Abstract
Mycobacterium avium, a slow‐growing nontuberculous mycobacterium, causes fever, diarrhoea, loss of appetite, and weight loss in immunocompromised people. We have proposed that endoplasmic reticulum (ER) stress‐mediated apoptosis plays a critical role in removing intracellular mycobacteria. In the present study, we investigated the role of the regulated IRE1‐dependent decay (RIDD) pathway in macrophages during M. avium infection based on its role in the regulation of gene expression. The inositol‐requiring enzyme 1 (IRE1)/apoptosis signal‐regulating kinase 1 (ASK1)/c‐Jun N‐terminal kinase (JNK) signalling pathway was activated in macrophages after infection with M. avium. The expression of RIDD‐associated genes, such as Bloc1s1 and St3gal5, was decreased in M. avium‐infected macrophages. Interestingly, M. avium‐induced apoptosis was significantly suppressed by pretreatment with irestatin (inhibitor of IRE1α) and 4μ8c (RIDD blocker). Macrophages pretreated with N‐acetyl cysteine (NAC) showed decreased levels of reactive oxygen species (ROS), IRE1α, and apoptosis after M. avium infection. The expression of Bloc1s1 and St3gal5 was increased in NAC‐pretreated macrophages following infection with M. avium. Growth of M. avium was significantly increased in irestatin‐, 4μ8c‐, and NAC‐treated macrophages compared with the control. The data indicate that the ROS‐mediated ER stress response induces apoptosis of M. avium‐infected macrophages by activating IRE1α‐RIDD. Thus, activation of IRE1α suppresses the intracellular survival of M. avium in macrophages.
Keywords: apoptosis, ER stress, IRE1α, Mycobacterium avium, RIDD
1. INTRODUCTION
Nontuberculous mycobacteria (NTM) do not cause leprosy or tuberculosis (McGrath, Blades, McCabe, Jarry, & Anderson, 2010). NTM infection causes lung damage, skin/soft rashes, disseminated disease, and defects in host defence (Nussbaum & Heseltine, 1990). The incidence of NTM lung disease is increasing worldwide (Arend, van Soolingen, & Ottenhoff, 2009). Mycobacterium avium complex, an NTM, is an important pathogen in pulmonary disease and in patients with acquired immunodeficiency syndrome (Lagrange, Wargnier, & Herrmann, 2000; Triccas et al., 1998).
Recent studies have focused on controlling mycobacteria by exploiting host innate immunity, principally apoptosis (Behar et al., 2011; Bhattacharyya et al., 2003; Parandhaman & Narayanan, 2014). In M. avium‐infected macrophages, reactive oxygen species (ROS) induce caspase‐dependent apoptosis by disrupting the mitochondria membrane potential, precipitating release of cytochrome c (Lee et al., 2016). Previously, we reported that endoplasmic reticulum (ER) stress‐mediated apoptosis through ROS regulates the intracellular survival of mycobacteria in macrophages (J. A. Choi et al., 2013). However, whether ER stress‐mediated apoptosis is involved in suppressing the growth of M. avium is unclear.
Accumulation of misfolded proteins and disruption of calcium homeostasis induce ER stress response (Oyadomari, Araki, & Mori, 2002; Song, 2012). ER stress‐mediated apoptosis activates inositol‐requiring enzyme 1 (IRE1)/apoptosis signal‐regulating kinase 1 (ASK1)/c‐Jun N‐terminal kinase (JNK) signalling, leading to expression of apoptosis‐related genes (H.‐H. Choi et al., 2010; Szegezdi, Logue, Gorman, & Samali, 2006). ER stress‐induced IRE1 activation induces the regulated IRE1‐dependent decay (RIDD) pathway (Hollien et al., 2009). RIDD‐mediated degradation of the mRNAs of ER chaperone proteins increases ER stress and sequentially induces apoptosis (Han et al., 2009). RIDD also induces apoptosis by suppressing the expression of anti‐apoptotic microRNAs (Upton et al., 2012). However, the role of RIDD in M. avium‐infected macrophages has never been reported.
In this study, we investigated the role of RIDD in M. avium‐infected macrophages. We found that several RIDD target genes, such as ST3 β‐galactoside α‐2,3‐sialyltransferase 5 (St3gal5) and biogenesis of lysosome‐related organelles complex‐1 subunit 1 (Bloc1s1), are involved in suppression of the growth of M. avium in macrophages.
2. RESULTS
2.1. Role of ER stress in M. avium‐mediated apoptosis
We analysed macrophage apoptosis by flow cytometry at 24 hr post‐infection with M. avium. Apoptosis was significantly increased by 23.05% in M. avium‐infected RAW 264.7 cells compared with the control (Figure 1a,b).
Figure 1.
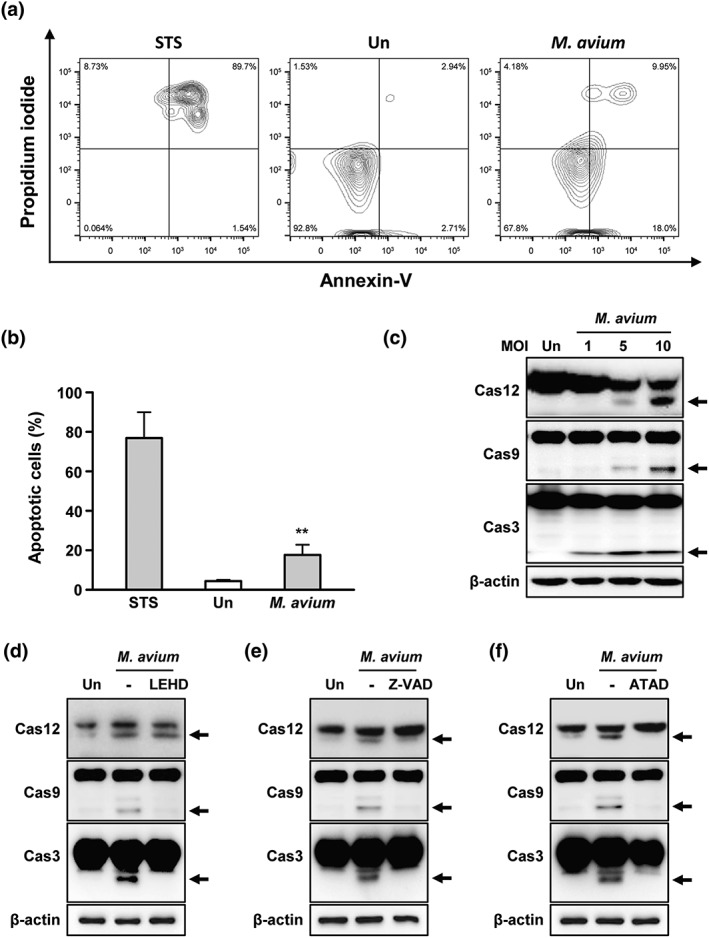
Mycobacterium avium‐induced apoptosis in macrophages. (a) RAW 264.7 cells were infected with M. avium at a multiplicity of infection (moi) of 5. Apoptosis of RAW 264.7 cells was assayed by flow cytometry with Annexin V/propidium iodide staining. RAW 264.7 cells were treated with staurosporine (STS, 1 μM) for 6 hr. (b) Percentage of apoptotic cells calculated from (a). (c) Activation of caspase‐12, caspase‐9, and caspase‐3 was determined in RAW 264.7 cells infected with M. avium in a moi‐dependent manner. RAW 264.7 cells were pretreated with (d) z‐ATAD‐fmk (20 μM), (e) z‐LEHD‐fmk (20 μM), or (f) z‐VAD‐fmk (20 μM) for 1 hr prior to M. avium infection. After 24 hr, induction of caspase‐12, caspase‐9, and caspase‐3 was analysed by Western blotting. Data are representative of at least three independent experiments with similar results (*p < .05, **p < .01, and ***p < .001)
Translocation of caspase‐12 to the ER membrane activates caspase‐9 and caspase‐3, leading to apoptosis (Rasheva & Domingos, 2009). Caspase‐12, caspase‐9, and caspase‐3 were activated in a multiplicity of infection (moi)‐dependent manner in RAW 264.7 cells after infection with M. avium (Figure 1c). M. avium‐induced caspase activation was reduced by z‐DEVD‐FMK (inhibitor of caspase‐12), z‐LEHD‐fmk (inhibitor of caspase‐9), or z‐VAD‐FMK (pan‐caspase inhibitor; Figure 1d–f). The activated forms of caspase‐9 and caspase‐3 were decreased by inhibition of caspase‐9 (Figure 1e). Pan‐caspase inhibition reduced M. avium‐induced caspase activation (Figure 1f). Interestingly, a caspase‐12 inhibitor reduced the M. avium‐induced activation of caspase‐9 and caspase‐3 (Figure 1d). These data suggest that caspase‐12 activation plays a key role in M. avium‐induced apoptosis.
Next, we analysed the production of ER stress‐related molecules in M. avium‐infected macrophages. M. avium infection increased X‐box binding protein 1 (XBP‐1) mRNA splicing from 12 hr and peaked at 24 hr in macrophages (Figure 2a). Increment of binding immunoglobulin protein (Bip), phosphorylated alpha subunit of eukaryotic initiation factor 2α (p‐eIF‐2α), and C/EBP homologous protein (CHOP) production were observed at 6 hr and peaked at 24 hr after M. avium infection (Figure 2b). Production of active caspase‐3 was peaked at 24 hr. In addition, the levels of activating transcription factor (ATF) 6 and protein kinase RNA‐like ER kinase (PERK) were increased at 12 hr (Figure 2b). M. avium‐induced Bip, p‐eIF‐2α, and CHOP levels were increased in a moi‐dependent manner (Figure 2c). To confirm that M. avium‐mediated ER stress induces apoptosis in macrophages, we used a chemical chaperone, 4‐PBA, before M. avium infection. As expected, the production levels of M. avium‐mediated ER stress sensor molecules and caspase‐3 activation were significantly reduced by 4‐PBA in a dose‐dependent manner (Figure 2d). In addition, M. avium‐induced phosphorylation of IRE1α was reduced by 4‐PBA (Figure 2e). M. avium‐induced apoptosis was significantly suppressed in 4‐PBA‐pretreated macrophages compared with the control (Figure 2f). These results suggest that ER stress plays a major role in M. avium‐induced apoptosis.
Figure 2.
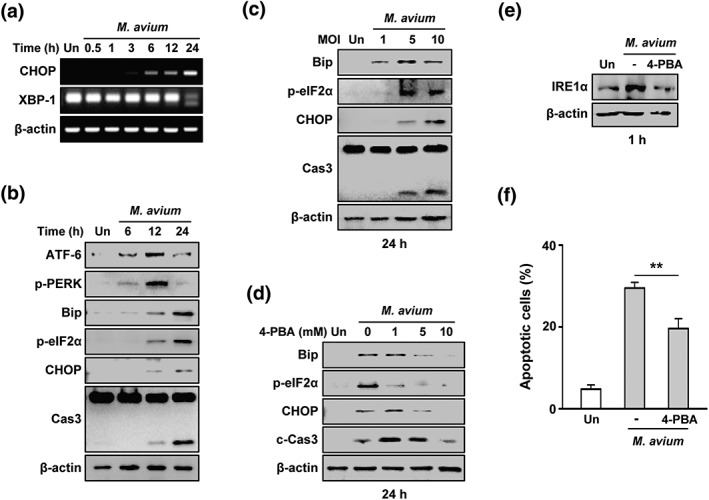
Endoplasmic reticulum (ER) stress plays a crucial role in Mycobacterium avium‐induced apoptosis. (a) Polymerase chain reaction (PCR) amplification of CHOP and XBP‐1 for 0–24 hr. (b) RAW 264.7 cells were infected M. avium (multiplicity of infection [moi] = 5) and incubated for 6–24 hr. (c) RAW 264.7 cells were infected with M. avium at moi of 1, 5, and 10 and incubated for 24 hr. ER stress factors were analysed by Western blotting. (d) RAW 264.7 cells were pretreated with the ER stress inhibitor 4‐PBA (1–10 mM) and infected with M. avium for 24 hr. (e) RAW 264.7 cells were pretreated with 4‐PBA (10 mM) and infected with M. avium. (f) Apoptosis of 4‐PBA‐pretreated cells following infection with M. avium for 24 hr. Data are representative of three independent experiments
2.2. Role of RIDD in M. avium‐mediated apoptosis
We next investigate the role of IRE1α in M. avium‐induced apoptosis. The levels of IRE1α, ASK‐1, and JNK were increased at 0.5–1 hr after M. avium infection (Figure 3a). Similarly, the levels of IRE1α, ASK‐1, and JNK were increased at 0.5–1 hr in bone marrow‐derived macrophages (BMDMs) during M. avium infection (Figure 3b). M. avium‐mediated production levels of IRE1α, CHOP, and caspase‐3 activation were decreased in irestatin‐ (an inhibitor of IRE1α) pretreated RAW 264.7 cells or BMDMs (Figure 3c–e). The number of apoptotic cells was significantly decreased by irestatin (Figure 3f). These data indicate that IRE1α pathway plays an important role in M. avium‐induced apoptosis.
Figure 3.

Inhibition of IRE1α reduces Mycobacterium avium‐mediated apoptosis of RAW 264.7 cells. (a) RAW 264.7 cells or (b) bone marrow‐derived macrophages (BMDMs) were infected with M. avium for 0–6 hr; and the inositol‐requiring enzyme 1 IRE1α, apoptosis signal‐regulating kinase 1 (ASK‐1), and (ASK1)/phospho‐c‐Jun N‐terminal kinase (JNK; p‐JNK) levels were determined by Western blotting. (c) RAW 264.7 cells or (d) BMDMs were pretreated with irestatin (10 mM) and infected with M. avium. (e) Cell lysates were subjected to Western blotting to determine the levels of C/EBP homologous protein (CHOP), cleaved caspase‐3, and β‐actin. (f) Apoptosis of irestatin‐pretreated cells following infection with M. avium for 24 hr. Data are representative of at least three independent experiments (*p < .05, **p < .01, and ***p < .001)
The IRE1α RNase domain activates the RIDD pathway during ER stress (Hollien et al., 2009). To assess the role of the RIDD pathway in M. avium‐infected macrophages, we analysed the RIDD target genes expression (Bloc1s1 and St3gal5). The expression of St3gal5 was decreased at 12 hr after M. avium infection and that of Bloc1s1 was downregulated in a time‐dependent manner (Figure 4a). In BMDMs, mRNA expression levels of St3gal5 and Bloc1s1 were reduced in a time‐dependent manner (Figure 4b). Similarly, expression levels of St3gal5 and Bloc1s1 were reduced at all time points in M. avium‐infected macrophages (Figure 4c). Interestingly, the M. avium‐mediated reduction in the expression of RIDD target genes was restored by 4μ8c (Figure 4d–f). The enhanced levels of CHOP and cleaved caspase‐3 by were markedly decreased in 4μ8c‐pretreated macrophages during M. avium infection (Figure 4g). Furthermore, M. avium‐induced apoptosis was significantly suppressed in 4μ8c‐pretreated macrophages (Figure 4h). These results suggest that RIDD pathway activation plays a key role in M. avium‐mediated apoptosis in macrophages.
Figure 4.
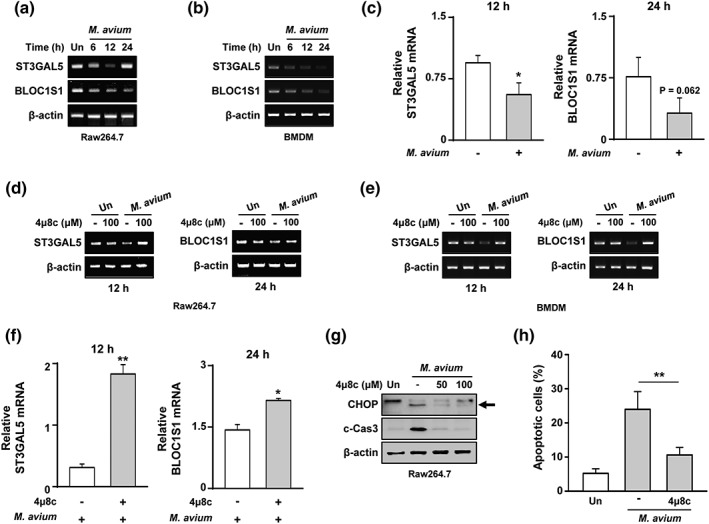
IRE1α‐dependent degradation of genes is involved in Mycobacterium avium‐mediated apoptosis. Polymerase chain reaction (PCR) amplification of Bloc1s1 and St3gal5 from M. avium‐infected (a) RAW 264.7 cells or (c) bone marrow‐derived macrophages (BMDMs). (b) Bloc1s1 and St3gal5 mRNA levels were identified by real‐time PCR. (d) RAW 264.7 cells or (f) BMDMs were pretreated with 4μ8c (100 μM) and infected with M. avium. (e) mRNA levels of St3gal5 and Bloc1s1 by real‐time PCR. (g) RAW 264.7 cells were pretreated with 4μ8c and infected with M. avium for 24 hr. (h) RAW 264.7 cells were incubated with 4μ8c prior to infection with M. avium. Data are representative of three independent experiments (*p < .05, **p < .01, and ***p < .001)
2.3. ROS degrade RIDD target genes through IRE1α pathway in M. avium‐infected macrophages
ROS are important to induce ER stress‐mediated apoptosis during mycobacterial infection (J. A. Choi et al., 2013). Initially, we assessed ROS production in M. avium‐infected macrophages. ROS levels peaked at 5 min (47.4%) after infection (Figure 5a). The enhanced levels of ROS were dramatically decreased by 39.4% by N‐acetyl cysteine (NAC; a scavenger of ROS; Figure 5b). NAC pretreatment reduced the levels of Bip, p‐eIF2α, CHOP, and cleaved caspase‐3 in RAW 264.7 cells (Figure 5c). In addition, activation of IRE1α was reduced in NAC‐pretreated macrophages during M. avium infection (Figure 5d,e). We next assessed how ROS affects RIDD pathway upon infection with M. avium. The macrophages were pretreated with NAC before M. avium infection and Bloc1s1 and St3gal5 expression was assayed. The expression levels of Bloc1s1 and St3gal5 in M. avium‐infected macrophages were significantly increased by NAC pretreatment (Figure 5f,g). To verify our standard polymerase chain reaction (PCR) results, we analysed the mRNA of Bloc1s1 and St3gal5 by real‐time PCR. Similarly, the expression levels of Bloc1s1 and St3gal5 mRNA were significantly increased in NAC‐pretreated RAW 264.7 cells during M. avium infection (Figure 5h). Interestingly, M. avium‐induced apoptosis was decreased in NAC‐pretreated macrophages (Figure 5i). These data indicate that ROS are important for RIDD pathway‐mediated apoptosis in M. avium‐infected macrophages.
Figure 5.
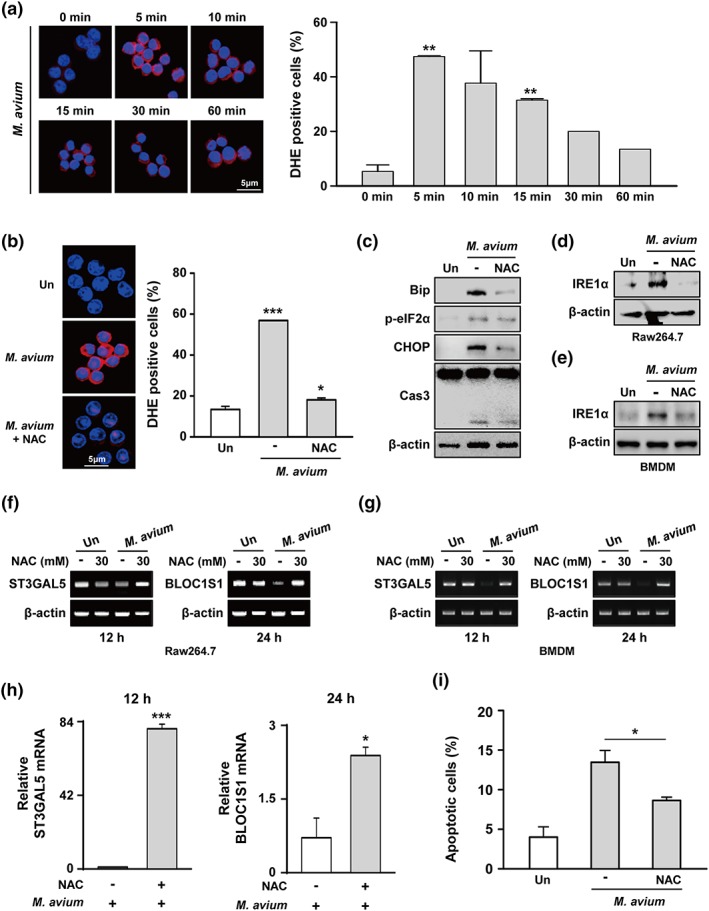
Mycobacterium avium‐induced reactive oxygen species (ROS) activates degradation of regulated IRE1‐dependent decay (RIDD) target genes via the endoplasmic reticulum (ER) stress‐IRE1α pathway. (a) RAW 264.7 cells were infected with M. avium at a moi of 5 for 0–60 min. Dihydroethidium (2 μM) assay of the intracellular ROS level. (b) RAW 264.7 cells were pretreated with N‐acetyl cysteine (NAC; 30 mM) and infected with M. avium for 5 min. (c) RAW 264.7 cells were pretreated with NAC and infected with M. avium for 24 hr. (d) RAW 264.7 cells or (e) bone marrow‐derived macrophages (BMDMs) were pretreated with NAC and infected with M. avium. IRE1α was determined by Western blotting. Bloc1s1 and St3gal5 mRNA levels were detected in NAC‐pretreated (f) RAW 264.7 cells or (g) BMDMs by PCR. (h) mRNA levels of RIDD target genes in NAC‐pretreated RAW 264.7 cells by real‐time PCR. (i) Apoptosis of NAC‐pretreated cells following infection with M. avium for 24 hr. Data are representative of at least three independent experiments (*p < .05, **p < .01, and ***p < .001)
2.4. ER stress‐mediated apoptosis suppresses the intracellular survival of M. avium
To analyse the involvement of the RIDD pathway in intracellular survival of M. avium, we pretreated cells with irestatin and 4μ8c before infection with M. avium. As expected, intracellular survival of M. avium was significantly enhanced in irestatin‐pretreated macrophages (Figure 6a). Similarly, viable count of M. avium was significantly increased in 4μ8c‐ and NAC‐pretreated macrophages (Figure 6b,c). The intracellular survival of M. avium was also induced in BMDMs pretreated with irestatin, 4μ8c, and NAC (Figure 6d–f). These data suggest that M. avium‐induced ROS activate the RIDD pathway, which influences the intracellular survival of M. avium.
Figure 6.
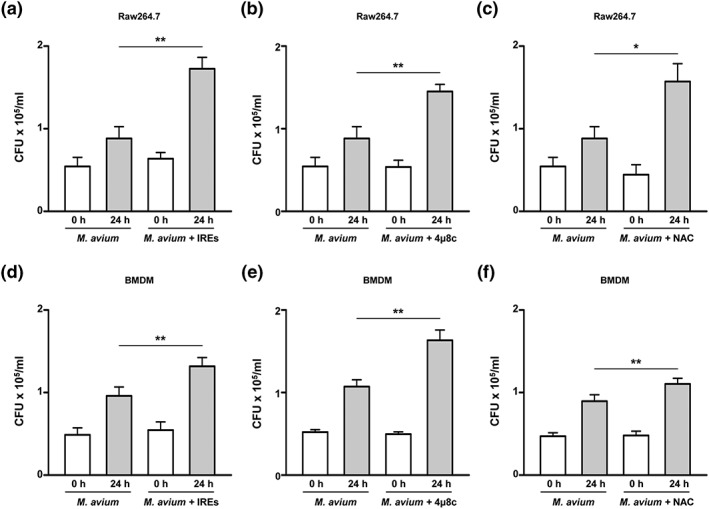
The endoplasmic reticulum (ER) stress response regulates the intracellular survival of Mycobacterium avium. Surviving intracellular M. avium were counted at 24 hr after infection. (a–c) RAW 264.7 cells or (d–f) BMDMs were pretreated with irestatin, NAC, or 4μ8c and infected with M. avium for 24 hr. Data are representative of at least three independent experiments (*p < .05, **p < .01, and ***p < .001)
3. DISCUSSION
Apoptosis regulates bacterial replication and growth (Behar et al., 2011; Faherty & Maurelli, 2008). In this study, ER stress‐mediated apoptosis inhibited intracellular growth of M. avium in macrophages. ER stress‐induced apoptosis modulates the survival of intracellular M. bovis (Cui et al., 2016) and suppresses the intracellular survival of mycobacteria in macrophages (H.‐H. Choi et al., 2010; Y.‐J. Lim et al., 2013; Y.‐J. Lim et al., 2011; Seimon et al., 2010). Our results suggest that ER stress plays a key role in suppression of M. avium through apoptosis induction.
In the present study, we showed that IRE1α‐RIDD pathway activation suppressed the growth of M. avium in macrophages. Under ER stress, IRE1α is involved in virus‐mediated apoptosis (Huang et al., 2016). The IRE1α activates the nuclear factor‐kappa B signalling pathway (Lencer, DeLuca, Grey, & Cho, 2015) and the expression of proinflammatory cytokines in immune cells (Junjappa, Patil, Bhattarai, Kim, & Chae, 2018). A previous study has demonstrated that the RIDD pathway promotes inflammation and apoptosis via the NLRP3 inflammasome (Lerner et al., 2012). A recent report suggests that activation of the IRE1 pathway plays an important role in removing Brucella abortus by inducing a proinflammatory response in macrophages (Grohmann, Christie, Waksman, & Backert, 2018). These findings provide clues to support our results why IRE1α‐RIDD pathway activation is involved in suppression of M. avium growth in macrophages. Furthermore, a previous report suggests that IRE1α‐RIDD pathway activation attenuates viral protein synthesis (Hollien et al., 2009). Our current results demonstrated that degradation of the mRNAs of RIDD target genes, including Bloc1s1 and St3gal5 in macrophages, is associated with M. avium‐mediated apoptosis. Degradation of Bloc1s1 induces apoptosis by activating PARP in Saccharomyces cerevisiae (Tam, Koong, & Niwa, 2014). St3gal5 is involved in cell differentiation and proliferation (Ishii et al., 1998). Thus, we propose that suppression of Bloc1s1 and St3gal5 modulates the intracellular survival of M. avium through induction of apoptosis.
ROS production is important for removing intracellular Mtb (Ehrt & Schnappinger, 2009; Y.‐J. Lim et al., 2011). In previous studies, we found that the ROS‐mediated ER stress response suppresses the growth of Mtb (J. A. Choi et al., 2013; Y. J. Lim et al., 2015). Here, we suggest that ROS production is important to induce IRE1α‐RIDD pathway activation in M. avium‐infected macrophages.
It remains to be identified which RIDD target genes are involved in suppression of M. avium growth. In summary, changes in the expression levels of Bloc1s1 and St3gal5 modulate the survival of intracellular M. avium in macrophages. Therefore, regulation of RIDD pathway offers a potential therapeutic regimen for suppression of intracellular M. avium in macrophages.
4. EXPERIMENTAL PROCEDURES
4.1. Cell culture and bacterial infection
RAW 264.7 (ATCC TIB‐71) and primary BMDMs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 100 IU mL−1 of penicillin, and 100 μg mL−1 of streptomycin (Welgene, Gyeongsan, South Korea). BMDMs were differentiated by growth for 5 days in medium containing macrophage colony‐stimulating factor (R&D Systems, Minneapolis, MN, USA). M. avium (ATCC 25291) was cultured in 7H9 Middlebrook medium supplemented with 10% OADC and 5% glycerol. M. avium was resuspended in PBS at 1 × 108/mL and stored at −80°C until use. For infection, bacteria were added to macrophages at required moi. RAW 264.7 cells and BMDMs, cultured in antibiotic‐free Dulbecco's modified Eagle's medium containing 2% FBS, were infected with M. avium (ATCC 25291) at a moi of 1, 5, and 10 for 3 hr and washed with fresh medium containing 2% FBS. RAW 264.7 cells and BMDMs were infected as described previously (Y.‐J. Lim et al., 2011). After infection with M. avium, the cells were washed with PBS to remove extracellular bacteria and lysed in sterile water at specified time points and appropriate serial dilutions seeded in Middlebrook 7H10 agar plates. Colony counts were performed based on triplicate plating.
4.2. Western blotting
After the indicated incubation period post‐infection, cells were lysed in radio‐immunoprecipitation assay buffer (ELPIS Biotech, Daejeon, South Korea) containing protease inhibitor cocktail. Western blot analysis was performed as previously described (Oh et al., 2018).
4.3. Reagents and antibodies
Irestatin (inhibitor of the endonuclease IRE1) was purchased from Axon Medchem (Groningen, The Netherlands). The inhibitor of IRE1 RNase activity, 4μ8c, was purchased from Millipore. NAC was purchased from Sigma‐Aldrich (St. Louis, MO, USA). Lipopolysaccharide was purchased from Invitrogen (Carlsbad, CA, USA). The following primary antibodies were used for Western blotting: anti‐GRP78/Bip, anti‐phospho‐eIF2α, anti‐CHOP, anti‐caspase‐3, anti‐PERK, and anti‐phospho‐JNK (p‐JNK; Cell Signaling, Danvers, MA, USA) and anti‐IRE1α, anti‐β‐actin, anti‐ATF6, and anti‐ASK1 (Santa Cruz Biotechnology, Dallas, TX, USA).
4.4. Reverse transcription quantitative real‐time PCR
Total RNA was isolated from RAW 264.7 cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions, and cDNA was prepared by reverse transcription using a Reverse Transcription Kit (ELPIS Biotech). CHOP, XBP‐1, St3gal5, Bloc1s1, and β‐actin cDNAs were generated using Prime Taq Premix (Genetbio, Nonsan, South Korea). PCR products were separated in a 1.5% agarose gel and subjected to real‐time PCR using SYBR Green Master Mix and a StepOnePlus™ Real‐Time PCR System with StepOne™ software (Applied Biosystems, Foster City, CA, USA). Data were analysed using Flow Jo software (Tree Star, Ashland, OR, USA).
4.5. ROS production assay
ROS production was assessed by dihydroethidium for 30 min at 37°C in darkness. After being washed with PBS, the cells were fixed with 4% paraformaldehyde for 20 min. Positive cells were analysed immediately for superoxide levels by confocal microscopy.
4.6. Apoptosis assay
The cells from each treatment condition were washed once with PBS, resuspended in Annexin‐binding buffer, and stained with FITC‐conjugated Annexin V and propidium iodide as per the manufacturer's instructions (BD Biosciences, Franklin Lakes, NJ, USA). After being stained, cells were washed with 300 μl of Annexin‐binding buffer and resuspended in 1% paraformaldehyde. The stained cells were analysed by flow cytometry on a FACS Canto II instrument (BD Biosciences) with Flow Jo software (Tree Star).
4.7. Statistical analysis
All experimental results were statistically evaluated using Student's t test or one‐way analysis of variance followed by Bonferroni's multiple comparison tests. Experiments were repeated at least three times. Differences were considered statistically significant when p values were <.05, and a difference with p < .001 was considered highly significant. Data are means ± standard deviation.
Contributions
D. G. and J. L. designed the study, performed the majority of the experiments, analysed the data, and wrote the manuscript. J. A. C., S. N. C., S. H. K., and S. H. S. performed the experiments and analysed data. C. H. S. designed the study, supervised the project, and wrote the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
STATEMENT OF ETHICS
The authors have no ethical conflicts to disclose.
FUNDING
This work was supported by the research fund of Chungnam National University and the Brain Korea 21 PLUS Project for Medical Science, Chungnam National University. The funders had no role in study design, data collection and analysis decision to publish, or preparation of the manuscript.
Go D, Lee J, Choi J‐A, et al. Reactive oxygen species‐mediated endoplasmic reticulum stress response induces apoptosis of Mycobacterium avium‐infected macrophages by activating regulated IRE1‐dependent decay pathway. Cellular Microbiology. 2019;21:e13094 10.1111/cmi.13094
Dam Go and Junghwan Lee contributed equally to this work.
REFERENCES
- Arend, S. M. , van Soolingen, D. , & Ottenhoff, T. H. (2009). Diagnosis and treatment of lung infection with nontuberculous mycobacteria. Current opinion in pulmonary medicine, 15(3), 201–208. 10.1097/MCP.0b013e3283292679 [DOI] [PubMed] [Google Scholar]
- Behar, S. , Martin, C. , Booty, M. , Nishimura, T. , Zhao, X. , Gan, H. X. , … Remold, H. G. (2011). Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis . Mucosal immunology, 4(3), 279–287. 10.1038/mi.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, A. , Pathak, S. , Basak, C. , Law, S. , Kundu, M. , & Basu, J. (2003). Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal‐regulating kinase 1/p38 mitogen‐activated protein kinase signaling and caspase 8 activation. Journal of Biological Chemistry, 278(29), 26517–26525. 10.1074/jbc.M300852200 [DOI] [PubMed] [Google Scholar]
- Choi, H.‐H. , Shin, D.‐M. , Kang, G. , Kim, K.‐H. , Park, J. B. , Hur, G. M. , … Song, C. H. (2010). Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT‐6‐mediated apoptosis. FEBS letters, 584(11), 2445–2454. 10.1016/j.febslet.2010.04.050 [DOI] [PubMed] [Google Scholar]
- Choi, J. A. , Lim, Y. J. , Cho, S. N. , Lee, J. H. , Jeong, J. A. , Kim, E. J. , … Song, C. H. (2013). Mycobacterial HBHA induces endoplasmic reticulum stress‐mediated apoptosis through the generation of reactive oxygen species and cytosolic Ca2+ in murine macrophage RAW 264.7 cells. Cell Death Dis, 4, e957 10.1038/cddis.2013.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Zhao, D. , Sreevatsan, S. , Liu, C. , Yang, W. , Song, Z. , … Zhou, X. (2016). Mycobacterium bovis induces endoplasmic reticulum stress mediated‐apoptosis by activating IRF3 in a murine macrophage cell line. Frontiers in cellular and infection microbiology, 6 10.3389/fcimb.2016.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt, S. , & Schnappinger, D. (2009). Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol, 11(8), 1170–1178. 10.1111/j.1462-5822.2009.01335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty, C. S. , & Maurelli, A. T. (2008). Staying alive: Bacterial inhibition of apoptosis during infection. Trends Microbiol, 16(4), 173–180. 10.1016/j.tim.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann, E. , Christie, P. J. , Waksman, G. , & Backert, S. (2018). Type IV secretion in Gram‐negative and Gram‐positive bacteria. Molecular microbiology, 107(4), 455–471. 10.1111/mmi.13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D. , Lerner, A. G. , Vande Walle, L. , Upton, J. P. , Xu, W. , Hagen, A. , … Papa, F. R. (2009). IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell, 138(3), 562–575. 10.1016/j.cell.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien, J. , Lin, J. H. , Li, H. , Stevens, N. , Walter, P. , & Weissman, J. S. (2009). Regulated Ire1‐dependent decay of messenger RNAs in mammalian cells. The Journal of cell biology, 186(3), 323–331. 10.1083/jcb.200903014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Xu, A. , Wu, X. , Zhang, Y. , Guo, Y. , Guo, F. , … Kong, L. (2016). Japanese encephalitis virus induces apoptosis by the IRE1/JNK pathway of ER stress response in BHK‐21 cells. Archives of virology, 161(3), 699–703. 10.1007/s00705-015-2715-5 [DOI] [PubMed] [Google Scholar]
- Ishii, A. , Ohta, M. , Watanabe, Y. , Matsuda, K. , Ishiyama, K. , Sakoe, K. , … Saito, M. (1998). Expression cloning and functional characterization of human cDNA for ganglioside GM3 synthase. Journal of Biological Chemistry, 273(48), 31652–31655. 10.1074/jbc.273.48.31652 [DOI] [PubMed] [Google Scholar]
- Junjappa, R. P. , Patil, P. , Bhattarai, K. R. , Kim, H.‐R. , & Chae, H.‐J. (2018). IRE1α implications in endoplasmic reticulum stress‐mediated development and pathogenesis of autoimmune diseases. Frontiers in immunology, 9, 1289–1289. 10.3389/fimmu.2018.01289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange, P. , Wargnier, A. , & Herrmann, J. (2000). Mycobacteriosis in the compromised host. Memorias do Instituto Oswaldo Cruz, 95, 163–170. 10.1590/S0074-02762000000700027 [DOI] [PubMed] [Google Scholar]
- Lee, K.‐I. , Whang, J. , Choi, H.‐G. , Son, Y.‐J. , Jeon, H. S. , Back, Y. W. , … Kim, H. J. (2016). Mycobacterium avium MAV2054 protein induces macrophage apoptosis by targeting mitochondria and reduces intracellular bacterial growth. Scientific reports, 6 10.1038/srep37804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer, W. I. , DeLuca, H. , Grey, M. J. , & Cho, J. A. (2015). Innate immunity at mucosal surfaces: The IRE1‐RIDD‐RIG‐I pathway. Trends Immunol, 36(7), 401–409. 10.1016/j.it.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner, A. G. , Upton, J. P. , Praveen, P. V. , Ghosh, R. , Nakagawa, Y. , Igbaria, A. , … Papa, F. R. (2012). IRE1alpha induces thioredoxin‐interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab, 16(2), 250–264. 10.1016/j.cmet.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y.‐J. , Choi, H.‐H. , Choi, J.‐A. , Jeong, J. A. , Cho, S.‐N. , Lee, J.‐H. , … Song, C.‐H. (2013). Mycobacterium kansasii‐induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis, 18(2), 150–159. 10.1007/s10495-012-0792-4 [DOI] [PubMed] [Google Scholar]
- Lim, Y.‐J. , Choi, J.‐A. , Choi, H.‐H. , Cho, S.‐N. , Kim, H.‐J. , Jo, E.‐K. , … Song, C.‐H. (2011). Endoplasmic reticulum stress pathway‐mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis . PloS one, 6(12), e28531 10.1371/journal.pone.0028531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y. J. , Choi, J. A. , Lee, J. H. , Choi, C. H. , Kim, H. J. , & Song, C. H. (2015). Mycobacterium tuberculosis 38‐kDa antigen induces endoplasmic reticulum stress‐mediated apoptosis via toll‐like receptor 2/4. Apoptosis, 20(3), 358–370. 10.1007/s10495-014-1080-2 [DOI] [PubMed] [Google Scholar]
- McGrath, E. E. , Blades, Z. , McCabe, J. , Jarry, H. , & Anderson, P. B. (2010). Nontuberculous mycobacteria and the lung: From suspicion to treatment. Lung, 188(4), 269–282. 10.1007/s00408-010-9240-9 [DOI] [PubMed] [Google Scholar]
- Nussbaum, J. M. , & Heseltine, P. (1990). Fatal pulmonary infection with Mycobacterium fortuitum . Western Journal of Medicine, 152(4), 423. [PMC free article] [PubMed] [Google Scholar]
- Oh, S. M. , Lim, Y. J. , Choi, J. A. , Lee, J. , Cho, S. N. , Go, D. , … Song, C. H. (2018). TNF‐alpha‐mediated ER stress causes elimination of Mycobacterium fortuitum reservoirs by macrophage apoptosis. The FASEB Journal, 32(7), 3993–4003. Faseb j, fj201701407R. 10.1096/fj.201701407R [DOI] [PubMed] [Google Scholar]
- Oyadomari, S. , Araki, E. , & Mori, M. (2002). Endoplasmic reticulum stress‐mediated apoptosis in pancreatic β‐cells. Apoptosis, 7(4), 335–345. 10.1023/A:1016175429877 [DOI] [PubMed] [Google Scholar]
- Parandhaman, D. K. , & Narayanan, S. (2014). Cell death paradigms in the pathogenesis of Mycobacterium tuberculosis infection. Frontiers in cellular and infection microbiology, 4 10.3389/fcimb.2014.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheva, V. I. , & Domingos, P. M. (2009). Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis, 14(8), 996–1007. 10.1007/s10495-009-0341-y [DOI] [PubMed] [Google Scholar]
- Seimon, T. A. , Kim, M.‐J. , Blumenthal, A. , Koo, J. , Ehrt, S. , Wainwright, H. , … Russell, D. G. (2010). Induction of ER stress in macrophages of tuberculosis granulomas. PloS one, 5(9), e12772 10.1371/journal.pone.0012772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C.‐H. (2012). Endoplasmic reticulum stress responses and apoptosis. Journal of Bacteriology and Virology, 42(3), 196–202. 10.4167/jbv.2012.42.3.196 [DOI] [Google Scholar]
- Szegezdi, E. , Logue, S. E. , Gorman, A. M. , & Samali, A. (2006). Mediators of endoplasmic reticulum stress‐induced apoptosis. EMBO reports, 7(9), 880–885. 10.1038/sj.embor.7400779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, A. B. , Koong, A. C. , & Niwa, M. (2014). Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell reports, 9(3), 850–858. 10.1016/j.celrep.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triccas, J. A. , Winter, N. , Roche, P. W. , Gilpin, A. , Kendrick, K. E. , & Britton, W. J. (1998). Molecular and immunological analyses of the Mycobacterium avium homolog of the immunodominant Mycobacterium leprae 35‐kilodalton protein. Infection and immunity, 66(6), 2684–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton, J.‐P. , Wang, L. , Han, D. , Wang, E. S. , Huskey, N. E. , Lim, L. , … Papa, F. R. (2012). IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase‐2. Science, 338(6108), 818–822. 10.1126/science.1226191 [DOI] [PMC free article] [PubMed] [Google Scholar]


