Abstract
The clinical significance of non‐HLA antibodies on renal allograft survival is a matter of debate, due to differences in reported results and lack of large‐scale studies incorporating analysis of multiple non‐HLA antibodies simultaneously. We developed a multiplex non‐HLA antibody assay against 14 proteins highly expressed in the kidney. In this study, the presence of pretransplant non‐HLA antibodies was correlated to renal allograft survival in a nationwide cohort of 4770 recipients transplanted between 1995 and 2006. Autoantibodies against Rho GDP‐dissociation inhibitor 2 (ARHGDIB) were significantly associated with graft loss in recipients transplanted with a deceased‐donor kidney (N = 3276) but not in recipients of a living‐donor kidney (N = 1496). At 10 years after deceased‐donor transplantation, recipients with anti‐ARHGDIB antibodies (94/3276 = 2.9%) had a 13% lower death‐censored covariate‐adjusted graft survival compared to the anti‐ARHGDIB‐negative (3182/3276 = 97.1%) population (hazard ratio 1.82; 95% confidence interval, 1.32‐2.53; P = .0003). These antibodies occur independently from donor‐specific anti‐HLA antibodies (DSA) or other non‐HLA antibodies investigated. No significant relations with graft loss were found for the other 13 non‐HLA antibodies. We suggest that pretransplant risk assessment can be improved by measuring anti‐ARHGDIB antibodies in all patients awaiting deceased‐donor transplantation.
Keywords: ARHGDIB, kidney transplantation, non‐HLA antibodies
Short abstract
From a multicenter evaluation of kidney transplants, the authors report that the pretransplant presence of autoantibodies against ARHGDIB are associated with long‐term graft loss in recipients transplanted with a deceased donor kidney, independent from donor‐specific HLA antibodies.
Abbreviations
- ABMR
Antibody‐mediated rejection
- AKME
Adjusted Kaplan‐Meier estimator
- APMAdonor-specificP
Adipocyte plasma membrane‐associated protein
- ARHGDIB
Rho GDP‐dissociation inhibitor 2
- ARHGEF6
Rho guanine nucleotide exchange factor 6
- AT1R
Angiotensin II type 1 receptor
- CDC‐XM
Complement‐dependent cytotoxicity crossmatch
- CI
Confidence interval
- CIT
Cold ischemia time
- DBD
Donation after brain death
- DCD
Donation after cardiac death
- DSA
Donor‐specific anti‐HLA antibodies
- ETAR
Endothelin type A receptor
- HLA
Human leukocyte antigen
- HR
Hazard ratio
- IL
Interleukin
- IPW
Inverse probability weighting
- LPLUNC1
BPI fold‐containing family B member 1
- MFI
Median fluorescence intensity
- MMF
Mycophenolate mofetil
- NOTR
Netherlands Organ Transplant Registry
- PECR
Peroxisomal trans‐2‐enoyl‐CoA reductase
- PLA2R
Phospholipase A2 receptor
- PRA
Panel reactive antibodies
- PRKCZ
Protein kinase C zeta type
- STBRs
Signal‐to‐background ratios
- Tubb4B
Tubulin beta‐4B
1. INTRODUCTION
Chronic kidney disease affects about 10% of the global population.1 Over 2 million people worldwide currently receive dialysis treatment, or are recipients of a kidney transplant. Kidney transplantation is the preferred treatment for end‐stage renal disease due to superior quality of life and survival rates. However, although short‐term renal allograft survival has improved considerably in the last 20 years, antibody‐mediated rejection (ABMR) remains one of the major causes of graft loss and of deterioration of graft function in the long‐term. Donor‐specific anti‐HLA antibodies (DSA) are well known to play an important role in this process. Nonetheless, (subclinical) ABMR occurs also in the absence of DSA, which has sparked interest in the short‐ and long‐term clinical relevance of donor‐reactive antibodies recognizing proteins other than HLA (ie, non‐HLA‐antibodies).2
The clinical relevance of non‐HLA antibodies on graft survival is not clear.3, 4 Although it has been reported that non‐HLA antibodies against Angiotensin II type 1 receptor (AT1R) are an independent risk factor for long‐term graft loss,5 others could not replicate these findings,6, 7 which may be caused by the inclusion or exclusion of patients with pretransplant DSA. However, antibody‐mediated rejection of renal allografts from HLA‐identical sibling donors and also from deceased donors in the absence of pretransplant DSA has been reported several times.8, 9 And recently it was reported that the development of circulating natural antibodies posttransplant is associated with poorer graft survival, worse graft function, and more microvascular injury.10
Non‐HLA antibodies have been described against a variety of targets, but large‐scale studies incorporating analysis of these antibodies simultaneously to assess their clinical relevance in kidney transplantation are lacking. In this retrospective study on a large national cohort of 4770 renal transplant recipients, we assessed the impact on graft survival of IgG autoantibodies against 14 previously identified target proteins.11
2. MATERIALS AND METHODS
2.1. Study population
Between January 1995 and December 2005, 6097 kidney transplantations with a negative complement‐dependent cytotoxicity crossmatch were performed in The Netherlands. Clinical data were obtained from the Dutch Organ Transplant Registry. The use of sera and experimental protocols was approved by the Research Ethics Committee for Biobanks and the Medical Ethics Committee of the University Medical Center Utrecht. Experimental protocols were performed in accordance with the Federation of Dutch Medical Scientific Societies Code of Conduct. The study was conducted in accordance with the 2013 Declaration of Helsinki and the 2008 Declaration of Istanbul. Of 4787/6097 (78%) transplantations, pretransplant serum was available. Seventeen transplantations were excluded due to lack of follow‐up, and the remaining 4770 transplantations were included in this analysis. Minimal follow‐up time was 10 years after transplantation. Data available on request due to privacy/ethical restrictions.
2.2. Assessment of non‐HLA antibodies
We selected 14 non‐HLA target proteins from the literature.11 Antibodies against the glomerular basement membrane protein agrin have been described in the context of transplant glomerulopathy.12 Antibodies against adipocyte plasma membrane‐associated protein (APMAP), Rho GDP‐dissociation inhibitor 2 (ARHGDIB), Rho guanine nucleotide exchange factor 6 (ARHGEF6), Lamin B1, BPI fold‐containing family B member 1 (LPLUNC1), protein kinase C zeta type (PRKCZ), and tubulin beta‐4B (Tubb4B) were all demonstrated either in chronic hemodialysis patients or patients awaiting kidney transplantation. Antibodies directed against targets expressed on the endothelium, that is, AT1R and endothelin type A receptor (ETAR), were reported to be involved directly or indirectly in renal disease. Antibodies against the intracellular proteins vimentin and peroxisomal trans‐2‐enoyl‐CoA reductase (PECR) have been reported to be associated with allograft failure.13, 14 Pre‐ and posttransplant endorepellin antibody levels were increased in patients with vascular rejection15 and phospholipase A2 receptor (PLA2R) antibodies are strongly associated with primary membranous nephropathy.16 We commercially purchased all proteins with the exception of PLA2R, which was produced in‐house, and coupled these proteins directly with carboxylated MagPlex Microspheres (Luminex Corp, Austin, TX) as recommended by Luminex.17 In addition, we produced 12 of the 14 proteins with a HaloTag, with the exception of AT1R and PECR. The HaloTag proteins were coupled via a HaloTag Amine (O4) Ligand (Promega, Madison, WI) to the carboxylated MagPlex microspheres. A detailed description of the proteins and the coupling methods is given elsewhere.11
A mix of 31 different microspheres was made containing 15 directly coupled proteins (transferrin and 14 target proteins), 13 in‐house produced HaloTag‐coupled proteins (transferrin and 12 target proteins), an IgG‐coupled microsphere as a positive control, and a HaloTag amine‐coupled and empty microsphere as additional negative controls. Transferrin (directly‐ or HaloTag‐coupled) also served as a negative control, since it is ubiquitously present and no autoantibodies against transferrin have been reported. Sera (1:25 dilution) were incubated overnight with the microsphere mix. Next, R‐phycoerythrin‐conjugated goat‐anti human antibody was added. After 30 minutes of incubation, wash buffer was added and samples were measured on a Luminex 200 flow analyzer (Luminex Corp).
2.3. Histology
Three‐micrometer formalin‐fixed paraffin‐embedded sections were used. After antigen retrieval in citrate solution pH 6, primary antibody against ARHGDIB (Biobyt, San Francisco, CA) was applied in a 1:2000 solution for 1 hour at room temperature, followed by HRP‐labeled polymer anti‐rabbit Ig detection antibody (BrightVision, VWR, Duiven). Bound antibody was visualized with Nova Red substrate (Vector labs), and finally nuclei were counterstained with hematoxylin.
2.4. Statistical analysis
Because the Kaplan‐Meier estimates were biased due to unbalanced distribution of confounders, death‐censored graft survival was assessed using the adjusted Kaplan‐Meier estimator (AKME) based on inverse probability weighting (IPW). Each observation is weighted by its inverse probability of being in a certain group.18 Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were derived using multivariable Cox regression. A Bonferroni correction was used to adjust for multiple comparisons when studying the effects of non‐HLA antibodies on graft survival and P < .002 (.05 divided by 25 non‐HLA antibodies) was considered as statistically significant (applied for analyses in Tables 1, S3, and S4). We adjusted in both the AKME and Cox regression for recipient age (quadratic) and donor age (quadratic), cold ischemia time (for donation after brain death or cardiac death), years on dialysis (quadratic), induction therapy with IL‐2 receptor blocker, and the presence of pretransplant single antigen bead‐defined DSA against HLA‐A/B/DR/DQ. (For more detailed descriptions see Kamburova et al.19) Statistical analyses were performed with R version 3.4.1 and SAS version 9.4 (SAS Institute, Cary, NC). Continuous data were analyzed with the Mann‐Whitney U test and categorical data with the chi‐square test.
Table 1.
Multivariable analyses of the effect of antibodies against ARHGDIB on 10‐year death‐censored graft failure
| No. (%) of transplantations with anti‐ARHGDIB antibodies | Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|---|
| Total cohort (N = 4770) | 134 (2.8) | 1.701 | 1.265‐2.288 | .0004 |
| Deceased donors (N = 3276) | 94 (2.9) | 1.820 | 1.318‐2.531 | .0003 |
| Living donors (N = 1494) | 40 (2.7) | 1.249 | 0.587‐2.657 | .5639 |
Note: In this multivariable analysis we evaluated the effect of the presence of pretransplant ARHGDIB on the 10‐year death‐censored graft failure and adjusted for differences in the following covariates: recipient age (quadratic), donor age (quadratic), donor type (living or deceased, only for the total cohort), cold ischemia time in hours for donation after brain death (DBD) and donation after cardiac death (DCD), time on dialysis in years (quadratic), induction therapy with Interleukin‐ 2 receptor–blocking antibody and the presence of pretransplant donor‐specific anti‐HLA antibodies against HLA‐A/B/DR/DQ. CI, confidence interval. A Bonferroni correction was used to adjust for multiple comparisons with P < .002 considered as statistically significant.
3. RESULTS
3.1. Determination of a clinically relevant cut‐off for the presence of non‐HLA antibodies
We analyzed the 4770 pretransplant sera using our multiplex non‐HLA assay, and the individual median fluorescence intensitiy (MFI) values with box and whisker plots are shown in Figure S1. As we observed relatively high background signals for some sera (Figure S1A), we decided to use signal‐to‐background ratios (STBRs) as a parameter to determine non‐HLA antibody positivity. The correlation between the MFI of transferrin (directly‐ or HaloTag‐coupled) and the MFIs of target microspheres was stronger than the correlation between MFI of other negative control microspheres and that of target microspheres (data not shown). Therefore, transferrin was selected as the most optimal negative control, and was used correspondingly to calculate the STBRs (Figure S2). To determine the clinically relevant cut‐off, we analyzed the impact on 1‐, 5‐, and 10‐year death‐censored graft survival of various cut‐offs for each antibody in a univariate analysis (Figure 1). For each non‐HLA antibody, we selected the ratio and absolute MFI cut‐off that resulted in the highest difference in graft survival between the antibody‐negative and antibody‐positive group. For ARHGDIB, a ratio of 10 in combination with an absolute MFI of 500 was chosen as cut‐off values using the directly coupled microspheres, resulting in 134/4770 (2.8%) positive patients (Figure 1). An overview of the selected cut‐offs for the other non‐HLA antibodies based on the maximal graft survival difference is summarized in Table S1. Depending on the type of non‐HLA antibody analyzed, percentages of positive sera ranged from 0.9% to 2.8%, and varying differences were observed in 1‐, 5‐, and 10‐year graft survival.
Figure 1.
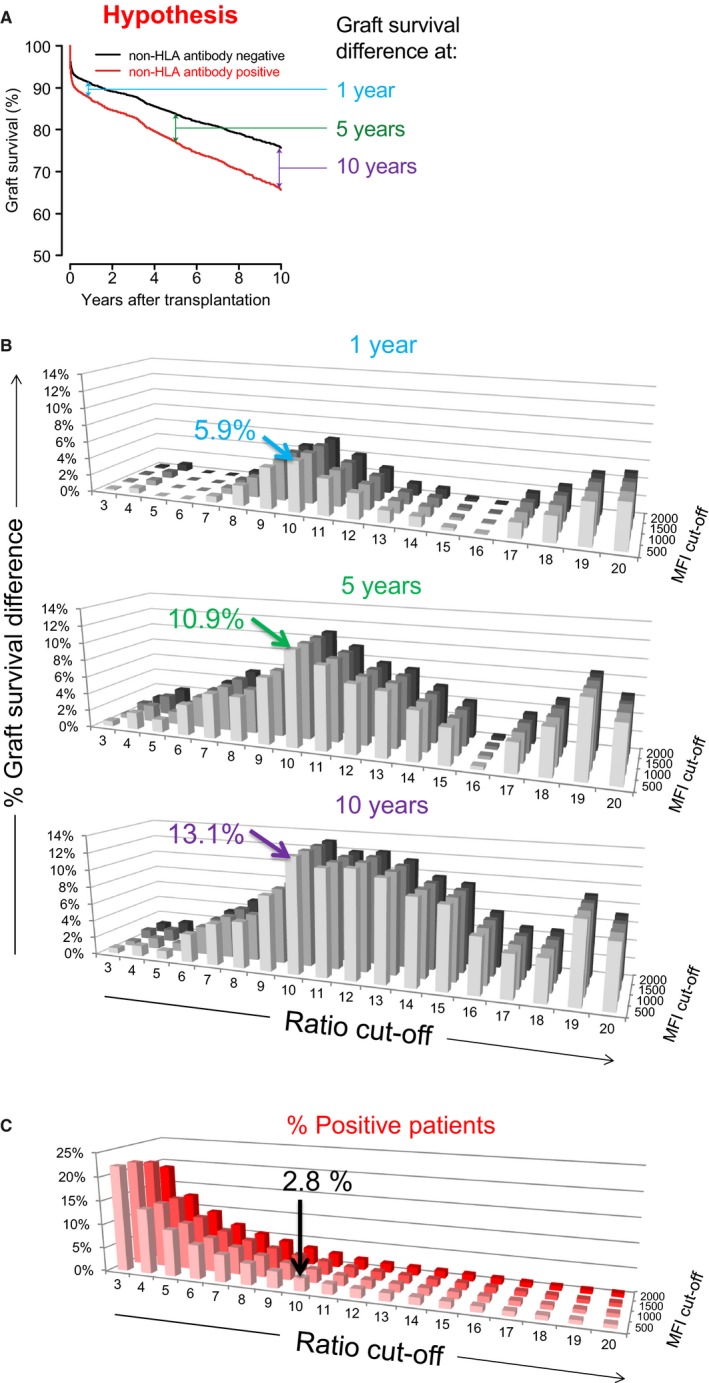
Impact of cut‐off for the presence of non‐HLA antibodies against ARHGDIB on graft survival. A, In this figure the hypothesis is displayed that the presence of a non‐HLA antibody is associated with graft failure. B, Using acquired data from non‐HLA measurements, we determined the difference in graft survival between the non‐HLA antibody negative and positive group for various cut‐off values at 1, 5, and 10 years after transplantation in a univariate Kaplan‐Meier analysis. Here, the results for directly coupled ARHGDIB are used as an example. The highest difference in graft survival between the ARHGDIB‐positive and ‐negative group was achieved with a cut‐off for signal‐to‐background ratio of 10 in combination with a cut‐off for absolute MFI of 500. The graft survival difference for this cut‐off between the ARHGDIB‐positive and ‐negative group was 5.9%, 10.9%, and 13.1% at 1, 5, and 10 years after transplantation, respectively. C, Shown are the percentages of ARHGDIB‐positive patients for each cut‐off. For the selected cut‐off there are 2.8% (134 of 4770) positive patients [Color figure can be viewed at http://www.wileyonlinelibrary.com]
3.2. Impact of pretransplant non‐HLA antibodies on long‐term graft survival
After Bonferroni correction, a significant difference in graft survival was observed between patients with pretransplant antibodies against ARHGDIB compared to patients without antibodies against this target (Table 1). No significant relation with graft loss was observed for the other non‐HLA antibodies. A summary of the impact of the other non‐HLA antibodies on 10‐year graft survival of the total cohort is summarized in Table S2.
Because our cohort contained a relatively high proportion of living donors and we previously found that DSA had mainly an impact on deceased‐donor transplantations with only a limited effect on living‐donor transplantations,19 we also analyzed the impact of non‐HLA antibodies on long‐term graft survival according to donor status (3276 deceased‐donor and 1494 living‐donor transplantations). After deceased‐donor transplantation, the AKME according to the presence of ARHGDIB antibodies showed a 10‐year death‐censored graft survival of 61% (95% CI 50%‐70%) for the 94 of 3276 patients with and 73% (95% CI 71%‐75%) for the 3182/3276 patients without ARHGDIB antibodies (Figure 2A; P = .017). Pretransplant DSA against HLA‐A/B/DR/DQ was found in only 10 of 94 patients (10.6%) with ARHGDIB antibodies and 423/3182 patients (13.3%) without (P = .454). A Kaplan‐Meier analysis in this small subgroup did not show an indication for a synergistic effect of DSA and anti‐ARHGDIB antibodies. In addition, we could find no indication for interaction between the presence of all studied autoantibodies, or an effect of their combined presence on graft survival. There were no significant differences between the patient, donor, and transplant characteristics between the anti‐ARHGDIB positive and negative groups transplanted with a deceased‐donor kidney, except the cold ischemia time was slightly longer in anti‐ARHGDIB‐positive patients (23.5 ± 7.7 hours) compared to anti‐ARHGDIB‐negative patients (21.8 ± 7.2) transplanted with a deceased donor (P = .043, Table 2). The multivariable analysis, adjusted for the same covariates, showed that the presence of ARHGDIB antibodies was associated with a higher risk of 10‐year graft failure after transplantation with a deceased‐donor kidney (Table 1, HR 1.82, 95% CI 1.32‐2.53, P = .0003). At 1 year after transplantation, the HR was 1.620 (95% CI, 0.993‐2.643) for the anti‐ARHGDIB‐positive group compared to the anti‐ARHGDIB‐negative group (Table S3). Furthermore, the rejection‐free survival was comparable for patients with and without antibodies against ARHGDIB (Figure S3). For the living‐donor transplantations, the presence of ARHGDIB antibodies was not associated with decreased graft survival (Figure 2B) or increased risk of graft failure in multivariable analysis (Table 1, HR 1.25, 95% CI 0.59‐2.66, P = .56). Finally, we also assessed the association between type of donor, anti‐ARHGDIB antibodies, and 10‐year graft survival in the same model. The hazard ratio for a living donor was 0.563 (95% CI 0.488‐0.650, P < .0001) compared to deceased donors, suggesting that that anti‐ARHGDIB was significantly associated with graft loss in recipients transplanted with a kidney from a deceased donor but not in recipients of a living‐donor kidney.
Figure 2.
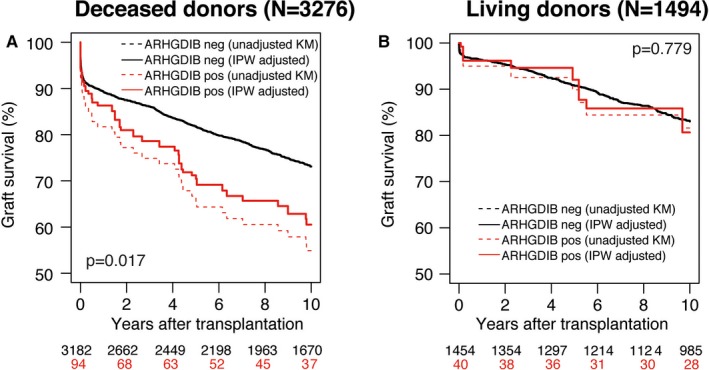
Graft survival according to the presence of pretransplant non‐HLA antibodies in deceased donor and living‐donor kidney transplantation. Inverse probability weighting (IPW) adjusted Kaplan‐Meier estimate (AKME) for death‐censored graft survival according to the presence of ARHGDIB in 3276 deceased (A) and 1494 living‐donor (B) transplantations. AKME was adjusted for the following covariates: recipient age (quadratic) and donor age (quadratic), cold ischemia time (for donation after brain death and donation after cardiac death), time on dialysis in years (quadratic), induction therapy with IL‐2 receptor blocker, and the presence of pretransplant donor‐specific anti‐HLA antibodies (DSA) [Color figure can be viewed at http://www.wileyonlinelibrary.com]
Table 2.
Characteristics of ARHGDIB‐positive and ARHGDIB‐negative patients transplanted with a deceased‐donor kidney
| Characteristics | ARHGDIB negative (N = 3182) | ARHGDIB positive (N = 94) | P‐value | Deceased‐donor transplantations (N = 3276) |
|---|---|---|---|---|
| Patient | ||||
| Age at transplantation (y, mean ± SD) | 46.9 ± 14.1 | 47.9 ± 13.4 | .462a | 46.1 ± 14.1 |
| Female sex ‐ no. (%) | 1286 (40.4) | 39 (41.5) | .834b | 1325 (40.5) |
| PRA at time of transplantation (%, mean ± SD) | 6.8 ± 18.7 | 9.2 ± 25.1 | .574a | 6.9 ± 18.9 |
| Highest PRA (%, mean ± SD) | 16.4 ± 28.2 | 15.4 ± 30.4 | .300a | 16.4 ± 28.2 |
| Dialysis, n (%) | .455b | |||
| No | 147 (4.6) | 3 (3.0) | 150 (4.6) | |
| Yes – hemodialysis | 1853 (58.2) | 49 (52.1) | 1902 (58.1) | |
| Yes – peritoneal dialysis | 1164 (36.6) | 41 (43.6) | 1205 (36.8) | |
| Unknown | 18 (0.6) | 1 (1.1) | 19 (0.6) | |
| Time on dialysis (y, mean ± SD) | 3.4 ± 2.6 | 3.4 ± 2.2 | .814a | 3.4 ± 2.6 |
| Donor | ||||
| Donor age (y, mean ± SD) | 42.7 ± 16.0 | 46.0 ± 14.6 | .062a | 42.8 ± 16.0 |
| Donor female sex – no. (%) | 1489 (46.8) | 46 (48.9) | .681b | 1535 (46.9) |
| Cold‐ischemia time (hours, mean ± SD) | 21.8 ± 7.2 | 23.5 ± 7.7 | .043a | 21.8 ± 7.2 |
| Transplant | ||||
| Repeat transplantation – no. (%) | 554 (17.4) | 16 (17.0) | .922b | 570 (17.4) |
| Pretransplant DSA against HLA‐A/B/DR/DQ – no. (%) | 423 (13.3) | 10 (10.6) | .454b | 433 (13.2) |
| Induction therapy | ||||
| IL‐2 receptor blocker – no. (%) | 651 (20.5) | 14 (14.9) | .186b | 665 (20.3) |
| T‐cell depleting antibodyc no. (%) | 134 (4.2) | 0 (0) | .042b | 134 (4.1) |
| Initial immunosuppression – no. (%) | ||||
| Steroids | 3120 (98.1) | 90 (95.7) | .117b | 3210 (98.0) |
| MMF/azathioprine | 2377 (74.7) | 63 (67.0) | .092b | 244 (74.4) |
| Cyclosporine/tacrolimus | 3000 (94.3) | 88 (93.6) | .785b | 3088 (94.3) |
| Sirolimus | 171 (5.4) | 9 (9.6) | .078b | 180 (5.4) |
| Other | 417 (13.1) | 11 (11.7) | .691b | 428 (13.1) |
| Unknown | 11 (0.4) | 1 (1.1) | .256b | 12 (0.4) |
DSA, Donor‐specific anti‐HLA antibodies; IL, interleukin; MMF, mycophenolate mofetil.
Mann‐Whitney U test for continuous variables.
Chi‐square test for categorical variables.
T cell‐depleting antibody therapy: ALG, ATG, OKT3 monoclonal antibodies.
3.3. ARHGDIB expression in the kidney
Finally, we were wondering where in the kidney ARHGDIB is expressed. Therefore, we stained biopsies of a transplanted kidney without histological abnormalities and a transplanted kidney with acute tubular necrosis using an anti‐ARHGDIB antibody. In a transplanted kidney without histological abnormalities, weak ARHGDIB expression was seen in endothelial cells of interlobular arteries, endothelial cells of peritubular capillaries, and endothelial cells of glomerular capillaries (Figure 3A). In a transplanted kidney with acute tubular necrosis, strong ARHGDIB expression was seen in endothelial cells of interlobular arteries, endothelial cells of peritubular capillaries, and endothelial cells of glomerular capillaries. In addition, positive staining for ARHGDIB is also seen in some podocytes and lymphocytes (Figure 3B).
Figure 3.
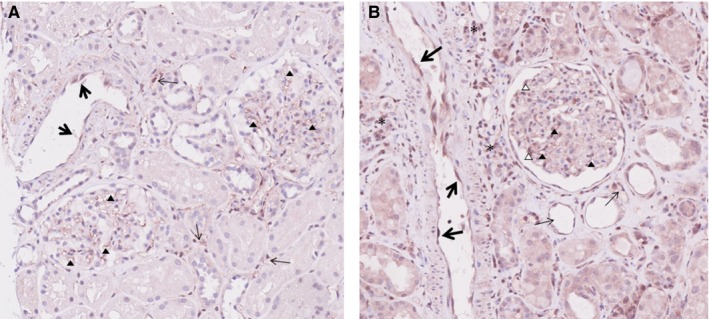
ARHGDIB expression in the kidney. A, In a transplanted kidney without histological abnormalities, weak ARHGDIB expression is seen in endothelial cells of interlobular arteries (large arrows), endothelial cells of peritubular capillaries (small arrows), and endothelial cells of glomerular capillaries (arrow heads). B, In a transplanted kidney with acute tubular necrosis, strong ARHGDIB expression is seen in endothelial cells of interlobular arteries (large arrows), endothelial cells of peritubular capillaries (small arrows), and endothelial cells of glomerular capillaries (solid arrow heads). In addition, positive staining for ARHGDIB is also seen in some podocytes (open arrow heads) and lymphocytes (asterisks) [Color figure can be viewed at http://www.wileyonlinelibrary.com]
4. DISCUSSION
In the present study, we determined the relation between graft failure and the presence of pretransplant non‐HLA antibodies in the sera of 4770 kidney transplantations performed in The Netherlands between 1995 and 2006. The results indicate that pretransplant antibodies against ARHGDIB represent a risk factor for graft loss in deceased‐donor transplantations.
The 14 non‐HLA target proteins included in our assay were selected based on reported antibody specificity in kidney transplant patients or patients with end‐stage renal disease, and their expression in the kidney. A number of these non‐HLA antibodies have been examined in relation to kidney transplantation. Antibodies against AT1R were associated with rejection and graft survival according to results from several large‐scale studies,5, 20 although a recent large study (n = 940) did not confirm this association.4 Pretransplant anti‐ETAR antibodies have been associated with higher serum creatinine values at 1 year posttransplantation and with more severe vascular rejection.21 Antibodies against LG3, a C‐terminal fragment of perlecan, were associated with acute tubulointerstitial rejection and long‐term renal allograft dysfunction.15, 22 Antibodies against PLA2R, LPLUNC, APMAP, and PRKCZ were described previously in small case‐series and have not been further evaluated.23, 24, 25 During the development of our non‐HLA antibody assay we found that MFI results on a specific bead was comparable between singleplex and multiplex measurement, indicating no occurrence of compromise by multiplex analysis. In general, non‐HLA antibodies frequently occur within the healthy population and for many autoantibodies a serum is considered positive when exceeding a cut‐off yielding a limited (<5%) frequency of positive results within the healthy population. This definition does not necessarily provide the most clinically relevant cut‐off value with regard to prognosis. To this end, we chose to define a clinically relevant cut‐off as it was previously described for HLA antibodies using a combination of STBR combined with a minimal MFI level.26 Using individual cut‐offs for each non‐HLA antibody, optimally discriminating patients with long‐term functioning grafts vs graft loss, we did not find associations between the presence of any of the abovementioned previously studied non‐HLA antibodies and graft loss, function, or rejection. We did not analyze the autoantibody MFI levels as continuous variables, because in the field of anti‐HLA antibodies it is well accepted that MFI is not an indication of antibody titer and is influenced by several factors such as (1) the antigen density, (2) the affinity of the antibody to the antigen, and (3) the amount/titer of the antibody (also dependent on the serum dilution used in the assay).
At present, no studies have been reported showing the association between anti‐ARHGDIB antibodies and graft loss. Here, we found significant associations between graft loss and the pretransplant presence of antibodies against ARHGDIB. The effect of these antibodies is observed predominantly in patients transplanted with a deceased‐donor kidney. This suggests an interaction between the presence of anti‐ARHGDIB antibodies and ischemia reperfusion injury, which is less prominent in living‐donor kidney transplantation.27 ARHGDIB is widely expressed, including in the renal pelvis and glomeruli. Increased expression of ARHGDIB has been reported in several solid tumors and hematological malignancies.28, 29, 30, 31 Bilalic et al32 first described that dialysis patients can have autoreactive antibodies against ARHGDIB. Further analysis in kidney biopsies showed cytoplasmic expression in endothelial cells of interlobular arteries and peritubular capillaries, and in podocytes. We consider it possible that cellular damage caused by ischemia reperfusion results in accessibility of ARHGDIB in endothelial cells to circulating auto‐antibodies. After binding, anti‐ARHGDIB antibodies may initiate complement activation causing local inflammation, which may explain why a relation between their presence and graft loss was observed only after deceased‐donor transplantation.
In our study, non‐HLA proteins were coated directly or indirectly (via HaloTag) to the microspheres. This strategy was chosen because the coupling process may influence the conformation and accessibility of epitopes predominantly recognized on non‐HLA beads.11 Previously we found that the correlation between results obtained with our test and with commercial assays depended on the coupling method of the proteins. This demonstrates the necessity of complete transparency and detailed description of methods when comparing the effects of non‐HLA antibodies in patient groups. Because there are no commercially available reference sera against the non‐HLA antibodies we selected, we were not able to properly compare our Luminex assay to other commercially available ELISA or Luminex autoantibody assays. In the sera used in our multiplex assay, we measured non‐HLA IgG antibodies. It is possible that some non‐HLA antibodies relevant to prognosis are of another isotype and were not detected here. In some autoimmune diseases, autoantibodies are of other isotypes, such as IgM‐RF or IgA‐anti‐tTG. However, most clinically relevant autoantibodies are of the IgG isotype. We also examined whether a reactivity pattern between non‐HLA antibodies can be distinguished, for instance due to cross‐reactivity, but we did not find significant associations between each of the 14 non‐HLA antibodies investigated and there was no relationship with pretransplant DSA.
In a large cohort studied by Opelz, a 9% difference in 10‐year graft survival was observed between recipients of HLA‐identical sibling donors with or without panel‐reactive HLA antibodies, which led to the conclusion that a high immunization grade against HLA may indicate an increased immunity against non‐HLA.33 In our study, the observed adverse effects of non‐HLA antibodies were independent of the presence of DSA as we adjusted for this covariable. Because ARHGDIB is considered to be a minor histocompatibility antigen, we examined the relation between antibody levels and potentially immunizing events, but found no link with repeat transplantation, female sex, pregnancies, or potentially confounding factors such as diabetes type 1, or several primary renal diseases. Therefore, mechanisms may be involved in the induction of non‐HLA antibody formation other than the well‐known sensitizing events stimulating HLA antibody production.
We were not able to include posttransplant samples (sera and/or biopsies) in our study, thereby limiting the use of results in pretransplant risk stratification. Future studies have to be performed to evaluate whether posttransplant monitoring of anti‐ARHGDIB is useful. Another limitation of the study was that we did not have detailed clinical information, such as autoimmune diseases or hypertension, to potentially link the presence of anti‐ARHGDIB antibodies to a clinical parameter. Due to lack of detailed rejection and histology information, we cannot directly link anti‐ARHGDIB antibodies with the rejection phenotype.
In conclusion, our study demonstrates that pretransplant non‐HLA antibodies against ARHGDIB are a significant risk factor in deceased‐donor transplantation. These antibodies occur independently from DSA or other non‐HLA antibodies investigated. It is currently unknown whether the presence of these antibodies is a biomarker, as is the case in many autoimmune diseases, or whether they play a role in the pathogenesis of graft loss. Although validation of our findings in independent cohorts is necessary, based on these results, we suggest that pretransplant risk assessment can be improved by measuring these antibodies in all patients awaiting deceased‐donor transplantation.
DISCLOSURE
The authors of this manuscript have no conflicts of interests to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
The authors thank Dr. Tri Nguyen (Department of Pathology, University Medical Center Utrecht, The Netherlands) for his assistance in performing and interpreting the anti‐ARGHDIB staining on kidney tissue. This study was supported by research funding from the Dutch Kidney Foundation Project code CP12.23 Risk assessment of kidney graft failure by HLA antibody profiling.
Kamburova EG, Gruijters ML, Kardol‐Hoefnagel T, et al. Antibodies against ARHGDIB are associated with long‐term kidney graft loss. Am J Transplant. 2019;19:3335‐3344. 10.1111/ajt.15493
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. World Kidney Day . Chronic kidney disease. 2018. https://www.worldkidneyday.org/faqs/chronic-kidney-disease. Accessed September 12, 2018.
- 2. Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post‐transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015;26(7):1721‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non‐HLA antibodies in transplantation. Transplantation. 2013;95(1):19‐47. [DOI] [PubMed] [Google Scholar]
- 4. Deltombe C, Gillaizeau F, Anglicheau D, et al. Is pre‐transplant sensitization against angiotensin II type 1 receptor still a risk factor of graft and patient outcome in kidney transplantation in the anti‐HLA Luminex era? A retrospective study Transpl Int. 2017;30(11):1150‐1160. [DOI] [PubMed] [Google Scholar]
- 5. Giral M, Foucher Y, Dufay A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13(10):2567‐2576. [DOI] [PubMed] [Google Scholar]
- 6. Gareau AJ, Wiebe C, Pochinco D, et al. Pre‐transplant AT1R antibodies correlate with early allograft rejection. Transpl Immunol. 2018;46:29‐35. [DOI] [PubMed] [Google Scholar]
- 7. Pinelli DF, Friedewald JJ, Haarberg KMK, et al. Assessing the potential of angiotensin II type 1 receptor and donor specific anti‐endothelial cell antibodies to predict long‐term kidney graft outcome. Hum Immunol. 2017;78(5‐6):421‐427. [DOI] [PubMed] [Google Scholar]
- 8. Kalil J, Guilherme L, Neumann J, et al. Humoral rejection in two HLA identical living related donor kidney transplants. Transplant Proc. 1989;21(1 Pt 1):711‐713. [PubMed] [Google Scholar]
- 9. Amico P, Hönger G, Mayr M, Schaub S. Detection of HLA‐antibodies prior to renal transplantation: prospects and limitations of new assays. Swiss Med Wkly. 2008;138(33‐34):472‐476. [DOI] [PubMed] [Google Scholar]
- 10. See SB, Aubert O, Loupy A, et al. Post‐transplant natural antibodies associate with kidney allograft injury and reduced long‐term survival. J Am Soc Nephrol. 2018;29(6):1761‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamburova EG, Kardol‐hoefnagel T, Wisse BW, Joosten I, Wil A. Development and validation of a multiplex non‐HLA antibody assay for the screening of kidney transplant recipients. Front Immunol. 2018;9:3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joosten SA, Sijpkens YWJ, van Ham V, et al. Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am J Transplant. 2005;5(2):383‐393. [DOI] [PubMed] [Google Scholar]
- 13. Dinavahi R, George A, Tretin A, et al. Antibodies reactive to non‐HLA antigens in transplant glomerulopathy. J Am Soc Nephrol. 2011;22(6):1168‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Besarani D, Cerundolo L, Smith JD, et al. Role of anti‐vimentin antibodies in renal transplantation. Transplantation. 2014. Jul 15;98(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 15. Cardinal H, Dieudé M, Brassard N, et al. Antiperlecan antibodies are novel accelerators of immune‐mediated vascular injury. Am J Transplant. 2013. Apr;13(4):861‐874. [DOI] [PubMed] [Google Scholar]
- 16. Dähnrich C, Komorowski L, Probst C, et al. Development of a standardized ELISA for the determination of autoantibodies against human M‐type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421:213‐218. [DOI] [PubMed] [Google Scholar]
- 17. De Jager W, Velthuis H, Prakken BJ, et al. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10(1):133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie J, Liu C. Adjusted Kaplan‐Meier estimator and log‐rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089‐3110. [DOI] [PubMed] [Google Scholar]
- 19. Kamburova EG, Wisse BW, Joosten I, et al. Differential effects of donor‐specific HLA antibodies in living versus deceased donor transplant. Am J Transplant. 2018;18(9):2274‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taniguchi M, Rebellato LM, Cai J, et al. Higher risk of kidney graft failure in the presence of anti‐angiotensin II type‐1 receptor antibodies. Am J Transplant. 2013;13(10):2577‐2589. [DOI] [PubMed] [Google Scholar]
- 21. Banasik M, Boratyńska M, Kościelska‐Kasprzak K, et al. The impact of non‐HLA antibodies directed against endothelin‐1 type A receptors (ETAR) on early renal transplant outcomes. Transpl Immunol. 2014;30(1):24‐29. [DOI] [PubMed] [Google Scholar]
- 22. Yang BHK, Hénault‐Rondeau M, Patey N, et al. Anti‐LG3 antibodies aggravate renal ischemia‐reperfusion injury and long‐term renal allograft dysfunction. Kidney Int. 2015;13866(11):1‐14. [DOI] [PubMed] [Google Scholar]
- 23. Sutherland SM, Li L, Sigdel TK, et al. Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int. 2009;76(12):1277‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otten HG, van Loon M, van Ginkel WGJ, van de Graaf EA, Hene RJ. Identification of 3 novel non‐HLA target antigens recognized after kidney transplantation. Tissue Antigens. 2007;69(5):375‐376. [Google Scholar]
- 25. Gupta G, Fattah H, Ayalon R, et al. Pre‐transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post‐kidney transplantation. Clin Transplant. 2016;30(4):461‐469. [DOI] [PubMed] [Google Scholar]
- 26. Wisse BW, Kamburova EG, Joosten I, et al. Toward a sensible single antigen bead cut‐off based on kidney graft survival. Transplantation. 2019;103(4):789‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364(9447):1814‐1827. [DOI] [PubMed] [Google Scholar]
- 28. Ma L, Xu G, Sotnikova A, et al. Loss of expression of LyGDI (ARHGDIB), a rho GDP‐dissociation inhibitor, in Hodgkin lymphoma. Br J Haematol. 2007;139(2):217‐223. [DOI] [PubMed] [Google Scholar]
- 29. Cho HJ, Baek KE, Yoo J. RhoGDI2 as a therapeutic target in cancer. Expert Opin Ther Targets. 2010;14(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 30. Pont MJ, Hobo W, Honders MW, et al. LB‐ARHGDIB‐1R as a novel minor histocompatibility antigen for therapeutic application. Haematologica. 2015;100(10):e419‐e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Von Klot CA, Dubrowinskaja N, Peters I, et al. Rho GDP dissociation inhibitor‐β in renal cell carcinoma. Oncol Lett. 2017;14(6):8190‐8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bilalic S, Veitinger M, Ahrer K‐H, et al. Identification of non‐HLA antigens targeted by alloreactive antibodies in patients undergoing chronic hemodialysis. J Proteome Res. 2010;9(2):1041‐1049. [DOI] [PubMed] [Google Scholar]
- 33. Opelz G. Non‐HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365(9470):1570‐1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


