Abstract
Background
Two randomized controlled trials (reSURFACE 1 and 2) have demonstrated the effectiveness of tildrakizumab, a high‐affinity, humanized, IgG1κ, anti‐interleukin‐23 monoclonal antibody, for treating moderate‐to‐severe plaque psoriasis in the first 28 weeks.
Objectives
To examine the efficacy of tildrakizumab and its impact on quality of life (QoL) in patients with different levels of week‐28 Psoriasis Area and Severity Index (PASI) improvement.
Methods
Patients treated with tildrakizumab 100 mg or 200 mg from baseline to week 28 were pooled from reSURFACE 1 and reSURFACE 2 and classified into five mutually exclusive week‐28 PASI improvement groups for each dose: PASI 0–49, 50–74, 75–89, 90–99 and 100. Mean PASI improvement and Dermatology Life Quality Index (DLQI) 0/1 over time were examined for each group.
Results
Of 1156 patients, 575 were in the 100‐mg and 578 in the 200‐mg cohorts, respectively. At week 28, 8.3%, 14.3%, 23.8%, 30.4% and 23.1% in the 100‐mg and 4.0%, 18.1%, 19.6%, 29.1% and 29.3% in the 200‐mg cohort achieved PASI < 50, 50–74, 75–89, 90–99 and 100, respectively. Patients with PASI < 50 at week 28 could be identified as early as week 8, and those with week‐28 PASI ≥ 90 had approximately 50% PASI improvement by week 4. Among patients achieving PASI > 50 at week 28 who continued the same dose of tildrakizumab to week 52, mean PASI improvement was maintained or improved over time. Similar results were observed for both doses. Higher proportions of patients achieved DLQI 0/1 in higher week‐28 PASI groups, and DLQI 0/1 was maintained or improved to week 52. However, not all patients with PASI 100 had DLQI 0/1.
Conclusion
Patients unlikely to respond to tildrakizumab could be identified by week 8, and those likely to achieve a PASI ≥ 90 response could be identified as early as week 4. Week‐28 PASI improvement level correlated with QoL improvement.
Introduction
Psoriasis is a chronic inflammatory skin disease characterized by itching, scaling and pain.1, 2 Patients with psoriasis are also at risk of psoriatic arthritis, infection, obesity, hypertension, diabetes mellitus, hypercholesterolaemia, cancer, depression, disfiguration and disability.3, 4, 5, 6 These clinical sequelae affect patients’ personal health, work productivity, quality of life (QoL) and interpersonal relationships throughout their lives.7, 8, 9
Recent advances in psoriasis immunology have led to the development of effective biologic agents that target the disease's key pathogenic components, such as interleukin (IL)‐23 and IL‐17A.10, 11, 12 One biologic agent recently approved by the US Food and Drug Administration and the European Medicines Agency is tildrakizumab, a high‐affinity, humanized, immunoglobulin G1κ, monoclonal antibody designed to treat moderate‐to‐severe plaque psoriasis by blocking the p19 subunit of IL‐23.12 Tildrakizumab is administered subcutaneously once every 12 weeks, following two initial injections administered at weeks 0 and 4. Two randomized controlled trials (reSURFACE 1 and reSURFACE 2) have proven the efficacy of tildrakizumab and its impact on QoL in adult patients with moderate‐to‐severe plaque psoriasis.12 In these two trials, more than 60% patients on tildrakizumab 100 or 200 mg achieved 75% or more improvement in Psoriasis Area and Severity Index (PASI) at week 12, following two doses of the drug, compared with 6% in the placebo group and 48% in the etanercept group. At week 28, after three doses of the drug, the proportion of tildrakizumab‐treated patients with 75% PASI improvement increased to 77–79%.
While 75%, 90% and 100% PASI improvements are valuable in assessing relative change from baseline, it is of potential interest to physicians, patients and payers to understand how different groups of patients have better or worse responses and how patients respond over time using different efficacy parameters. In this investigation, we conducted a post hoc analysis of the two phase‐3 trial data sets to better understand the onset of tildrakizumab efficacy over the first 28 weeks, to assess the durability and improvability of this efficacy between weeks 28 and 52, and to examine the impact of tildrakizumab on patients’ QoL over 52 weeks by different levels of week‐28 PASI response.
Materials and methods
Study design
Both phase‐3 trials (reSURFACE 1 and reSURFACE 2) used a three‐part, double‐blinded, randomized, and placebo‐controlled study design with a 64‐week (reSURFACE 1) or 52‐week (reSURFACE 2) treatment period.12 In part 1 (weeks 0–12), adult patients with moderate‐to‐severe chronic plaque psoriasis were randomized to receive placebo, tildrakizumab 100 mg or 200 mg (at weeks 0, 4, then every 12 weeks), or etanercept 50 mg twice a week (reSURFACE 2 only). In part 2 (weeks 12–28), placebo patients were re‐randomized to receive tildrakizumab 100 mg or 200 mg, patients randomized to tildrakizumab continued therapy, and etanercept patients continued 50 mg weekly dosing. In part 3 (weeks 28–64 for reSURFACE 1, weeks 28–52 for reSURFACE 2), patients with 50% or greater improvement in PASI from baseline at week 28 were re‐randomized to receive the same, a higher or lower dose of tildrakizumab, or placebo (randomized withdrawal in reSURFACE 1); also, patients on etanercept (reSURFACE 2 only) with less than 75% PASI improvement from baseline at week 28 were switched to tildrakizumab 200 mg.
Together, the two trials enrolled 1862 adult patients from 250 sites in 16 countries from 2012 through 2015.12, 13, 14 These patients had a 6‐month or longer history of moderate‐to‐severe chronic plaque psoriasis at baseline and were candidates for phototherapy or systemic therapy. More detailed inclusion and exclusion criteria of these trials have been published elsewhere.12, 13, 14
Sample
This analysis only included psoriasis patients who were randomized to receive the same dose of tildrakizumab (100 mg or 200 mg) continuously from baseline to week 28. A subgroup analysis was conducted among patients who continuously received tildrakizumab 100 mg or 200 mg from baseline to 52 weeks and who achieved 50% or more reduction in PASI from baseline at week 28.
Study measures
Baseline characteristics
Patients’ demographic and clinical characteristics were collected at baseline. These characteristics included age, gender, race, bodyweight, body mass index (BMI), body surface area (BSA), duration of psoriasis and patient‐reported conditions such as psoriasis arthritis, cardiovascular diseases, diabetes and psoriasis‐related treatment history.
Psoriatic severity and efficacy measures
Psoriatic severity was measured in both trials using changes in PASI from baseline at weeks 4, 8, 12, 16, 22, 28, 32, 36, 40 and 52.12 The efficacy of tildrakizumab treatment in the current analysis was determined by categorization of the percentage of PASI reduction from baseline: PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100.
QoL measure
QoL was measured in both trials at baseline, and at weeks 12, 28, 40 and 52 via the Dermatology Life Quality Index (DLQI) questionnaire,12 which has been widely used in clinical trials to assess dermatology‐specific QoL in adult patients with psoriasis, acne, eczema and warts.15, 16 The DLQI questionnaire has 10 items (each item scores between 0 and 3) and a total score ranging from 0 to 30, with 0 or 1 indicating no impairment/impact on patient's QoL and 30 indicating significant impairment.15, 16 To assess the impact of tildrakizumab treatment on patients’ dermatology‐specific QoL, the proportion of patients with a DLQI total score of 0 or 1 (DLQI 0/1) was used in this analysis.
Analysis
All patients continuously treated with tildrakizumab 100 mg or 200 mg from baseline to week 28 were pooled from both trials to form the 100‐mg cohort and 200‐mg cohort, respectively. Within each dose cohort, biologic‐naïve and biologic‐experienced sub‐cohorts were created based on patients’ prior use of biologic agents for psoriasis (no or yes). Within each cohort and sub‐cohort, patients were further categorized into five mutually exclusive week‐28 PASI response groups: PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100.
Patients’ baseline demographic and clinical characteristics, mean percentage and 95% confidence interval (CI) of PASI reduction from baseline, and the proportion of patients with DLQI 0/1 were analysed for each week‐28 PASI response group within each dose cohort and biologic‐naïve or biologic‐experienced sub‐cohort, from baseline to week 28. Additional subgroup analyses were conducted among patients who continuously received tildrakizumab 100 mg or 200 mg from baseline to 52 weeks and who achieved at least 50% improvement in PASI from baseline at week 28.
Results
Baseline characteristics
A total of 1156 patients were pooled from the two trials. Of these patients, 575 were in the 100‐mg cohort; 69.6% were male, 81.4% were Caucasian, and the mean age was 45.6 years. An additional 581 patients were in the 200‐mg cohort; 73.0% were male, 79.4% were Caucasian, and the mean age was 45.9 years (Table 1). Overall, 100 of the tildrakizumab 100‐mg patients were biologic‐experienced and 475 were biologic‐naïve, while 99 of the tildrakizumab 200‐mg patients were biologic‐experienced and 482 were biologic‐naïve.
Table 1.
Baseline characteristics of patients treated with tildrakizumab by week 28 PASI groups
| Week‐28 PASI groups: tildrakizumab 100 mg (n = 575) | Week‐28 PASI groups: tildrakizumab 200 mg (n = 581) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PASI < 50 | PASI 50–74 | PASI 75–89 | PASI 90–99 | PASI 100 | PASI < 50 | PASI 50–74 | PASI 75–89 | PASI 90–99 | PASI 100 | |
| Number of patients | 48 | 82 | 137 | 175 | 133 | 23 | 105 | 114 | 169 | 170 |
| Age, years, mean (SD) | 49.2 (13.8) | 46.9 (12.9) | 46.5 (13.1) | 45.7 (13.3) | 42.6 (13.3) | 47.2 (15.2) | 47.2 (13.1) | 46.3 (12.1) | 46.1 (13.6) | 44.4 (13.7) |
| Male (%) | 81.3 | 70.7 | 68.6 | 67.4 | 68.4 | 87.0 | 67.6 | 64.9 | 76.3 | 76.5 |
| Race (%) | ||||||||||
| White | 83.3 | 73.2 | 84.7 | 81.7 | 82.0 | 82.6 | 75.2 | 73.7 | 83.4 | 81.2 |
| Black | 0.0 | 0.0 | 3.7 | 2.3 | 2.3 | 0.0 | 2.9 | 3.5 | 2.4 | 1.8 |
| Asian | 10.4 | 25.6 | 9.5 | 14.3 | 7.5 | 17.4 | 21.9 | 19.3 | 12.4 | 12.4 |
| Others | 6.3 | 1.2 | 2.2 | 1.7 | 8.3 | 0.0 | 0.0 | 3.5 | 1.8 | 4.7 |
| Bodyweight (kg), mean (SD) | 99.5 (25.5) | 84.4 (22.6) | 94.3 (24.3) | 87.3 (23.1) | 86.4 (20.1) | 93.3 (16.9) | 89.9 (24.1) | 89.2 (22.4) | 90.2 (23.1) | 85.0 (21.6) |
| Body mass index (kg/m2), mean (SD) | 32.0 (6.4) | 29.0 (7.3) | 31.8 (7.6) | 29.4 (7.2) | 29.3 (6.3) | 31.3 (9) | 29.9 (6.6) | 30.7 (7.7) | 30.2 (7.9) | 28.3 (6.9) |
| % body surface area affected, mean (SD) | 34.3 (16.9) | 35.7 (20.1) | 30.7 (17.5) | 33.2 (19.2) | 27.3 (15.1) | 33.5 (18) | 32.7 (19.2) | 31.6 (18.7) | 30.8 (16.9) | 30.2 (16.4) |
| Years of psoriasis, mean (SD) | 22.8 (15.5) | 18.6 (14.9) | 18.3 (13.6) | 16.5 (11.9) | 13.8 (10.6) | 17.8 (12.5) | 17.5 (11.5) | 18.1 (12) | 17.7 (13) | 15.7 (11.6) |
| PASI, mean (SD) | 20.2 (6.4) | 21.8 (9.7) | 20.1 (7.8) | 21 (8.3) | 17.9 (5.4) | 19.1 (7.6) | 20 (8.8) | 20.3 (9.1) | 20.6 (7.5) | 19.9 (7.6) |
| DLQI total score, mean (SD) | 13.9 (7.0) | 13.6 (7.3) | 13.8 (6.8) | 15.0 (7.0) | 14.4 (7.0) | 12.7 (5) | 13 (6.8) | 13 (7.3) | 13.1 (7.0) | 13.7 (7.0) |
| Previous medical conditions (%) | ||||||||||
| Psoriatic arthritis | 14.6 | 17.1 | 15.3 | 16.0 | 18.1 | 26.1 | 22.9 | 11.4 | 16.0 | 12.9 |
| Cardiovascular diseases | 37.5 | 31.7 | 29.2 | 22.9 | 21.8 | 39.1 | 29.5 | 28.1 | 31.4 | 19.4 |
| Diabetes | 14.6 | 7.3 | 11.0 | 8.6 | 7.5 | 13.0 | 14.3 | 13.2 | 11.8 | 8.2 |
| Previously treated with biologics (%) | 31.3 | 15.9 | 16.1 | 18.9 | 12.8 | 26.1 | 17.1 | 18.4 | 14.8 | 17.1 |
DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; SD, standard deviation.
PASI improvement from baseline to week 28
Among patients treated with tildrakizumab 100 mg, the numbers and percentages of patients with week‐28 PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100 responses were 48 (8.3%), 82 (14.3%), 137 (23.8%), 175 (30.4%) and 133 (23.1%), respectively (Fig. 1). At week 4 (second dose), the mean percentages of PASI improvement from baseline for the week‐28 PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100 groups were 16.0%, 30.0%, 38.4%, 45.6% and 52.6%, respectively. Starting from week 8 (4 weeks after the second dose), the 95% CIs for mean change from baseline among the week‐28 PASI response groups were non‐overlapping, indicating very different and unique response curves. Overall, percentages of PASI improvement continued over time for all cohorts except for the group with eventual week‐28 PASI < 50. The mean percentages of PASI improvement from baseline were similar for patients who had previously been treated with biologics and those who were biologic‐naïve. The percentage of patients in the week‐28 PASI < 50 group was numerically higher among biologic‐experienced patients (15.0% vs. 6.9%), although this may have been due to the smaller sample size in this subgroup (data not shown).
Figure 1.
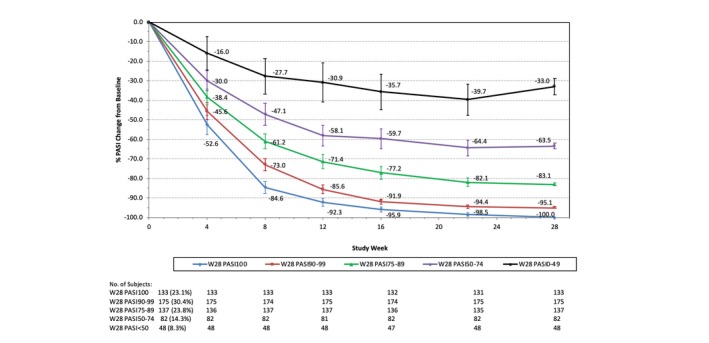
% PASI change from baseline by week‐28 PASI response groups: patients on tildrakizumab 100 mg from baseline to week 28. PASI, Psoriasis Area and Severity Index; W, week. 95% confidence interval is shown for each data point.
Similar trends were observed for the 200‐mg cohort, with 23 (4.0%), 105 (18.1%), 114 (19.6%), 169 (29.1%) and 170 (29.3%) patients achieving PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100 at week 28, respectively (Fig. 2). At week 4, the mean percentages of PASI improvement from baseline for the week‐28 PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100 groups were 24.0%, 26.4%, 34.6%, 46.4% and 51.6%, respectively. Biologic‐naïve and biologic‐experienced patients had similar percentages of PASI improvement over time, except that again there were numerically more biologic‐experienced patients in the week‐28 PASI < 50 group (6.1% vs. 3.5%; data not shown).
Figure 2.
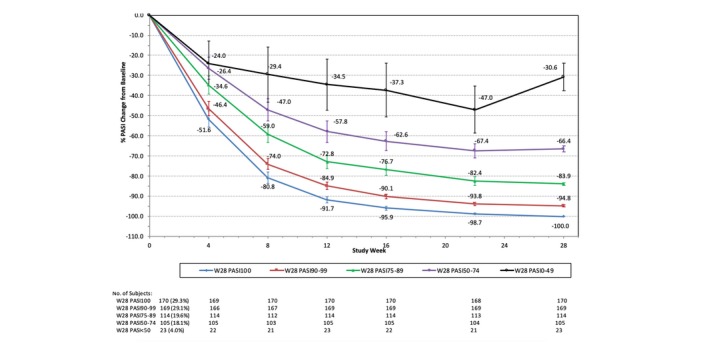
% PASI change from baseline by week‐28 PASI response groups: patients on tildrakizumab 200 mg from baseline to week 28. PASI, Psoriasis Area and Severity Index; W, week. 95% confidence interval is shown for each data point.
PASI improvement from baseline to week 52
Figure 3 delineates the subgroup analyses among patients who continuously received tildrakizumab 100 mg or 200 mg from baseline to 52 weeks and achieved at least 50% PASI improvement from baseline at week 28. Overall, the mean percentage of PASI improvement from baseline was maintained or improved over time. Specifically, for patients in the week‐28 PASI 50–74 group, the mean percentage of PASI improvement continued to improve from week 28 (100 mg: 63.3%; 200 mg: 66.5%) to week 52 (100 mg: 76.5%; 200 mg: 75.2%). Similar trends were observed for both the 100‐mg and 200‐mg cohorts. At week 52, the mean percentage of PASI improvement from baseline for the week‐28 PASI 50–74 group was over 75% (100 mg: 76.5%; 200 mg: 75.2%).
Figure 3.
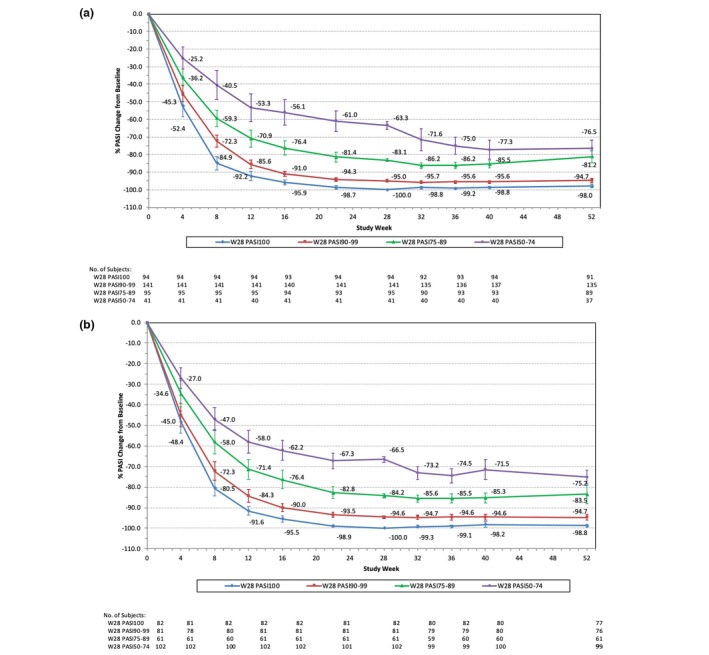
% PASI change from baseline by week‐28 PASI response groups from baseline to week 52. (a) Patients continuously on tildrakizumab 100 mg from baseline to week 52 (n = 371). (b) Patients continuously on tildrakizumab 200 mg from baseline to week 52 (n = 326). PASI, Psoriasis Area and Severity Index; W, week. 95% confidence interval is shown for each data point.
DLQI changes over time
Overall, patients achieving higher week‐28 PASI responses were more likely to achieve DLQI 0/1 at week 28 than those with lower PASI responses. In addition, the proportions of patients with DLQI 0/1 in each week‐28 response group continuously increased from baseline to week 28, except for those treated with tildrakizumab 100 mg who were in the week‐28 PASI < 50 group. For patients in the week‐28 PASI < 50, PASI 50–74, PASI 75–89, PASI 90–99 and PASI 100 response groups, the proportions of patients achieving DLQI 0/1 at week 28 were 8.3%, 22.0%, 40.9%, 66.3% and 86.5%, respectively, in the 100‐mg cohort, and 8.7%, 35.2%, 43.9%, 70.4% and 85.9%, respectively, in the 200‐mg cohort.
Similar trends were observed for the subgroup analysis among patients who were continuously treated with tildrakizumab 100 mg or 200 mg from baseline to week 52 (Fig. 4). Specifically, for patients in the week‐28 PASI 50–74 response group, the percentage of patients achieving DLQI 0/1 improved from 29.3% at week 28 to 34.2% at week 52 in the 100‐mg cohort, and from 38.2% to 40.4% in the 200‐mg cohort.
Figure 4.
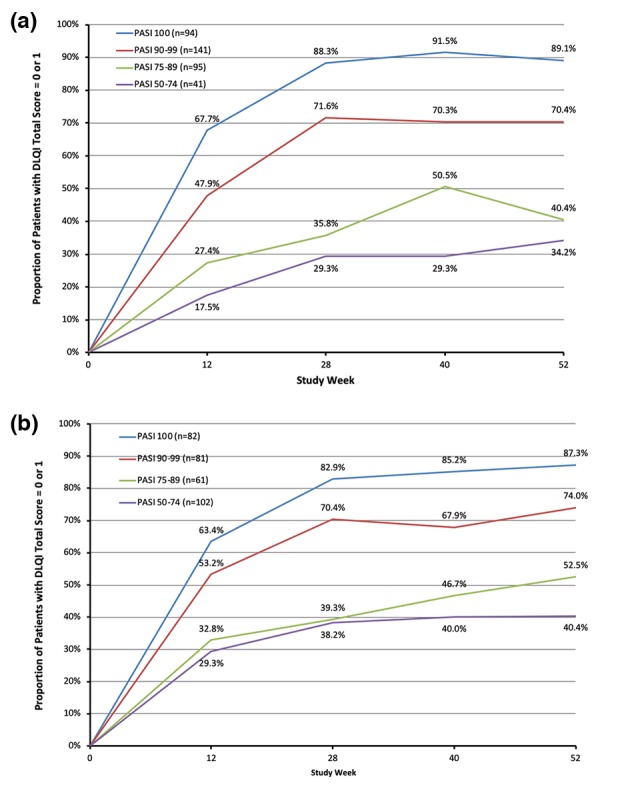
Proportion of patients with DLQI 0/1 from baseline to week 52 by week‐28 PASI response groups. (a) Patients continuously on tildrakizumab 100 mg from baseline to week 52. (b) Patients continuously on tildrakizumab 200 mg from baseline to week 52. DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; W, week.
Discussion
In this pooled post hoc analysis, we examined the efficacy of tildrakizumab treatment and its impact on QoL over 52 weeks among different week‐28 PASI response groups. This approach not only allowed examination of the robustness of tildrakizumab's efficacy and its impact on QoL, but also enabled a focus on the durability and improvability of outcomes over time. The early response curve of patients who achieved PASI < 50 at week 28 appeared to be different from the response curves of patients who achieved higher levels of week‐28 PASI response. More importantly, differences in responses between patients who eventually achieved PASI improvement <50% and ≥50% could be differentiated as early as week 8 (i.e. after two doses of tildrakizumab).
These results are clinically important as they suggest that suboptimal response may be apparent before the third dose of tildrakizumab (at week 16), allowing clinicians to opt for alternative treatment at an earlier time point. Nonetheless, only 8.3% of patients treated with tildrakizumab 100 mg did not achieve at least 50% PASI improvement from baseline at week 28, while many patients who achieved a ≥50% PASI improvement from baseline at week 28 appeared to have continued improvement beyond week 28. Similar patterns of response were observed with the 200‐mg dose. The patterns of response to tildrakizumab in patients by prior biologic exposure were also similar, regardless of the tildrakizumab dose.
Between weeks 28 and 52, efficacy was sustained or improved among all subgroups of patients who achieved at least PASI 50 improvement from baseline at week 28. At week 28, over 50% of patients treated with tildrakizumab achieved PASI ≥ 90. Importantly, these patients experienced approximately 50% PASI improvement from baseline by week 4, regardless of their previous biologic exposure. This could be clinically relevant as it suggests that early rapid improvement (i.e. reducing the disease burden by 50% after a single dose and before the second dose) may be indicative of an excellent clinical outcome by week 28.
Better skin clearance was associated with a greater likelihood of patients achieving DLQI 0/1 (i.e. no impairment/impact on QoL) over 52 weeks. Particularly, separate DLQI 0/1 response curves were observed for patients achieving week‐28 PASI 100 vs. those with PASI 90–99 and PASI 75–89, indicating the importance of aiming for greater PASI improvement and the impact of this improvement on patient QoL. More importantly, DLQI 0/1 responses were improved or maintained beyond week 28 for patients continuously treated with tildrakizumab (both 100 and 200 mg) between week 28 and week 52, highlighting the long‐term sustained efficacy of tildrakizumab on patients’ lives beyond skin clearance.
However, achieving complete skin clearance (i.e. PASI 100) was not necessarily associated with achieving DLQI 0/1, as 11–14% of patients with 100% skin clearance reported impairment of QoL. Therefore, both efficacy and QoL improvement need to be evaluated to provide a complete picture of treatment success. The lack of absolute concordance between PASI 100 and no impact on QoL has been reported previously17 and may reflect the fact that skin clearance is only one of the domains that affect patient QoL. For example, other aspects not captured in the PASI, such as itching and pain, can affect QoL.
This study has several limitations. First, we examined outcomes following tildrakizumab treatment only from the perspectives of PASI and DLQI, and not from other perspectives, such as symptom control, BSA improvement or treatment satisfaction. Therefore, the results may not provide a full perspective on outcomes following tildrakizumab therapy. Future studies examining outcomes from other perspectives may provide additional informative and useful insights. Secondly, the patients included in this post hoc analysis were selected from two randomized clinical trials and may have different characteristics from patients treated in clinical practice. Future studies using real‐world data may provide additional insights into the outcomes of tildrakizumab treatment. Finally, we did not explore factors that may influence the sustainability and improvability of tildrakizumab efficacy. These factors could include, but are not limited to, patient demographic and clinical characteristics, polypharmacy, environmental factors and personal behaviour. Future studies using advanced statistical methods to identify these factors might be important and useful for personalized psoriasis care.
Conclusion
Only 8.3% and 4% of psoriasis patients treated with tildrakizumab 100 and 200 mg, respectively, failed to achieve at least 50% PASI improvement at week 28. Importantly, these patients could be differentiated as early as week 8 by their lower percentage of PASI improvement. By continuing the same dose of tildrakizumab 100 mg or 200 mg from week 28 to week 52, patients achieving week‐28 PASI ≥ 90 sustained their clinical responses, while those with week‐28 PASI 50–74 continued to improve. Tildrakizumab treatment had similar efficacy in patients with and without prior use of biologic agents. Among patients achieving PASI ≥ 90 at week 28, tildrakizumab 100 and 200 mg were associated with rapid PASI improvement from baseline (approximately 50%) by week 4. Patients with a higher PASI response demonstrated better QoL improvement, although achieving PASI 100 was not necessarily associated with achieving DLQI 0/1. These results are important because they suggest that patients and their healthcare providers can make an educated decision as to whether to continue tildrakizumab therapy or not by week 12 or 16 (i.e. before the third dose).
Conflicts of interest
The authors were either clinical trial investigators or researchers sponsored or employed by Sun Pharmaceutical Industries Inc.
Funding sources
This study was funded by Sun Pharmaceutical Industries Inc. (Princeton, NJ, USA).
References
- 1. Lowes MA, Suarez‐Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014; 32: 227–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conrad C, Gilliet M. Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol 2018; 54: 102–113. [DOI] [PubMed] [Google Scholar]
- 3. Mease PJ, Gladman DD, Papp KA et al Prevalence of rheumatologist‐diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013; 69: 729–735. [DOI] [PubMed] [Google Scholar]
- 4. Han C, Lofland JH, Zhao N, Schenkel B. Increased prevalence of psychiatric disorders and health care‐associated costs among patients with moderate‐to‐severe psoriasis. J Drugs Dermatol 2011; 10: 843–850. [PubMed] [Google Scholar]
- 5. Hsu S, Papp KA, Lebwohl MG et al Consensus guidelines for the management of plaque psoriasis. Arch Dermatol 2012; 148: 95–102. [DOI] [PubMed] [Google Scholar]
- 6. Richard MA, Barnetche T, Horreau C et al Psoriasis, cardiovascular events, cancer risk and alcohol use: evidence‐based recommendations based on systematic review and expert opinion. J Eur Acad Dermatol Venereol 2013; 27(Suppl 3): 2–11. [DOI] [PubMed] [Google Scholar]
- 7. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014; 70: 512–516. [DOI] [PubMed] [Google Scholar]
- 8. Pariser D, Schenkel B, Carter C et al A multicenter, non‐interventional study to evaluate patient‐reported experiences of living with psoriasis. J Dermatolog Treat 2016; 27: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO . Global report on psoriasis. 2016. URL http://apps.who.int/iris/bitstream/10665/204417/1/9789241565189_eng.pdf (last accessed: 26 November 2017).
- 10. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 11. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 12. Reich K, Papp KA, Blauvelt A et al Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390: 276–288. [DOI] [PubMed] [Google Scholar]
- 13. FDA . A study to evaluate the efficacy and safety of subcutaneous MK‐3222, followed by an optional long‐term safety extension study, in participants with moderate‐to‐severe chronic plaque psoriasis (MK‐3222‐010). 2012. URL https://clinicaltrials.gov/ct2/show/NCT01722331 (last accessed: 20 February 2018).
- 14. FDA . A study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab (SCH 900222/MK‐3222) in participants with moderate‐to‐severe chronic plaque psoriasis followed by a long‐term extension study (MK‐3222‐011). 2012. URL https://clinicaltrials.gov/ct2/show/NCT01729754 (last accessed: 20 February 2018).
- 15. Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004; 9: 169–180. [DOI] [PubMed] [Google Scholar]
- 16. Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994‐2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
- 17. Elewski BE, Puig L, Mordin M et al Psoriasis patients with Psoriasis Area and Severity Index (PASI) 90 response achieve greater health‐related quality‐of‐life improvements than those with PASI 75‐89 response: results from two phase 3 studies of secukinumab. J Dermatolog Treat 2017; 28: 492–499. [DOI] [PubMed] [Google Scholar]


