Abstract
Introduction
Chest ultrasound is increasingly used to radiologically diagnose childhood pneumonia, but there are limited data on its use for pulmonary tuberculosis (PTB).
Aim
Compare chest ultrasound with a chest X‐ray (CXR) findings.
Methods
Children (up to 13 years) with suspected PTB were enrolled. Bedside chest ultrasound findings were compared to CXR. The analysis was stratified by PTB category: confirmed PTB (microbiologically confirmed), unconfirmed PTB (clinical diagnosis with negative microbiological tests), or unlikely PTB (other respiratory diseases with improvement without tuberculosis treatment).
Results
One hundred fifty‐nine children were enrolled (57% boys, median age 26.6 months [interquartile range 15.1‐59.3]). Ultrasound detected abnormalities in 72% (n = 114), CXR in 56% (n = 89), P < .001. Pleural effusion was detected on ultrasound in 15% (n = 24) compared 9% (n = 14) on CXR, P = .004, more in confirmed PTB (33%, n = 12 vs 8%, n = 4 unlikely PTB, P = .013). Ultrasound detected enlarged mediastinal lymph nodes more commonly (22%, n = 25) than CXR (6%, n = 10, P = .001); the size of lymph nodes in the unlikely category (1.0 cm) was smaller than the other two PTB categories (1.4 and 1.5 cm, P = .001). Inter‐reader agreement (kappa Cohen) was higher for ultrasound than CXR for several findings (consolidation 0.67 vs 0.47, pleural effusion 0.86 vs 0.56, enlarged lymph nodes 0.56 vs 0.27).
Conclusion
Ultrasound detected abnormalities more frequently than CXR with the higher inter‐reader agreement; ultrasound abnormalities were most common in children with confirmed PTB. Ultrasound is a promising modality for detecting abnormalities in PTB. Further studies should evaluate the diagnostic accuracy of ultrasound against a gold standard.
Keywords: chest ultrasound, CXR, diagnostics, pediatric pulmonary tuberculosis
1. INTRODUCTION
Tuberculosis (TB) remains a global major public health issue in children with an estimated one million incident cases in 2017.1 Pulmonary TB (PTB) is the most common presentation but diagnosis can be challenging due to nonspecific signs and symptoms, paucibacillary disease, and difficulties in obtaining adequate samples.2 Chest X‐ray (CXR) is the imaging tool of choice, with perihilar or mediastinal lymphadenopathy being the most characteristic findings.3, 4, 5 However, the intra‐ and inter‐reader agreement for lymphadenopathy on CXR is poor (κ 0.00‐0.40).5, 6, 7 Other common CXR findings described in children with PTB are consolidation or pleural effusion,3, 5 but these are common findings in other respiratory infections as well.
Chest ultrasound is increasingly used for the diagnosis of pediatric lung disease including pneumonia, bronchiolitis, or respiratory distress syndrome.8 Advantages of chest ultrasound are that it is free of ionizing radiation; can be performed by the clinician at the bedside and is easily reproducible; making it a suitable tool for diagnosis, prompt management decisions, and monitoring of treatment response. Training in chest ultrasound is relatively easy, and clinicians can be expected to perform good quality chest ultrasounds after 30 supervised examinations.9 Furthermore, due to the recent development of portable, low‐cost ultrasound machines, bedside chest ultrasound may become a cost‐effective tool especially in resource‐limited settings.
Data on the use of chest ultrasound for the diagnosis of PTB in children are limited.10, 11, 12, 13 Previous studies showed that chest ultrasound demonstrated pleural effusion more commonly in children with microbiologically confirmed PTB than in children with other respiratory diseases.13 Mediastinal ultrasound was feasible in visualizing lymphadenopathy,10, 11, 12 and children with confirmed PTB had larger lymph nodes than children with other respiratory diseases.13 Consolidation was as common in children diagnosed with PTB compared to those with other respiratory diseases, but the resolution of consolidation was slower in children with confirmed PTB.13
The aim of this study was to compare chest ultrasound findings with CXR findings in children with suspected PTB.
2. METHODS
This was a prospective study of children hospitalized with suspected PTB at a tertiary children's hospital in Cape Town, South Africa from July 2014 to October 2015. Children from up to 13 years (the maximum age of children being admitted in our hospital) with suspected PTB were consecutively enrolled. Inclusion criteria were cough plus one of the following: (a) weight loss or failure to thrive over the last 3 months; (b) a positive tuberculin skin test; (c) a CXR suggestive of PTB; or (d) a household TB contact. Children on TB treatment or TB prophylaxis for more than 72 hours, those unable to provide an adequate sample, or when parents were unable to provide informed consent, were excluded. Children with exclusively extra‐pulmonary TB (EPTB) or if the time between chest ultrasound and CXR was more than 7 days were excluded from this analysis.
The study was approved by the Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town. Written informed consent was obtained from a parent or legal guardian; assent was obtained in children older than 7 years.
2.1. Investigations
Two induced sputum samples were collected for liquid mycobacterial culture (BACTEC MGIT; Becton Dickinson, Sparks, MD) and Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA), HIV testing, chest ultrasound, and CXR were performed on all enrolled children. Children were categorized into one of three PTB categories14; (a) confirmed PTB, Mycobacterium tuberculosis detected by either culture or GeneXpert; (b) unconfirmed PTB, clinically diagnosed but microbiological testing negative; or (c) unlikely PTB, not clinically or microbiologically diagnosed, and improvement of respiratory disease without TB treatment.14
All children underwent chest ultrasound, including mediastinal ultrasound, and had an anterior‐posterior and lateral CXR.
2.2. Chest ultrasound
A portable, low‐cost, greyscale ultrasound machine (Mindray DP10, Mindray, Shenzhen, China) with a 5 to 10 MHz linear probe was used to scan the chest. A clinician (CCH) without prior ultrasound experience, who attended 4‐day ultrasound training before the start of the study, performed the ultrasound scans. The children were scanned either sitting or supine plus in left and right lateral decubitus position, to examine the lungs and pleura. The chest was divided into 14 regions; the left and right, anterior, lateral and posterior, superior and inferior region, and the two apices. All regions were scanned in the longitudinal and transverse (intercostal) planes.15 In addition, mediastinal ultrasound was performed through the suprasternal notch with a 5.0 to 8.5 MHz micro‐convex probe. The child was placed in the supine position with the neck slightly extended to improve access to the suprasternal notch: transverse and oblique views were obtained.16 All cine clips were saved. The clinician performing the ultrasound recorded the findings “consolidation less than 0.5 cm” (E‐Image 1), “consolidation ≥0.5 cm” (E‐Image 2a), “pleural effusion” (E‐Image 3a), “enlarged mediastinal lymph nodes ≥1.0 cm” (E‐Image 4a) on a standardized reporting form. Another clinician (SB), with 1 year of pediatric ultrasound experience, reviewed the saved ultrasound cine clips and independently reported on these using the same standardized reporting form. In case the sonographer and the clinician reviewing the saved ultrasound cine clips disagreed on a chest ultrasound finding, the saved cine clips were reviewed by both clinicians again and their findings were discussed to come to an agreement. If no consensus was met the findings of the sonographer were used for comparison with CXR. A senior ultrasound specialist was consulted to resolve any disagreement on mediastinal ultrasound.
2.3. CXR
To ensure the highest achievable quality of CXR reports, the posterior‐anterior and lateral CXRs were independently reviewed by two pediatric radiologists reporting on a standardized record sheet “consolidation” (E‐Image 2b), “pleural effusion” (E‐Image 3b), “enlarged mediastinal lymph nodes” (E‐Image 4b), and “final diagnosis”: TB or no TB. In case of disagreement on the final diagnosis, a third pediatric radiologist was consulted, and the final diagnosis was based on majority opinion. In case of disagreement for the other findings (consolidation, pleural effusion, or enlarged mediastinal lymph nodes), the finding of the most experienced TB radiologist (SA) was used for comparison with ultrasound. The inter‐reader agreement of clinicians analyzing the chest ultrasound was compared with the inter‐reader agreement between the first two pediatric radiologists reporting on the CXRs.
The sonographer, the clinician reviewing the saved ultrasound clips, and the radiologists were blinded to each other's findings and clinical or microbiological data.
2.4. Data analysis
Ultrasound findings were compared to CXR findings for the presence of consolidation, pleural effusion, or enlarged mediastinal lymph nodes. Descriptive statistics were used for participant characteristics; mean and standard deviation (SD) were used for normally distributed data, median and interquartile range (IQR) were used for nonnormally distributed data. McNemar's test or χ 2 test was used to calculate the P value.
Cohen's kappa coefficient (κ) was used to analyze the inter‐reader agreement for chest ultrasound and for CXR. The following interpretation of the result was used: less than zero no agreement, 0 to 0.20 slight, 0.21 to 0.40 fair, 0.41 to 0.60 moderate, 0.61 to 0.80 substantial, and 0.81 to 1 almost perfect agreement.17 STATA 14.2 (StataCorp; 2015, TX) was used for analysis.
3. RESULTS
3.1. Demographics
One hundred seventy‐eight children underwent chest ultrasound and CXR. Eight children were excluded from analysis because of an exclusive EPTB diagnosis, and a further 11 children were excluded because the interval between chest ultrasound and CXR was more than 7 days. Of the 159 children remaining, 36 (23%) had confirmed PTB, 73 (46%) had unconfirmed PTB, and 50 (31%) had unlikely PTB, Figure 1. Ninety children (57%) were boys; median age was 26.6 months (IQR = 15.1‐58.1) and 14% (n = 22) were HIV infected, Table 1.
Figure 1.
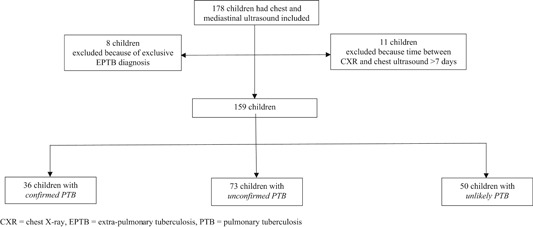
Flow diagram of participant enrolment
Table 1.
Demographics
| Male, n (%) | Median age (IQR), in mo | HIV infected, n (%) | Mean interval chest US and CXR, d (SD) | |
|---|---|---|---|---|
| All enrolled patients, n = 159 | 90 (57) | 26.6 (15.1‐58.1) | 22 (14) | 2.3 (1.7) |
| Confirmed PTB, n = 36 | 23 (64) | 48.6 (18.3‐65.9) | 6 (17) | 2.3 (1.6) |
| Unconfirmed PTB, n = 73 | 42 (58) | 25.0 (14.0‐43.0) | 10 (17) | 2.4 (1.6) |
| Unlikely PTB, n = 50 | 25 (50) | 23.9 (16.4‐56.2) | 6 (12) | 2.1 (1.8) |
Abbreviations: CXR, chest X‐ray, d, day; HIV, human immunodeficiency virus; IQR, interquartile range; mo, month; n, number; PTB, pulmonary tuberculosis; SD, standard deviation; US, ultrasound.
The mean time between chest ultrasound and CXR was 2.3 days (SD 1.7 days). The mean duration for performing a chest ultrasound was 6 minutes (SD 5‐8 minutes).
3.2. Chest ultrasound vs CXR findings
One hundred fourteen (72%) children had an abnormal finding (consolidation ≥0.5 cm, pleural effusion, or enlarged mediastinal lymph node ≥1 cm) on chest ultrasound vs 89 (56%) children who had an abnormal finding on CXR, P < .001. Children in the confirmed PTB category had the highest proportion with an abnormal finding on ultrasound (83%) compared to 72% in the unlikely PTB category, P = .220, Table 2.
Table 2.
Abnormal chest ultrasound and chest X‐ray findings by TB category
| Abnormal ultrasound a | Abnormal CXR b | Abnormal CXR or ultrasound | P value | |
|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | Ultrasound vs CXR | |
| Total | 114/159 (72) | 89/159 (56) | 130/159 (82) | <.001 |
| Confirmed PTB | 30/36 (83) | 22/36 (61) | 32/36 (89) | .021 |
| Unconfirmed PTB | 49/73 (67) | 37/73 (51) | 55/73 (75) | <.001 |
| Unlikely PTB | 36/50 (72) | 30/50 (60) | 44/50 (88) | .201 |
Abbreviations: CXR, chest X‐ray; n, number of children with abnormal finding; N, number of children investigated; PTB, pulmonary tuberculosis.
Abnormal CXR means: consolidation, pleural effusion, or enlarged mediastinal lymph nodes detected.
Abnormal chest ultrasound means: consolidation ≥0.5 cm, pleural effusion, or enlarged mediastinal lymph nodes detected.
Consolidation was the most common abnormal finding, with a consolidation ≥0.5 cm occurring in 75 (47%) children on chest ultrasound and in 83 (52%) on CXR, P = .267, Table 3. Consolidation was as common in children with confirmed PTB (56%) as in children with unlikely PTB (52%, P = .744). CXR and chest ultrasound performed similarly for detecting large consolidation (≥0.5 cm) across the three PTB categories, Table 3. In contrast, a small consolidation (<0.5 cm) was reported on chest ultrasound in 78 (49%) children (15/36 confirmed PTB, 41/73 unconfirmed PTB, 22/50 unlikely PTB, P = .250). CXR reported consolidation of any size in 44 (28%) of those children. In six (five unlikely PTB and one unconfirmed PTB) children, a consolidation was reported on CXR but no consolidation (not ≥0.5 cm nor <0.5 cm) was seen on chest ultrasound. In three of those six children, the radiologists did not agree on this finding, a third radiologist was consulted.
Table 3.
Positive findings on chest ultrasound findings versus chest X‐ray
| Finding PTB category | Positive chest ultrasound finding | Positive CXR finding | Agreement chest ultrasound and CXR positive | Positive chest ultrasound or CXR finding | P value |
|---|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | n/N (%) | Ultrasound vs CXR | |
| Consolidation (≥0.5 cm) | 75/159 (47) | 83/159 (52) | 53/159 (33) | 105/159 (66) | .267 |
| Confirmed PTB | 20/36 (56) | 20/36 (56) | 15/36 (42) | 25/36 (69) | 1.000 |
| Unconfirmed PTB | 29/73 (40) | 33/73 (45) | 18/73 (25) | 44/73 (60) | .433 |
| Unlikely PTB | 26/50 (52) | 30/50 (60) | 20/50 (40) | 36/50 (72) | .317 |
| Pleural effusion | 24/159 (15) | 14/159 (9) | 13/159 (8) | 25/159 (16) | .004 |
| Confirmed PTB | 12/36 (33) | 4/36 (11) | 4/36 (11) | 12/36 (33) | .005 |
| Unconfirmed PTB | 8/73 (11) | 7/73 (10) | 6/73 (8) | 9/73 (12) | .564 |
| Unlikely PTB | 4/50 (8) | 3/50 (6) | 3/50 (6) | 4/50 (8) | .317 |
| Enlarged mediastinal lymph nodes ≥1 cm | 25/112 (22) | 10/159 (6) | 3/112 (3) | 29/159 (18) | .001 |
| Confirmed PTB | 7/24 (29) | 4/36 (11) | 0/24 (0) | 11/36 (31) | .206 |
| Unconfirmed PTB | 8/51 (16) | 6/73 (8) | 3/51 (6) | 8/73 (11) | .257 |
| Unlikely PTB | 10/37 (27) | 0/50 (0) | 0/37 (0) | 10/50 (20) | .002 |
Abbreviations: CXR, chest X‐ray; n, number of children with a positive finding; N, number of children who underwent the investigation; PTB, pulmonary tuberculosis.
Pleural effusion was found on ultrasound in 24/159 (15%) children compared to 14/159 (9%) children on CXR, P = .004, Table 3. Both chest ultrasound and CXR detected pleural effusion in 13 children; chest ultrasound detected 11 (7%) additional children with a pleural effusion that was not seen on CXR, mainly in the confirmed PTB category where eight additional children were detected (in 7/8 children the pleural effusion was accompanied by a consolidation; in five of those seven children CXR reported only a consolidation; in the remaining children the CXR was reported to be normal). In one of the 14 children with a reported pleural effusion on CXR, a large consolidation was reported on chest ultrasound rather than a pleural effusion. Pleural effusion on ultrasound was significantly more commonly detected in children with confirmed PTB (33%) than in children with unlikely PTB (8%), P = .013. In the confirmed PTB category the pleural effusion was accompanied by consolidation in 8/12 (75%) children, in the unconfirmed PTB category in 4/8 (50%) children and in the unlikely PTB category in 1/4 (25%) children.
Mediastinal ultrasound could be evaluated in 112/159 (70%) children as the clips were of poor quality or ultrasound could not be performed due to poor compliance in 30%. Enlarged mediastinal lymph nodes were detected in 25/112 (22%) children on ultrasound (of whom 10 were in the unlikely PTB category) and in 10/159 (6%) children on CXR, P = .001, Table 3. CXR did not detect any enlarged lymph nodes in the unlikely PTB category. The enlarged lymph nodes in the unlikely PTB category were significantly smaller than in the other two categories (median size enlarged lymph nodes unlikely PTB 1.0 cm [range 1.0‐1.1 cm], unconfirmed PTB category 1.4 cm [range 1.0‐2.8 cm], confirmed PTB category 1.5 cm [range 1.0‐1.6 cm], P = .001).
Twenty‐two children were HIV infected; ultrasound detected abnormalities in similar numbers of HIV infected (73%) and uninfected children (58%), P = .181, Table 4.
Table 4.
Positive chest ultrasound and CXR findings in HIV infected and noninfected children with suspected PTB
| Imaging finding | HIV+ | HIV− | P value |
|---|---|---|---|
| (n = 22) | (n = 137) | ||
| Abnormal chest ultrasound, n (%) | 16 (73) | 79 (58) | .181 |
| Consolidation ≥0.5 cm, n (%) | 14 (64) | 61 (45) | .096 |
| Pleural effusion, n (%) | 5 (23) | 19 (14) | .281 |
| Enlarged mediastinal lymph nodes, n/N (%) | 3/16 (19) | 22/96 (23) | .711 |
| Abnormal CXR, n (%) | 14 (64) | 75 (55) | .435 |
| Consolidation, n (%) | 14 (64) | 69 (50) | .247 |
| Pleural effusion, n (%) | 1 (5) | 13 (9) | .448 |
| Enlarged mediastinal lymph nodes, n (%) | 0 (0) | 10 (7) | .191 |
Abbreviations: CXR, chest X‐ray; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis.
Chest ultrasound had “substantial” and “almost perfect” inter‐reader agreement for consolidation ≥0.5 cm (κ = 0.67) or pleural effusion (κ = 0.86), respectively, and a moderate agreement for enlarged mediastinal lymph nodes (κ = 0.56). The inter‐reader agreement for a consolidation less than 0.5 cm on chest ultrasound was fair (κ = 0.39). The inter‐reader agreement for CXR was fair to moderate for consolidation (κ = 0.47), pleural effusion (κ = 0.56), and enlarged mediastinal lymph nodes (κ = 0.27), Table 5.
Table 5.
Inter‐reader agreement of chest X‐ray and chest ultrasound
| Imaging finding | Kappa Cohen chest X‐ray | Kappa Cohen chest ultrasound |
|---|---|---|
| Consolidation <0.5 cm | … | 0.39 |
| Consolidation ≥0.5 cm | 0.47 | 0.67 |
| Pleural effusion | 0.56 | 0.86 |
| Enlarged mediastinal lymph nodes | 0.27 | 0.56 |
4. DISCUSSION
In this study of children with suspected PTB, an abnormal finding was seen more frequently on chest ultrasound than on CXR for all three PTB categories. Children in the confirmed PTB category had an abnormal finding on chest ultrasound more frequently. Furthermore, ultrasound had a higher inter‐reader agreement than CXR for consolidation ≥0.5 cm, pleural effusion, or enlarged mediastinal lymph nodes. The ultrasound had the additional advantages in that it could be performed relatively easily as a bedside investigation, was quick to perform and was done by a clinician.
This is the first study comparing chest ultrasound findings with CXR findings in children with suspected PTB. A recent systematic review showed that there are limited data on the use of chest ultrasound in patients with PTB.18 This review identified 12 small (1‐87 participants) studies, of which three were pediatric (each with 30‐57 participants), using mediastinal ultrasound.10, 11, 12 We found that a large consolidation (≥0.5 cm) was nearly as commonly detected on ultrasound as on CXR and that a small consolidation (<0.5 cm) was predominantly identified on chest ultrasound. This is consistent with previous pneumonia studies,19, 20 in which a consolidation less than 1 cm was more commonly detected on chest ultrasound than CXR.19, 20 A study performed in adults with PTB reported that subpleural nodules (consolidation <1 cm, close to the pleural line) were present in almost all patients21; however, a control group was lacking. In our study, a small consolidation was found on ultrasound in 49% of children and was present in all three TB categories, including the unlikely PTB category. The clinical importance of small consolidation is unclear, as this may represent early disease, but has also been reported in healthy people. Further, the inter‐reader agreement for a small consolidation was only fair. Further studies are needed to evaluate the clinical relevance and natural history of a small consolidation.
Pleural effusion was significantly more common on ultrasound than on CXR, especially in the confirmed PTB category. This is consistent with prior studies that have shown ultrasound to be superior to CXR for pleural effusion,22, 23 and for differentiating consolidation from pleural effusion.24 In the confirmed PTB category, pleural effusion was often accompanied by a consolidation. Although pleural effusion and consolidation can occur in children with pneumonia caused by other bacteria or viruses, PTB should be considered as a possible diagnosis when a pleural effusion is detected in the right setting and accompanied by a consolidation.
Enlarged mediastinal lymph nodes, the diagnostic hallmark for PTB in children,3, 4, 5 were more commonly detected by ultrasound but were also found in the unlikely PTB category, but the size of nodes was smaller than in the other two categories. In contrast, CXR did not detect enlarged lymph nodes in unlikely TB; as the size of lymph nodes in the unlikely PTB category ranged from 1.0 to 1.1 cm, these might have been too small to be detected on CXR. A previous study in children with a positive tuberculin skin test showed that mediastinal ultrasound detected enlarged lymph nodes in 67% of the children with a normal CXR.10 It can be difficult to detect enlarged mediastinal lymph nodes on the posterior‐anterior CXR as vascular and thymic structures might be confused for enlarged lymph nodes and vice versa. In addition, the wide inter‐reader variability of reporting enlarged lymph nodes on CXR makes the interpretation difficult. Detection of enlarged mediastinal lymph nodes on ultrasound in the unlikely PTB category makes it difficult to distinguish PTB from other respiratory infections of other causes; based on the difference in size measured between the two PTB category and the unlikely PTB category, we recommend that future studies should determine the cut‐off for enlarged lymph nodes in children with TB on ultrasound.
Chest ultrasound had a better inter‐reader agreement for consolidation, pleural effusion, or enlarged mediastinal lymph nodes than CXR. This is in line with previous studies showing poor inter‐reader agreement for the detection of mediastinal lymphadenopathy on CXR5, 6, 7 and a higher inter‐reader agreement amongst chest ultrasound readers compared to CXR readers for the detection of consolidation in children with pneumonia.25
Limitations of this study include comparing chest ultrasound with CXR. We found a limited inter‐reader agreement on CXR findings; therefore, we considered CXR as an imperfect comparison to ultrasound. Because of that, we chose not to calculate the sensitivity or specificity of ultrasound vs CXR. Until advances in technology make CT or MRI a safe, ethical choice, we are limited to using CXR. A further limitation was the lack of prior ultrasound experience by the sonographer; previous chest ultrasound studies on children with pneumonia found that the diagnostic accuracy was higher when the sonographer was more experienced.8
Chest ultrasound is limited as only findings close to the pleural line can be detected, as the ultrasound beam will be scattered and impeded by the aerated lung. Therefore, abnormalities that do not reach the pleural line may be missed by ultrasound. Mediastinal ultrasound could not be evaluated in 30% of the children, due to low‐quality imaging or noncompliance. This is a known limitation inherent in the use of mediastinal ultrasound especially in young children with short necks.
A further limitation is that a third radiologist was only consulted when there was a disagreement on the final diagnosis. Finally, ultrasound images were interpreted by clinicians, whereas CXR images were interpreted by pediatric radiologists; despite this, an ultrasound revealed abnormalities more commonly and with a higher inter‐reader agreement.
Further prospective, larger‐scale, multicenter studies evaluating the diagnostic accuracy of chest ultrasound compared to a gold standard such as CT or MRI should be conducted, using experienced sonographers.
5. CONCLUSIONS
Chest ultrasound detected abnormalities more frequently than CXR, and ultrasound abnormalities were most common in the confirmed PTB category. Chest ultrasound also showed higher inter‐reader agreement between clinicians for all categories of findings than CXR read by subspecialist pediatric radiologists. Chest ultrasound may, therefore, be useful to identify lung disease including PTB and other respiratory diseases. Lymph node size appears to differentiate PTB from other lower respiratory tract infections. However, microbiologic diagnosis remains important for confirming the diagnosis. Further studies should evaluate if chest ultrasound detects enlarged mediastinal lymph nodes in children with other respiratory diseases, and evaluate the diagnostic accuracy and significance of chest ultrasound compared to a valid imaging gold standard, like chest CT or MRI.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
CCH, SB, MPG, and HJZ conceived the ultrasound protocol. CCH performed and recorded chest and mediastinal ultrasound. SB reviewed the saved ultrasound cine clips. SA, HL, and HM recorded the CXRs. CCH analyzed the data and prepared the manuscript. MPG and HJZ supervised CCH; HJZ designed and oversaw the TB diagnostic study. CCH drafted the manuscript. CCH, SB, SA, MPG, and HJZ were involved in data interpretation and manuscript revision. All authors contributed to and approved the final version of the manuscript.
Supporting information
E‐image 1: Small consolidation (<0.5 cm) on chest ultrasound
E‐image 2A: Large consolidation (>0.5 cm) on chest ultrasound
E‐image 2B: Consolidation on CXR
E‐image 3A: Pleural effusion on chest ultrasound
E‐image 3B: Pleural effusion on CXR
E‐image 4A: Enlarged mediastinal lymph node (LN) on chest ultrasound
E‐image 4B: Enlarged lymph node on CXR
ACKNOWLEDGMENTS
We would like to thank the study participants and their parents/legal guardians as well as the staff at the SA‐MRC Research Unit for Child and Adolescent Health, UCT and the hospital staff at Red Cross War Memorial Children's Hospital in Cape Town South Africa.
We thank Dr Tom Heller, senior ultrasound specialist, for reviewing the mediastinal ultrasound cine clips as a third reviewer and for the ultrasound training. The study was funded by the National Institute of Health (NIH) RO1 HD058971, MRC South Africa, NRF South Africa. SB and CH were funded by a Marie Curie People grant and SB is currently a participant in the BIH‐Charité Clinician Scientist Program funded by Charité‐Universiätsmedizin Berlin and the Berlin Institute of Health. HJZ is funded by MRC South Africa.
Heuvelings CC, Bélard S, Andronikou S, et al. Chest ultrasound compared to chest X‐ray for pediatric pulmonary tuberculosis. Pediatric Pulmonol. 2019;54:1914‐1920. 10.1002/ppul.24500
Presentation: Preliminary data were presented at the 66th Annual Meeting of the American Society of Tropical Medicine and Hygiene in Baltimore, United States of America (November 5‐9 2017).
References
REFERENCES
- 1. World Health Organization (WHO) . Global tuberculosis report 2018. Geneva: WHO; 2018. [Google Scholar]
- 2. Information about Tuberculosis ; TB in Children – Getting, diagnosing & preventing TB. Global Health education. http://www.tbfacts.org/tb-children/#sthash.S93Elcix.dpuf. Last assessed 26 March 2018.
- 3. Marais BJ, Gie RP, Schaaf HS, et al. A proposed radiological classification of childhood intra‐thoracic tuberculosis. Pediatr Radiol. 2004;34:886‐894. [DOI] [PubMed] [Google Scholar]
- 4. García‐Basteiro AL, López‐Varela E, Augusto OJ, et al. Radiological findings in young children investigated for tuberculosis in mozambique. PLoS One. 2015;10(5):e0127323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richter‐Joubert L, Andronikou S, Workman L, Zar HJ. Assessment of airway compression on chest radiographs in children with pulmonary tuberculosis. Pediatr Radiol. 2017;47(10):1283‐1291. 10.1007/s00247-017-3887-9 [DOI] [PubMed] [Google Scholar]
- 6. du Toit G, Swingler G, Iloni K. Observer variation in detecting lymphadenopathy on chest radiography. Int J Tuberc Lung Dis. 2002;6:814‐817. [PubMed] [Google Scholar]
- 7. Swingler GH, du Toit G, Andronikou S, van der Merwe L, Zar HJ. Diagnostic accuracy of chest radiography in detecting mediastinal lymphadenopathy in suspected pulmonary tuberculosis. Arch Dis Child. 2005;90:1153‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heuvelings CC, Bélard S, Familusi MA, Spijker R, Grobusch MP, Zar HJ. Chest ultrasound for the diagnosis of paediatric pulmonary diseases: a systematic review and meta‐analysis of diagnostic test accuracy. BMB. 2018;129:35‐51. 10.1093/bmb/ldy041 [DOI] [PubMed] [Google Scholar]
- 9. Carrié C, Biais M, Lafitte S, Grenier N, Revel P, Janvier G. Goal‐directed ultrasound in emergency medicine: evaluation of a specific training program using an ultrasonic stethoscope. Eur J Emerg Med. 2015;22(6):419‐425. [DOI] [PubMed] [Google Scholar]
- 10. Bosch‐Marcet J, Serres‐Creixams X, Zuasnabar‐Cotro A, Codina‐Puig X, Catala‐Puigbo M, Simon‐Riazuelo L. Comparison of ultrasound with plain radiography and CT for the detection of mediastinal lymphadenopathy in children with tuberculosis. Pediatr Radiol. 2004;34:895‐900. [DOI] [PubMed] [Google Scholar]
- 11. Bosch‐Marcet J, Serres‐Créixams X, Borrás‐Pérez V, Coll‐Sibina MT, Guitet‐Juliá M, Coll‐Rosell E. Value of sonography for follow‐up of mediastinal lymphadenopathy in children with tuberculosis. J Clin Ultrasound. 2007;35:118‐124. [DOI] [PubMed] [Google Scholar]
- 12. Moseme T, Andronikou S. Through the eye of the suprasternal notch: point‐of‐care sonography for tuberculous mediastinal lymphadenopathy in children. Pediatr Radiol. 2014;44:681‐684. [DOI] [PubMed] [Google Scholar]
- 13. Heuvelings CC, Bélard S, Andronikou S, Jamieson‐Luff N, Grobusch MP, Zar HJ. Chest ultrasound findings in children with suspected pulmonary tuberculosis. Pediatr Pulmonol. 2019;54:463‐470. 10.1002/ppul.24230 [DOI] [PubMed] [Google Scholar]
- 14. Graham SM, Cuevas LE, Jean‐Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis. 2015;61(S3):S179‐S187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol Med. 2008;113(2):190‐198. [DOI] [PubMed] [Google Scholar]
- 16. Pool KL, Heuvelings CC, Bélard S, et al. Technical aspects of mediastinal ultrasound for pediatric pulmonary tuberculosis. Pediatr Radiol. 2017;47(13):1839‐1848. [DOI] [PubMed] [Google Scholar]
- 17. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 18. Di Gennaro F, Pisani L, Veronese N, et al. Potential diagnostic properties of chest ultrasound in thoracic tuberculosis—a systematic review. Int J Environ Res Public Health. 2018;15(10):2235 10.3390/ijerph15102235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Claes AS, Clapuyt P, Menten R, Michoux N, Dumitriu D. Performance of chest ultrasound in pediatric pneumonia. Eur J Radiol. 2017;88:82‐87. [DOI] [PubMed] [Google Scholar]
- 20. Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point‐of‐care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167:119‐125. [DOI] [PubMed] [Google Scholar]
- 21. Agostinis P, Copetti R, Lapini L, Badona Monteiro G, N'Deque A, Baritussio A. Chest ultrasound findings in pulmonary tuberculosis. Trop Doct. 2017;47(4):320‐328. 10.1177/0049475517709633 [DOI] [PubMed] [Google Scholar]
- 22. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9‐15. [DOI] [PubMed] [Google Scholar]
- 23. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions: ultrasound and pleural effusions. J Hosp Med. 2015;10(12):811‐816. 10.1002/jhm.2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acunas B, Celik L, Acunas A. Chest sonography: differentiation of pulmonary consolidation from pleural disease. Acta Radiol. 1989;30(3):273‐275. [PubMed] [Google Scholar]
- 25. Ellington LE, Gilman RH, Chavez MA, et al. Lung ultrasound as a diagnostic tool for radiographically‐confirmed pneumonia in low‐resource settings. Respir Med. 2017;128:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
E‐image 1: Small consolidation (<0.5 cm) on chest ultrasound
E‐image 2A: Large consolidation (>0.5 cm) on chest ultrasound
E‐image 2B: Consolidation on CXR
E‐image 3A: Pleural effusion on chest ultrasound
E‐image 3B: Pleural effusion on CXR
E‐image 4A: Enlarged mediastinal lymph node (LN) on chest ultrasound
E‐image 4B: Enlarged lymph node on CXR


