ABSTRACT
Background
Preclinical studies have hypothesized a possible immunological reponse to allogeneic materials due to detection of remnants of potential immunogenic molecules. However, their impact on integration, bone remodeling and immunological reaction after the augmentation procedure is largely unknown and a direct correlation of analytical data and evaluation of human biopsies is missing.
Purpose
The present study aimed to compare two commercially available allogeneic materials regarding their content of cellular remnants as well as the bone remodeling, and integration and potential immunologic reactions on a histological and immunohistochemical level, integrating also in vitro analytical evaluation of the specific batches that were used clinically.
Materials and Methods
Twenty patients were randomly assigned to treatment with Maxgraft or Puros for lateral ridge augmentation in a two‐stage surgery. After a mean healing period of 5 months, implants were placed and biopsies were taken for histological, immunhistochemical, and histomorphometrical evaluation regarding bone remodeling and inflammation, protein concentrations in vitro and the presence of MHC molecules of the same batches used clinically.
Results
No differences in clinical outcome, histological, immunohistochemical, and in vitro protein analysis between the two bone grafting materials were observed. Active bone remodeling, amount of newly formed bone, and residual grafting material was independent of the materials used, but varied between subjects. MHC1 residues were not detected in any sample.
Conclusions
Within the limitations of this study, both tested materials yielded equivalent results in terms of clinical outcome, new bone formation, and lack of immunological potential on a histological and immunohistochemical level.
Keywords: guided bone regeneration, histomorphometry, human histology, lateral ridge augmentation, particulated bone allograft
1. INTRODUCTION
Loss of bone volume leading to alveolar atrophy results from different pathogenic processes related to tooth loss, periodontitis, dental trauma, or tumors.1 Dimensional changes of the alveolar ridge often lead to unfavorable local conditions, for example, for implant placement. Therefore, bone grafting in order to obtain sufficient bone volume is one of the most widespread administered therapies in oral and maxillofacial surgery.2 Autologous bone application is still considered the gold standard,3, 4 although this technique can be associated with several disadvantages, for example, donor site morbidity, pain, impaired function or limitations in quantity and quality of available bone.5
In recent years, the use of allogeneic human bone has been favored worldwide, and the number of reports of its use for several indications in oral surgery is increasing.6, 7 This may be due to superior remodeling potential compared to xenogenic materials and increasing safety guidelines regarding standardized procedures for the screening of donors as well as harvesting, processing, and storing of allogeneic human bone. Extensive serological screening for potential infectious diseases, graft sterilization and establishment of a global biovigilance programme in combination with a long‐term traceability of allografts have significantly improved safety, resulting only in a theoretical risk of transmission, for example, human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) today.6, 8, 9
Recent evidence for the detection of cellular remnants, such as cartilage tissues, cells or proteins in allogeneic substitutes,10, 11 raised the question if immunological side effects may occur, resulting for example in sensitization12 against the allogeneic substitute. In a very recent study, Fretwurst et al. showed that major histocompatibility complex molecules (MHC) were detectable in allogeneic bone blocks. The authors conclude that despite thorough processing, a potential antigenicity might not be totally eliminated in allografts, probably inducing a T cell‐mediated immune response against the allograft.13
Due to the controversial discussion of immunological aspects in allogeneic graft healing, in the present study the hypothesis was tested whether MHC molecules can be detected in both materials tested, a solvent dehydrated allogeneic material from a single donor (Puros Allograft) and in a freeze‐dried material pooled from multiple donors (Maxgraft), eliciting an immunological response in the patients. Therefore, five different batches of each material were screened for soluble protein content and residues of MHC molecules according to methods reported previously.13 The patients receiving those allografts were clinically closely monitored for signs of inflammatory reactions. Furthermore, biopsy cores were harvested prior to implant placement and analyzed using histological and immunohistochemical methods in order to relate these findings to potential signs of inflammation, T‐cell mediated cellular reactions or other histological signs indicating adverse effects on graft incorporation in the human recipients. As a secondary objective we also analyzed the healing of the grafts histomorphometrically and histologically by investigating anabolic and catabolic immunohistochemical markers for bone remodeling in order to explore influences of probable inflammation on osteogenesis.
2. MATERIALS AND METHODS
2.1. Study design, bone allograft material, and participants
As a randomized clinical trial, the publication was written on the basis of CONSORT guidelines.14 Twenty systemically healthy, non‐smoking, partially edentulous patients with alveolar ridge defects and the desire for implant treatment were recruited and treated in a single periodontal office in Hamburg, Germany, between March 2016 and June 2017. Alveolar ridge defects were classified according to the index published by Seibert in 1983.15 Only patients with Seibert class I defects were included in the present study. Lateral augmentation procedures of the alveolar ridge were performed in a parallel trial design in a two‐stage surgery with two different commercially available particulate allograft materials that were rehydrated in the second phase of the PRGF system (PRGF‐2; BTI, Vittoria, Spain) and the same type of collagen membranes. Ten patients for each group were randomly assigned to the type of bone grafting material by a blinded clinician not involved in this study and not involved in the periodontal office by drawing sealed envelopes. As allografts for guided bone regeneration, we used Maxgraft Allograft Spongiosa Particle (botiss biomaterials GmbH, Berlin, Germany, part of Straumann Group, Basel, Switzerland) or Puros Allograft Spongiosa Particle (Zimmer Biomet, Warsaw, Indiana) each in 10 of 20 individuals included in this study. If more than one implant was placed in one patient, only one biopsy was taken for histological analysis in order to prevent bias due to multiple biopsy analysis from one individual. Both bone grafting materials are treated in a multistep chemical cleaning process to inactivate potential pathogens. Maxgraft is a pooled allogeneic material from multiple donors and finally dehydrated by freeze‐drying, whereas Puros is an allogeneic material harvested from one single donor per batch and is dehydrated using a solvent dehydration process prior to packaging and gamma‐irradiation. Each process has been validated to inactivate viruses and bacteria and preserve the natural collagen‐bone mineral composition which prevents disease transmission by removing and/or inactivating cells, viruses, antigens, and pathogens.16
Biopsy collection and experiments were performed in compliance with the World Medical Association Declaration of Helsinki (version 2008) and were approved by an ethics committee (Hamburg Medical Association, Germany, no. PV5211) and the study was registered with the German Register for Clinical Trials (DRKS no.: 00013010). All patients gave their informed consent and all patients completed the study successfully and were available for follow‐up visits. No adverse events were recorded. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Consent was obtained from all patients for publication of this study and any accompanying images.
2.2. Surgical procedure
Grafting and subsequent implant placement was performed under local anesthesia using Ultracain‐DS Forte (Sanofi‐Aventis, Frankfurt/Main, Germany). Prior to surgical intervention, venous blood was collected from the patients for preparation of plasma rich in growth factors (PRGF) according to the manufacturer's specifications using the PRGF‐Endoret technology (BTI, Miñano, Spain). PRGF was added to both of the previously in sterile saline rehydrated allogeneic graft materials in order to improve postoperative healing.17 After deflection of a mucoperiosteal flap (Figure 1A), a cortical perforation was done (Figure 1B), and bone grafting material was inserted (Figure 1C). The allografts were covered with a Jason membrane for guided bone regeneration, according to the manufacturer's recommendations (botiss biomaterials GmbH, Zossen, Germany) (Figure 1D). A periosteal releasing incision of the mucoperiosteal flap was performed (Figure 1E) in order to mobilize the flap for a tension‐free primary closure of the surgical site. Flap‐fixation was performed using a horizontal and vertical mattress suture with 5.0 Goretex filaments (W. L. Gore & Associates GmbH, Putzbrunn, Germany). A 2.0% chlorhexidine rinsing solution was administered for post‐operative oral hygiene. Postoperative appointments were scheduled after 1 to 2 days, 2, 6, and 12 weeks. Sutures were removed 2 weeks after augmentation. Implants were inserted in an open flap approach after ~4 to 5 months of healing. Biopsies were taken from the augmented sites before implant insertion using a trephine bur with a core diameter of 3.0 mm (Komet Dental, Gebr. Brasseler GmbH & Co. KG, Lemgo, Germany) and a speed of 600 rpm with external cooling using sterile saline (Figure 1F). Drilling was performed to a maximum depth of 6.0 mm exactly at the position where implant placement was intended. Implants with a diameter of at least 3.3 mm and a minimum length of 8.0 mm (Straumann Group; Camlog GmbH, Wimsheim, Germany; Astra Implant System, Dentsply Sirona Implants, Mannheim, Germany) were inserted according to the manufacturer's recommendations with a mean insertion torque of 35 Ncm and with an additional administration of PRGF on the implant surface.18 After implant placement, the mucoperiosteal flap was readapted and fixed with Goretex sutures. Uncovering and prosthetic restauration of the implants was carried out after the healing period of ~4 months (Figure 1G). Two‐dimensional radiographs, using the parralleling technique with a Rinn holder (Dentsply Rinn, Smile WayYork, Pennsylvania) were taken immediately following the bone augmentation procedure (not shown), immediately after implant insertion (not shown), and after final prosthetic reconstruction in order to visualize the final result as a baseline for future radiographic comparison (Figure 1H).
Figure 1.
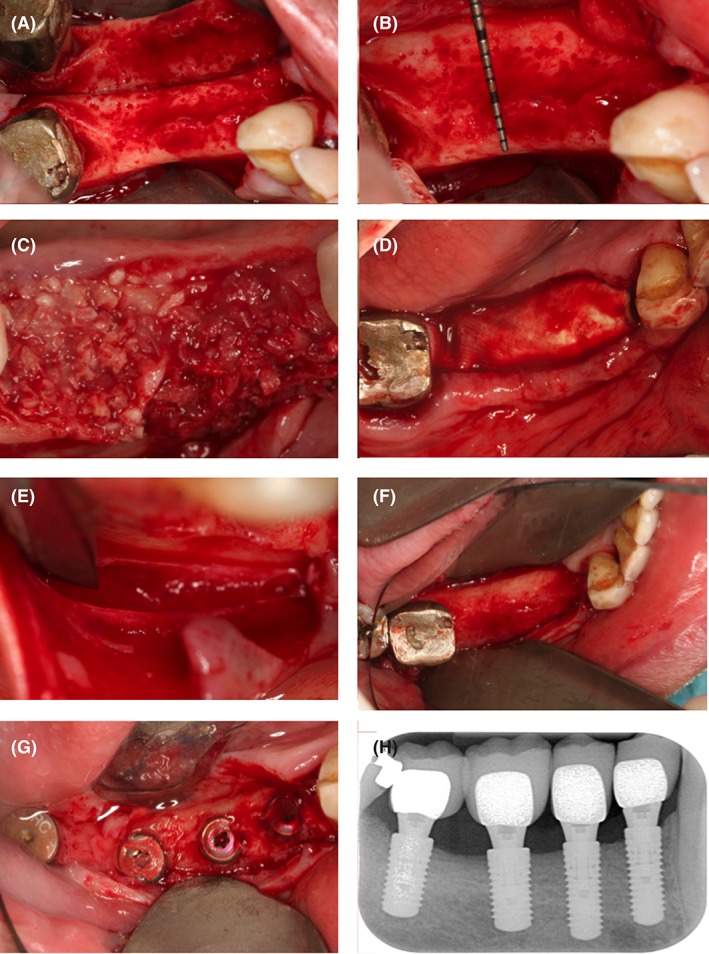
Overview of the surgical procedure. A very thin alveolar crest appeared after reflection of a mucoperiosteal flap (A). The alveolar ridge was then prepared with cortical perforations (B). Thereafter, the bone allograft material soaked in the second phase of the PRGF solution was applied in order to build up the alveolar bone volume necessary for future implant placement (C). It was then covered with a resorbable collagen membrane (D). The surgical site was primarily closed by means of a periosteal incision (E), the use of horizontal mattress sutures and a continuous half‐hitch suture. The second surgical procedure took place after a healing period of 4 months. The significant gain in alveolar ridge volume can be appreciated (F). Four implants were placed according to the manufacturer's recommendations (Straumann Group, Basel Switzerland). Four months later, the implants were uncovered (G). Postoperative two‐dimensional radiographs demonstrate stable integration of the implants 36 months after final restauration (H)
2.3. Protein extraction
Extraction of proteins was performed as previously reported.13 We dialyzed 4 × 150 mg bone material of each allograft batch using the Slide A Lyzer Mini Dialysis Filter Devices 3.5 kDa (Thermo Fisher Scientific, Darmstadt, Germany), corresponding to the manufacturer's protocol. Briefly, 44.5 mL PBS (phosphate buffered saline) were filled in the part below the filter of the device. After wetting the filter with 4 mL PBS, 2 mL lysis buffer (0.3 M sucrose, 10 mM Tris pH 7.5, 5 mM MgCl2, 1% Triton X‐100) and 150 mg bone material were added to the filter device. Three incubation steps were performed for 2 hours, overnight and 2 hours by orbital shaking at 200 rpm, 4°C. To narrow the volume, the pooled dialyzed (about 10 mL) protein extract of each batch was added to the Amicon Ultra‐4 10 K Centrifugal Filter Device (Merck, Darmstadt, Germany) and centrifuged twice at 4000g, 4°C for 20 minutes. Protein concentration was determined spectrophotometrically using the Qubit Protein Assay kit (Thermo Fisher Scientific).
2.4. ELISA measurements for detection of MHC1
To perform the ELISA, leukocytes served as a positive control. Leukocytes were extracted from 7.5 mL EDTA blood with 750 μL lysis buffer. After incubation on ice for 5 minutes and centrifugation at 400g, 4°C for 5 minutes, the pellet contained leukocytes and residues of erythrocytes. Incubation of the pellet with 1.5 mL lysis buffer on ice for 5 minutes and centrifugation at 400g, 4°C for 5 minutes were repeated until the erythrocytes were completely removed from the leukocyte pellet. Then the purified leukocyte pellet was resuspended in 1 mL PBS and centrifuged at 400g, 4°C for 5 minutes. These washing steps were repeated three times. Finally, the leukocyte pellet solved in 200 μL PBS was sonicated three times by the Sonificator UP50H (Hielscher Ultrasonics GmbH, Teltow, Germany) and centrifuged at 1000g for 15 minutes. The quantity of the leukocyte proteins in the supernatant was determined spectrophotometrically using the Qubit Protein Assay kit (Thermo Fisher Scientific).
Quantification of MHC1 was carried out by an MHC1 ELISA kit (BlueGene Biotec, Shanghai, China) corresponding to the manufacturer's protocol. Briefly, 5 μL Balance Solution were mixed in 2 × 50 μL of the protein solutions and added to each well. The microplates were incubated with 100 μL Conjugate for 1 hour at 37°C and washed with Wash Solution five times. Next, 50 μL Substrate A and 50 μL Substrate B were added to each well, also to the wells containing 50 μL blank (PBS) and 50 μL positive (leukocytes) control, and incubated in the dark for 15 minutes at 37°C. After adding 50 μL Stop Solution to each well, the color change was spectrophotometrically determined as the Optical Density (OD) at 450 nm using a microplate reader (Tecan Infinite 200Pro, Männerdorf, Switzerland).
2.5. Histology
Each biopsy sample was fixed by immersion in 4% buffered formaldehyde (Sörensen buffer) at room temperature (RT) for at least 1 day and subsequently decalcified for about 2 to 3 weeks in 4.1% disodium ethylenediaminetetraacetic acid (EDTA) solution, which was changed every 24 hours. After hydration, tissues were dehydrated in an ascending series of ethanol and embedded in paraffin. Serial longitudinal sections of 2 to 3 μm were cut and representative slides were stained with hematoxylin‐eosin (HE). In order to identify osteoclasts, selected tissue sections were stained for tartrate‐resistant acid phosphatase (TRAP).
2.6. Immunohistochemistry
Representative slides from the median parts of the sample series were deparaffinized, rehydrated and rinsed for 10 minutes in tris‐buffered saline (TBS). Endogenous peroxidase was blocked in a methanol/H2O2 (Merck, Darmstadt, Germany) solution for 45 minutes in the dark. Sections were pretreated with PBS containing 1% bovine serum albumin (BSA) for 20 minutes at RT, digested with 0.4% pepsin for 10 minutes at 37°C and then incubated with the primary antibodies in a humid chamber. The following markers were investigated: bone matrix and differentiation markers (alkaline phosphatase [ALP], osteocalcin [OC], osteopontin [OP], [runx2]), immunological markers (CD3, CD4, CD8, and ED1), and the vessel marker von Willebrand factor (vWF). Collagen type I was also stained. Antibody details and incubation protocols are listed in Table 1. Antibody binding was detected with the peroxidase‐conjugated EnVision anti‐mouse system or the EnVision anti‐rabbit/anti‐goat HRP‐conjugated secondary antibodies (Dako, Glostrup, Denmark), diluted 1:50 and incubated for 30 minutes at RT. Peroxidase activity was visualized using diaminobenzidine (DAB) yielding a brown staining product and slides were counterstained with Mayer's hematoxylin.
Table 1.
Antibody details and incubation protocols
| Antibody | Isotype | Manufacturer | Incubation protocol |
|---|---|---|---|
| Alkaline phosphatase (ALP) | Rabbit polyclonal | Quartett (Berlin, Germany) | ready to use, o/N, 4°C |
| CD3 | Rabbit polyclonal | Dako (Glostrup, Denmark) | HP, citrate buffer, 1:50, 1 hour, RT |
| CD4 | Rabbit monoclonal | Abcam (Cambridge, UK) | HP, EDTA buffer, 1:50, o/N, RT |
| CD8 | Mouse monoclonal | Dako (Glostrup, Denmark) | HP, citrate buffer, 1:50, 1 hour, RT |
| Collagen type I (COL I) | Rabbit monoclonal | Abcam (Cambridge, UK) | 1:400, 1 hour, RT |
| ED1 (CD68) | Mouse monoclonal | Dako (Glostrup, Denmark) | 1:100, 1 hour, RT |
| Osteocalcin (OC) | Mouse monoclonal | Takara (Otsu, Shiga, Japan) | 1:100, 1 hour, RT |
| Osteopontin (OP) | Rabbit polyclonal | Abcam (Cambridge, UK) | 1:200, 1 hour, RT |
| Runx2 | Goat polyclonal | Santa Cruz (Santa Cruz, CA) | 1:30, o/N, 4°C |
| von Willebrand Factor (vWF) | Rabbit polyclonal | Linaris (Wertheim, Germany) | 1:200, 1 hour, RT |
Abbreviations: HP, heat pretreatment; o/N, overnight; RT, room temperature.
Specificity controls were run by (a) omitting primary antibodies and applying TBS or normal horse serum instead, (b) omitting primary antibodies or bridge and secondary antibodies, respectively. Mandibular bone or fetal human bone tissues carrying known antigens were used as positive controls.
2.7. Histological and immunohistochemical evaluation
Histological specimens were evaluated qualitatively and semi‐quantitatively on the basis of established scoring methods in bone histology and pathology19 or own published methods as well as methods from the literature on certain parameters investigated in similar studies on the healing of bone replacement materials.20, 21, 22 The assessment was always performed in a blinded way by two independent, histologically experienced examiners on three different sections of the section series (central, lateral). The sections were analyzed using a light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Three representative regions of interest (ROI) were determined at a lens magnification of ×40. These were always in the center of the area with proven bone substitute material and at two apical or coronal or lateral margins to the autochthonous tissue. Osteogenesis was qualitatively evaluated over the entire section according to the following scheme: 0 = negative; 1 = bone formation around/in bone substitute material (eg, granules): appearance of osteoblasts, detection of osteoid deposits; 2 = bone formation around/in bone substitute material (eg, granules): evidence of fibrous bone with osteoblasts, incipient remodeling in lamellar bone, detection of early osteocytes; 3 = bone formation around/in bone substitute material (eg, granules): detection of lamellar bone with primary osteons and osteocytes, vascular detection, fibrous bone remnants in the lamellar bone, remains of bone replacement material embedded in/on bone; 4 = poorly incorporated/attached residues of bone substitute material in mature lamellar bone, appearance of true osteons and cement lines.
Infiltrates were semi‐quantitatively evaluated according to the following scheme: 0 = none; 1 = loose infiltrates, disseminated or focal; 2 = dense, moderately extensive round cell infiltrates; 3 = extensive, dense round cell infiltrates with highly endothelial venules, edema, focal giant cells; 4 = pronounced inflammatory reaction including giant cells, necrosis.
Histochemical and immunohistochemical findings with purely cellular localization (TRAP, ALP, runx2, CD8, and CD3) were semi‐quantitatively evaluated as follows: 0 = negative; 1 = weak; 2 = moderate; 3 = strong; 4 = very strong. In evaluating ALP immune responses, staining in vessel walls was not considered. The semi‐quantitative evaluation of vWF was performed by evaluating the density of immune‐reactive vessel cross‐sections or sections: 0 = negative; 1 = weak; 2 = moderate; 3 = strong/dense; 4 = very strong/dense. Bone matrix proteins like collagen type I (COL 1), osteocalcin (OC), and osteopontin (OP), which were detectable both cellularly and extracellularly (connective tissue, bone matrix), were semi‐quantitatively evaluated according to the following scheme: 0 = negative; 1 = detection only in cells (eg, osteoblasts, fibroblasts); 2 = detection in cells as well as extracellular with onset of bone formation (osteoid); 3 = detection both in cells and in bone matrix/connective tissue; 4 = strong detection in cells and extracellular; 5 = detection only extracellular.
2.8. Histomorphometrical analysis
Histomorphometrical analysis was conducted with Leica Application Suite (LAS) software (Leica Microsystems GmbH). The areas to be measured were bypassed using area measurement, and the results were stored in the LAS report and calculated as percentages using Excel.
2.9. Statistical analysis
Due to the prospectice randomized nature of this clinical trial in a private practice setting the pre‐specified statistical null hypothesis, that no significant differences between the two tested allogeneic bone grafting materials will be found, a formal sample size calculation was not necessary to carry out. For continuous data, the mean, SD, as well as the minimum and maximum were calculated.
Statistical analyses were performed using the SPSS software package, version 22.0 (SPSS Inc., Chicago, Illinois). Statistical differences in the measured data were calculated using ANOVA with Tukey's HSD test for all pairwise comparisons that correct for experiment‐wise error rate. Two‐sample comparisons were performed using Student's t‐test for equal or unequal variance where appropriate. Due to the small size of the variables, the Holm‐Bonferroni method was not employed for multiple test correction. A P‐value ≤.05 was considered as statistically significant. All P‐values are two‐sided.
3. RESULTS
3.1. Patient demographics and characteristics
Patient demographics and characteristics are summarized in Table 2. Mean age of the 20 patients (10 male, 10 female) at the point of augmentation was 59.6 years (SD 10.5; range 38.8‐78.3). Ten individuals (3 male, 7 female) received treatment with Maxgraft allograft material and 10 individuals (7 male, 3 female) with Puros allograft material. In the majority of patients, the maxilla was treated, and treatment of posterior teeth was much more common than treatment of anterior teeth. There were no statistically significant differences in the regions where the biopsy was harvested. Of the 20 patients, 11 had suffered from periodontitis, that was previously treated. The mean healing period before implantation was 5.0 months (SD 1.1; range: 4.0‐7.0). All 20 patients completed the treatment and no patients were lost to follow‐up and no adverse events were observed.
Table 2.
Patient demographics and characteristics
| Patient demographics and characteristics | |||
|---|---|---|---|
| Maxgraft (N = 10) | Puros (N = 10) | Total (N = 20) | |
| Sex, n | |||
| Male | 7 | 3 | 10 |
| Female | 3 | 7 | 10 |
| Age, years | |||
| Mean ± SD | 57.1 ± 13.4 | 62.1 ± 6.4 | 59.6 ± 10.5 |
| Min; max | 38.8; 78.3 | 52.8; 73.6 | 38.8; 78.3 |
| Treated region, n | |||
| Maxilla | 7 | 8 | 15 |
| Mandible | 3 | 2 | 5 |
| Treated tooth/teeth, n | |||
| Anterior | 1 | 1 | 2 |
| Posterior | 9 | 9 | 18 |
| Healing period, months | |||
| Mean ± SD | 5.3 ± 1.1 | 4.7 ± 1.1 | 5.0 ± 1.1 |
| Min; max | 4.0; 7.0 | 4.0; 7.0 | 4.0; 7.0 |
| Periodontitis, n | |||
| Yes | 7 | 4 | 11 |
| No | 3 | 6 | 9 |
SD, standard deviation; min, minimum; max, maximum.
3.2. Clinical findings
After the healing period, all augmented areas in all patients showed a sufficient bone volume during the re‐entry for biopsy retrieval and implant placement without major bone resorption as assessed by periapical radiographs. No postoperative complications such as membrane exposure or infections occurred in any of the patients. Healing was uneventful, just slightly delayed in three patients, which experienced a slight wound dehiscence. The sutures were left in place for another week and patients were placed under chlorhexidine (CHX) gel application twice daily. The sutures were removed after a total healing time of 3 weeks and the healing was uneventful. Patients did not complain about post‐operative pain and no soft‐tissue augmentation was necessary before insertion of final restorations.
3.3. Protein measurements and results of the MHC1 ELISA
Measurements of the soluble protein content in the tested graft materials revealed concentrations ranging from 0.38 to 1.50 μg/mg dry mass (Maxgraft) and 0.47 to 1.70 μg/mg dry mass (Puros) (Table 3). Using the MHC1 ELISA method, no MHC1 antigens could be detected in any allograft sample tested, whereas high concentrations of MHC1 could be measured in leukocytes, which served as a positive control (data not shown).
Table 3.
Total soluble protein in graft materials
| Sample | Brand | μg protein/mg bone powder (μg) |
|---|---|---|
| X15‐013 | Maxgraft | 0.38 |
| X15‐003 | Maxgraft | 0.95 |
| X15‐004 | Maxgraft | 1.31 |
| L15‐037 | Maxgraft | 1.31 |
| L15‐034 | Maxgraft | 1.50 |
| 30 328 244 | Puros | 0.47 |
| 20 315 452 | Puros | 1.05 |
| 20 329 284 | Puros | 1.34 |
| 20 328 187 | Puros | 1.39 |
| 20 315 450 | Puros | 1.70 |
3.4. Histological, histochemical, and immunohistochemical findings
Representative overviews of biopsies are shown as Figures S1‐S4.
In lower magnification, the core biopsies appeared as cylindrical specimens composed of mostly cancellous bone consisting of interconnected trabecules of different diameters, allogeneic granules, and intertrabecular connective tissue (Figure 2). Artificial ecchymosis and bone or connective tissue fragmentation due to trephanation could be observed in nearly all specimens. All biopsies showed the formation of a network of cancellous bony trabeculae by appositional membranaceous osteogenesis of different stages around or connecting allogenic particles or larger spongy or even compact ossicles with minor or no allogenic remnants (Figure 2A‐F). Allogenic particles from both manufacturers could be clearly identified as mostly basophilic lamellar bone fragments containing empty osteocyte lacunae (Figure 2A,B,E). Only very occasionally, organic remnants in these lacunae could be identified for both types of material. Freshly formed bone was of fibrous type. Most surfaces of newly formed bone were covered by osteoblasts with underlying osteoid. In some specimens, fibrous bone was already remodeled into mature cancellous or compact bone appearing as lamellar bone with fibrous bone remnants incorporated (Figure 2C,D). The bone surfaces were covered by lining cells. Focally, allogenic remnants of different sizes were embedded in new bone (Figure 2A,B,D,E). Biopsies of patients with a longer healing time appeared to show a higher amount of bone formation and/or bone remodeling into lamellar bone (Figure 2B,C). Intertrabecular tissue consisted of loose or fibrous connective tissue with fibroblasts and moderate to strong vascularization (Figure 2B,C,D). Osteoclasts appeared on the surface of newly formed bone and allogenic granules. No foreign body giant cells were detected. Infiltrations were observed in six specimens equally distributed to both groups and mostly appeared as small areas of loose round cell aggregations located in the connective intertrabecular tissue or at the periphery of the specimens (Figure 2E,F). Semi‐quantitatively, osteogenesis was mostly graded as 2 or 3 on the scale, while infiltration was judged as not present or grade 1 in the majority of patients (Table 4).
Figure 2.
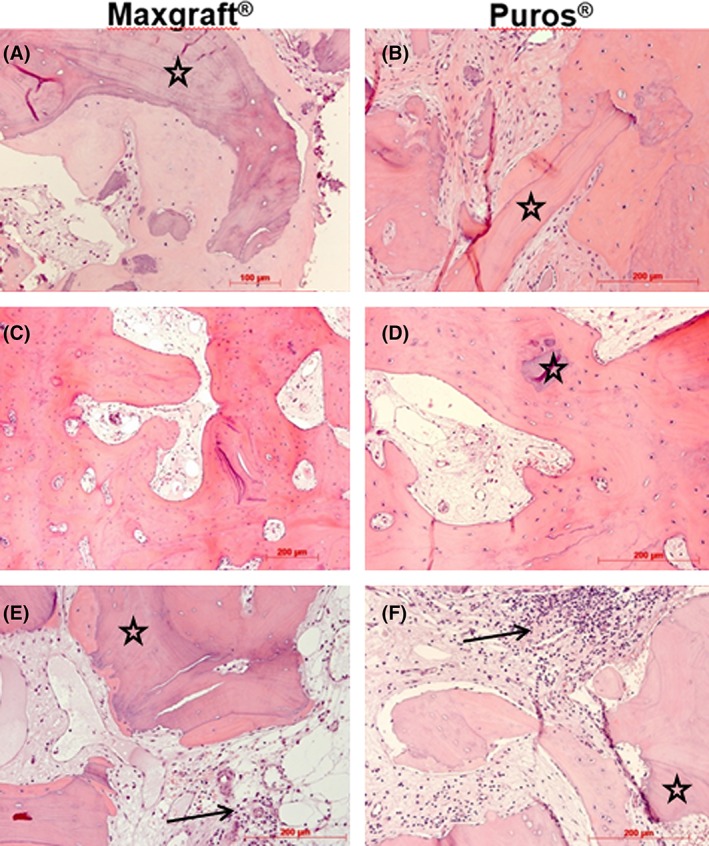
Histology. Representative photomicrographs of biopsies; Maxgraft shown in (A), (C), and (E), Puros in (B), (D) and (F); osteogenesis around allogenic particles (stars, A, B, E, F); progressed osteogenesis without allogenic remnants (C) and embedded small allogenic remnant (D); small infiltrates (arrows, E, F); HE staining, original magnification ×20 except 2E (×10)
Table 4.
Comparative histological and immunohistochemical evaluation of core biopsies
| Histological findings | ||||
|---|---|---|---|---|
| Grading | Maxgraft (N = 10) | Puros (N = 10) | Total (N = 20) | P‐valuea |
| Osteogenesis, n | … | … | … | .120 |
| 1 | 0 | 1 | 1 | … |
| 2 | 5 | 7 | 12 | … |
| 3 | 5 | 2 | 7 | … |
| Infiltration, n | … | … | … | .714 |
| 0 | 8 | 6 | 14 | … |
| 1 | 1 | 4 | 5 | … |
| 2 | 1 | 0 | 1 | … |
| Osteoclastic activity (TRAP), n | … | … | … | 1.000 |
| 0 | 2 | 1 | 3 | … |
| 1 | 6 | 6 | 12 | … |
| 3 | 1 | 3 | 4 | … |
| 4 | 1 | 0 | 1 | … |
| ED1, n | … | … | … | .777 |
| 0 | 2 | 2 | 4 | … |
| 1 | 7 | 5 | 12 | … |
| 2 | 0 | 3 | 3 | … |
| 3 | 1 | 0 | 1 | … |
| Osteoblastic activity (runX2), n | … | … | … | 1.000 |
| 0 | 7 | 5 | 12 | … |
| 1 | 0 | 4 | 4 | … |
| 2 | 3 | 1 | 4 | … |
| ALP, n | … | … | … | .806 |
| 0 | 3 | 0 | 3 | … |
| 1 | 2 | 7 | 9 | … |
| 2 | 4 | 2 | 6 | … |
| 3 | 1 | 1 | 2 | … |
| Collagen type I, n | … | … | … | .556 |
| 1 | 1 | 2 | 3 | … |
| 2 | 9 | 8 | 17 | … |
| Osteocalcin, n | … | … | … | .363 |
| 1 | 3 | 5 | 8 | … |
| 2 | 5 | 4 | 9 | … |
| 3 | 2 | 1 | 3 | … |
| Osteopontin, n | … | … | … | 1.000 |
| 0 | 2 | 3 | 5 | … |
| 1 | 7 | 6 | 13 | … |
| 2 | 1 | 0 | 1 | … |
| 3 | 0 | 1 | 1 | … |
| vWF, n | … | … | … | .424 |
| 0 | 0 | 1 | 1 | … |
| 1 | 2 | 0 | 2 | … |
| 2 | 6 | 4 | 10 | … |
| 3 | 2 | 5 | 7 | … |
| CD3, n | … | … | … | .722 |
| 0 | 6 | 6 | 12 | … |
| 1 | 4 | 3 | 7 | … |
| 2 | 0 | 1 | 1 | … |
| CD4, n | … | … | … | .736 |
| 0 | 9 | 8 | 17 | … |
| 1 | 0 | 1 | 1 | … |
| 2 | 1 | 1 | 2 | … |
| CD8, n | … | … | … | .331 |
| 0 | 10 | 9 | 19 | … |
| 1 | 0 | 1 | 1 | … |
ANOVA Tukey's HSD test for comparison of Maxgraft vs Puros. TRAP, tartrate‐resistant acid phosphatase; runX2, runt‐related transcription factor 2; ALP, bone‐specific alkaline phosphatase; vWF, von Willebrand factor.
TRAP‐positive osteoclasts were localized on the surfaces of newly formed bone but also of allogenic particles (Figure 3A,B). In the specimens of four patients, no TRAP activity was visible. Focally osteoblasts, lining cells and a few fibroblasts showed immunoreactivity for ALP in nearly all specimens (Figure 3c,d). CD3‐positive lymphocytes could be detected in eight of the specimens investigated. Most of the CD3‐positive cells were found within infiltrates or perivascularly (Figure 3G,H). CD4‐positive lymphocytes were seen in only three of the investigated specimens. In one case, they were located within an infiltration; in two cases, aggregation was observed close to bone surfaces (Figure 3E,F). For CD8, only one case showed few positive lymphocytes within an infiltration.
Figure 3.
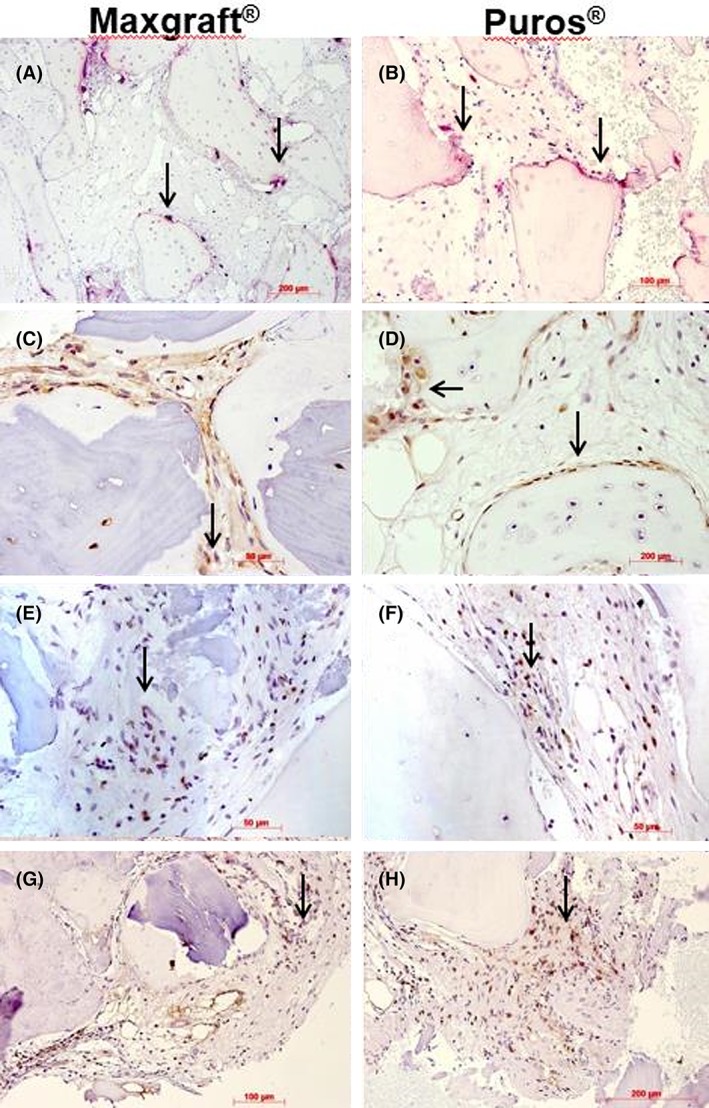
Histochemistry and immunohistochemistry I. Representative photomicrographs of biopsies; Maxgraft shown in (A), (C), (E), and (G), Puros in (B), (D), (F) and (H); osteoclasts on bone surfaces (arrows), TRAP staining, original magnification ×10 (A, B); alkaline phosphatase immunohistochemistry, arrows indicate immunoreactive osteoblasts; DAB, ×40; CD4 immunohistochemistry, arrows indicate very few immunoreactive cells, DAB, ×40 (E, F); CD3 immunohistochemistry, arrows indicate few immunoreactive cells, DAB ×20, ×40 (H)
COL I immunostaining in a weak to moderate manner could be seen in the matrix of newly formed bone with stronger staining in osteoid seams and osteocytes in most cases. Focally, osteoblasts were immunoreactive (Figure 4A,B). Additionally, connective tissue staining could be observed. COL I was not present in allogenic particles (Figure 4C,D).
Figure 4.
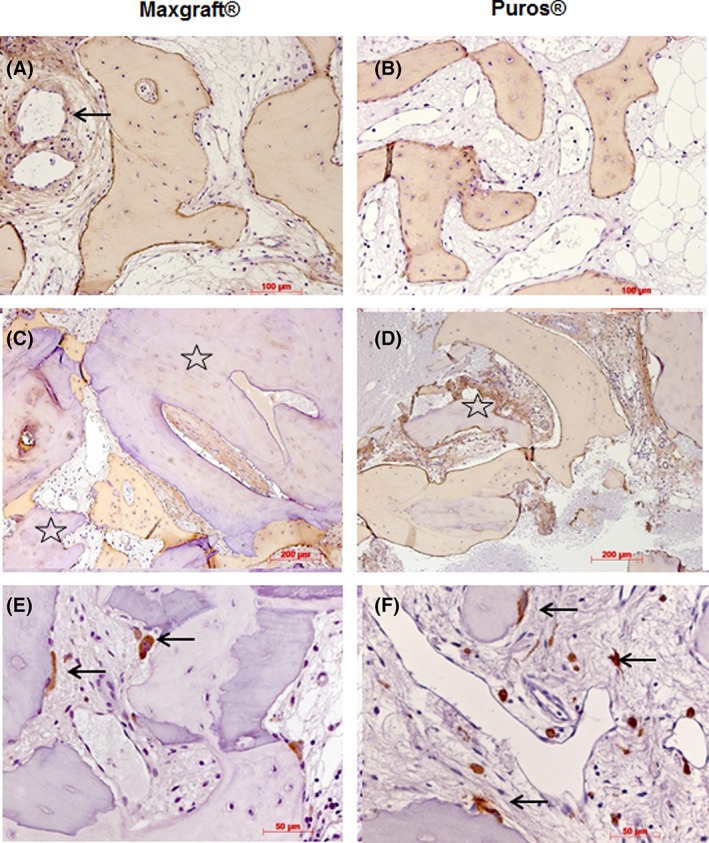
Immunohistochemistry II. Representative photomicrographs of biopsies; Maxgraft shown in (A) and (C), Puros in (B) and (D); collagen type I immunohistochemistry; immunoreactive newly formed bone matrix and osteoblasts, focally reactive connective tissue and vessel walls (arrow, A, B, C, D), no immunostaining in allogenic remnants (stars, C, D), DAB, original magnification ×20; ED1 immunohistochemistry, immunoreactive osteoclasts on bone and allogenic surfaces (arrows, E, F), DAB, ×40
All osteoclasts showed ED1 immunoreactivity. Additionally, macrophages were positive. (Figure 4E,F).
The newly formed bone matrix showed weak OC immunostaining, while most osteoblasts, osteocytes and some fibroblasts near to bone surfaces were stained more intensively. Also, interfaces between allogenic particles and newly formed bone were reactive (Figure 5A,B).
Figure 5.
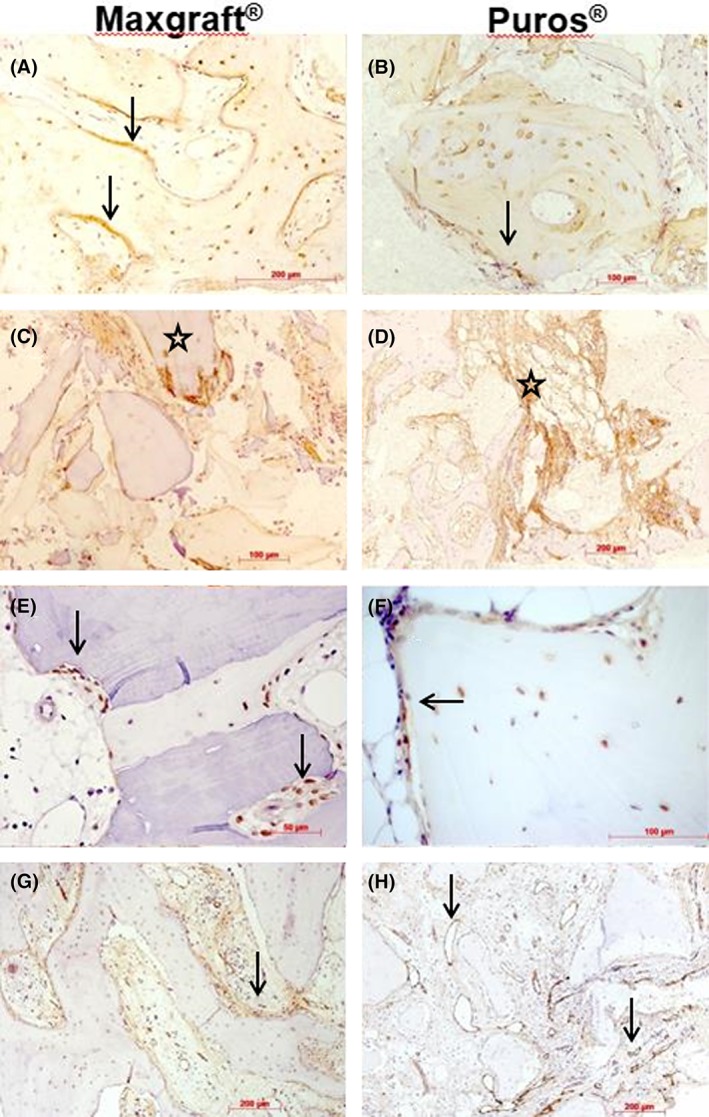
Immunohistochemistry III. Representative photomicrographs of biopsies; Maxgraft shown in (A), (C), (E), and (G), Puros in (B), (D), (F), and (H); osteocalcin immunohistochemistry, immunoreactive osteoid (arrows) and osteocytes, DAB, original magnification ×20 (A, B); osteopontin immunohistochemistry, immunoreactive connective tissue (stars, C, D), DAB, x20; runx2 immunohistochemistry, immunoreactive pre‐osteoblasts and osteoblasts on the surfaces of newly formed bone (arrows, E, F), DAB, ×40; vWF immunohistochemistry, immunoreactive vessels near bone and among allogenic granules (arrows, G, H), DAB, ×10
Immunoreactivity for OP was weak or absent. Focally, connective tissue areas and interfaces between allogenic material and newly formed bone were stained (Figure 5C,D).
In terms of runx2, immunoreactive cells were observed only in eight of the total cases. Staining was restricted to osteoblasts and lining cells and some fibroblasts near to bone surfaces (Figure 5E,F).
Staining for vWF revealed a moderate to good vessel density in most of the specimens. Capillaries, small arterioles, and large sinuosoids were the predominant vessel type located between the bone trabecules and allogenic granules (Figure 5g,h).
The statistical evaluation of the histological and immunohistochemical findings that are listed in Table 4 did not reveal any significant differences between the allografts tested.
3.5. Histomorphometrical findings
Table 5 lists the histomorphometrical findings of relevance. In the total patient population, the mean proportion of newly formed bone in the total amount of mineralized tissue was 41% for Maxgraft and 27% or Puros (SD 20.0 for Maxgraft and 17.0 for Puros; total range 7.0‐74.0) and the mean proportion of remaining allograft was 14% for Maxgraft and 13% for Puros (SD 10.0 for Maxgraft and 8.0 for Puros) and the mean proportion of soft tissue was 47% for Maxgraft and 60% for Puros (SD 14.0 for Maxgraft and 18.0 for Puros). Differences between Maxgraft and Puros were not statistically significant (P = .101 for newly formed bone, P = .866 for remaining allograft, P = .085 for soft tissue; Table 5), largely due to heterogenous findings within the individual groups.
Table 5.
Histomorphometrical findings
| Parameter | Maxgraft (N = 10) | Puros (N = 10) | P‐valuea |
|---|---|---|---|
| Newly formed bone, % | … | … | .101 |
| Mean ± SD | 41 ± 20 | 27 ± 17 | … |
| Min; max | 13; 74 | 7; 63 | … |
| Remaining allograft, % | … | … | .866 |
| Mean ± SD | 14 ± 10 | 13 ± 8 | … |
| Min; max | 0; 27 | 0; 22 | … |
| Soft tissue, % | … | … | .085 |
| Mean ± SD | 47 ± 14 | 60 ± 18 | … |
| Min; max | 26; 63 | 17; 75 | … |
ANOVA Tukey's HSD test for comparison of Maxgraft vs Puros. SD, standard deviation; min, minimum; max, maximum.
4. DISCUSSION
Histologically and immunohistochemically, there were only minor signs of an inflammatory reaction in both groups. Small cellular infiltrations, as seen in some specimens investigated, may be due to transient weak inflammatory reactions, which are considered to be part of the normal process of bone fracture and bone substitute healing.23, 24 Especially CD4+ T‐cells, which we detected in some of our specimens (Figure 3), are involved in bone healing and remodeling.25, 26 Immunohistochemical findings for vWF showed a good vascularization of the augmented areas for both allogeneic grafts (Figure 5).
Further indications for the lack of antigenicity of the materials used are evident from the histological observations: nearly no proteinaceous remnants, for example, osteocyte remnants, could be observed and the graft granules showed no collagen type I as revealed by immunohistochemistry. Furthermore, multinucleated foreign body giant cells as it was reported after application of an allogeneic spongious bone block (Tutobone) for alveolar ridge augmentation,27 were not observed in our study. Other authors reported that MHC molecules have been detected in some but not all allogeneic bone blocks processed by peracetic‐acid‐ethanol‐sterilization (PES) and the authors elucidated the significance of such remnants on graft incorporation and long‐term survival.13 PES treatment has been extensively validated and was classified as an effective and safe processing method.28, 29 Transplants sterilized by this method have been in clinical use for decades. A retrospective study following up on several thousand recipients of large transplants in orthopedic surgery including cortical and cancellous bone, tendon, amniotic membrane, and skin confirmed not only excellent primary integration, but also lack of late complications or even rejections.30 This may suggest that trace amounts of MHC molecules are clinically not relevant. Despite the frequent use of human tissue products worldwide in a variety of medical specialties, formation of alloantibodies complicating future solid organ transplantation has rarely been reported for fresh‐frozen or cryopreserved bone.12, 31 After using freeze‐dried or solvent dehydrated bone allografts from single or multiple donors for dental applications, such incidences have not been reported at all. In our study, we did not detect any MHC1 molecules in the individual batches of bone allograft materials using ELISA.
According to the findings for TRAP histochemistry and ED1 immunohistochemistry, both allogeneic materials are resorbed by osteoclasts located on the surface of graft particles. Since in the present study there was no evidence for the occurrence of larger multinucleated cells indicating a foreign body reaction, as it was observed after augmentation with allogeneic cancellous bone blocks (Puros),27 these findings may lead to the assumption that the efficiency of decellularization negatively correlates with the size of the allograft that is subject to chemical processing. In other words, cellular remnants are fewer in particulate material than in solid blocks. Although the procedures remove the vast majority of cellular matter, denatured traces can be detected.11 As shown by thermogravimetric measurements, Maxgraft only contains around 62% anorganic matter, while the rest is composed of residual moisture (~5%) and natural bone proteins resulting in improved biomechanical properties compared to purely anorganic materials.32
While ED1 marks all macrophages and osteoclasts, TRAP staining is also a functional marker for resorptive osteoclast activity. This may explain the missing TRAP staining in sections from a subgroup of our patients (Table 4), indicating insufficient activity or maturity of the osteoclasts during the period of biopsy harvesting.33 The detection of TRAP‐positive osteoclasts, which are also found on the surfaces of newly formed bone, indicates an ongoing remodeling process. Resorption of allogeneic graft materials is considered to be slower than that of autogenous bone but faster than that of xenogeneic and alloplastic substitutes.34
The bone grafting materials used in our study were allogeneic mineralized bone grafts, which have shown a progressive transformation into vital new bone. After a mean healing period of 5.0 months, bone replacement materials were either converted or incorporated into vital new bone. We are aware of the fact that studying only 10 subjects per grafting material does not allow for a generalized statement, but our work may serve as a clinical pilot study with a promising outcome and confirms the results of already published data using the same materials for the augmentation of maxillary sinuses and alveolar ridges.35, 36, 37, 38, 39 However, to our knowledge, the present study is the first investigation showing not only histological and histomorphometrical findings, but also detailed histochemical and immunohistochemical results characterizing the healing pattern of bone allografts after lateral alveolar ridge augmentation in humans, even at the level of the individual batches of bone grafting material. To our knowledge, such studies have only been published for other bone substitutes, for example, alloplastic bone ceramics.40, 41
The histological findings for both materials tested in this study showed similar processes typical for osteoconductive phenomena of bone healing: membranaceous osteogenesis around allogenic graft material forming a bony cancellous network, remodeling of the newly formed bone from fibrous into mature lamellar bone tissue, degradation of the graft by osteoclastic activity. Furthermore, no significant differences between the investigated grafting materials could be found histomorphometrically (Table 5), indicating a similar healing process or osteogenic activity of both products. Allogeneic grafts or their remnants could be clearly identified as more basophilic stained bone fragments with empty osteocyte lacunae histologically. This histological picture resembled that of other histological studies in humans on the healing of different allograft materials.42, 43, 44, 45, 46, 47 Similar findings could also be obtained in animal studies, although they are not directly comparable with the human situation as any use of human‐derived material in animals is classified as xenogeneic. Additionally, the clinical procedures in most animal studies using human bone grafts have been undertaken in long bones or for critical size defects in jaw bones.48 In both groups, no abnormal tissues like cartilage or pathological alterations like necrosis or microbes could be seen histologically, except for the usual artifacts due to trephination (Figures S1‐S4). This was in contrast to a study using fresh‐frozen cortical and cortico‐cancellous allogeneic block grafts for augmentation of the anterior maxilla, which found necrotic areas as well as reduced incorporation, remodeling, and increased resorption of these grafts.49
There are only very few studies comparing histomorphometric data obtained after sinus lift procedures using allogeneic bone substitutes for the same healing time as ours. Since the healing pattern following sinus lift procedures differs from that of lateral ridge augmentation, the results are not directly comparable. Gapski et al. in 2006 found a mean percentage of 73.3% newly formed bone after implantation of Puros for sinus elevation after 6 months, albeit in four patients only.36 In another study comparing Puros with other bone substitutes after 5 months healing in sinus lift procedures in 30 patients, the mean bone volume fraction was 30.28%.45 Stacchi et al. (2008) used fresh frozen bone in sinus lift in 10 patients and found a mean percentage of newly formed bone of 48.15% after 5 months.43 For fresh frozen bone, new bone formation was 8.26% after a healing time of 6 months in sinus lift.50 Monje et al. (2017) compared the outcome of solvent dehydrated allograft Puros with a freeze‐dried human allograft used in sinus lift after a healing time of 6 months. However, the allogeneic graft material was mixed with autogenous bone in a ratio of 1:1.51 This was also the case in a sinus augmentation study of Galindo‐Moreno et al. (2018).47 Recently, a study on 14 patients calculated 18.65% of new bone when using allogeneic spongious bone blocks (Tutobone) for vertical and horizontal ridge augmentation prior to implant placement.27 As mentioned above, a direct comparison to our study is not possible since we used those materials for lateral ridge augmentation. Another factor that may be responsible for the heterogenous findings in the present study could be the addition of PRGF after rehydration. It remains unclear how even the distribution of particulate material and PRGF was established within the grafting material in the individual patient and especially when comparing multiple patients. Since PRGF has been applied as a supplement for the grafts in both groups, we cannot give any statement concerning a specific influence of this fibrin preparation on the graft and wound healing. However, there are indications from pre‐clinical and clinical studies as well as systematic reviews that PRGF may improve bone healing.17, 52, 53
For osteogenic markers, no obvious differences in their immunostaining pattern and intensity could be obtained between biopsies of both grafts. Runx2, ALP, COL 1, OC, and OP are cellular and bone matrix marker proteins, that reflect increasing stages of osteogenesis and bone tissue formation, which have been detected during bone substitute healing.40, 41 In a rabbit experiment using bone allograft for critical size defects, an increasing immunostaining for these markers was found48 and those staining patterns were similar to our findings. These osteogenic markers were also upregulated in a mouse calvarium defect model using human DFDBA, where gene expression was investigated after 1 and 3 months of healing.54 From allograft studies in rhesus monkeys, it is known that non‐decalcified freeze‐dried human allograft is superior to decalcified allografts in stimulating new bone formation.55 OC‐immunoreactive cells were also observed after application of fresh frozen bone for sinus lift after 6 months.56
As mentioned before, the current study is limited by its low sample size. Therefore, the statistical findings should be viewed critically. To support our statistical data, analyses of larger sample sizes are required and planned. Nevertheless, our data indicate no significant differences between the Maxgraft and the Puros group for all parameters and a similar biological behavior after implantation.
5. CONCLUSION
Based on our results, both allogeneic bone materials, a freeze‐dried bone allograft material from multiple donors (Maxgraft) and a solvent dehydrated single donor bone allograft material (Puros), demonstrated a high regeneration potential and no signs for inflammatory reactions or adverse events based on our clinical evaluation. Histomorphometrical, histological, and immunohistochemical evaluation confirmed natural remodeling events, new bone formation rate, and excessive revascularization of the regenerated area and excluded a foreign body reaction. Because detectable MHC residues and cellular reactions were absent in the evaluated biopsies, an immunogenic response to the allogeneic particles is unlikely, confirming and supporting the well‐established use in daily dental practice. Again, the sample size of the present study is too small to draw any comparative conclusions between the two allogeneic materials used.
CONFLICT OF INTEREST
All authors have no conflicts of interest to report.
Supporting information
Supplemental Figure 1 Overview of biopsy from Puros allograft
Supplemental Figure 2 Overview of biopsy from Maxgraft allograft
Supplemental Figure 3 Overview of biopsy from Puros allograft
Supplemental Figure 4 Overview of biopsy from Maxgraft allograft
ACKNOWLEDGMENTS
The authors would like to thank all patients who participated in this study as well as all the staff who contributed directly or indirectly to the study. Further, the authors acknowledge I. Müller‐Bay and S. van Dyck (both Bonn, Germany) for technical assistance and Maren Hasper (IHK Hannover, Germany) for histomorphometric analysis.
Solakoglu Ö, Götz W, Heydecke G, Schwarzenbach H. Histological and immunohistochemical comparison of two different allogeneic bone grafting materials for alveolar ridge reconstruction: A prospective randomized trial in humans. Clin Implant Dent Relat Res. 2019;21:1002–1016. 10.1111/cid.12824
REFERENCES
- 1. Tan WL, Wong TLT, Wong MCM, Lang NP. A systematic review of post‐extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23(Suppl 5):1‐21. 10.1111/j.1600-0501.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 2. Sanz M, Vignoletti F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent Mater. 2015;31:640‐647. 10.1016/j.dental.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3. Baj A, Trapella G, Lauritano D, Candotto V, Mancini GE, Giannì AB. An overview on bone reconstruction of atrophic maxilla: success parameters and critical issues. J Biol Regul Homeost Agents. 2016;30:209‐215. [PubMed] [Google Scholar]
- 4. Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology‐is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017;3:23 10.1186/s40729-017-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol. 2014;7(Suppl 2):S203‐S217. [PubMed] [Google Scholar]
- 6. Kolk A, Handschel J, Drescher W, et al. Current trends and future perspectives of bone substitute materials ‐ from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40:706‐718. 10.1016/j.jcms.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 7. Smeets R, Hanken H, Jung O, et al. KnochenersatzmaterialienBone substitute materials. Der MKG‐Chirurg. 2014;7:53‐67. [Google Scholar]
- 8. Krasny K, Kaminski A, Krasny M, et al. Preparation of allogeneic bone for alveolar ridge augmentation. Cell Tissue Bank. 2017;18:313‐321. 10.1007/s10561-017-9631-8. [DOI] [PubMed] [Google Scholar]
- 9. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36:633‐641. 10.1007/s00264-011-1391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson D, Kakarala G, Hampson K, Steele N, Ashton B. Viable cells survive in fresh frozen human bone allografts. Acta Orthop. 2007;78:26‐30. 10.1080/17453670610013385. [DOI] [PubMed] [Google Scholar]
- 11. Fretwurst T, Spanou A, Nelson K, Wein M, Steinberg T, Stricker A. Comparison of four different allogeneic bone grafts for alveolar ridge reconstruction: a preliminary histologic and biochemical analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:424‐431. 10.1016/j.oooo.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 12. O'Sullivan ED, Battle RK, Zahra S, Keating JF, Marson LP, Turner DM. Allosensitization following bone graft. Am J Transplant. 2017;17:2207‐2211. [DOI] [PubMed] [Google Scholar]
- 13. Fretwurst T, Gad LM, Steinberg T, et al. Detection of major histocompatibility complex molecules in processed allogeneic bone blocks for use in alveolar ridge reconstruction. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:16‐21. [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495‐1499. 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 15. Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. technique and wound healing. Compend Contin Educ Dent. 1983;4:549‐562. [PubMed] [Google Scholar]
- 16. Ramachandra SS, Rana R, Reetika S, Jithendra KD. Options to avoid the second surgical site: a review of literature. Cell Tissue Bank. 2014;15:297‐305. 10.1007/s10561-013-9395-8. [DOI] [PubMed] [Google Scholar]
- 17. Eda T, Takahashi K, Kanao S, et al. Comparison study between plasma rich in growth factors and platelet‐rich plasma for osteoconduction in rat calvaria. J Oral Maxillofac Surgery, Med Pathol. 2017;29:563‐569. [Google Scholar]
- 18. Anitua EA. Enhancement of osseointegration by generating a dynamic implant surface. J Oral Implantol. 2006;32:72‐76. 10.1563/736.1. [DOI] [PubMed] [Google Scholar]
- 19. Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue ‐ a review. Diagn Pathol. 2014;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koerdt S, Siebers J, Bloch W, Ristow O, Kuebler AC, Reuther T. Immunohistochemial study on the expression of von Willebrand factor (vWF) after onlay autogenous iliac grafts for lateral alveolar ridge augmentation. Head Face Med. 2013;9 10.1186/1746-160X-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wälivaara D‐å, Abrahamsson P. Evaluation of 4 different bone graft substitutes and autogenous bone grafting in root‐end resection osteotomies after retrograde root‐filling with intermediate restorative material (IRM): an experimental study in dogs. Open J Stomatol. 2013;3:203‐208. [Google Scholar]
- 22. Konermann A, Gotz W, Le M, et al. Histopathological verification of osteoimmunological mediators in peri‐implantitis and correlation to bone loss and implant functional period. J Oral Implantol. 2016;42:61‐68. 10.1563/aaid-joi-D-13-00355. [DOI] [PubMed] [Google Scholar]
- 23. Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1‐R9. 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt‐Bleek K, Schell H, Schulz N, et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. 2012;347:567‐573. 10.1007/s00441-011-1205-7. [DOI] [PubMed] [Google Scholar]
- 25. Bozec A, Zaiss MM. T regulatory cells in bone remodelling. Curr Osteoporos Rep. 2017;15:121‐125. 10.1007/s11914-017-0356-1. [DOI] [PubMed] [Google Scholar]
- 26. Kalyan S. It may seem inflammatory, but some T cells are innately healing to the bone. J Bone Miner Res. 2016;31:1997‐2000. 10.1002/jbmr.2875. [DOI] [PubMed] [Google Scholar]
- 27. Lorenz J, Kubesch A, Al‐Maawi S, et al. Allogeneic bone block for challenging augmentation‐a clinical, histological, and histomorphometrical investigation of tissue reaction and new bone formation. Clin Oral Investig. 2018;22:3159‐3169. 10.1007/s00784-018-2407-0. [DOI] [PubMed] [Google Scholar]
- 28. Pruss A, Baumann B, Seibold M, et al. Validation of the sterilization procedure of allogeneic avital bone transplants using peracetic acid‐ethanol. Biologicals. 2001;29:59‐66. 10.1006/biol.2001.0286. [DOI] [PubMed] [Google Scholar]
- 29. Pruss A, Göbel UB, Pauli G, et al. Peracetic acid‐ethanol treatment of allogeneic avital bone tissue transplants–a reliable sterilization method. Ann Transplant. 2003;8:34‐42. [PubMed] [Google Scholar]
- 30. Pruss A, Perka C, Degenhardt P, et al. Clinical efficacy and compatibility of allogeneic avital tissue transplants sterilized with a peracetic acid/ethanol mixture. Cell Tissue Bank. 2002;3:235‐243. 10.1023/A:1024697515420. [DOI] [PubMed] [Google Scholar]
- 31. Mosconi G, Baraldi O, Fantinati C, et al. Donor‐specific anti‐HLA antibodies after bone‐graft transplantation. Impact on a subsequent renal transplantation: a case report. Transplant Proc. 2009;41:1138‐1141. 10.1016/j.transproceed.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 32. Trajkovski B, Jaunich M, Muller W‐D, et al. Hydrophilicity, viscoelastic, and physicochemical properties variations in dental bone grafting substitutes. Mater (Basel, Switzerland). 2018;11 10.3390/ma11020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diepenhorst NA, Nowell CJ, Rueda P, et al. High throughput, quantitative analysis of human osteoclast differentiation and activity. Anal Biochem. 2017;519:51‐56. [DOI] [PubMed] [Google Scholar]
- 34. Danesh‐Sani SA, Engebretson SP, Janal MN. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: a systematic review and meta‐analysis. J Periodontal Res. 2017;52:301‐312. 10.1111/jre.12402. [DOI] [PubMed] [Google Scholar]
- 35. Froum SJ, Tarnow DP, Wallace SS, et al. The use of a mineralized allograft for sinus augmentation: an interim histological case report from a prospective clinical study. Compend Contin Educ Dent. 2005;26:259‐260. 261‐266, 262–264. [PubMed] [Google Scholar]
- 36. Gapski R, Neiva R, Oh T‐J, Wang H‐L. Histologic analyses of human mineralized bone grafting material in sinus elevation procedures: a case series. Int J Periodontics Restorative Dent. 2006;26:59‐69. [PubMed] [Google Scholar]
- 37. Froum SJ, Wallace SS, Elian N, Cho SC, Tarnow DP. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (bio‐Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006;26:543‐551. [PubMed] [Google Scholar]
- 38. Soardi CM, Spinato S, Zaffe D, Wang H‐L. Atrophic maxillary floor augmentation by mineralized human bone allograft in sinuses of different size: an histologic and histomorphometric analysis. Clin Oral Implants Res. 2011;22:560‐566. 10.1111/j.1600-0501.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- 39. Block MS. Horizontal ridge augmentation using particulate bone. Atlas Oral Maxillofac Surg Clin North Am. 2006;14:27‐38. 10.1016/j.cxom.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 40. Friedmann A, Gissel K, Konermann A, Gotz W. Tissue reactions after simultaneous alveolar ridge augmentation with biphasic calcium phosphate and implant insertion–histological and immunohistochemical evaluation in humans. Clin Oral Investig. 2015;19:1595‐1603. 10.1007/s00784-014-1385-0. [DOI] [PubMed] [Google Scholar]
- 41. Gotz W, Gerber T, Michel B, et al. Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone[r]) osteogenesis: a study on biopsies from human jaws. Clin Oral Implants Res. 2008;19:1016‐1026. 10.1111/j.1600-0501.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- 42. Noumbissi SS, Lozada JL, Boyne PJ, et al. Clinical, histologic, and histomorphometric evaluation of mineralized solvent‐dehydrated bone allograf (Puros) in human maxillary sinus grafts. J Oral Implantol. 2005;31:171‐179. [DOI] [PubMed] [Google Scholar]
- 43. Stacchi C, Orsini G, Di Iorio D, et al. Clinical, histologic, and histomorphometric analyses of regenerated bone in maxillary sinus augmentation using fresh frozen human bone allografts. J Periodontol. 2008;79:1789‐1796. 10.1902/jop.2008.070649. [DOI] [PubMed] [Google Scholar]
- 44. Beck TM, Mealey BL. Histologic analysis of healing after tooth extraction with ridge preservation using mineralized human bone allograft. J Periodontol. 2010;81:1765‐1772. 10.1902/jop.2010.100286. [DOI] [PubMed] [Google Scholar]
- 45. Schmitt CM, Doering H, Schmidt T, Lutz R, Neukam FW, Schlegel KA. Histological results after maxillary sinus augmentation with Straumann(R) BoneCeramic, bio‐Oss(R), Puros(R), and autologous bone. A randomized controlled clinical trial. Clin Oral Implants Res. 2013;24:576‐585. 10.1111/j.1600-0501.2012.02431.x. [DOI] [PubMed] [Google Scholar]
- 46. Spin‐Neto R, Stavropoulos A, Coletti FL, Faeda RS, Pereira LAVD, Marcantonio E Jr. Graft incorporation and implant osseointegration following the use of autologous and fresh‐frozen allogeneic block bone grafts for lateral ridge augmentation. Clin Oral Implants Res. 2014;25:226‐233. 10.1111/clr.12107. [DOI] [PubMed] [Google Scholar]
- 47. Galindo‐Moreno P, de Buitrago JG, Padial‐Molina M, Fernández‐Barbero JE, Ata‐Ali J, O′Valle F. Histopathological comparison of healing after maxillary sinus augmentation using xenograft mixed with autogenous bone versus allograft mixed with autogenous bone. Clin Oral Implants Res. 2018;29:192‐201. 10.1111/clr.13098. [DOI] [PubMed] [Google Scholar]
- 48. Hawthorne AC, Xavier SP, Okamoto R, Salvador SL, Antunes AA, Salata LA. Immunohistochemical, tomographic, and histological study on onlay bone graft remodeling. Part III: allografts. Clin Oral Implants Res. 2013;24:1164‐1172. 10.1111/j.1600-0501.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- 49. Spin‐Neto R, Stavropoulos A, Coletti FL, Pereira LAVD, Marcantonio E Jr, Wenzel A. Remodeling of cortical and corticocancellous fresh‐frozen allogeneic block bone grafts–a radiographic and histomorphometric comparison to autologous bone grafts. Clin Oral Implants Res. 2015;26:747‐752. 10.1111/clr.12343. [DOI] [PubMed] [Google Scholar]
- 50. Xavier SP, Dias RR, Sehn FP, Kahn A, Chaushu L, Chaushu G. Maxillary sinus grafting with autograft vs. fresh frozen allograft: a split‐mouth histomorphometric study. Clin Oral Implants Res. 2015;26:1080‐1085. 10.1111/clr.12404. [DOI] [PubMed] [Google Scholar]
- 51. Monje A, O'Valle F, Monje‐Gil F, et al. Cellular, vascular, and Histomorphometric outcomes of solvent‐dehydrated vs freeze‐dried allogeneic graft for maxillary sinus augmentation: a randomized case series. Int J Oral Maxillofac Implants. 2017;32:121‐127. 10.11607/jomi.4801. [DOI] [PubMed] [Google Scholar]
- 52. Dragonas P, Schiavo JH, Avila‐Ortiz G, Palaiologou A, Katsaros T. Plasma rich in growth factors (PRGF) in intraoral bone grafting procedures: a systematic review. J Craniomaxillofac Surg. 2019;47:443‐453. 10.1016/j.jcms.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 53. Moraschini V, Barboza ESP. Effect of autologous platelet concentrates for alveolar socket preservation: a systematic review. Int J Oral Maxillofac Surg. 2015;44:632‐641. 10.1016/j.ijom.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 54. Kangwannarongkul T, Subbalekha K, Vivatbutsiri P, Suwanwela J. Gene expression and microcomputed tomography analysis of grafted bone using Deproteinized bovine bone and freeze‐dried human bone. Int J Oral Maxillofac Implants. 2018;33:541‐548. 10.11607/jomi.6234. [DOI] [PubMed] [Google Scholar]
- 55. Yukna RA, Vastardis S. Comparative evaluation of decalcified and non‐decalcified freeze‐dried bone allografts in rhesus monkeys. I. Histologic findings. J Periodontol. 2005;76:57‐65. 10.1902/jop.2005.76.1.57. [DOI] [PubMed] [Google Scholar]
- 56. De Ponte FS, Cutroneo G, Falzea R, et al. Histochemical and morphological aspects of fresh frozen bone: a preliminary study. Eur J Histochem. 2016;60:2642 10.4081/ejh.2016.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Overview of biopsy from Puros allograft
Supplemental Figure 2 Overview of biopsy from Maxgraft allograft
Supplemental Figure 3 Overview of biopsy from Puros allograft
Supplemental Figure 4 Overview of biopsy from Maxgraft allograft


