ and class‐specific qPCR (Bacchetti De Gregoris et al. 2011)] will also be evaluated.
Objective 2: To assess whether ingestion of the dietary emulsifier lecithin in controlled diets induces bacterial translocation and affects selected biomarkers of gut and metabolic health in healthy volunteers
The FADiets study primarily aims to determine whether short‐term (2 week), high intake of lecithin alters gut function, as indicated by the increased presence of bacterial DNA in the circulation (venous blood sampling), increased gut inflammation (faecal sampling for white blood cell components such as calprotectin), gut microbiota and metabolic activity [bacterial diversity, faecal short‐chain fatty acids (SCFAs) and volatile organic compounds] and altered glucose metabolism (assessed by oral glucose tolerance test).
The study will implement a 5‐week randomised crossover dietary intervention trial (https://clinicaltrials.gov/ Identifier: NCT03842514), in overweight or obese (but otherwise healthy) adults (BMI ranging from 27 to 40 kg/m2), comparing a ‘low‐calorie, low‐emulsifier’ diet, with a ‘low‐calorie, high‐emulsifier’ diet (Fig. 4). This population was chosen to increase recruitment and retainment of subjects throughout the study by offering the opportunity to lose weight should they follow our diets. Both diets are weight loss (low‐calorie) diets, fed to basal energy requirements with below average amounts of red meat, fish and eggs [compared with amounts consumed by the UK population according to National Diet and Nutrition Survey data (Bates et al. 2014)] to ensure a relatively low lecithin intake, so participants are likely therefore to be more compliant and consume all the food provided. Low‐calorie diets provided are identical in all aspects (energy, macronutrients and foods) so as to counter any possible microbiota effects driven by the energy content of the diet, except for the addition of 15 g (2 x 7.5 g/day) soya lecithin to the high‐emulsifier diet. The low‐emulsifier diet consisting of commercially available foods (of verified ingredient composition) will provide ~ 0.3 g/day choline [which is below the EFSA adequate daily intake (ADI) and US Department of Agriculture (USDA) guidance minimum for adults of 0.4 g/day (EFSA NDA Panel 2016; USDA 2019)] from food, and the emulsifier supplemented diet will provide 3.7 g/day choline (1.73 g choline twice daily in the form of the soya lecithin granules – which is 3.46 g/day), giving 3.7 g in total from food and the supplement (Table 4).
Figure 4.
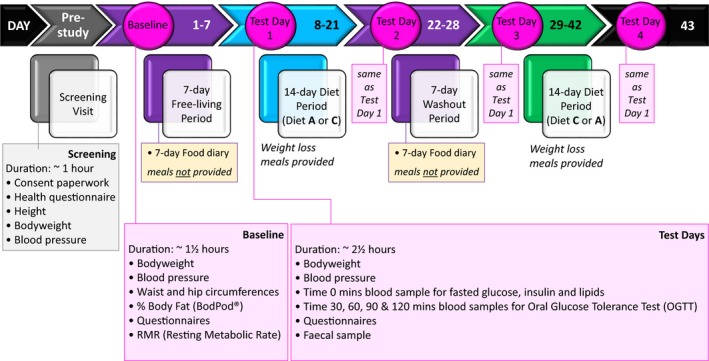
The FADiets study protocol summary. The research question: ‘Does dietary intake of soy lecithin alter the intestinal lining and the microbes that normally exist in the intestinal lumen, in healthy subjects, consumed over a 2‐week period (in comparison to a low‐emulsifier diet)?’ [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Table 4.
Low‐calorie intervention diets provided to volunteers on the FADiets study
| Low calorie, high emulsifier diet * | |||||
|---|---|---|---|---|---|
| Day | Energy † (kcal) | Fat (%) | Protein (%) | Carbohydrates/fibre (%) | Choline ‡ (from diet & lecithin supplement) (mg) |
| 1 | 2000 | 30 | 15 | 55 | 3717.4 |
| 2 | 2000 | 30 | 15 | 55 | 3698.2 |
| 3 | 2000 | 30 | 15 | 55 | 3810.1 |
| 4 | 2000 | 30 | 15 | 55 | 3707.7 |
| 5 | 2000 | 30 | 15 | 55 | 3783.2 |
| 6 | 2000 | 30 | 15 | 55 | 3722.7 |
| 7 | 2000 | 30 | 15 | 55 | 3725.7 |
| Average | 3737.9 | ||||
| Low calorie, low emulsifier diet | |||||
| Day | Energy † (kcal) | Fat (%) | Protein (%) | Carbohydrates/fibre (%) | Choline ‡ (from diet only) (mg) |
| 1 | 2000 | 30 | 15 | 55 | 270.4 |
| 2 | 2000 | 30 | 15 | 55 | 263.1 |
| 3 | 2000 | 30 | 15 | 55 | 367.1 |
| 4 | 2000 | 30 | 15 | 55 | 261.6 |
| 5 | 2000 | 30 | 15 | 55 | 339.9 |
| 6 | 2000 | 30 | 15 | 55 | 273.1 |
| 7 | 2000 | 30 | 15 | 55 | 265.6 |
| Average | 291.5 | ||||
Low‐calorie test diet supplemented with 2 daily servings of 7.5 g Lamberts® soya lecithin granules in fruit smoothies (total 15 g/day).
Energy intakes matched to the closest calorie to each volunteer resting metabolic rate assessed at baseline by indirect calorimetry (range 1500 to 3000 kcal). Example 2000 kcal matched diets are shown.
The dietary intervention will consist of two 14‐day diet periods, with either no added emulsifier or 15 g soya lecithin per day, with a 7‐day baseline habitual diet (‘free‐living’) period [i.e. to reflect normal eating patterns of participants] and a 7‐day mid‐study washout [i.e. a return to normal (habitual) diet], so that both periods prior to intervention are the same (habitual food intake will be recorded using food diaries). A high‐profile human dietary intervention study (David et al. 2014) used a similar period of washout to ensure recovery of microbial diversity following each study arm examined, and we have also previously shown that the microbiota responds quickly to changes in diet and that these changes are rapidly reversed by intervention with a subsequent diet (Walker et al. 2011). All food and drinks for the intervention diets will be prepared by the Human Nutrition Unit at the Rowett Institute for volunteers to collect, reheat and consume at home. Volunteers will undergo initial health screening and confirmation of eligibility to participate and will be randomised for treatment order, to be conducted by computer generation by our statistical support at Biomathematics and Statistics Scotland. All volunteers will be matched to the closest calorie to their resting metabolic rate (assessed at baseline by indirect calorimetry). The study will be conducted in a non‐blinded method with diets colour‐coded.
The lecithin supplement to be used is a commercial source of soya lecithin granules [Lamberts® – a food‐grade product, manufactured to the stringent pharmaceutical standards of Good Manufacturing Practice (GMP); composition summarised in Table 5]. It will be ingested twice daily (at a dose of 7.5 g), incorporated into a fruit smoothie matrix for stability, consistency of preparation and consumer acceptability. The total dose (15 g/day) is substantially greater than the typical dietary intake of up to 7 g/day, so is expected to be enough to test for possible effects in what is a relatively short‐term study. Previous work has reported that 7.5 g of lecithin, ingested three times daily for 4 weeks and had no adverse effects in human volunteers (Cobb et al. 1980), but this study did not measure bacterial translocation or changes to the gut microbiota.
Table 5.
Fat and phospholipid composition of Lamberts® soya lecithin granules used to supplement the FADiets study low‐calorie, high‐emulsifier test diet
| Component | Amount (g) per 7.5 g serving* |
|---|---|
| Phosphatidyl choline | 1.7 |
| Phosphatidyl ethanolamine | 1.5 |
| Phosphatidyl inositol | 1.1 |
| Phosphatidic acid | 0.6 |
| Phosphatidyl serine | 0.075 |
| Fatty acids | 3.8 |
|
of which: – saturated |
0.9 |
| – monounsaturated | 0.3 |
| – polyunsaturated | 2.5 |
Full composition can be found at http://www.lambertshealthcare.co.uk
Participants on low‐calorie, high‐emulsifier diet will ingest within food a 7.5 g serving twice daily.
All study participants will keep a daily weighed food intake diary during the 7‐day baseline and 7‐day washout periods, and complete a gastrointestinal discomfort questionnaire (Storey et al. 2007). Stool samples will be collected during both arms and during the washout period, with faecal 16S rRNA gene sequencing and volatile organic compound analysis (measuring alterations in microbiota genes and metabolic activity, respectively) performed on all available samples.
The primary objective will be to assess bacterial translocation in response to a diet containing soya lecithin emulsifier, in comparison with the control period of a low‐emulsifier diet. Bacterial DNA in venous blood sampled at the start and end of each diet period will be measured by qPCR (Bacchetti De Gregoris et al. 2011). In support, serum lipopolysaccharide‐binding protein and soluble CD14 will also be measured as secondary markers of bacterial translocation to the circulation (Landmann et al. 1995; Blairon et al. 2003; Knapp et al. 2003; Uhde et al. 2016).
Secondary endpoints of the FADiets study include changes in bacterial diversity and metabolic activity, gut inflammation and glucose metabolism. Alterations to the composition of the gut microbiota have been observed in animal models following exposure to emulsifiers (Table 3), with some reporting increased Proteobacteria (Chassaing et al. 2015; Furuhashi et al. 2019). We will monitor for any differences in faecal bacterial community structure between habitual diet, and the high‐ and low‐emulsifier diets by Illumina MiSeq sequencing of the V1–V2 regions of bacterial 16S rRNA genes isolated from participant faecal samples. Taxonomic profiles as well as microbiota diversity measures such as Shannon and inverse Simpson indices will be made as previously described (Chung et al. 2016; Reichardt et al. 2018). Short‐chain fatty acids, such as acetate, butyrate and propionate, generated by the majority of bacteria in the intestine, are a major fuel source for epithelial cells lining the bowel (Reichardt et al. 2018), and some, such as butyrate, may have anti‐inflammatory and anti‐carcinogenic effects (Hamer et al. 2008). Levels of SCFAs are therefore indicative of bowel health, and participant faecal samples will be analysed for key SCFAs by gas chromatography profiling (Richardson et al. 1989). Volatile organic compounds present in faeces, resulting from the combined metabolism of the gut mucosa and microbiota, can be indicative of intestinal infection and inflammation, so participant sample volatile organic compounds will be measured using optimised methods for analysis of faecal headspace gases by gas chromatography‐mass spectrometry (Ahmed et al. 2016). Faecal calprotectin levels will also be monitored as these have been found to be significantly increased in stools of patients with IBD, whereas they are not elevated in patients with non‐inflammatory functional diseases such as irritable bowel syndrome (Burri & Beglinger 2014). Plasma trimethylamine‐N‐oxide, as a measure of cardiovascular risk (Tang et al. 2013), will also be monitored. Plasma fasting glucose and glucose tolerance will be assessed in study participants using a standard 75 g 2‐hour oral glucose tolerance test at the start and end of each dietary intervention period. In addition, circulating lipids and levels of insulin will be measured, with appropriate calculations performed to estimate insulin sensitivity (Blesso et al. 2013).
Proposed mechanism of action
Our proposed mechanism of action for some commonly used dietary emulsifiers, consumed daily at low levels, on gut and metabolic health is that they may (1) cause alterations to the gut microbiota and (2) disrupt the intestinal mucosal barrier. Together, these (3) contribute to increased permeability of the intestinal layer and (4) promote the increased translocation of bacteria from the gut to the bloodstream. This results in (5) a state of low‐grade inflammation, (6) glucose intolerance and (7) increased risk of IBD (Fig. 5). Within the series of experiments proposed above, we will study all the proposed constituents, apart from the end result of (7) increased risk of IBD, due to the chronic nature of this outcome.
Figure 5.
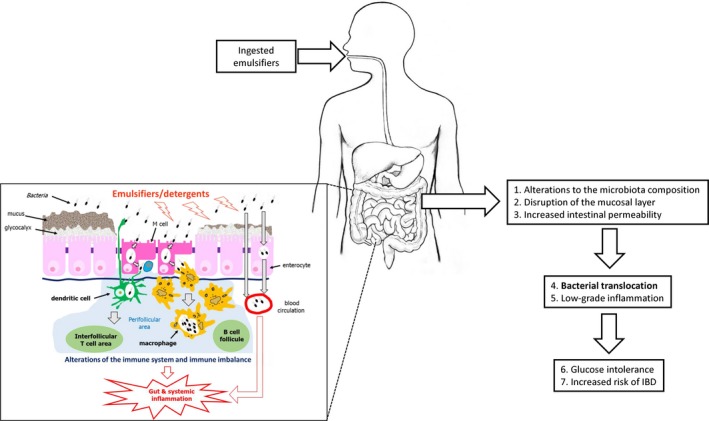
Overview of proposed adverse health effects of ingested emulsifiers. The insert shows the hypothesised mechanisms of action of emulsifiers on altering the microbiota, causing disruption of the mucosal barrier, enhancing bacterial translocation through and between intestinal epithelial cells and increasing uptake across M (microfold)‐cells of the follicle‐associated epithelium. This may lead to immune imbalance, increased levels of bacteria in the circulation and increased gut and systemic inflammation. IBD, inflammatory bowel disease [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Beneficiaries and public health message
The expectation is that the results of these proposed studies will lead to greater public awareness and reduction where need is identified, in the use of certain emulsifiers in the food chain that have potential to cause harm. The academic nutrition and public health communities, with the scientific advice of key independent food safety authorities (e.g. EFSA and JECFA, the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives) examining food additives, food processing and changing consumption patterns across populations, may then be better placed to support informed choice for healthier diet planning, not only to better develop during infancy and enjoy a healthy old age, but also including support towards dietary strategies for combating IBD, cardiovascular risk and metabolic disorders, such as metabolic syndrome, type 2 diabetes and obesity.
The data obtained would be of interest to those working in basic scientific fields of nutrition and intestinal biology, and those working in the fields of gut ‘omics’ and gut health. Outcomes would also be of significant interest to formulation scientists working in and around the food industry (e.g. in food composition, surfactant and natural biopolymer formulation science), at leading companies supplying the food, nutrition and pharmaceutical (drug formulation and delivery) emulsifier market, and to key providers of further education in agri‐food science, food industry development, technologies and practice. As an example, the Food Additives and Ingredients Association works with both the food industry and consumers, to promote a better understanding of the role of food additives and functional food ingredients in a healthy and safe diet.
Dissemination
Overall, the outcomes of this research proposal will add to the literature on whether excessive exposure to emulsifiers in the food chain could be harmful to health; data relevant to the key international health and food safety regulatory authorities, the scientific food research community and the food industry (both processing and additive formulation).
Conclusions
Emerging in vitro and animal evidence suggests that food additives such as emulsifiers may contribute to gut and metabolic disease development through alterations to the gut microbiota, intestinal mucus layer, increased bacterial translocation and associated inflammatory response. The MECNUT Emulsifier project aims to further explore the mechanistic basis for these relationships across a wide range of permitted dietary emulsifiers and detergents in vitro. As part of this work package, the FADiets study aims to determine the impact of soy lecithin on gut and metabolic health in vivo using a controlled dietary intervention. This growing area of nutritional science may lead to innovative knowledge, which could pave new ways of addressing gut and metabolic health via implementing dietary guidelines directed at food additives.
Conflicts of interest
No conflicts of interest have been declared by any author.
Funding
This study is funded by the Medical Research Council (MR/P023606/1).
Ethics
The human volunteer FADiets study (https://clinicaltrials.gov/ Identifier: NCT03842514) will be conducted in compliance with the ‘principles of Good Clinical Practice’ for non‐CTIMP/MHRA studies. In addition to Sponsorship approval, ethical approval has been obtained from the Rowett Institute Ethics Committee (approval date 11/02/2019, internal study number 810). Ex vivo studies using freshly resected macro/microscopically human ileum tissue removed during routine surgery (e.g. for colon cancer) will be obtained following informed written consent and with approval of the Regional Human Ethics Committee; Linköping, Sweden.
Author Contributions
All authors contributed to the writing and preparation of the manuscript.
Acknowledgements
Additional members of the academic team including Dr Carrie Duckworth, Professor John Wilding, Professor Mark Pritchard and Professor Chris Probert (University of Liverpool, UK), Professor Harry Flint (Rowett Institute, UK), Dr Graham Horgan (Biomathematics and Statistics Scotland), Professor Johan Söderholm and Dr Åsa Keita (University Hospital Linköping, Sweden).
Contributor Information
A. M. Johnstone, Email: Alex.Johnstone@abdn.ac.uk.
B. J. Campbell, Email: B.J.Campbell@liverpool.ac.uk.
References
- Abou‐Donia MB, El‐Masry EM, Abdel‐Rahman AA et al (2008) Splenda alters gut microflora and increases intestinal p‐glycoprotein and cytochrome p‐450 in male rats. Journal of Toxicology and Environmental Health, Part A 71: 1415–29. [DOI] [PubMed] [Google Scholar]
- Adams J & White M (2015) Characterisation of UK diets according to degree of food processing and associations with socio‐demographics and obesity: cross‐sectional analysis of UK National Diet and Nutrition Survey (2008–12). International Journal of Behavioral Nutrition and Physical Activity 12: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I, Greenwood R, Costello B et al (2016) Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 43: 596–611. [DOI] [PubMed] [Google Scholar]
- Amar J, Chabo C, Waget A et al (2011) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Molecular Medicine 3: 559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S, Patin A, Giuffrida F et al (2014) In vitro digestion of citric acid esters of mono‐and diglycerides (CITREM) and CITREM‐containing infant formula emulsions. Food & Function 5: 1409–21. [DOI] [PubMed] [Google Scholar]
- American Lecithin Company (2009) Lecithins & phospholipids. Available at: http://www.americanlecithin.com/lecithin_2009 (accessed 3 April 2019).
- Arnold AR & Chassaing B (2019) Maltodextrin, Modern Stressor of the Intestinal Environment. Cellular and Molecular Gastroenterology and Hepatology 7: 475–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta M, Stiemsma LT, Amenyogbe N et al (2014) The intestinal microbiome in early life: health and disease. Frontiers in Immunology 5: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Perez‐Chanona E, Mühlbauer M et al (2012) Intestinal inflammation targets cancer‐inducing activity of the microbiota. Science 338: 120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti De Gregoris T, Aldred N, Clare AS et al (2011) Improvement of phylum‐and class‐specific primers for real‐time PCR quantification of bacterial taxa. Journal of Microbiological Methods 86: 351–6. [DOI] [PubMed] [Google Scholar]
- Bates B, Lennox A, Prentice A et al (2014) National Diet and Nutrition Survey: results from Years 1 to 4 (combined) of the rolling programme for 2008 and 2009 to 2011 and 2012. Available at: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed 3 April 2019).
- Bian X, Chi L, Gao B et al (2017a) The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD‐1 mice". PLoS ONE 12: e0178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X, Chi L, Gao B et al (2017b) Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Frontiers in Physiology 8: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X, Tu P, Chi L et al (2017c) Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food and Chemical Toxicology 107: 530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blairon L, Wittebole X & Laterre P (2003) Lipopolysaccharide‐binding protein serum levels in patients with severe sepsis due to Gram‐positive and fungal infections. The Journal of Infectious Diseases 187: 287–91. [DOI] [PubMed] [Google Scholar]
- Blesso CN, Andersen CJ, Barona J et al (2013) Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk‐free egg substitute in individuals with metabolic syndrome. Metabolism 62: 400–10. [DOI] [PubMed] [Google Scholar]
- Boyer JL (2013) Bile formation and secretion. Comprehensive Physiology. Comprehensive Physiology 3: 1035–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branen AL, Davidson PM, Salminen S et al (2001) Food additives, 2nd edn, pp. 1–952. CRC Press: London. [Google Scholar]
- Burgess JW, Neville TA, Rouillard P et al (2005) Phosphatidylinositol increases HDL‐C levels in humans. Journal of Lipid Research 46: 350–5. [DOI] [PubMed] [Google Scholar]
- Burri E & Beglinger C (2014) The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Review of Gastroenterology & Hepatology 8: 197–210. [DOI] [PubMed] [Google Scholar]
- Cani PD & Everard A (2015) Keeping gut lining at bay: impact of emulsifiers. Trends in Endocrinology & Metabolism 26: 273–4. [DOI] [PubMed] [Google Scholar]
- Canty DJ & Zeisel SH (1994) Lecithin and choline in human health and disease. Nutrition Reviews 52: 327–39. [DOI] [PubMed] [Google Scholar]
- Carocho M, Barreiro MF, Morales P et al (2014) Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Comprehensive Reviews in Food Science and Food Safety 13: 377–99. [DOI] [PubMed] [Google Scholar]
- Cediel G, Reyes M, Costa Louzada ML et al (2018) Ultra‐processed foods and added sugars in the Chilean diet (2010). Public Health Nutrition 21: 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Rolhion N, de Vallee A et al (2011) Crohn disease‐associated adherent‐invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. The Journal of Clinical Investigation 121: 966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK et al (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Van de Wiele T, De Bodt J et al (2017) Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 66: 1414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WSF, Walker AW, Louis P et al (2016) Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biology 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M, Turkki P, Linscheer W et al (1980) Lecithin Supplementation in Healthy Volunteers Effect on Cholesterol Esterification and Plasma, and Bile lipids. Annals of Nutrition and Metabolism 24: 228–37. [DOI] [PubMed] [Google Scholar]
- Costa Louzada ML, Martins APB, Canella DS et al (2015) Ultra‐processed foods and the nutritional dietary profile in Brazil. Revista de Saúde Pública 49: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couedelo L, Amara S, Lecomte M et al (2015) Impact of various emulsifiers on ALA bioavailability and chylomicron synthesis through changes in gastrointestinal lipolysis. Food & Function 6: 1726–35. [DOI] [PubMed] [Google Scholar]
- Csáki KF (2011) Synthetic surfactant food additives can cause intestinal barrier dysfunction. Medical Hypotheses 76: 676–81. [DOI] [PubMed] [Google Scholar]
- Darfeuille‐Michaud A, Boudeau J, Bulois P et al (2004) High prevalence of adherent‐invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127: 412–21. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran V, Rathinavel A, Pushpanathan M et al (2014) Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS ONE 9: e105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan B, Suzuki H, Herlekar D et al (2014) Inflammation‐associated adherent‐invasive Escherichia coli are enriched in pathways for use of propanediol and iron and M‐cell translocation. Inflammatory Bowel Diseases 20: 1919–32. [DOI] [PubMed] [Google Scholar]
- EFSA NDA Panel (European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies) (2016) Dietary reference values for choline. EFSA Journal 14: e04484. [Google Scholar]
- European Parliament (2008) Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (Text with EEA relevance). Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%253A32008R1333 (accessed 16 July 2019).
- Everard A & Cani PD (2013) Diabetes, obesity and gut microbiota. Best Practice & Research Clinical Gastroenterology 27: 73–83. [DOI] [PubMed] [Google Scholar]
- Fardet A (2018) Characterization of the degree of food processing in relation with its health potential and effects. Advances in Food and Nutrition Research 85: 79–129. [DOI] [PubMed] [Google Scholar]
- Fennema OR (1987) Food additives ‐ an unending controversy. The American Journal of Clinical Nutrition 46: 201–3. [DOI] [PubMed] [Google Scholar]
- FSA (Food Standards Agency) (2018a) EU Approved additives and E Numbers. Available at: http://www.food.gov.uk/print/pdf/node/847 (accessed 16 July 2019).
- FSA (Food Standards Agency) (2018b) Food Additives: Different food additives and advice on regulations and the safety of additives in food. Available at: http://www.food.gov.uk/print/pdf/node/279 (accessed 16 July 2019).
- Furuhashi H, Higashiyama M & Okada Y et al (2019) Dietary emulsifier polysorbate‐80 induces small‐intestinal vulnerability to indomethacin‐induced lesions via dysbiosis. Journal of Gastroenterology and Hepatology. 10.1111/jgh.14808. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gevers D, Kugathasan S, Denson LA et al (2014) The treatment‐naive microbiome in new‐onset Crohn’s disease. Cell Host & Microbe 15: 382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney MJ. (2018) Ultra‐processed foods: definitions and policy issues. Current Developments in Nutrition 3: nzy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Martucci NJ, Moreno‐Olivas F et al (2017) Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact 5: 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez A, Scharl M, Sempere L et al (2014) Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn's disease. Gut 63: 272–80. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A, Zapater P, Juanola O et al (2016) Gut bacterial DNA translocation is an independent risk factor of flare at short term in patients with Crohn’s disease. The American Journal of Gastroenterology 111: 529–40. [DOI] [PubMed] [Google Scholar]
- Halmos EP, Mack A & Gibson PR (2019) Review article: emulsifiers in the food supply and implications for gastrointestinal disease. Alimentary Pharmacology & Therapeutics 49: 41–50. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K et al (2008) Review article: the role of butyrate on colonic function. Alimentary Pharmacology & Therapeutics 27: 104–19. [DOI] [PubMed] [Google Scholar]
- Hasenhuettl GL & Hartel RW (2008) Food emulsifiers and their applications 2nd edn, pp. 1–426. Springer: New York. [Google Scholar]
- Huvaere K, Vandevijvere S, Hasni M et al (2012) Dietary intake of artificial sweeteners by the Belgian population. Food Additives & Contaminants: Part A 29: 54–65. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) (1974a) Lecithin. In, Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents . Seventeenth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), June 25‐July 4, 1973, Geneva, Switzerland. WHO Food Additives Series no 5. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO)/Geneva, Switzerland: World Health Organization (WHO). Available at: http://www.inchem.org/documents/jecfa/jecmono/v05je42.htm (accessed 16 July 2019).
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) (1974b) Polyoxyethylene (20) sorbitan monoesters of lauric oleic, palmitic and stearic acid and triester of stearic acid. In, Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Seventeenth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), June 25‐July 4, 1973, Geneva, Switzerland. WHO Food Additives Series no 5. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO)/ Geneva, Switzerland: World Health Organization (WHO). Available at: http://www.inchem.org/documents/jecfa/jecmono/v05je47.htm (accessed 16 July 2019).
- Jiang Z, Zhao M, Zhang H et al (2018) Antimicrobial emulsifier–glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis, and systemic low‐grade inflammation in low‐fat diet fed mice. Molecular Nutrition & Food Research 62: 1700547. [DOI] [PubMed] [Google Scholar]
- Johansson ME, Gustafsson JK, Holmen‐Larsson J et al (2014) Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63: 281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner M, Kocjan A, Stein J et al (2014) First multicenter study of modified release phosphatidylcholine “LT‐02” in ulcerative colitis: a randomized, placebo‐controlled trial in mesalazine‐refractory courses. The American Journal of Gastroenterology 109: 1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Gu W, Lee IA et al (2012) High fat diet‐induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 7: e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanjui T, Artz W & Mahungu S (2003) Emulsifiers: Organic Emulsifiers In: Encyclopedia of Food Sciences and Nutrition (Caballero B, Trugo L, Finglas PM. eds), 2nd edn, pp. 2070–77. Elsevier Science Ltd: Amsterdam. [Google Scholar]
- Knapp S, de Vos AF, Florquin S et al (2003) Lipopolysaccharide binding protein is an essential component of the innate immune response to Escherichia coli peritonitis in mice. Infection and Immunity 71: 6747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop KA, McDonald KG, Kulkarni DH et al (2016) Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65: 1100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann R, Zimmerli W, Sansano S et al (1995) Increased circulating soluble CD14 is associated with high mortality in Gram‐negative septic shock. Journal of Infectious Diseases 171: 639–44. [DOI] [PubMed] [Google Scholar]
- Langhans R & Thalheimer W (1971) Polysorbate 60: Effects in bread. Cereal Chemistry 48: 283–91. [Google Scholar]
- Laudisi F, Di Fusco D, Dinallo V et al (2019) The food additive maltodextrin promotes endoplasmic reticulum stress ‐ driven mucus depletion and exacerbates intestinal inflammation. Cellular and Molecular Gastroenterology and Hepatology 7: 457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte M, Couedelo L, Meugnier E et al (2016) Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high‐fat fed mice. Molecular Nutrition & Food Research 60: 609–20. [DOI] [PubMed] [Google Scholar]
- Lee D, Swan CK, Suskind D et al (2018) Children with Crohn’s disease frequently consume select food additives. Digestive Diseases and Sciences 63: 2722–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn SR, Britton GJ, Contijoch EJ et al (2018) Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 154: 1037–046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla MA, Dubey A, Kumar A et al (2018) Exploring the human microbiome: The potential future role of next‐generation sequencing in disease diagnosis and treatment. Frontiers in Immunology 9: 2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HM, Campbell BJ, Hart CA et al (2004) Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 127: 80–93. [DOI] [PubMed] [Google Scholar]
- Mepham B (2011) Food additives: an ethical evaluation. British Medical Bulletin 99: 7–23. [DOI] [PubMed] [Google Scholar]
- Mercurius‐Taylor LA, Jayaraj AP & Clark CG (1984) Is chronic detergent ingestion harmful to the gut? British Journal of Industrial Medicine 41: 279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merga Y, Campbell BJ & Rhodes JM (2014) Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Digestive Diseases 32: 475–83. [DOI] [PubMed] [Google Scholar]
- Michalak A, Mosińska P & Fichna J (2016) Common links between metabolic syndrome and inflammatory bowel disease: current overview and future perspectives. Pharmacological Reports 68: 837–46. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Moubarac JC, Cannon G et al (2013) Ultra‐processed products are becoming dominant in the global food system. Obesity Reviews 14: 21–8. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Cannon G, Moubarac JC et al (2017) The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra‐processing. Public Health Nutrition 21: 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen H & Bas H (2004) Mono‐ and Diglycerides In: Emulsifiers in Food Technology, (Whitehurst RJ. ed), pp. 40–58. Oxford: Blackwell Publishing Ltd. [Google Scholar]
- Moubarac J, Martins APB, Claro RM et al (2013) Consumption of ultra‐processed foods and likely impact on human health. Evidence from Canada. Public Health Nutrition 16: 2240–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad A, de Carvalho Pincinato E, Mazzola P et al (2010) Influence of soy lecithin administration on hypercholesterolemia. Cholesterol 2010: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch A, Ström M & Söderholm JD (2007) Dihydroxy bile acids increase mucosal permeability and bacterial uptake in human colon biopsies. Scandinavian Journal of Gastroenterology 42: 1167–74. [DOI] [PubMed] [Google Scholar]
- Münch A, Söderholm JD, Öst Å et al (2011) Low levels of bile acids increase bacterial uptake in colonic biopsies from patients with collagenous colitis in remission. Alimentary Pharmacology & Therapeutics 33: 954–60. [DOI] [PubMed] [Google Scholar]
- National Geographic/FAOSTAT (Food and Agriculture Organisation of the United Nations) (2011) What the world eats. Available at: http://www.nationalgeographic.com/what-the-world-eats/ (accessed 29 June 2019).
- Nieuwdorp M, Gilijamse PW, Pai N et al (2014) Role of the microbiome in energy regulation and metabolism. Gastroenterology 146: 1525–33. [DOI] [PubMed] [Google Scholar]
- Nigro G, Hanson M, Fevre C, et al (2016) Intestinal Organoids as a Novel Tool to Study Microbes-Epithelium Interactions In: Organoids, Methods in Molecular Biology, Vol. 1576, (Turksen K. ed), pp. 1–12. Humana Press: New York. [DOI] [PubMed] [Google Scholar]
- Palacios LE & Wang T (2005) Extraction of egg‐yolk lecithin. Journal of the American Oil Chemists' Society 82: 565–9. [Google Scholar]
- Palmnäs MS, Cowan TE, Bomhof MR et al (2014) Low‐dose aspartame consumption differentially affects gut microbiota‐host metabolic interactions in the diet‐induced obese rat. PLoS ONE 9: e109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prorok‐Hamon M, Friswell MK, Alswied A et al (2014) Colonic mucosa‐associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63: 761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley EM (2011) Passing the bug–translocation, bacteremia, and sepsis in the intensive care unit patient: is intestinal decontamination the answer? Critical Care Medicine 39: 1202–03. [DOI] [PubMed] [Google Scholar]
- Raimondi F, Santoro P, Barone MV et al (2008) Bile acids modulate tight junction structure and barrier function of Caco‐2 monolayers via EGFR activation. American Journal of Physiology‐Gastrointestinal and Liver Physiology 294: G906–13. [DOI] [PubMed] [Google Scholar]
- Reichardt N, Vollmer M, Holtrop G et al (2018) Specific substrate‐driven changes in human faecal microbiota composition contrast with functional redundancy in short‐chain fatty acid production. The ISME Journal 12: 610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JM (2018) Dietary exposure to emulsifiers and detergents and the prevalence of cardiovascular disease. QJM: An International Journal of Medicine 111: 283–6. [DOI] [PubMed] [Google Scholar]
- Richardson A, Calder A, Stewart C et al (1989) Simultaneous determination of volatile and non‐volatile acidic fermentation products of anaerobes by capillary gas chromatography. Letters in Applied Microbiology 9: 5–8. [Google Scholar]
- Roberts CL, Keita AV, Duncan SH et al (2010) Translocation of Crohn's disease Escherichia coli across M‐cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 59: 1331–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CL, Rushworth SL, Richman E et al (2013) Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. Journal of Crohn's and Colitis 7: 338–41. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Palacios A, Harding A, Menghini P et al (2018) The artificial sweetener Splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease–like ileitis. Inflammatory Bowel Diseases 24: 1005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, Kanazawa A, Ikeda F et al (2014) Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes. Diabetes Care 37: 2343–50. [DOI] [PubMed] [Google Scholar]
- Scanu A, Oriente P, Szajewski JM et al (1961) Triton hyperlipemia in dogs. II. Atheroscieross, diffuse lipidosis, and deletion of fat stores produced by prolonged administration of the non‐tonic surface‐active agent. The Journal of Experimental Medicine 114: 279–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaubeck M & Haller D (2015) Reciprocal interaction of diet and microbiome in inflammatory bowel diseases. Current Opinion in Gastroenterology 31: 464–70. [DOI] [PubMed] [Google Scholar]
- Shah R, Kolanos R, DiNovi MJ et al (2017) Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food Additives & Contaminants Part A 34: 905–17. [DOI] [PubMed] [Google Scholar]
- Sigall‐Boneh R, Pfeffer‐Gik T, Segal I et al (2014) Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflammatory Bowel Diseases 20: 1353–60. [DOI] [PubMed] [Google Scholar]
- Simon GL & Gorbach SL (1984) Intestinal flora in health and disease. Gastroenterology 86: 174–93. [PubMed] [Google Scholar]
- Simpson HL & Campbell BJ (2015) Review: dietary fibre–microbiota interactions. Alimentary Pharmacology & Therapeutics 42: 158–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Ye A & Horne D (2009) Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Progress in Lipid Research 48: 92–100. [DOI] [PubMed] [Google Scholar]
- Singh RK, Wheildon N & Ishikawa S (2016) Food additive P‐80 impacts mouse gut microbiota promoting intestinal inflammation, obesity and liver dysfunction. SOJ Microbiology & Infectious Diseases 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimani N, Deharveng G, Southgate D et al (2009) Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle‐aged populations in the European Prospective Investigation into Cancer and Nutrition study. European Journal of Clinical Nutrition 63(Suppl 4): S206–25. [DOI] [PubMed] [Google Scholar]
- Srour B, Fezeu LK, Kesse‐Guyot E et al (2019) Ultra‐processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet‐Santé). British Medical Journal 365: l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele EM, Baraldi LG, Costa Louzada ML et al (2016) Ultra‐processed foods and added sugars in the US diet: evidence from a nationally representative cross‐sectional study. British Medical Journal Open 6: e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LJ, Burgess JR, Stochelski MA et al (2014) Amounts of artificial food colors in commonly consumed beverages and potential behavioral implications for consumption in children. Clinical Pediatrics 53: 133–40. [DOI] [PubMed] [Google Scholar]
- Storey D, Lee A, Bornet F et al (2007) Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. European Journal of Clinical Nutrition 61: 349–54. [DOI] [PubMed] [Google Scholar]
- Suez J, Korem T, Zeevi D et al (2014) Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514: 181–6. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Khilkin M, Kerjaschki D et al (1998) Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 115: 281–6. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Ung V, Sydora BC et al (2009) Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflammatory Bowel Diseases 15: 359–64. [DOI] [PubMed] [Google Scholar]
- Szuhaj BF. (Ed.) (1989) Lecithins: Sources, Manufacture and Uses (AOCS Monograph). American Oil Chemists' Society: Urbana, IL. [Google Scholar]
- Tadros TF (2005) Applied Surfactants: Principles and Applications, pp. 1–634. Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim. [Google Scholar]
- Tang WW, Wang Z, Levison BS et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine 368: 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins RK, Burke LG, Zollinger RM et al (1970) Relationship of biliary phospholipid and cholesterol concentrations to the occurrence and dissolution of human gallstones. Annals of Surgery 172: 936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde M, Ajamian M, Caio G et al (2016) Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 65: 1930–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K, Olofsson G, Fae A et al (2002) In vitro determination of active bile acid absorption in small biopsy specimens obtained endoscopically or surgically from the human intestine. European Journal of Clinical Investigation 32: 115–21. [DOI] [PubMed] [Google Scholar]
- USDA (US Department of Agriculture) Agricultural Research Service (2019) FoodData Central, 2019. Available at: https://fdc.nal.usda.gov/ (accessed 10 September 2019).
- Van Den Brûle S, Ambroise J, Lecloux H et al (2016) Dietary silver nanoparticles can disturb the gut microbiota in mice. Particle and Fibre Toxicology 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennois E, Merlin D, Gewirtz AT et al (2017) Dietary Emulsifier‐Induced Low‐Grade Inflammation Promotes Colon Carcinogenesis. Cancer Research 77: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince A, Dyer N, O'Grady F et al (1972) Bacteriological studies in Crohn's disease. Journal of Medical Microbiology 5: 219–29. [DOI] [PubMed] [Google Scholar]
- Vo TD, Lynch BS & Roberts A (2019) Dietary Exposures to Common Emulsifiers and Their Impact on the Gut Microbiota: Is There a Cause for Concern? Comprehensive Reviews in Food Science and Food Safety 18: 31–47. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F et al (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143: 913–6. [DOI] [PubMed] [Google Scholar]
- Walker AW, Ince J, Duncan SH et al (2011) Dominant and diet‐responsive groups of bacteria within the human colonic microbiota. The ISME Journal 5: 220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Duckworth CA, Watson AJ et al (2013) A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Disease Models & Mechanisms 6: 1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu L, Chen X et al (2018) Effects of Food‐Additive‐Information on consumers’ willingness to accept food with additives. International Journal of Environmental Research and Public Health 15: E2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinöcker M & Lindseth I (2018) The Western diet–microbiome‐host interaction and its role in metabolic disease. Nutrients 10: E365. [DOI] [PMC free article] [PubMed] [Google Scholar]


