Abstract
The limited effectiveness of rituximab plus intravenous immunoglobulin (IVIG) in desensitization may be due to incomplete B cell depletion. Obinutuzumab is a type 2 anti‐CD20 antibody that induces increased B cell depletion relative to rituximab and may therefore be more effective for desensitization. This open‐label phase 1b study assessed the safety, pharmacokinetics, and pharmacodynamics of obinutuzumab in highly sensitized patients with end‐stage renal disease. Patients received 1 (day 1, n = 5) or 2 (days 1 and 15; n = 20) infusions of 1000‐mg obinutuzumab followed by 2 doses of IVIG on days 22 and 43. Eleven patients received additional obinutuzumab doses at the time of transplant and/or at week 24. The median follow‐up duration was 9.4 months. Obinutuzumab was well tolerated, and most adverse events were grade 1‐2 in severity. There were 11 serious adverse events (SAEs) in 9 patients (36%); 10 of these SAEs were infections and 4 occurred after kidney transplant. Obinutuzumab plus IVIG resulted in profound peripheral B cell depletion and appeared to reduce B cells in retroperitoneal lymph nodes. Reductions in anti‐HLA antibodies, number of unacceptable antigens, and the calculated panel reactive antibody score as centrally assessed using single‐antigen bead assay were limited and not clinically meaningful for most patients (NCT02586051).
Keywords: alloantibody, B cell biology, clinical research/practice, clinical trial, immunosuppressant — fusion proteins and monoclonal antibodies: B cell specific, immunosuppression/immune modulation, kidney transplantation/nephrology, pharmacology
Short abstract
The authors assess the safety, pharmacokinetics, and pharmacodynamics of obinutuzumab in combination with high‐dose intravenous immunoglobulins in highly sensitized patients with end‐stage renal disease awaiting kidney transplantation and find that obinutuzumab is tolerated well and effectively depletes B cells, but has limited effects on reducing pre‐existing anti‐HLA alloantibody levels.
Abbreviations
- AE
adverse event
- CLL
chronic lymphocytic leukemia
- CPRA
calculated panel reactive antibody
- CV
coefficient of variation
- DSA
donor‐specific anti‐HLA antibody
- eGFR
estimated glomerular filtration rate
- ESRD
end‐stage renal disease
- FCM
flow‐cytometry–mediated
- HLA
human leukocyte antigen
- IRR
infusion‐related reaction
- IVIG
intravenous immunoglobulin
- MFI
mean fluorescence intensity
- PD
pharmacodynamic
- PI
proteasome inhibitors
- PK
pharmacokinetics
- PRA
panel reactive antibodies
- SAB
single‐antigen bead
- SAE
serious adverse event
- UA
unacceptable antigen
1. INTRODUCTION
Antihuman leukocyte antigen (HLA) alloantibodies are a major barrier to successful kidney transplantation in patients with end‐stage renal disease (ESRD) and can lead to substantial reductions in renal allograft survival.1, 2 HLA‐sensitized patients represent approximately 30% of patients on the waitlist for kidney transplant3 and historically have faced longer wait times for a suitable donor graft—as well as inferior long‐term outcomes following transplant—than patients who are not sensitized.4, 5, 6 To improve access to transplant for this population, several approaches have been attempted, including treatment with the anti‐CD20 monoclonal antibody rituximab to deplete CD20+ B cells, which play a central role in humoral immunity.
A seminal study combining rituximab with intravenous immunoglobulin (IVIG) reported reduction of the level of sensitization (as measured by panel reactive antibodies [PRA]) that enabled most patients (16/20; 80%) to receive a kidney graft with excellent posttransplant outcomes.7 These effects were not replicated in subsequent similarly uncontrolled case series, however, raising the question whether inconsistent effects might be due to the patient populations studied. Alternatively, this could be due to incomplete tissue B cell depletion.8, 9, 10, 11, 12 B cell depletion would be expected to reduce anti‐HLA alloantibody levels if anti‐HLA alloantibodies were produced by a plasma‐cell population that required ongoing replenishment by production of new plasmablasts from memory B cell populations. Despite enabling peripheral blood B cell depletion, rituximab incompletely depletes lymphoid organ B cells.8, 11, 12, 13 Although the exact reasons for the inconsistent effects of rituximab on desensitization are not known, one hypothesis is that achieving more extensive B cell depletion than is achieved with rituximab, particularly in tissue, may improve results in highly sensitized patients with ESRD.
Obinutuzumab is a glycoengineered type 2 anti‐CD20 monoclonal antibody that has been shown to be more efficacious than rituximab in depleting B cells in peripheral blood and lymphoid tissue, in both animal models and patients with chronic lymphocytic leukemia (CLL);13, 14 in particular, significantly greater B cell depletion in bone marrow was observed with obinutuzumab than with rituximab in patients with CLL.14 We hypothesized that the greater capacity of obinutuzumab to induce depletion of tissue‐resident B cells may overcome the limitations of rituximab and provide greater anti‐HLA antibody reduction in highly sensitized patients. We present here the results of an open‐label study of obinutuzumab in combination with high‐dose IVIG to evaluate the safety, tolerability, and pharmacokinetics (PK) of obinutuzumab and its effect on B cell depletion and anti‐HLA antibodies in highly sensitized patients with ESRD awaiting transplant.
2. MATERIALS AND METHODS
2.1. Study design
THEORY was a phase 1b, open‐label, sequential 2‐cohort study comparing single and repeated doses of obinutuzumab with high‐dose IVIG in highly sensitized candidates for renal transplant in the United States (NCT02586051). Twenty‐five patients were enrolled between November 2015 and August 2016 at 7 centers in the United States. Patients in cohort 1 received a single intravenous infusion of 1000‐mg obinutuzumab on day 1 followed by 2 g/kg IVIG (maximum 140 g) at weeks 3 and 6. Following a 28‐day observation period for adverse events (AEs), patients were enrolled in cohort 2 and received 1000‐mg obinutuzumab infusions on days 1 and 15, and an optional 1000‐mg infusion at week 24, plus an IVIG regimen identical to cohort 1. All patients were monitored for 12 months following the last obinutuzumab infusion (Figure S1). Patients who underwent kidney transplant (per local center practices) before week 52 received an additional obinutuzumab infusion at the time of transplant (peritransplant) and 24 weeks after transplant and were followed up for 52 weeks after their last obinutuzumab dose to further assess safety and tolerability. The transplant population is still being followed at the time of this report and will be described separately. Analyses are based on the data available as of March 10, 2017, unless otherwise stated. Statistical methods are described in the Data S1.
This study was conducted in accordance with local institutional review board ethical standards, good clinical practices, and the Declaration of Helsinki. All patients provided written informed consent before study participation.
2.2. Study objectives
The primary objective assessed the safety and tolerability of the obinutuzumab/IVIG regimen at week 24 of the desensitization phase. The secondary objective characterized the PK and pharmacodynamic (PD) profiles of obinutuzumab. PD markers included peripheral blood CD19+ B cells and anti‐HLA alloantibodies. Exploratory objectives aimed to quantify the impact of obinutuzumab on sensitization as measured by the number of unacceptable antigens (UAs), the calculated panel reactive antibody (CPRA) score, and anti‐HLA antibodies strength at week 24, as well as posttransplant estimated glomerular filtration rate (eGFR), calculated using Chronic Kidney Disease Epidemiology Collaboration equation.15
2.3. Patient selection
Patients were 18‐65 years old, had ESRD, were awaiting renal transplant, and had both a history of sensitizing events and CPRA ≥ 50% at screening. CPRA was calculated based on input from the local transplant team and incorporated site‐generated data (Data S1). Patients were required to be United Network for Organ Sharing listed for a deceased donor kidney transplant and have an estimated high likelihood of receiving an offer 12‐18 months after screening, as evidenced by presence on ≥1 match run for a deceased donor kidney during the past year or CPRA ≥98%. The number of patients who had received a prior transplant was capped at 6. Further details are available in the Data S1.
2.4. Blood B cell enumeration and measurement in tissue
B cells were measured using a validated 6‐color, lyse/no‐wash flow cytometry assay. Cells from peripheral blood or lymph nodes were stained with antibodies to identify B cells. Total B cells were defined using light scatter characteristics, CD45+, CD3−/CD14−, CD56−/CD33−, and CD19+. Further details are available in the Data S1.
2.5. Anti‐HLA antibodies
For patient clinical management, participating sites continued to use antibody screening data generated at their local laboratories. Serum samples were collected in parallel for central study analyses. Anti‐HLA alloantibodies were quantified using Luminex‐based single‐antigen bead (SAB) Lab Screen assays at the UCLA Immunogenetics Center (Clinical Laboratory Improvement Amendments certified and American Society for Histocompatibility and Immunogenetics accredited). Anti‐HLA serum reactivity was calculated by measurement of HLA antibody bound to each bead, expressed as mean fluorescence intensity (MFI).16 The desensitization assessment scientific panel and criteria for UAs are described in the Data S1.
2.6. Laboratory assessments
All laboratory safety assessments, including IgA, IgM, IgG, vaccination titers, and the schedule of assessments, are depicted in Tables S1 and S2.
3. RESULTS
3.1. Patient disposition and baseline characteristics
Twenty‐five patients were enrolled and received ≥1 dose of obinutuzumab (cohort 1, n = 5; cohort 2, n = 20). As of the data cutoff of March 10, 2017, 8 patients had received a transplant (cohort 1, n = 1; cohort 2, n = 7). One of the 7 patients in cohort 2 was withdrawn before day 15 because the patient received a transplant 6 days after the baseline visit and therefore received only 1 dose of obinutuzumab and no IVIG infusion; this patient was followed for safety only and not included in the exploratory efficacy assessment. Overall, 6 of the 8 patients had received 2 doses of obinutuzumab prior to transplantation, and the remaining 2 received only 1 dose each (Figure 1). Per protocol, patients undergoing transplant received additional doses of obinutuzumab around the time of and 24 weeks after renal transplant. Patient baseline characteristics are presented in Table 1.
Figure 1.
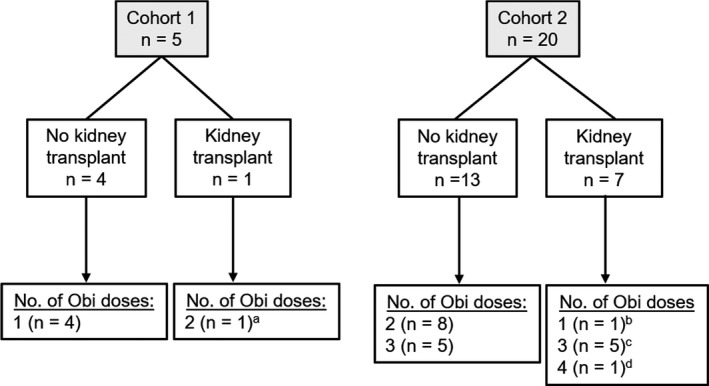
Patient disposition and number of obinutuzumab (Obi) doses. aThis patient received 1 obinutuzumab dose before transplant and 1 dose after. bThis patient received a transplant at week 1 and was withdrawn from study treatment. cThese 5 patients received 2 obinutuzumab doses before transplant and 1 dose after. dThis patient received 2 obinutuzumab doses before transplant and 2 doses after
Table 1.
Baseline patient demographics and clinical characteristics
| Cohort 1 (n = 5) | Cohort 2 (n = 20) | All patients (N = 25) | |
|---|---|---|---|
| Age, median (range), y | 46 (34‐54) | 51 (29‐65) | 48 (29‐65) |
| Female, n (%) | 4 (80) | 18 (90) | 22 (88) |
| Race, n (%) | |||
| White | 2 (40) | 14 (70) | 16 (64) |
| Black/African American | 3 (60) | 4 (20) | 7 (28) |
| Asian | 0 | 2 (10) | 2 (8) |
| Primary ESRD diagnosis, n (%) | |||
| Hypertension | 1 (20) | 0 | 1 (4) |
| Cystic disease | 2 (40) | 7 (35) | 9 (36) |
| Glomerulonephritis | 0 | 4 (20) | 4 (16) |
| Diabetes | 0 | 6 (30) | 6 (24) |
| Other | 2 (40) | 3 (15) | 5 (20) |
| Years on waitlist, median (range) | 8.0 (3.2‐12) | 4.9 (0.63‐12) | 5.3 (0.63‐12) |
| Immunizing events, n (%) | |||
| Pregnancy [para] | 4 (80) [1‐2] | 18 (90) [1‐8] | 22 (84) [1‐8] |
| Blood transfusion | 3 (60) | 13 (65) | 16 (64) |
| Prior transplant | 3 (60) | 3 (15) | 6 (24) |
| CPRA, median (range), %a | 99.95 (72.29‐99.96) | 91.77 (49.44‐99.99) | 94.51 (49.44‐99.99) |
| No. of UAs, median (range) | 33 (7‐56) | 24 (2‐67) | 26.5 (2‐67) |
Abbreviations: CPRA, calculated panel reactive antibody level; ESRD, end‐stage renal disease; para, number of pregnancies reaching viable gestational age; UA, unacceptable antigen.
CPRA data presented here are those generated at a central laboratory, not the local site laboratories.
3.2. Safety
A total of 193 AEs were reported in 22 of 25 patients (88%). Of those AEs, 38 occurred at a median of 0.5 (range, 0‐18) weeks after transplant in the 8 patients who had received a transplant.
No deaths were reported. The most common adverse drug reactions were nonserious protocol‐defined infusion‐related reactions (IRRs) (Data S1), occurring in 13 patients (52%). IRRs occurred mainly after the first infusion only, except in 3 patients who experienced an IRR at both the first and second infusions. No IRRs led to dose reduction or discontinuation; the most common IRR symptoms were chills (5 patients), nausea (4 patients), and hypotension (3 patients).
Eleven patients (44%) experienced 21 infection AEs a median (range) of 9.4 (6.5‐15.6) weeks after the initial dose. Eight of those 21 infections occurred after kidney transplant in 3 of the 8 patients who received a transplant.
Eleven serious AEs (SAEs) were reported in 9 patients (36%) (Table 2); 10 were infections reported in 8 patients (32%) with a median (range) onset of 6 (4‐34) weeks after last obinutuzumab infusion. Four of the 10 infection SAEs occurred after kidney transplant in 3 patients; these were pneumonia and nocardiosis in 1 patient, incision infection in another, and postoperative wound infection in a third patient. All SAEs resolved with standard medical care, without sequelae. Of the 11 SAEs, 3 reported in 2 patients were assessed by the investigators as related to study drug (pneumonia and nocardiosis in 1 patient and pneumonia in another patient). The case of pneumonia and nocardiosis was in a 61‐year‐old female patient, 63 days after receiving a deceased donor renal transplant and a T cell–depleting induction regimen with alemtuzumab. The nocardia infection resolved after imipenem and oral sulfamethoxazole/trimethoprim.
Table 2.
Serious adverse events
| Patient no. | Obinutuzumab doses | Time from last obinutuzumab dose to SAE onset, wk | SAE, preferred term |
|---|---|---|---|
| 102 | 1 | 4 | Pneumonia |
| 17 | Device‐related infection | ||
| 104 | 1 | 34 | Diverticulitis |
| 110 (Tx) | 3 | 8 | Pneumonia (posttransplant) |
| 9 | Nocardiosis (posttransplant) | ||
| 112 | 2 | 6 | Sepsis |
| 117 (Tx) | 3 | 5 | Incision‐site infection (posttransplant) |
| 118 (Tx)a | 1 | 6 | Postoperative wound infection (posttransplant) |
| 121 | 2 | 5 | Escherichia coli UTI |
| 123 | 3 | 6 | Angina pectoris |
| 125 | 2 | 4 | Peritonitis |
Abbreviations: SAE, serious adverse event; Tx, received transplant; UTI, urinary tract infection.
This patient was withdrawn, followed up only for safety, and not considered in the desensitization analysis.
As of the data cutoff date of March 10, 2017, the 8 patients who received a transplant on study displayed a median eGFR15 of 76 mL/min/1.73 m2, ranging from 43 to 104 mL/min/1.73 m2 by week 12 after transplant, and there were no signs of antibody‐mediated rejection.
Whereas serum IgA and IgM levels slightly decreased from baseline to week 12 to a mean (median) of 96.6% (95.6%) and 91.7% (87.9%), respectively, serum IgG increased to a mean (median) of 114% (111%). From baseline to week 24, IgA, IgM, and IgG decreased to a mean (median) of 93.3% (86.5%), 94.0% (68.2%), and 88.0% (88.6%), respectively. Changes in cohort 1 and cohort 2 were comparable. No correlation between the onset of infection and immunoglobulin levels was observed.
Vaccination titers were stable throughout the study. There were no changes in serostatus of antibodies to mumps, rubella, or varicella‐zoster. Antipneumococcal capsular polysaccharide and antitetanus toxoid antibody titers fluctuated, but the median concentration did not change between screening and week 24 and all titers remained in the protective range.
3.3. Pharmacodynamic analyses
3.3.1. Peripheral B cells
By week 3 after first obinutuzumab dose, 60% and 100% of patients in cohorts 1 and 2 (with reported results), respectively, had CD19+ B cells depleted to <0.441 cells/μL. By week 24, 80% of patients in cohort 1 had detectable CD19+ B cell levels, and in cohort 2, 90% of patients with reported results still had fully depleted CD19+ B cells (Figure 2). A patient in cohort 2 had rapid repopulation of B cells. Subsequent analysis revealed reduced obinutuzumab exposure and the presence of antidrug antibodies (data not shown). Obinutuzumab appeared to reduce peripheral CD4+ and CD8+ T cells on average by 15%‐36% (Figure S2).
Figure 2.
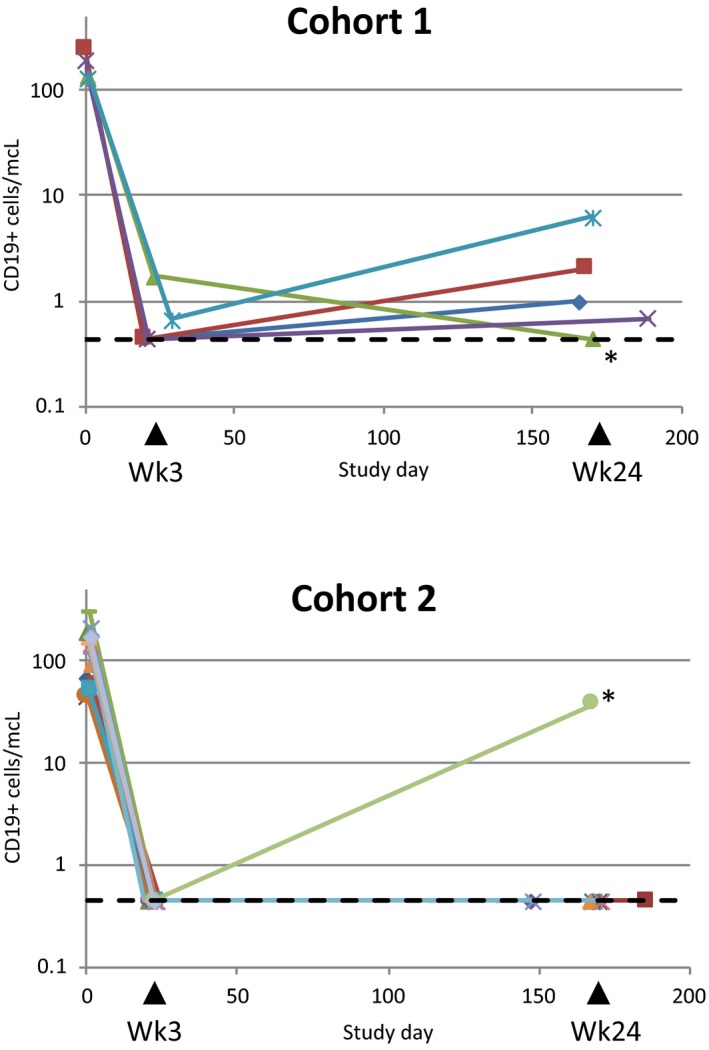
CD19+ peripheral B cell counts in patients from cohort 1 and cohort 2 during the desensitization phase. Cell counts are given in number of cells per μL as a function of study days. The lower limit of quantification of the high‐sensitivity flow cytometry assay was 0.441 cells/μL and is indicated with a dashed line. Traces for the 2 patients with detectable antidrug antibodies are marked with an asterisk
3.3.2. CD19+ B cells in lymph nodes
In patients who received a transplant, the level of CD19+ B cells in retroperitoneal lymph nodes procured at time of transplant appeared reduced in the majority (5/7) relative to observational comparator cohorts of peritransplant patients who had not been exposed to obinutuzumab (Data S1; Figure 3). The 2 patients whose B cell percentages in lymph nodes were not reduced relative to nonstudy comparator nodes were fully depleted in blood by high‐sensitivity flow cytometry at the time of transplant.
Figure 3.
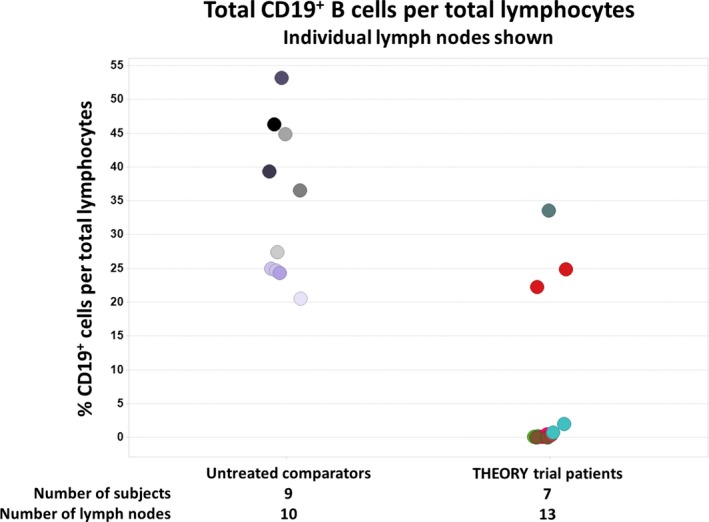
CD19+ B cell frequency in peritoneal lymph nodes of patients who received a transplant (7 patients, 13 nodes)
3.3.3. Anti‐HLA antibody profile
Mean changes in MFI over all alleles were modest for most patients and largely within the assay variability margin. Post‐hoc individual patient bootstrapping analyses revealed that 3 patients (all cohort 2) had reductions in overall mean MFI values by ≥25% and that no patient had increase of ≥25% (Figure 4). Mean MFI posttreatment changes from baseline ranged from −17.1% to −3.24% in cohort 1 and from −31.4% to 20.6% in cohort 2 (Figure 4, exemplary patient profiles in the Figure S3). Only four patients had posttreatment MFI increases or decreases from baseline of >50% in a subset of their HLA reactivities (data not shown), a level of change thought to be significant relative to assay variability.16 Two of these patients (1 each in cohorts 1 and 2) had decreases of >50% from baseline, consistent with desensitization, in 2 of 52 alleles and 24 of 58 alleles, respectively. The patient from cohort 2 with a significant reduction in 24 of 58 alleles exhibited a parallel pronounced MFI increase in another 18 alleles (Figure 4 inset). Two patients in cohort 2 had unexpected MFI increases >50% in 1 of 38 alleles and 5 of 51 alleles, respectively. According to results with corresponding diluted baseline samples, MFI increases were not due to the attenuation of a prozone effect, except for possibly in 1 patient in whom baseline MFI signals for 8 of 58 alleles indeed displayed a hook effect upon dilution (Data S1). There were no obvious sensitizing or infectious events that coincided with the signal increase in these 6 patients.
Figure 4.
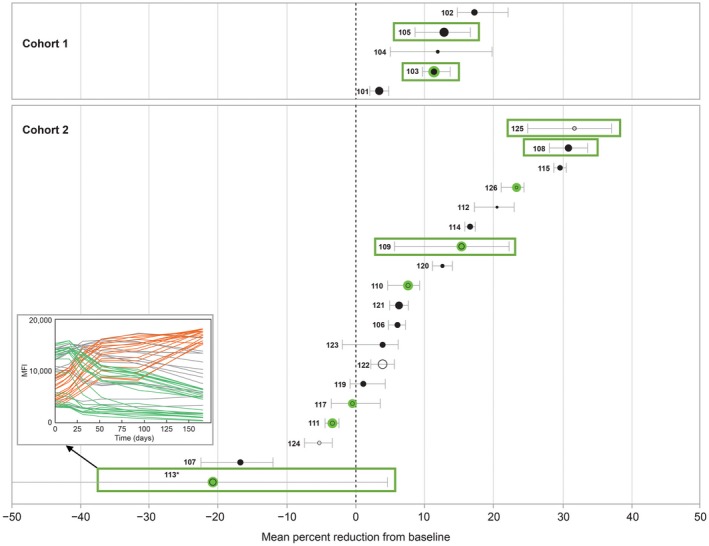
Mean percent reduction in MFI and 95% CIs from baseline to week 24 (filled circles) or last valid MFI measurement (open circles); limited to alleles with MFI > 3000 at any time point and baseline or posttreatment MFI > 500. Marker size is proportional to the number of alleles per patient. Mean and 95% CIs were calculated using bootstrapping with bias‐corrected CI. Patients who have received a transplant are encircled in green; patients identified as biological responders by the DASP are boxed. Abbreviations: CI, confidence interval; DASP, desensitization assessment scientific panel; MFI, mean fluorescence intensity
3.3.4. Unacceptable antigens and CPRA over time
The number of UA ranged from 2 to 67 (median, 27) per patient at baseline. From baseline to a median posttreatment time point of 24 weeks, 14 of 24 patients had a numerical decrease in UA (mean, −4.5), whereas 2 of 24 had an increase (mean, +2.5; Figure 5A). Of the 16 patients with changes in the number of UAs, only 4 had changes that resulted from a significant MFI change of >50% on top of crossing the threshold; 2 patients had increases (+2 and +3) and 2 had decreases in the number of UAs (−1 and −5) (Figure 5B).
Figure 5.
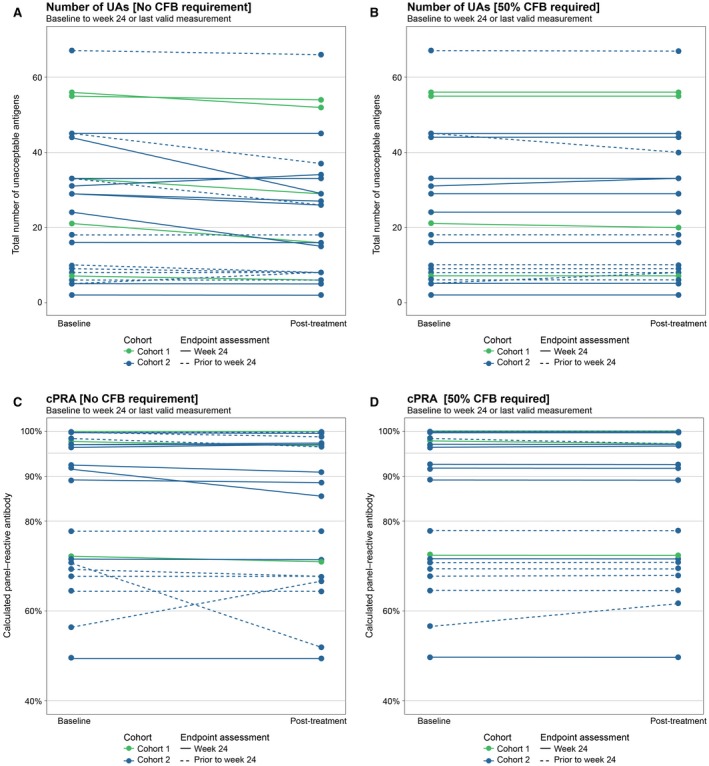
Change in the number of UAs (A, B) and CPRA score (C, D) from baseline to week 24 or last valid MFI measurement. For UAs and CPRA presented in panels B and D, change in acceptability of a given antigen needed not only to surpass the MFI threshold of 3000 but also display a robust change in MFI of 50%. Abbreviations: CFB, change from baseline; CPRA, calculated panel reactive antibody; MFI, mean fluorescence intensity; UA, unacceptable antigen
Of the 24 patients analyzed, changes in UA (defined by crossing MFI threshold only) resulted in CPRA reductions in 12 patients and increases in 2 patients (Figure 5C). Mean change in CPRA was −0.98% (range, −18.8% to +10.4%). When posttreatment CPRA accounted for changes in UA that were additionally accompanied by a minimum 50% MFI change from baseline, 2 of the 24 patients had increases and 2 had decreases in CPRA, with a mean change of 0.15% (range, −1.31% to +5.11%) (Figure 5D).
3.4. On‐study kidney transplants
At the time of the primary analysis, 8 of the 25 patients (cohort 1, n = 1; cohort 2, n = 7) had received a deceased‐donor kidney transplant, occurring at week 1 (in a patient from cohort 2 who was withdrawn from study treatment and not considered for exploratory efficacy analysis) and at weeks 15, 16, 19, 21, 21, 22, and 40. The 8 patients who received a transplant up until the time of the primary analysis had baseline characteristics similar to those in patients who had not received a transplant, including ESRD history and level of sensitization. Two of the 8 transplanted patients had a prior kidney transplant, consistent with the proportion of patients with prior transplants in the study (6/25). In addition to obinutuzumab, all patients received a center‐specific standard‐of‐care multidrug immunosuppressive induction regimen: mycophenolic acid or its prodrug mycophenolate mofetil, tacrolimus, and corticosteroids. Five patients received thymoglobulin and 3 patients received alemtuzumab; 2 patients additionally received high‐dose IVIG. None of the mismatched donor antigens were listed as UAs by the patient's program. Two of 7 patients displayed donor‐specific anti‐HLA antibodies (DSAs) at time of transplant based on central laboratory SAB results: anti‐A24 at MFI 4700 in 1 patient from cohort 2 (reduced from 13 860 at baseline) and anti‐DR8 at MFI 2350 in another patient from cohort 1 (reduced from 3030 at baseline). Virtual crossmatch was negative in the 8 transplanted patients based on center‐specific criteria. Flow cytometric crossmatch tests against donor B cells using serum collected immediately before kidney transplant was positive for 3 of the 6 evaluable patients (2 of these 3 were the patients with preexisting DSAs), equivocal for 1, and negative for 2; the corresponding flow cytometric crossmatch against donor T cells was negative for 7 of 7 evaluable patients. All those crossmatches were done locally in presence of pronase to attenuate potential interference from residual anti‐CD20 therapeutic antibody and were assessed based on center‐defined thresholds.
3.5. Pharmacokinetic analyses
PK data were available from all 25 patients using limited PK sampling. Obinutuzumab serum concentration data were analyzed using nonlinear mixed effect modeling.17 The concentration‐time course of obinutuzumab was well described by a linear 2‐compartment PK model with time‐dependent clearance. Clearance and volume of distribution parameters increased with body weight and were higher in male patients, although data from only 3 male patients were included; body weight and gender are known to affect obinutuzumab PK in hematological indications.18 Main PK parameters following 2 doses of 1000‐mg obinutuzumab (cohort 2) are shown in Table 3.
Table 3.
Predicted PK parameters in patients from cohort 2
| Parameter | Time after first dose | ||
|---|---|---|---|
| Initial time (Time = 0) | Month 6 | Month 12 | |
| CL, L/d | |||
| Geometric mean (CV%) | 0.176 (0.312) | 0.126 (0.312) | 0.0895 (0.312) |
| Median (range) | 0.176 (0.0752‐0.292) | 0.126 (0.0536‐0.208) | 0.0895 (0.0382‐0.148) |
| Effective half‐life, day | |||
| Geometric mean (CV%) | 14.3 (0.2) | 20.1 (0.2) | 28.2 (0.2) |
| Median (range) | 14.8 (9.3‐19.8) | 20.8 (13.0‐27.8) | 29.2 (18.3‐39.1) |
| C max, μg/mL | |||
| Geometric mean (CV%) | 419.9 (0.23) | ||
| Median (range) | 408.5 (278.6‐807.3) | ||
| C trough, μg/mL | |||
| Geometric mean (CV%) | 0.7 (1.37) | ||
| Median (range) | 0.9 (0.0‐5.8) | ||
| AUC168d, μg × d/mL | |||
| Geometric mean (CV%) | 11 958 (0.32) | ||
| Median (range) | 11 981 (7147‐28 229) | ||
CV was computed as the standard deviation of the log‐transformed data.
Abbreviations: AUC168d, cumulative area under the concentration‐time curve over 168 d; CL, clearance of obinutuzumab; C max, maximum observed serum concentration after second dose of obinutuzumab; C trough, minimum serum concentration (before dose on day 168); CV, coefficient of variation; PK, pharmacokinetic.
In the population PK model in patients with ESRD, after the last dose in cohort 2 the estimated median C max was 409 μg/mL and AUC168d was 11 981 μg × day/mL. Following IV administration, the median volume of distribution at steady state was 3.77 L, which approximates total blood volume, indicating that distribution was largely restricted to plasma and interstitial fluid. The clearance of obinutuzumab was approximately 0.18, 0.13, and 0.09 L/d at time 0, month 6, and month 12, respectively. The 1 male patient in cohort 2 with antidrug antibody who had low exposure had rapid recovery of peripheral B cells (Figure 2).
4. DISCUSSION
At the time this study was designed, highly sensitized patients with ESRD made up 30% of the kidney transplant waitlist. These patients have longer wait times for a suitable donor graft and inferior long‐term outcomes following transplant than patients who are not sensitized.4, 5, 6, 19 With no approved pharmacological therapy to reduce the degree of sensitization before transplant, new approaches for effective desensitization to control HLA‐specific alloantibody production remain an unmet medical need. This study assessed the safety, PK/PD, and exploratory efficacy of obinutuzumab/IVIG in highly sensitized patients with ESRD.
This is the first study assessing obinutuzumab outside of oncology. No new safety signals were identified in patients with ESRD awaiting renal transplant. The most common AEs were IRRs. In contrast to previous experience with obinutuzumab in oncology—where 20% of patients with CLL experienced grade 3 or 4 IRRs, frequently leading to treatment modification or discontinuation—all IRRs in this study were mild to moderate and none resulted in treatment withdrawal or incomplete drug administration.
In patients with B cell malignancies, a 2‐compartment PK model with linear and time‐dependent clearance components accurately described the concentration‐time course of obinutuzumab, with steady‐state PK parameter values typical of monoclonal antibodies.18 In those patients, time‐dependent clearance components reflected the depletion of the target or changes in the target expression levels with time. In patients with ESRD, the clearance of obinutuzumab was at the lower end of the range known for IgG clearance;20 why obinutuzumab clearance continued to depend on time in these patients remains unclear. The volume of distribution at steady‐state approximated total blood volume, indicating distribution was largely restricted to plasma and interstitial fluid.
Based on high‐sensitivity flow cytometry, peripheral blood CD19+ B cell depletion after one 1000‐mg obinutuzumab dose was rapid and strong, with repopulation by 24 weeks. B cell depletion after 2 obinutuzumab doses appeared longer lasting, with the majority of patients still fully depleted at week 24. CD19+ B cells in lymph nodes collected at the time of transplant appeared to also be effectively reduced by obinutuzumab/IVIG in the majority of patients tested, including memory B cells and plasmablasts (Looney et al, in preparation). Future desensitization studies with obinutuzumab may warrant initial treatment with 2 doses of obinutuzumab to reliably deplete B cells over several months.
Rituximab has had limited success in reducing anti‐HLA antibody levels in patients with ESRD, with some uncontrolled studies showing no effect,8, 9, 10, 11, 12 possibly due to incomplete tissue B cell depletion.8, 11, 12, 13 The current study was carried out based on the improved B cell depletion observed in blood and secondary lymphoid organs with obinutuzumab compared with rituximab.13, 14 Obinutuzumab resulted in extensive peripheral blood and tissue B cell depletion; however, anti‐HLA antibody reductions were inconsistent and limited. Although a few patients did display pronounced reduction in some anti‐HLA reactivities, the results of this study do not suggest a clinically meaningful desensitizing effect across most patients. The fact that depletion of CD20+ B cells alone does not significantly affect HLA antibody production indicates that HLA antibodies are produced by CD20−, mature, long‐lived plasma cells. These plasma cells may not depend on constant replenishment from a CD20+ B cell population but rather may exist for the remainder of the host's life, only gradually outcompeted by newly produced plasma cells. Consistent with this, vaccination Ig titers were also not significantly affected by obinutuzumab.
Based on these findings, one may expect plasma‐cell targeting to have greater effects on the reduction of anti‐HLA antibody titers. However, despite its ability to significantly deplete bone marrow plasma cells, treatment with proteasome inhibitors (PI) results in variable reductions in anti‐HLA antibodies in transplant patients and autoantibodies in patients with autoimmune disease,21, 22, 23, 24 likely due to the frequent occurrence of rebound in antibody production.25 Potential mechanisms underlying these observations have been recently described in Rhesus macaques by Kwun et al26 and suggest a compensatory increase in memory B cells following PI treatment that may then replenish plasma cells and help maintain anti‐HLA alloantibody levels. Alternatively, niche‐resident plasma cells may exhibit varying degrees of PI resistance.
Targeting long‐lived plasma cells while also inhibiting their renewal via effective B cell depletion with obinutuzumab may represent a promising avenue forward. Newer‐generation PIs, eg carfilzomib, may provide improved tolerability and effectiveness over bortezomib.27, 28 Alternatively, future therapeutic regimens could combine obinutuzumab with antibodies targeting plasma‐cell survival factors like IL‐6, B cell activating factor (BAFF), and/or a proliferation‐inducing ligand (APRIL).29 The benefit of suppressing anti‐HLA alloantibodies via sustained plasma‐cell depletion with combination therapy will need to be weighed against the safety risk of depleting protective humoral response and vaccinations.
HLA alloantibodies were assessed in a central laboratory using a state‐of‐the‐art fluorescence‐based SAB‐based assay. In contrast, earlier positive studies with rituximab/IVIG or IVIG alone had employed a cytotoxic lymphocyte panel assay and suggested more meaningful and consistent desensitization based on PRA.7, 30 Although antibody strength as reflected in SAB MFI signals is typically considered a prerequisite for identifying clinically relevant anti‐HLA alloantibodies, assessing desensitization based on all positive MFI reactivities may neglect a clinically meaningful desensitizing effect on specific alloantigens. The ideal endpoint in desensitization remains to be defined because each technique for evaluation of efficacy presents challenges and opportunities; this difficulty in finding an appropriate endpoint is common in transplant.31 The main limitations of this study are the lack of an IVIG control arm and the small study size, which do not allow for a more clinically meaningful endpoint, eg the rate of transplantation. The limited follow‐up at the time of the data cutoff limits conclusions on longer‐term safety.
By the data cutoff of March 10, 2017, 8 patients had received a transplant (1 who received the transplant at week 1 was withdrawn, only followed up for safety, and not considered in the exploratory efficacy or tissue B cell analyses). For the remaining 7, the flow‐cytometry–mediated (FCM) crossmatch experiments did not result in any positive T cell crossmatch but did result in a positive B cell crossmatch for 3 patients. Of those 3 patients, 2 had B cell crossmatch based on pre‐treatment baseline samples that were already positive, and 1 had B cell crossmatch converted from negative at baseline to positive at the time of transplant. Although B cell crossmatch was performed after treatment with pronase, a false negative result through residual anti‐CD20 antibody in recipients’ serum cannot completely be ruled out. It is difficult to conclusively determine the impact of obinutuzumab/IVIG on the rate of transplant owing to the contemporary implementation of the new Kidney Allocation System, which prioritizes allocation to highly sensitized patients—and may alter the need for desensitization treatment in deceased‐donor transplant recipients in the United States. B cell depletion therapy may improve posttransplant outcomes by controlling amnestic DSA expansion and preventing antibody‐mediated rejection.32, 33 In our study, obinutuzumab/IVIG resulted in profound and lasting depletion of peripheral CD19+ B cells. The follow‐up of patients who received transplants will allow assessment of any potential prevention of humoral rejection in this high‐risk population.
In summary, obinutuzumab was well tolerated in patients with ESRD awaiting renal transplant. Although obinutuzumab resulted in profound peripheral blood B cell depletion and appeared to reduce B cells substantially in lymph nodes, the effect of obinutuzumab on anti‐HLA alloantibodies, UAs, and CPRA was limited and inconsistent.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. RRR is a consultant and has served on advisory boards for Genentech, Inc., has received consultant fees from GlaxoSmithKline, served on advisory boards for Veloxis, and has received research support from EMD Serono. SCJ has received grants from Genentech, Inc. and Novartis and has received grant/consultancy fees from Hansa Medical, Vitaeris, and Care Dx. SB has received consultancy fees from Genentech, Inc. FV has received grant/clinical trial funding from Genentech, Inc., Angion, Bristol‐Myers Squibb, and Pfizer. ND has received a grant from F. Hoffmann‐La Roche and served on an advisory board for Merck. EFR has received consultancy fees from F. Hoffmann‐La Roche. AAZ has received fees from Genentech, Inc. RF has received consultancy fees from Novartis and Veloxis. TS is an employee of F. Hoffmann‐La Roche. HT, CL, CJ, CG, AM, RR, AS, and MDC are employees of Genentech, Inc. PB and DB were employees of Genentech, Inc. at the time of this study. The other authors have no conflicts of interest to disclose.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank Wida Cherikh, Nicole Justies, Stephanie Derbyshire, Kirsten Jones, and the study management team for their contribution to the study, and Jay Garg, Nicolas Frey, Christian Klein, and Pablo Umana for discussions. This study was funded by F. Hoffmann‐La Roche. Support for medical writing, furnished by Ellen Mercado, PhD, of Health Interactions, was provided by F. Hoffmann‐La Roche.
Redfield RR, Jordan SC, Busque S, et al. Safety, pharmacokinetics, and pharmacodynamic activity of obinutuzumab, a type 2 anti‐CD20 monoclonal antibody for the desensitization of candidates for renal transplant. Am J Transplant. 2019;19:3035‐3045. 10.1111/ajt.15514
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform (http://www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
REFERENCES
- 1. Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4(3):438‐443. [DOI] [PubMed] [Google Scholar]
- 2. Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1(3):421‐432. [DOI] [PubMed] [Google Scholar]
- 3. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data Report: kidney. Am J Transplant. 2018;18(suppl 1):18‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meier‐Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58(3):1311‐1317. [DOI] [PubMed] [Google Scholar]
- 5. Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451‐1462. [DOI] [PubMed] [Google Scholar]
- 6. Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 annual data report: kidney. Am J Transplant. 2015;15(suppl 2):1‐34. [DOI] [PubMed] [Google Scholar]
- 7. Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242‐251. [DOI] [PubMed] [Google Scholar]
- 8. Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B‐cell populations in vivo. Am J Transplant. 2007;7(2):402‐407. [DOI] [PubMed] [Google Scholar]
- 9. Marfo K, Ling M, Bao Y, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation. 2012;94(4):345‐351. [DOI] [PubMed] [Google Scholar]
- 10. Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6(10):2418‐2428. [DOI] [PubMed] [Google Scholar]
- 11. Thaunat O, Patey N, Gautreau C, et al. B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation. 2008;85(11):1648‐1653. [DOI] [PubMed] [Google Scholar]
- 12. Kamburova EG, Koenen HJ, Borgman KJ, ten Berge IJ, Joosten I, Hilbrands LB. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant. 2013;13(6):1503‐1511. [DOI] [PubMed] [Google Scholar]
- 13. Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti‐CD20 antibody with enhanced direct and immune effector cell‐mediated B‐cell cytotoxicity. Blood. 2010;115(22):4393‐4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101‐1110. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reed EF, Rao P, Zhang Z, et al. Comprehensive assessment and standardization of solid phase multiplex‐bead arrays for the detection of antibodies to HLA. Am J Transplant. 2013;13(7):1859‐1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beal S, Sheiner L, Boeckmann A, Bauer RJ. NONMEM Users Guides (1989–2011). Ellicott City, MD: Icon Development Solutions; 2011. [Google Scholar]
- 18. Gibiansky E, Gibiansky L, Carlile DJ, Jamois C, Buchheit V, Frey N. Population pharmacokinetics of obinutuzumab (GA101) in chronic lymphocytic leukemia (CLL) and non‐Hodgkin's lymphoma and exposure‐response in CLL. CPT Pharmacometrics Syst Pharmacol. 2014;3:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gosset C, Lefaucheur C, Glotz D. New insights in antibody‐mediated rejection. Curr Opin Nephrol Hypertens. 2014;23(6):597‐604. [DOI] [PubMed] [Google Scholar]
- 20. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633‐659. [DOI] [PubMed] [Google Scholar]
- 21. Alexander T, Sarfert R, Klotsche J, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. 2015;74(7):1474‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The impact of proteasome inhibition on alloantibody‐producing plasma cells in vivo. Transplantation. 2011;91(5):536‐541. [DOI] [PubMed] [Google Scholar]
- 23. Moreno Gonzales MA, Gandhi MJ, Schinstock CA, et al. 32 doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti‐HLA antibody. Transplantation. 2017;101(6):1222‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Driscoll J, Tremblay S, Dasgupta N, Aronow B, Singh H, Woodle ES. Genomic and biochemical proteasomal adaptations in carfilzomib‐resistant plasma cells that limit HLA‐desensitization in transplantation candidates. Blood. 2017;130(suppl 1):5448. [Google Scholar]
- 25. Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor‐based desensitization. Am J Transplant. 2015;15(1):101‐118. [DOI] [PubMed] [Google Scholar]
- 26. Kwun J, Burghuber C, Manook M, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017;28(7):1991‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van de Donk NW. Carfilzomib versus bortezomib: no longer an ENDEAVOR. Lancet Oncol. 2017;18(10):1288‐1290. [DOI] [PubMed] [Google Scholar]
- 28. Tremblay S, Shields AR, Alloway R, et al. A prospective iterative trial of carfizomib‐based desensitization trial: initial comparative observations. Am J Transplant. 2017;17(suppl 3). https://atcmeetingabstracts.com/abstract/a-prospective-iterative-trial-of-carfizomib-based-desensitization-trial-initial-comparative-observations/ [Google Scholar]
- 29. Chu VT, Beller A, Nguyen TT, Steinhauser G, Berek C. The long‐term survival of plasma cells. Scand J Immunol. 2011;73(6):508‐511. [DOI] [PubMed] [Google Scholar]
- 30. Jordan SC, Tyan D, Stablein D, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end‐stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15(12):3256‐3262. [DOI] [PubMed] [Google Scholar]
- 31. Tambur AR, Campbell P, Claas FH, et al. Sensitization in Transplantation: assessment of Risk (STAR) 2017 Working Group meeting report. Am J Transplant. 2018;18(7):1604‐1614. [DOI] [PubMed] [Google Scholar]
- 32. Vo AA, Choi J, Cisneros K, et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation. 2014;98(3):312‐319. [DOI] [PubMed] [Google Scholar]
- 33. Zachary AA, Lucas DP, Montgomery RA, Leffell MS. Rituximab prevents an anamnestic response in patients with cryptic sensitization to HLA. Transplantation. 2013;95(5):701‐704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform (http://www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.


