Abstract
Background
The first International Society of Atopic Dermatitis (ISAD) global meeting dedicated to atopic dermatitis (AD) in Sub‐Saharan Africa (SSA) was held in Geneva, Switzerland in April 2019. A total of 30 participants were present at the meeting, including those from 17 SSA countries, representatives of the World Health Organization (WHO), the International Foundation for Dermatology (IFD) (a committee of the International League of Dermatological Societies, ILDS http://www.ilds.org), the Fondation pour la Dermatite Atopique, as well as specialists in telemedicine, artificial intelligence and therapeutic patient education (TPE).
Results
AD is one of the most prevalent chronic inflammatory skin diseases in SSA. Besides neglected tropical diseases (NTDs) with a dermatological presentation, AD requires closer attention from the WHO and national Departments of Health.
Conclusions
A roadmap has been defined with top priorities such as access to essential medicines and devices for AD care, in particular emollients, better education of primary healthcare workers for adequate triage (e.g. better educational materials for skin diseases in pigmented skin generally and AD in particular, especially targeted to Africa), involvement of traditional healers and to a certain extent also patient education, bearing in mind the barriers to effective healthcare faced in SSA countries such as travel distances to health facilities, limited resources and the lack of dermatological expertise. In addition, several initiatives concerning AD research in SSA were discussed and should be implemented in close collaboration with the WHO and assessed at follow‐up meetings, in particular, at the next ISAD meeting in Seoul, South Korea and African Society of Dermatology and Venereology (ASDV) meeting in Nairobi, Kenya, both in 2020.
Introduction
In 2018, the ISAD recognized the need to actively involve more sub‐Saharan African dermatologists in the society activities after it was realized that the African continent was under‐represented at the well‐attended 9thISAD Georg Rajka Symposium in Utrecht. In retrospect, Africa has been an important target continent for the Society.
In order to advance the involvement of the ISAD in Africa, a meeting was organized in Geneva (Fig. 1) to identify research and intervention priorities in Africa and initiate African‐led projects for AD (research and care) with the help of international health organizations as the main objectives. Dr Matshidiso Moeti, the WHO regional representative for Africa (Brazzaville, Republic of Congo), appointed Prof Mamadou Ball (Nouakchott, Mauritania), a dermatologist and former WHO staff member to present the inaugural lecture; ‘Overview of health challenges including skin health in SSA’. The IFD/ILDS was represented by its chair, Dr Claire Fuller (United Kingdom). The Fondation pour la Dermatite Atopique (Laboratoires Pierre Fabre), already active in western/central francophone Africa, was an early and essential supporter of the ISAD initiative.
Figure 1.

Group photo, Novotel Genève Centre.
The WHO position on the African Region (AFRO) neglected tropical diseases (NTDs) with skin manifestations and chronic skin non‐communicable diseases including AD
WHO/AFRO countries are characterized by an increase in healthy life expectancy from 50.9 to 53.8 years between 2012 and 2015 (highest increase in any WHO Region). The burden of disease is driven by communicable and non‐communicable conditions and violence/injuries. Lower respiratory conditions, HIV/AIDS and diarrhoeal diseases represent the top causes of both morbidity and mortality.1 The health system performance assessment indicates that AFRO countries are performing suboptimally at 49% of their possible level of functionality.2 The challenging situation of NTD control was illustrated by the story of a young shepherd with a mycetoma seen with a large, thick plaque after a disease course of 10 years duration. NTDs are typically diseases of poverty, affecting underserved people with poor health service coverage, emphasizing inaccessibility (geographical and financial), delay in reporting to health facilities and scarcity of tools (medicines and diagnostic tests). The WHO list of NTDs includes 20 conditions amongst which 5 are amenable to control by preventive chemotherapy through mass medicine administration (MMA) (onchocerciasis; lymphatic filariasis; schistosomiasis; soil‐transmitted helminthiasis; and blinding trachoma) with the 15 other diseases requiring case management (CM). Amongst this CM list, nine are considered as skin NTDs (including leprosy, Buruli ulcer, leishmaniasis, scabies, mycetomas and yaws). Co‐endemicity is the rational basis for the ‘integrated’ approach to control skin‐related NTDs, since combining the management of related diseases leads to more efficient use of resources.3
The WHO is supporting countries to ensure (1) the availability and the affordability of existing tools and intensifying their use; (2) the field level capacities to manage target diseases is provided; and (3) the rapid development and implementation of innovative control tools (diagnostics, treatment tools and strategies for public health interventions). In addition, the WHO is giving support for countries to develop master plans and to adapt regional strategies and guidelines. The WHO is providing manuals to help health workers recognize NTDs through changes on the skin.4, 5, 6
For other non‐NTD chronic skin conditions such as AD, the lack of dermatologists and dermatology clinics, the probable underestimated low burden of disease and low mortality in this group precludes WHO prioritization. For AD as well as asthma and allergic rhinitis, the available data indicate prevalence rates for some countries similar to European Union countries. AD and asthma are common inflammatory disease seen both in primary care and especially dermatology clinics/hospitals. There is a strong need of better epidemiological and burden of disease data and of programs of training for common dermatological conditions seen in primary care. The grouping of AD under ‘eczemas’ in the WHO non‐NTD chronic conditions list, which probably includes many cases of contact allergy and other eczematous conditions, remains a problem, compounded by the lack of validated diagnostic criteria for AD adapted to black African skin and environments. In addition to difficulties with diagnosis, barriers to the implementation of therapeutic education include, as for NTDs, poverty, inadequate facilities, shortage of skilled health workers, illiteracy, language and cultural barriers. The possibility of following an ‘integrated’ approach, as for NTDs, by better education of primary healthcare workers, including using digital technologies, may improve appropriate primary patient care and patient referral to specialists when necessary.7
Whilst recognizing the validity of the WHO NTD ‘integrated’ approach, participants favoured a greater emphasis on dermatological conditions in general and also discussed AD integration with common‐related disorders such as asthma and rhinitis. For the WHO NTDs integrative approach, special attention should be paid to campaigns against scabies to differentiate it from AD.
The discussion following the presentation suggested that the WHO should support country's plans to develop AD prevention and education strategies, including access to cheaper emollient therapies as well as innovative digital technologies such as telemedicine. The discussion also emphasized that improving the knowledge of primary care providers, including traditional healers, should be considered as a primary target, a point also emphasized by the IDF. For implementation of this policy, the WHO requests a bottom‐up approach with the involvement of national African dermatologists, lobbying national Ministers of Health to raise the issue of neglected common chronic skin diseases at future WHO Regional Committee meetings. Ministers should be briefed about the high prevalence of skin diseases and paradoxically the lack of WHO publications on this subject.
Survey on AD in Sub‐Saharan Africa
In order to assess the current situation of AD in SSA, an online questionnaire‐based survey was conducted between Oct 2018 and Jan 2019. An invitation to complete the survey was sent to 105 colleagues from African countries through the personal networks of the organizers. The survey was done in three different languages: English, French and Portuguese. Data were collected using SurveyMonkey™. A total of 90 responses from 23 countries (52 Francophone, 23 Anglophone and 15 Lusophone) were obtained (Fig. 2a) corresponding to an 85% response rate. Fifty‐two responders were board certified dermatologists (58%) and resident dermatologists in training (11 participants, 12%), but nine paediatricians and six general practitioners, most with training in allergy, two allied health workers and a patient serving as an ambulance paramedic (South Africa) also completed the survey. Some of the responders had a mixed training (paediatrics and allergy 4, dermatology and allergy 3, dermatology and paediatric dermatology 2).
Figure 2.
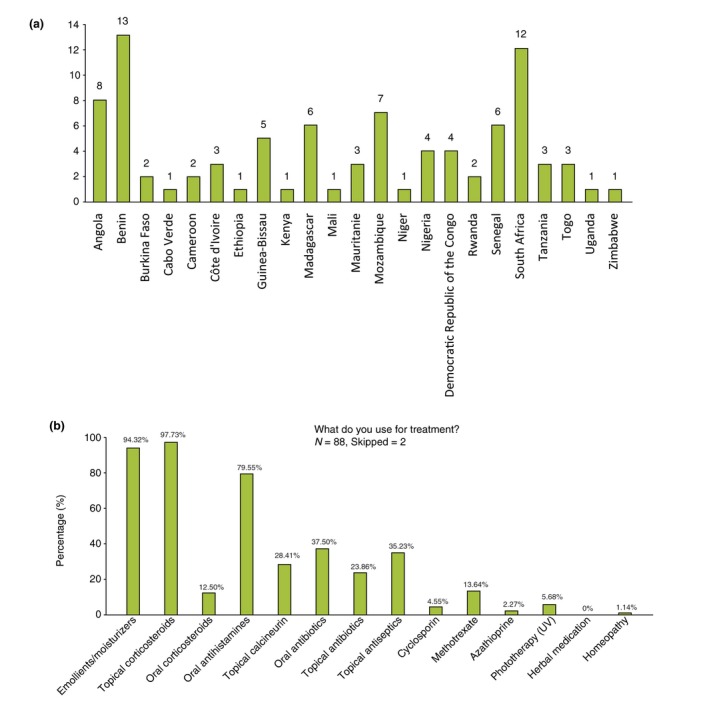
(a) survey responses by country and (b) treatments used by survey responders.
The majority of responders (69.7%) were seeing 1–10 AD patients/week in the setting of a regional/central centre. Most were using emollients/moisturizers and topical corticosteroids. For systemic therapies, oral antihistamines ranked first before oral antibiotics (given mostly for secondary infections), systemic corticosteroids and methotrexate (Fig. 2b). Guidelines from dermatology or allergy societies (South Africa, foreign countries) were used by roughly half of the practitioners.
Problems related to AD that were considered common or very common in Africa included: lack of medication, lack of information about the disease amongst the local population and primary care workers, limited access to medical care and lack of proper training of healthcare professionals. A striking feature was the lack of education materials and educational programs for patients with AD in most African countries.
The survey supported the impression that AD is a growing problem in Africa.8 The acceptance of a chronic disease that needs continuous care is a difficult concept for patients and their carers.
The discussion that followed revealed that a common problem was the unavailability of first‐line treatments such as emollients, because of the dependence on expensive imported products. Self‐administered alternative medicines including mostly herbal medications were commonly used, as well as consulting traditional healers. It was highlighted that the widespread use of alternative/traditional medicines, combined low cost, more patient‐practitioner dialogue and better cultural acceptability. Participants reported cases of severe erythrodermic presentations of AD after traditional herbal medicines applications (Fig. 3a). Some additional aspects were highlighted in the SSA context: self‐medication with high doses of vitamins, lack of availability of bathing facilities and limiting the use of petrolatum‐based products on the skin due to polycyclic aromatic hydrocarbon contamination fears and cancer risk, but also due to the fire hazard with paraffin emollients.9, 10
Figure 3.
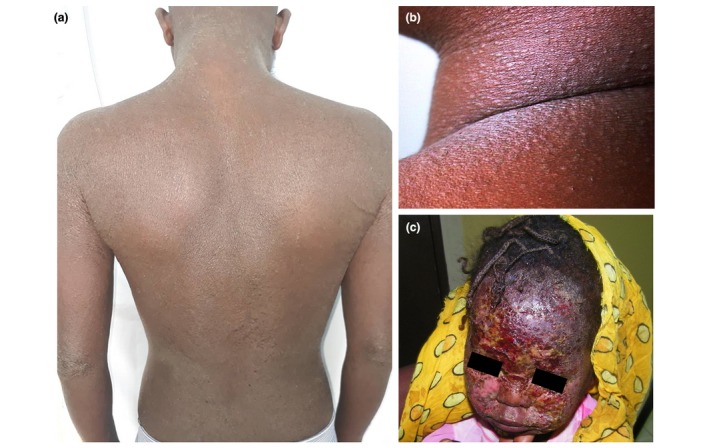
(a) erythroderma after herbal therapy, (b) papular AD and (c) severe bacterial superinfection in childhood AD.
Summary of the major issues concerning AD in SAA
Clinical aspects
In general, the clinical aspects shown by the different presenters were similar to what is observed in the rest of the world. However, some clinical features were highlighted as more common such as a higher frequency of palmar hyperlinearity and lichenoid papular lesions (papular/follicular AD) (Fig. 3b), prurigo‐type lesions, extensor involvement (reverse pattern), frequent facial involvement and severe bacterial superinfection (Fig. 3c). The impact of neglected superinfection on morbidity/mortality is not known.
The most used diagnostic criteria are those of the UK working party, which have not been validated for African populations except in two studies done in Ethiopia and South Africa, both of which found poor positive predictive values for the criteria.11, 12 Adapted, validated and clinician‐friendly criteria are needed for research and clinical use.13 Adaptation of existing scoring systems such as SCORAD14, 15 is being developed for black skin.16
The major differential diagnoses cited by participants besides contact eczema are scabies, insect bites, actinic lichen planus, HTLV1 infective dermatitis, HIV infection, hand foot and mouth disease (for flares).
Several participants discussed the difficulty of long‐term AD management of African patients seeking a cure, who do not accept the concept of chronic disease, a problem already noted worldwide. Corticosteroid phobia also exists in SSA, but the extent of the problem needs evaluation.17
During the discussion of the clinical aspects of AD, the common use of rubbing with abrasive ‘washing nets’ to exfoliate and smoothen the skin was described and may explain some cases of severe lichenification and possibly hyperpigmentation. There was an expressed need for more in‐depth studies of the AD phenotype in black skin in African populations,18, 19 the expectations of the African populations, and allergy testing to identify local relevant allergens, differentiate AD from contact dermatitis or to diagnose additional contact dermatitis in AD patients.20
Genetic risk mechanisms and environmental effects
Despite reduced filaggrin breakdown products having been shown in one study,21 no equivalent of the null mutations in the gene encoding filaggrin, seen in European and Asian people, can to date explain a skin barrier defect in African black skin. Strong genetic risks for AD in Africa have not yet been identified.22 Other skin barrier components associated or not with pigmentation such as redox status, which has been shown to be increased in high versus low phototype skin,23 were discussed.
The patient environment at the onset of AD in African populations is clearly different from that of populations of African ancestry born and living in developed countries. The following habits and facts were cited in the discussion: removal of vernix in neonates, shea butter massage in infants, insecticide use and exposures, endemic parasitic infections, type of foods,24 use of irritating plants, overall bad sanitation and scarce access to water but increased use of harsh detergents, differences in prevalence between urban and rural areas with AD more prevalent in urban areas as noted in developed nations.25
The spectrum of clinically relevant common allergens/atopens across the SSA is poorly documented. Patch/prick testing with standard commercial series, developed for northern regions such as the European series, may be inadequate and provide unreliable data.
Concerning cytokine imbalance and microbiome, studies are lacking in African skin. Early life changes in skin, gut and airway microbiome can precede AD, food allergy and asthma.26 As the skin microbiome is also influenced by such factors as ethnicity, climate, UV exposure and lifestyle factors, future studies of African AD skin microbiome may yield novel regional variations. It was also noted that a butyrate‐rich diet (enriched in fibre‐rich foods and milk fat) shaped the gut microbiome and reduced AD incidence.27 A recent study in South African children demonstrated differences in gut microbiome composition between rural children with AD compared with their healthy counterparts.28
Recent reports have indicated a multipolarity of immune axes in AD. Notably, Asian AD skin phenotypes included psoriatic features that were associated with a strong Th17 immune axis.29 It was noted by some participants that a similar phenotype in African patients has been observed in clinical practice, indicating the need for identifying possibly novel African immune endotypes.
Epidemiology, health resources, telemedicine and therapeutic patient education (TPE)
As investigated in a recent systematic review, only four studies have addressed changes in population prevalence of AD in Africa using validated instruments such as those used by the International Study of Asthma and Allergies in Childhood (ISAAC). The period prevalence of AD in adolescents increased in some SSA countries (Ethiopia, Kenya and South Africa) with a decrease in prevalence in Nigeria over the same period.30 Overall, the existing figures for life prevalence of AD are comparable to those found in developed countries. Relevant atopen/allergen exposures are largely unknown. Sensitization to birch pollen has been detected in parts of Africa (Zimbabwe, Tanzania and South Africa) where there are no birch trees.31 At a molecular level, these reactions are directed against profilin Bet v 2 an ubiquitous plant allergen cross‐reacting with local plants and not to Bet v 1 as seen in clinically relevant allergies in Europe. Mouse sensitization seems common in AD patients when assessed at a molecular level by Mus m1 detection,32 but its relevance to AD has not yet been established.
Access to specialists in dermatology is difficult in nearly all countries, with an average of one dermatologist for 1–2 million inhabitants. Public health systems are usually articulated in three levels; local, regional and national and most dermatologists practice in the capital cities, with some exceptions such as Burkina Faso. In general, there is inadequate public health infrastructure and either insufficient resources or poor management of natural resources.
Concerning the training of dermatologists, the most endowed country represented was South Africa with 171 certified dermatologists followed by Ethiopia (approximately 100) and Côte d'Ivoire with 72 (Table 1).
Table 1.
Countries represented at the meeting and the number of trained dermatologists
| Country | Population (M)/population density in h/km2 | Number of dermatologists | Comments on organization of dermatology in health system |
|---|---|---|---|
| Burkina Faso | 20/75 | 30 | 14 dermatologists in Ouagadougou, 4 in Bobo‐Dioulasso |
| Mauritania | 3.6/3 | 15 | 14 dermatologists in Nouakchott |
| Côte d'Ivoire | 25/70 | 72 | 65 dermatologists in Abidjan |
| Mali | 18/15 | 30 | 23 dermatologists in Bamako |
| Togo | 7/123 | 15 | 13 dermatologists in Lome |
| Niger | 21/17 | 9 | 6 dermatologists in Niamey |
| Benin | 11/98 | 14 | 12 in Cotonou 2 in Parakou |
| Madagascar | 25/43 | 13 | Mostly in Antanarivo |
| RDC | 81/36 | 18 | Dermatologists mostly in Kinshasa |
| Mozambique | 28.8/3.1 | 15 | 13 dermatologists in Maputo, 1 in Beira and 1 in Nampula |
| Rwanda | 12.7/486 | 8 | 7 dermatologists in Kigali |
| Senegal | 16.2/82 | 55 | 42 dermatologists in Dakar |
| Tanzania | 61/64 | 21 | 10/21 dermatologists in the two major cities Dar es Salaam and Mwanza |
| South Africa | 58/48 | 171 | Dermatologist in urban areas |
| Ethiopia | 110/99 | 99 | Most dermatologist in Addis Ababa and few in other cities |
| Uganda | 45/183 | 16 registered | Only 13 practicing dermatologists |
The question of training abroad was discussed, but local training was found preferable if young trainees can stay in their country of origin. Training programs and societies for the represented countries were surveyed and are shown in the Table S1. The discussion moved to the need to train non‐dermatologists such as local nurses and general practitioners. In South Africa, training programs are active at improving the status and number of nurse practitioners (160 nurses have been trained from all over sub‐Saharan Africa). These nurses provide primary care services, which have improved referrals patterns at tertiary level and improved patient skin care generally at local level.33, 34, 35 Another example from South Africa was a patient‐run eczema society (South African National Eczema Society; SANEA) offering direct patient communication and discussion including simple basic techniques such as how to wash the skin and apply medication. The case for training traditional healers was also discussed as some traditional healers are amenable to training in the essentials of dermatology for working in conjunction with health services.
It was agreed that telemedicine could be particularly helpful in the context of SSA.36 Madagascar was shown as a highly connected African country. Established international examples included the RAFT network (Switzerland)37 delivering tele‐expertise and e‐learning to 11 French speaking countries in Africa since 2000 and the Africa Teledermatology Project (ATP), which includes 12 countries in Africa.38 Local programs included examples in Mauritania, Mali and South Africa.39 In Sao Tome, a project in collaboration with Portugal focuses initially on albinism. Potential telemedicine applications in AD projects were discussed including its use for atopic schools, e‐learning, webinars with AD experts with local knowledge of medication and local practices, apps adjusted for pigmented skin and user‐friendly video tutorials of therapeutic education for patients and families. Existing apps for tele‐expertise–store and forward (Bogou, RAFT),40 e‐learning (Dudal, RAFT)41 and M‐health (PO SCORAD, Dermatite atopique HD) are already providing help. TPE was shown to be effective in a feasibility study for diabetes in Mozambique. Healthcare professionals and education specialists helped in the design of the project, which was adapted to local populations. It was implemented through local training of educators. Overall, TPE objectives (patient empowerment, achievement of competencies of disease knowledge, diagnosis and treatment, understanding and acceptance of the disease and patient autonomy) fit perfectly the AD context, but need local field adaptation.42, 43 The benefit of TPE in terms of reducing medical costs in chronic diseases was debated.44
Access to essential medicines including emollients
The use of emollients is an important 1st line, basic and crucial step45 in long‐term AD control. In the SSA setting, the use of local long‐term treatments, particularly emollients, is variable. In some areas, access to essential drugs is assured but in others it is difficult or impossible, especially if imported products from branded cosmetic houses are used (e.g. one emollient tube represents 15% of local minimum monthly salary in Madagascar). In addition, AD care is hampered by the absence of coverage by health insurance in some countries. Possible solutions were discussed such as local emollient production with glycerol, shea butter or coconut oil.
Topical corticosteroid supplies were reported as variably available. They can be found as skin lightening/depigmenting creams, at cheaper prices, on many street vendor stalls or small corner shops. Their quality cannot be guaranteed, and they may pose a potential risk of superinfection if used for AD.
For severe cases, systemic steroids are sparsely used for short periods of time. Methotrexate is available, but in some countries, the supply is just enough for rheumatology. Cyclosporin is available in some countries, but rarely used.
Newer drugs to manage AD such as biologicals and small molecules are currently out of reach of most SSA public medical systems but are variably available to private medical systems. As many of the drug trials evaluating the efficacy and safety of these biologics and small molecule for AD treatments are not undertaken in Africa, their effects and adverse effects are extrapolated from European and North American findings, which may not be applicable to the region. New vaccination strategies, still in experimental stages, might become useful and affordable tools in the future.46
Design thinking as a possible approach in SSA
Design thinking is a creative innovation process originally used in the area of design, to improve the look and functionality of products in line with what the customers want. More recently, this process is being used to tackle complex social and healthcare problems. Design thinking has already been used in many projects in Africa47 (available at http://siteresources.worldbank.org/WBI/Resources/2137981278955272198/Design_Thinking_for_SocialInnovation2.pdf).
Design thinking is a person‐centred approach that, when applied to healthcare, involves listening closely to patient's problems, desires and needs. Design thinking allows creative solutions to appear from the patients themselves, rather than these being imposed by the healthcare professional. It also involves working in collaboration with a multidisciplinary team, which includes patients and their families, doctors, psychologists, social workers, nurses and others. The multidisciplinary team must be trained and have homogeneity in knowledge, attitudes and perceptions. Some skills can be developed and adapted for this purpose, like empathy, integrative thinking, creativity and experimentation.
A workshop session used design thinking to propose a roadmap for AD.
Summary and roadmap
The meeting highlighted, by field experience, the importance and burden of AD in SSA, even with limited published data. AD is one of the most common inflammatory dermatological diseases in primary, secondary and especially tertiary care in SSA and has many clinical presentations. Because of the chronicity of AD and difficulty with treatment availability, meeting participants acknowledged the burden of AD in dermatological practice and considered AD as a significant emerging public health problem in SSA. Therefore, international health organizations such as the WHO, dermatology foundations such as the IFD and international learned societies such as the ISAD should encourage and support studies on AD in Africa for better recognition, understanding and management of the uniqueness of SSA AD. Whilst acknowledging the approach of integrating skin diseases with other conditions, participants stressed the importance of dermatological conditions specifically.
A design thinking approach could be used to create effective solutions for the region.
Participants agreed that the difficulties in diagnosis and treatment of AD are related to the lack of medical infrastructure, in particular, trained primary care workers and certified dermatologists. They also agreed on the need to develop telemedicine in order to compensate for the low number and uneven distribution of dermatologists. Research integrating artificial intelligence to support primary care management with appropriate feedback, and documenting its effectiveness was also suggested.
Particularly important measures should be implemented in the SSA context for AD:
Lower taxes on emollients or investigate alternate suppliers, include emollients in the list of essential medicines at all levels of healthcare and, in the longer term, produce regional products. The implementation of a regional survey of products currently available in SSA was deemed urgent, in order to make practical proposals.
For topical corticosteroids, the risk of using over the counter preparations or those used as skin lightening/depigmenting agents has not been thoroughly assessed. Until this is done, the products should be discouraged for use in AD and the WHO should promote the use of licensed products by ensuring their availability at all levels of healthcare as essential drugs.
The availability of methotrexate as an essential drug at a tertiary level or under specialist supervision should be surveyed in all SSA countries, with toxicity adequately managed clinically and biologically.
Development of AD prevention and education activities including TPE through e‐learning and dedicated apps
Improving the knowledge of primary healthcare providers including traditional healers (some already involved for HIV prevention): publication of an iconographic document on the main dermatoses diagnosed in pigmented African skin for primary care workers (as an example see the booklet ‘Skin diseases, case management algorithms & atlas’ from Mekelle, Tigray, Ethiopia).
Concerning research initiatives, this first ISAD meeting devoted to SSA AD has listed the following as top priorities:
Make specific pan African surveys on emollients, education programmes for primary care workers, AD scoring scales on pigmented skin, AD prevalence comparing various published diagnostic criteria and patient desires/needs
Skin barrier function in black African skin
Impact of AD superinfection on morbidity/mortality
Keloids and AD (test the hypothesis of less keloids in AD, TH2 cytokines‐fibrosis)
Scabies/AD (test the hypothesis of TH2 cytokines as limiting parasitic infections)
To improve exchanges between SSA countries with language barriers, a digital platform, which should be operated in English, French and Portuguese, could be set up and adapted for e‐learning, telemedicine and research projects.
All initiatives should be implemented in close collaboration with African dermatologists, the WHO and the IFD and assessed at follow‐up meetings, in particular, at the next ISAD Meeting in Seoul, South Korea and at the African Society of Dermatology and Venereology (ASDV) Meeting and Nairobi, Kenya, both in 2020.
Supporting information
Table S1. African Dermatological Societies and training programs amongst represented countries.
Acknowledgements
The meeting was sponsored by ISAD and WHO; the participants were invited on a personal basis by the organizers. We thank warmly Prof Mamadou Ball for his participation on behalf of WHO. The help of Caroline Blondel‐Baldassarre (European Society for Dermatological Research), Georges Farah, Sophie Mery and Helène Passerini (Fondation pour la Dermatite Atopique) and Kira Weirum Dalgaard (LEO Foundation) is warmly acknowledged. We thank Michaela Kocjancic for her very effective secretarial support.
Conflicts of interest
None declared.
Funding sources
None declared.
References
- 1. The work of WHO in the African Region‐Report of the Regional Director: 2017‐2018 URL https://www.afro.int/node/10291 (last accessed: 28 February 2019).
- 2. The state of health in the WHO African Region: an analysis of the status of health services and health systems in the context of Sustainable Development Goals. URL: https://www.afro.who.int/sites/default/files/sessions/documents/State%20of20African Region.pdf (last accessed: 28 February 2019).
- 3. Integrating neglected tropical diseases in global health and development . Fourth WHO Report on neglected tropical diseases ISBN 978‐92‐4‐156544‐8 WHO/HTM/NTD/2017.1.
- 4. WHO . Recognizing neglected tropical diseases through the changes of skin: a training guide for front line health workers. 2018, ISBN 978‐92‐4‐151353‐1.
- 5. Engelman D, Fuller LC, Solomon AW et al Opportunities for integrated control of neglected tropical diseases that affect the skin. Trends Parasitol 2016; 32: 843–854. [DOI] [PubMed] [Google Scholar]
- 6. Mitjà O, Marks M, Bertran L et al Integrated control and management of neglected tropical skin diseases. PLoS Negl Trop Dis 2017; 11: e0005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barogui YT, Diez G, Anagonou E et al Integrated approach in the control and management of skin neglected tropical diseases in Lalo Benin. PLoS Negl Trop Dis 2018; 12: e0006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Afif KAM, Buraik MA, Buddenkotte J et al Understanding the burden of atopic dermatitis in Africa and the Middle East. Dermatol Ther (Heidelb) 2019; 9: 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pratt MM, John K, MacLean AB, Afework S, Phillips DH, Poirier MC. Polycyclic aromatic hydrocarbon (PAH) exposure and DNA adduct semi‐quantitation in archived human tissues. Int J Environ Res Public Health 2011; 8: 2675–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campaign for Safe Cosmetics . URL http://www.safecosmetics.org/get-the-facts/chemicals-of-concern/petrolatum. (last accessed: 26th May 2019).
- 11. Haileamlak A, Lewis SA, Britton J et al Validation of the International Study of Asthma and Allergies in Children (ISAAC) and U.K. criteria for atopic eczema in Ethiopian children. Br J Dermatol 2005; 152: 735–741. [DOI] [PubMed] [Google Scholar]
- 12. Chalmers DA, Todd G, Saxe N et al Validation of the U.K. Working Party diagnostic criteria for atopic eczema in a Xhosa‐speaking African population. Br J Dermatol 2007; 156: 111–116. [DOI] [PubMed] [Google Scholar]
- 13. Chalmers JR, Thomas KS, Apfelbacher C et al Report from the fifth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2018; 178: e332–e341. [DOI] [PubMed] [Google Scholar]
- 14. Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997; 195: 10–19. [DOI] [PubMed] [Google Scholar]
- 15. Stalder JF, Barbarot S, Wollenberg A et al Patient‐Oriented SCORAD (PO‐SCORAD): a new self‐assessment scale in atopic dermatitis validated in Europe. Allergy 2011; 66: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 16. Faye O, Meledie N'djong AP, Diadie S et al. Patient‐Oriented SCOring for Atopic Dermatitis (PO‐SCORAD) for black skin is a validated tool to evaluate atopic dermatitis severity and correlates with SCORAD. J Eur Acad Dermatol Venereol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aubert‐Wastiaux H, Moret L, Le Rhun A et al Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol 2011; 165: 808–814. [DOI] [PubMed] [Google Scholar]
- 18. Bieber T, D'Erme AM, Akdis CA et al Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J Allergy Clin Immunol 2017; 139: S58–S64. [DOI] [PubMed] [Google Scholar]
- 19. Ayanlowo O, Puddicombe O, Gold‐Olufadi S. Pattern of skin diseases amongst children attending a dermatology clinic in Lagos, Nigeria. Pan Afr Med J 2018; 29: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campana R, Dzoro S, Mittermann I et al Molecular aspects of allergens in atopic dermatitis. Curr Opin Allergy Clin Immunol 2017; 17: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thawer‐Esmail F, Jakasa I, Todd G et al South African amaXhosa patients with atopic dermatitis have decreased levels of filaggrin breakdown products but no loss‐of‐function mutations in filaggrin. J Allergy Clin Immunol 2014; 133: 280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winge MC, Bilcha KD, Liedén A et al Novel filaggrin mutation but no other loss‐of‐function variants found in Ethiopian patients with atopic dermatitis. Br J Dermatol 2011; 165: 1074–1080. [DOI] [PubMed] [Google Scholar]
- 23. Bessou‐Touya S, Picardo M, Maresca V, Surlève‐Bazeille JE, Pain C, Taïeb A. Chimeric human epidermal reconstructs to study the role of melanocytes and keratinocytes in pigmentation and photoprotection. J Invest Dermatol 1998; 111: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 24. Hooper R, Calvert J, Thompson RL, Deetlefs ME, Burney P. Urban/rural differences in diet and atopy in South Africa. Allergy 2008; 63: 425–431. [DOI] [PubMed] [Google Scholar]
- 25. Schram ME, Tedja AM, Spijker R, Bos JD, Williams HC, Spuls PI. Is there a rural/urban gradient in the prevalence of eczema? A systematic review Br J Dermatol 2010; 162: 964–973. [DOI] [PubMed] [Google Scholar]
- 26. Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O'Mahony L. Recent developments and highlights in mechanisms of allergic diseases: microbiome Allergy 2018; 73: 2314–2327. [DOI] [PubMed] [Google Scholar]
- 27. Roduit C, Frei R, Ferstl R et al High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019; 74: 799–809. [DOI] [PubMed] [Google Scholar]
- 28. Mahdavinia M, Rasmussen HE, Botha M et al Effects of diet on the childhood gut microbiome and its implications for atopic dermatitis. J Allergy Clin Immunol 2019; 143: 1636–1637. [DOI] [PubMed] [Google Scholar]
- 29. Brunner PM, Guttman‐Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad‐spectrum and targeted therapies. J Allergy Clin Immunol 2017; 139: S65–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deckers IA, McLean S, Linssen S et al Investigating international time trends in the incidence and prevalence of atopic eczema 1990‐2010: a systematic review of epidemiological studies. PLoS ONE 2012; 7: e39803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westritschnig K, Sibanda E, Thomas W et al Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy 2003; 33: 22–27. [DOI] [PubMed] [Google Scholar]
- 32. Thawer‐Esmail F, Irvine A, Carrara H, Todd G, Schmid‐Grendelmeier P. Sensitization to mouse (Mus m 1) is a leading pattern in amaXhosa atopic dermatitis patients in Cape Town Region, South Africa. Abstract presented at EAACI Annual Meeting 2016, Vienna.
- 33. Ersser SJ, Kaur V, Kelly P et al The contribution of the nursing service worldwide and its capacity to benefit within the dermatology field. Int J Dermatol 2011; 50: 582–589. [DOI] [PubMed] [Google Scholar]
- 34. Cloete D. Dermatology nursing in a rural area – the Overberg experience. Community dermatology in a rural area in the Western Cape. CME 2013; 31: 254–258. [Google Scholar]
- 35. Stevens J. Dermatology nursing in the community: the Mitchells Plain experience. Dermatology is an important element of community nursing. CME 2013; 31: 250–253. [Google Scholar]
- 36. Schmid‐Grendelmeier P, Masenga EJ, Haeffner A, Burg G. Teledermatology as a new tool in sub‐saharan Africa: an experience from Tanzania. J Am Acad Dermatol 2000; 42: 833–835. [DOI] [PubMed] [Google Scholar]
- 37. Bediang G, Perrin C, Ruiz de Castañeda R et al The RAFT telemedicine network: lessons learnt and perspectives from a decade of educational and clinical services in low‐ and middle‐incomes countries. Front Public Health 2014; 2: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lipoff JB, Cobos G, Kaddu S, Kovarik CL. The Africa Teledermatology Project: a retrospective case review of 1229 consultations from sub‐Saharan Africa. J Am Acad Dermatol 2015; 72: 1084–1085. [DOI] [PubMed] [Google Scholar]
- 39. Colven R, Shim MH, Brock D, Todd G. Dermatological diagnostic acumen improves with use of a simple telemedicine system for underserved areas of South Africa. Telemed J E Health 2011; 17: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faye O, Bagayoko CO, Dicko A et al A Teledermatology Pilot programme for the management of skin diseases in primary health care centres: experiences from a resource‐limited country (Mali, West Africa). Trop Med Infect Dis 2018; 3: Pii: E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bagayoko CO, Neaf JM, Maiga H, Traoré AK, Geissbuhler A. Réseau de télé‐enseignement médical: identification des barrières technologiques, organisationnelles, et humaines. J Health Inform Afr 2015; 3: 1–13. [Google Scholar]
- 42. Lagger G, Pataky Z, Golay A. Efficacy of therapeutic patient education in chronic diseases and obesity. Patient Educ Couns 2010; 79: 283–286. [DOI] [PubMed] [Google Scholar]
- 43. Stenberg U, Haaland‐Øverby M, Fredriksen K, Westermann KF, Kvisvik T. A scoping review of the literature on benefits and challenges of participating in patient education programs aimed at promoting self‐management for people living with chronic illness. Patient Educ Couns 2016; 99: 1759–1771. [DOI] [PubMed] [Google Scholar]
- 44. Heratizadeh A, Werfel T, Wollenberg A. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol 2017; 140: 845–853. [DOI] [PubMed] [Google Scholar]
- 45. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. [DOI] [PubMed] [Google Scholar]
- 46. Bachmann MF, Zeltins A, Kalnins G et al Vaccination against IL‐31 for the treatment of atopic dermatitis in dogs. J Allergy Clin Immunol 2018; 142: 279–281. [DOI] [PubMed] [Google Scholar]
- 47. Brown T, Wyatt J. Design thinking for social innovation. Development Outreach 2010; 12: 29–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. African Dermatological Societies and training programs amongst represented countries.


